Abstract
We investigated the relationship of discrepancies between VIQ and NVIQ (IQ split) to autism symptoms and adaptive behavior in a sample of high-functioning (mean FSIQ = 98.5) school-age children with autism spectrum disorders divided into three groups: discrepantly high VIQ (n = 18); discrepantly high NVIQ (n = 24); and equivalent VIQ and NVIQ (n = 36). Discrepantly high VIQ and NVIQ were associated with autism social symptoms but not communication symptoms or repetitive behaviors. Higher VIQ and NVIQ were associated with better adaptive communication but not socialization or Daily Living Skills. IQ discrepancy may be an important phenotypic marker in autism. Although better verbal abilities are associated with better functional outcomes in autism, discrepantly high VIQ in high-functioning children may also be associated with social difficulties.
Keywords: Autism, Cognitive profiles, IQ, Symptomatology, Adaptive functioning, Asperger syndrome
Introduction
Identification of specific cognitive profiles that may arise from brain abnormalities and drive behavioral symptoms in autism can illuminate meaningful subtypes in this heterogeneous disorder (Frith 2001; Pennington 2002). Defining specific autism subtypes may lead to identification of distinct genetic etiologies (Gottesman and Gould 2003) and provide new directions for treatment intervention (Fisher and Happé 2005; Riggs et al. 2006). Autism spectrum disorders (ASDs) are associated with a highly variable cognitive profile (e.g., de Bruin et al. 2006; Fein et al. 1986; Ghaziuddin and Mountain-Kimchi 2004; Siegel et al. 1996). Recent work suggests that a discrepancy between verbal and nonverbal intellectual abilities may be a particularly informative phenotype of the disorder. Joseph et al. (2002) found that school-aged ASD children with significantly better (≥9 points) nonverbal IQ (NVIQ) than verbal IQ (VIQ) had more social symptoms, as measured by the Autism Diagnostic Observation Schedule (ADOS), than children with either evenly developing VIQ and NVIQ or better developed VIQ than NVIQ. The authors suggested that the IQ discrepancy might mark a meaningful phenotype within the autism spectrum (Joseph et al. 2002), a claim that was reinforced by two subsequent papers that linked discrepantly higher NVIQ with a larger head circumference in autism (Deutsch and Joseph 2003; Lainhart et al. 2006).
Klin et al. (2007) argue that in addition to social symptoms, or disability, it is important to investigate the adaptive social abilities associated with possible cognitive phenotypes, as they represent fundamental outcome variables that may be distinct from autism symptoms in their response to treatment intervention and in their relationship to other markers of functioning such as IQ. There is ample evidence that adaptive behavior deficits in high-functioning ASDs are greater than would be predicted based on IQ alone. For example, Liss et al. (2001) found that children with high-functioning autism are more impaired in areas of socialization and daily living than IQ matched controls. Klin et al. (2007) reported correlations of .25–.45 (small to medium effect sizes) between both VIQ and NVIQ and overall adaptive functioning in two separate samples, with adaptive communication most strongly related to VIQ. Bolte and Poustka (2002) reported similar findings in a sample of high-functioning children with ASDs. To our knowledge, no group has examined the relationship between VIQ–NVIQ discrepancy and adaptive outcome.
The current investigation seeks to replicate and expand the findings of Joseph et al. (2002) by investigating links between VIQ–NVIQ discrepancy with autism symptoms and adaptive skills among high-functioning school-aged children. In order to achieve these aims, we examine the relationship between VIQ–NVIQ discrepancy and autism symptoms indexed by both the ADOS and the Autism Diagnostic Interview (ADI), and the relationship between VIQ–NVIQ discrepancy and parent-reported adaptive functioning using the Vineland Adaptive Behavior Scales (VABS). Based on previous findings, we predict that autism social symptoms in particular (not communication symptoms or restricted/repetitive behaviors) will be related to discrepancies between VIQ and NVIQ. While we have no hypotheses concerning the relationship between VIQ/NVIQ discrepancies and adaptive behavior, we expect VIQ and NVIQ separately to be positively related to adaptive functioning, as has been previously demonstrated.
Methods
Participants
The study included a clinically referred sample of 78 children (mean age = 9.7 years, range 6–17 years; 82% male) with an autism spectrum diagnosis (high-functioning autism n = 31; Asperger Syndrome n = 29; PDD-NOS n = 18) evaluated by a multidisciplinary team at the Center for Autism Spectrum Disorders at Children’s National Medical Center in the Washington, DC metropolitan area. All participants had NVIQs ≥ 70, and the group’s mean NVIQ was in the average range (M = 102.4, SD = 16.7) as measured by the Wechsler Intelligence Scale for Children—3rd Edition (WISC-III), Wechsler Intelligence Scale for Children—4th Edition (WISC-IV), or the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler 1991,1999,2003).
All participants met criteria for an ASD on the ADI or ADI-R (Le Couteur et al. 1989; Lord et al. 1994) and/or the ADOS (Lord et al. 1999) following the criteria established by the NICHD/NIDCD Collaborative Programs for Excellence in Autism (see Lainhart et al. 2006) as well as by expert clinical impression using the DSM-IV (APA 1994). Participant characteristics for the total sample, as well as the three different IQ split groups (see below) are described in Table 1.
Table 1.
Sample characteristics
| Total sample (n = 78) | VIQ > NVIQ (n = 18) | Even IQ (n = 36) | NVIQ > VIQ (n = 24) | Statistic | p | |
|---|---|---|---|---|---|---|
| Age | 9.7 ± 2.8 | 9.2 ± 2.9 | 10.3 ± 3.1 | 9.3 ± 2.3 | F = 1.3 | .28 |
| Gender (% male) | 82 | 78 | 83 | 83 | χ2 = 0.3 | .87 |
| Hollingshead SES | 18.6 ± 7.7 | 15.5 ± 5.2 | 19.5 ± 8.49 | 19.7 ± 7.5 | F = 1.6 | .22 |
| Verbal IQ | 98.7 ± 23.16 | 117.8 ± 20.5a | 103.1 ± 17.9b | 77.7 ± 14.8c | F = 28.5 | < .001 |
| Performance IQ | 102.4 ± 16.8 | 101.4 ± 17.9 | 105.4 ± 15.9 | 98.7 ± 17.0 | F = 1.2 | .30 |
| Full scale IQ | 98.5 ± 20.8 | 109.5 ± 20.6a | 102.9 ± 17.5a | 83.0 ± 17.5b | F = 12.5 | < .001 |
| IQ split (VIQ–NVIQ) | −0.3 ± 17.5 | 24.9 ± 11.4 | −0.7 ± 6.3 | −18.5 ± 5.1 | – | – |
Within row, different subscripts letters indicate statistically significant differences
Measures
Verbal and Nonverbal Ability Measures
VIQ and NVIQ were assessed using one of three intelligence scales, the WISC-III (n = 27), WISC-IV (n = 35), or the WASI (n = 16). In order to create comparable cross test measures, VIQ was estimated using the Vocabulary and Similarities subtests from each of the three IQ scales and NVIQ was estimated using the Block Design and Matrix Reasoning subtests from the WISC-IV and WASI and the Block Design and Object Assembly subtests from the WISC-III (in which Matrix Reasoning was not included). VIQ and NVIQ are presented in norm-referenced standardized scores (M = 100; SD = 15; Table 1). A significant IQ discrepancy was defined as a difference between calculated VIQ and NVIQ of at least 11 points. This difference was based on the IQ discrepancies that reach statistical significance (p < .05) according to the administration manuals of all three intelligence scales (the actual IQ difference reported in the manuals ranged from 10.73 to 11.80; selecting a cutoff between 10 and 12 did not change the group assignment of any participant). Three groups were created: VIQ > NVIQ (discrepantly high VIQ), NVIQ > VIQ (discrepantly high NVIQ), and Even IQ (equivalent IQ).
Autism Diagnostic Measures
Autism symptoms were assessed using the Autism Diagnostic Interview (ADI/ADI-R; Le Couteur et al. 1989; Lord et al. 1994) and Autism Diagnostic Observation Schedule (ADOS; Lord et al. 1999). The ADI is a detailed parent or caregiver interview of developmental history and autism symptoms. Scores are aggregated into symptom clusters that correspond to DSM-IV criteria for a diagnosis of autism. The ADOS is a structured play and conversational interview that includes a series of social presses and other opportunities to elicit symptoms of an ASD.
Scores from the ADI and ADOS were combined to create composite measures of autism symptoms for each domain (communication, reciprocal social interaction, and restricted and repetitive behaviors) that incorporate both parent report and clinician observation (Kenworthy et al. in press; Lopez et al. 2005). This was done by first converting domain raw scores of the ADI and ADOS into standardized z-scores and then taking the mean of the ADI and ADOS z-scores to create composite scores. This approach provides an index of autism symptoms based on multiple observations (parents, clinician), conserves statistical power, and makes the contribution of ADOS and ADI scores comparable. Unstandardized raw scores for the ADOS and ADI/ADI-R are presented in Table 2.
Table 2.
Autism symptoms and adaptive abilities
| Total sample | VIQ > NVIQ | Even IQ | NVIQ > VIQ | |
|---|---|---|---|---|
| n = 78 | n = 18 | n = 36 | n = 24 | |
| ADI/ADI-R (raw scores) | ||||
| Communication | 16.3 ± 4.2 | 15.2 ± 4.3 | 16.3 ± 4.2 | 17.2 ± 4.1 |
| Social | 19.9 ± 5.4 | 17.9 ± 6.0 | 19.9 ± 5.0 | 21.3 ± 5.2 |
| Repetitive behaviors | 6.9 ± 2.9 | 7.0 ± 2.9 | 6.8 ± 2.9 | 7.0 ± 2.7 |
| n = 78 | n = 18 | n = 36 | n = 24 | |
| ADOS-3 (raw scores) | ||||
| Communication | 4.3 ± 1.9 | 4.2 ± 1.8 | 3.9 ± 1.7 | 4.7 ± 2.2 |
| Social | 8.2 ± 3.2 | 7.7 ± 3.5 | 7.9 ± 3.2 | 9.0 ± 3.0 |
| Repetitive behaviors | 1.9 ± 1.7 | 2.0 ± 1.3 | 1.7 ± 1.7 | 2.0 ± 2.0 |
| n = 55 | n = 15 | n = 23 | n = 17 | |
| VABS (std. scores; M = 100 ± 15) | ||||
| Communication | 81.2 ± 20.5 | 92.3 ± 18.6 | 82.9 ± 18.4 | 69.3 ± 19.6 |
| Socialization | 70.6 ± 13.6 | 73.5 ± 10.8 | 70.4 ± 15.8 | 68.3 ± 12.7 |
| Daily living skills | 75.3 ± 18.6 | 80.1 ± 16.2 | 77.0 ± 18.2 | 68.9 ± 20.2 |
ADI Autism Diagnostic Interview/Revised, ADOS Autism Diagnostic Observation Schedule, VABS Vineland Adaptive Behavior Scales
Adaptive Functioning
The Vineland Adaptive Behavior Scales (VABS; Sparrow et al. 1984; Sparrow et al. 2005) is a standardized, structured parent interview of adaptive behaviors. This measure assesses adaptive functioning across the following domains: Communication Skills, Daily Living Skills, and Socialization Skills. Both the first (n = 43) and second (n = 12) editions of the VABS were used in the study. There were no significant differences for any domain or the composite score between the first and second editions of the VABS for this sample (all ts[54] < 1.3; all ps > .1) and thus Vineland edition was not included in any statistical model. Vineland standard scores (M = 100; SD = 15) are presented in Table 2.
Socioeconomic Status (SES)
Socioeconomic Status was based on the four-factor Hollingshead Index of Social Position (Hollingshead 1975), which utilizes marital status, gender, education, and occupation to compute a household SES score. Lower scores reflect higher SES.
Data Analytic Procedure
Differences between the extreme groups (VIQ > NVIQ; NVIQ > VIQ) in autism symptoms (communication, reciprocal social interaction, repetitive behaviors) and adaptive skills (VABS communication, socialization, Daily Living Skills) were analyzed using independent samples t-tests. To examine whether group differences could be explained exclusively by VIQ or NVIQ and to examine whether results of the extreme group analysis applied to the entire sample, Spearman correlations were calculated between VIQ, NVIQ, and the difference between VIQ and NVIQ (IQ Split = VIQ–NVIQ) and each measure of autism symptoms and adaptive functioning. Spearman correlations were selected as the appropriate statistic because ADOS and ADI data are primarily categorical and using ranks rather than absolute distance between scores reduces the potential influence of extreme scores.
As described in the results section, the three groups had significantly different full-scale IQ (FSIQ) scores. To address concerns that differences in FSIQ between the IQ split groups (VIQ > NVIQ and NVIQ > VIQ; reported below) explains group differences in the primary outcome measures, we re-ran all of the primary analyses with a FSIQ matched sub-sample of subjects from each of the IQ split groups. Because the sample sizes for the extreme groups were much smaller (n = 12 per group), Cohen’s d effect sizes for the follow-up analyses were compared to findings for the overall sample. Only results that were significant in the primary analyses are reported in the follow-up analyses.
Results
Descriptive Statistics
As shown in Table 1, the VIQ > NVIQ, Even IQ, and NVIQ > VIQ groups differed significantly from each other on VIQ and FSIQ scores. The VIQ of the VIQ > NVIQ group was significantly greater than that of the Even IQ group, which was significantly higher than that of the NVIQ > VIQ group. The NVIQ > VIQ group had significantly lower FSIQ than the Even IQ and VIQ > NVIQ groups, which did not differ from each other. There were no group differences on NVIQ or in age, gender ratio, or SES.
Extreme Groups
Autism Symptoms
The VIQ > NVIQ group showed fewer social symptoms than the NVIQ > VIQ group (t[1,40] = 2.45, p = .02). There were no differences in autism communication or repetitive behavior symptoms between groups (ts[1,40] < 1.3, all ps > .2).
Adaptive Functioning
The VIQ > NVIQ group scored better on VABS communication than did the NVIQ > VIQ group (t[1,30] = 3.39, p < .01). No group differences in VABS Daily Living Skills (t[1,30] = 1.72, p = .09) or socialization skills were found (t[1,30] = 1.24, p = .20).
FSIQ Matched Subsample
Results with the FSIQ matched subsample were consistent with our initial findings in that the VIQ > NVIQ group continued to show fewer social symptoms than the NVIQ > VIQ group (Cohen’s d = 0.74 vs. d = 0.60 in the full sample and FSIQ matched subsample, respectively) suggesting that differences in FSIQ did not account for group differences in social symptoms. By contrast, the effect size for differences in VABS communication in the full sample was 1.00 compared to just 0.43 in the FSIQ matched subsample, suggesting that group differences in FSIQ contributed to differences in adaptive communication.
Correlations Using Full Sample
VIQ–NVIQ (IQ Split)
As shown in Table 3, consistent with the extreme group analysis, when examining the full sample, the VIQ–NVIQ discrepancy was associated with fewer autism social symptoms but not communication symptoms or repetitive behaviors. With regard to adaptive functioning, VIQ–NVIQ discrepancy was correlated with stronger adaptive communication skills but not socialization or Daily Living Skills.
Table 3.
Spearman’s rho correlations between IQ profiles and both autism symptoms and adaptive functioning
| VIQ | NVIQ | VIQ–NVIQ | |
|---|---|---|---|
| Autism social symptoms | −.24* | −.02 | −.34** |
| Autism comm. symptoms | −.32** | −.29* | −.20 |
| Autism restrict/repet. beh. | .10 | .12 | −.06 |
| VABS socialization | .17 | .07 | .15 |
| VABS communication | .65** | .37** | .43** |
| VABS daily living skills | .20 | −.01 | .24 |
n = 78 for autism symptoms; n = 55 for adaptive functioning
p < .05;
p < .01
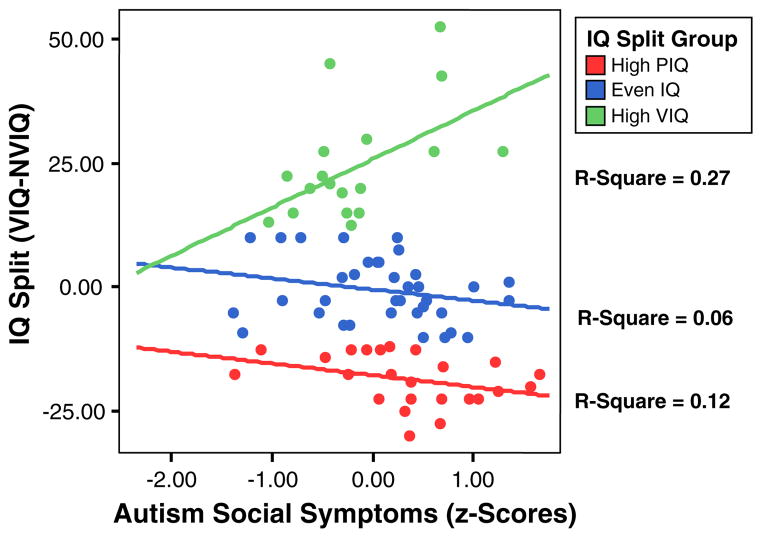
A scatterplot was used to further examine the relationship between IQ split and autism social symptoms by calculating separate regression lines for each IQ split group. Figure 1 shows the Even IQ and NVIQ > VIQ groups had similar regression lines indicating that discrepantly higher NVIQ was associated with more autism social symptoms. By contrast, the regression line for the VIQ > NVIQ group was in the opposite direction indicating that discrepantly higher VIQ was associated with more social symptoms. Thus, these data suggest that as the discrepancy between VIQ and NVIQ increases in either direction, autism social symptoms also increase.
Fig. 1.
Scatterplot showing autism social symptoms and IQ split (VIQ–NVIQ) scores
A follow-up analysis examining whether the correlation between IQ split and autism social symptoms was significantly different by group was conducted. Correlations were converted to z-scores using Fisher’s z-transformation for correlations and differences in z-scores were tested. Results indicated the correlation between IQ split and autism social symptoms for the VIQ > NVIQ group was significantly different from the Even IQ and NVIQ > VIQ groups (all ps < .01), which did not differ from each other (p = .69; VIQ > NVIQ r = −.52, Even IQ r = .25, NVIQ > VIQ r = .35).
VIQ and NVIQ
Stronger VIQ and stronger NVIQ scores each were associated with fewer autism communication symptoms and stronger VIQ was associated with fewer social symptoms. NVIQ was not associated with social symptoms and neither IQ measure was associated with repetitive behaviors. Stronger VIQ and NVIQ scores each were associated with better adaptive communication skills. Neither IQ measure was significantly correlated with adaptive socialization or Daily Living Skills.
FSIQ Matched Subsample
When the correlations were recalculated for the FSIQ matched subsample, the correlation between VIQ–NVIQ discrepancy and autism social symptoms was similar to the correlation for the full sample (ρ = −.28 vs. ρ = −.34, respectively). By contrast, the correlation between VIQ and social symptoms was much smaller in the FSIQ matched subsample compared to the full sample (ρ = −.09 vs. ρ = −.24, respectively). This pattern corroborates findings above suggesting that the discrepancy between VIQ and NVIQ is related to autism social symptoms independent of level of FSIQ.
The correlation between VIQ–NVIQ discrepancy and adaptive communication was much lower in the FSIQ matched subsample compared to the full sample (ρ = .20 vs. ρ = .43, respectively) whereas the correlations of VIQ and NVIQ separately with adaptive communication were similar in the two samples (VIQ and adaptive communication ρ = .55 vs. ρ = .65; NVIQ and adaptive communication ρ = .27 vs. ρ = .37, respectively). These results suggest that the relationship between IQ split and adaptive communication may be explained by FSIQ.
Discussion
We investigated the relationship of discrepancies between VIQ and NVIQ (IQ split) to autism symptoms and adaptive behavior in a sample of high-functioning school-age children with ASDs. We used two methods: comparison of extreme groups of children with VIQ > NVIQ and NVIQ > VIQ; and using the full sample, examination of correlations of the IQ indices (VIQ, NVIQ, and VIQ–NVIQ discrepancy) with autism symptoms and adaptive behavior. Using both methods, we find that discrepantly high NVIQ is associated with greater social symptoms in ASD. This result replicates and extends the findings of Joseph et al. (2002) to a higher IQ sample of children with ASDs (mean FSIQ = 98.5 in the current study compared to 84.5 in the Joseph et al. school-age sample).
Our findings potentially qualify Joseph et al.’s (2002) original findings that discrepantly higher NVIQ was associated with more autism social symptoms. We found the same pattern in our overall findings, but when using a scatterplot to examine the relationship between VIQ–NVIQ discrepancy and autism social symptoms within each of the IQ discrepancy groups, we found that the discrepancy between VIQ and NVIQ in either direction (favoring either VIQ or NVIQ) was associated with more autism social symptoms. These findings suggest that although strong verbal abilities are often associated with better overall functioning in autism (particularly for autism communication symptoms), as VIQ becomes increasingly discrepant from NVIQ it is also associated with considerable social difficulties as well.
Our group analysis was complicated by the fact that FSIQ, driven by VIQ, was significantly higher in the VIQ > NVIQ group than in the NVIQ > VIQ group. To address differences in FSIQ as a possible explanation for our primary findings, we re-ran our primary analyses in a subsample with all groups matched on FSIQ. These findings were comparable to our original findings and suggest that group differences in social symptoms were not due to differences in FSIQ. In addition, it was the split between VIQ and NVIQ that was related to social symptoms in the correlations within the full sample, underscoring the importance of the discrepancy in IQ scores to social symptoms in this sample. That IQ split was not correlated with autism communication symptoms, while both VIQ alone and NVIQ alone were, suggests that VIQ–NVIQ discrepancy is uniquely related to autism social symptoms.
Similar to Joseph et al.’s (2002) findings but in contrast to Klin et al.’s (2007) more recent report on two separate samples, we find that higher VIQ and NVIQ were associated with less severe communication symptoms. Reasons for discrepant findings among these four samples are not immediately clear. Both Klin et al.’s samples have IQ ranges similar to the current study whereas mean IQ in Joseph et al.’s school-age sample is about one standard deviation lower. In comparing symptom severity (reflected in the ADOS scores), Joseph et al.’s sample may have somewhat more symptoms while the study presented here was more similar to Klin et al.’s samples. The lack of consensus among these studies concerning the relationship between IQ and autism communication symptoms requires additional research, perhaps with a longitudinal design to more definitively address this question.
Our investigation of social, communication, and daily living abilities, as measured by the Vineland Adaptive Behavior scales (VABS), reveals a different pattern. IQ split was not related to socialization skills, nor were the extreme IQ split groups different in their social abilities. Consistent with previous reports (Bolte and Poustka 2002; Klin et al. 2007), VIQ and NVIQ were strongly correlated with adaptive communication abilities. Taken together, these findings suggest that both VIQ and NVIQ are related to adaptive communication, arguing that communication skills are related to overall cognitive ability among children with ASDs and average range IQ.
There are a number of limitations to the current study. Specifically, differences in FSIQ across the three IQ split groups qualifies interpretation of our primary finding that as the discrepancy between VIQ and NVIQ increases autism social symptoms increase. We have tried to address this by re-analyzing our data using a subset of subjects from each group that is matched on FSIQ. Because this was a smaller sample, limiting statistical power, we compared the effect size of these results to the original findings as described above. Second, this is a cross-sectional study, which limits any speculation about causal mechanisms. Rather, we can only conclude that among children with ASDs, those with a significant discrepancy between VIQ and NVIQ are also likely to have more social symptoms. Future studies using prospective longitudinal designs quantitatively assessing autism symptoms and neurocognitive profiles over time are needed to address questions concerning causality. Finally, our reliance on a clinical sample for this investigation introduced three different versions of the Wechsler Intelligence Scales into the data set, each with somewhat different subtests. As a result, we relied on two subtests to determine VIQ and NVIQ, respectively. Although these abbreviated scores are highly correlated with more comprehensive Wechsler IQ scores (verbal measures correlate with VCI/VIQ > .7 and non-verbal measures correlate with PRI/PIQ > .56 across Wechsler scales [Wechsler 1991, 1999, 2003]), future investigations using one comprehensive IQ measure may be useful.
These findings have several implications. They support earlier investigations proposing IQ split as a potentially informative marker for subgroups in ASD. The findings also raise the importance of scrutinizing social skills in children with ASD and high VIQ who, due to large vocabularies and fluent speech, may appear to be more competent in social settings than is actually the case. Finally, these data support Klin et al.’s (2007) argument that social ability and disability are independent constructs and should both be studied in ASD.
Acknowledgments
The authors thank Jennifer A. Silvers, Laura Case, and Anne Della Rosa for their work compiling data and the many children with ASD and their families who made this research possible. The authors also thank Alex Martin for his helpful comments on an earlier draft of this paper. This research was supported in part by the Intramural Program of the NIH, National Institutes of Mental Health. This work was supported by the Studies for the Advancement of Autism Research and Treatment (STAART: NIMH U54 MH066417) for supporting data collection.
Contributor Information
David O. Black, Email: blackdavid@mail.nih.gov, Pediatric Developmental Neuroscience Branch, National Institute of Mental Health, 10 Center Drive, MSC 1255 Building 10, Room 4N208, Bethesda, MD 20892-1255, USA
Gregory L. Wallace, Laboratory of Brain & Cognition, National Institute of Mental Health, Bethesda, MD, USA
Jennifer L. Sokoloff, Center for Autism Spectrum Disorders, Children’s National Medical Center, Rockville, MD, USA
Lauren Kenworthy, Center for Autism Spectrum Disorders, Children’s National Medical Center, Rockville, MD, USA.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Bolte S, Poustka F. The relation between general cognitive level and adaptive behavior domains in individuals with autism with and without comorbid mental retardation. Child Psychiatry and Human Development. 2002;33:165–172. doi: 10.1023/a:1020734325815. [DOI] [PubMed] [Google Scholar]
- De Bruin EI, Verheij F, Ferdinand RF. WISC-R subtest but no overall VIQ–PIQ difference in Dutch children with PDD-NOS. Journal of Abnormal Child Psychology. 2006;34:263–271. doi: 10.1007/s10802-005-9018-3. [DOI] [PubMed] [Google Scholar]
- Deutsch DK, Joseph RM. Brief Report: Cognitive correlates of enlarged head circumference in children with autism. Journal of Autism and Developmental Disorders. 2003;3:209–214. doi: 10.1023/a:1022903913547. [DOI] [PubMed] [Google Scholar]
- Fein D, Pennington B, Markowitz P, Braverman M, Water-house L. Toward a neuropsychological model of infantile autism: Are the social deficits primary? Journal of the American Academy of Child and Adolescent Psychiatry. 1986;25:198–212. doi: 10.1016/s0002-7138(09)60227-2. [DOI] [PubMed] [Google Scholar]
- Fisher N, Happé F. A training study of theory of mind and executive function in children with autistic spectrum disorders. Journal of Autism and Developmental Disorders. 2005;35:757–771. doi: 10.1007/s10803-005-0022-9. [DOI] [PubMed] [Google Scholar]
- Frith U. What framework should we use for understanding developmental disorders? Developmental Neuropsychology. 2001;20:555–563. doi: 10.1207/S15326942DN2002_6. [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Mountain-Kimchi K. Defining the intellectual profile of Asperger syndrome: Comparison with high-functioning autism. Journal of Autism and Developmental Disorders. 2004;34:279–284. doi: 10.1023/b:jadd.0000029550.19098.77. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven: Yale University; 1975. [Google Scholar]
- Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2002;46:807–821. doi: 10.1111/1469-7610.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Black DO, Harrison B, Della-Rosa A, Wallace GL. Are executive control functions related to autism symptoms in high-functioning children? Child Neuropsychology. doi: 10.1080/09297040802646983. in press. [DOI] [PubMed] [Google Scholar]
- Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, Lord C. Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: The Vineland and the ADOS. Journal of Autism and Developmental Disorders. 2007;37:748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, et al. Head circumference and height in autism: A study by the Collaborative Program of Excellence in Autism. American Journal of Medical Genetics. 2006;140:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, et al. Autism diagnostic interview: A standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Liss M, Fein D, Allen D, Dunn M, Feinstein C, Morris R, et al. Executive functioning in high-functioning children with autism. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42:261–270. [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism diagnostic observation schedule. Los Angeles, CA: Western Psychological Services; 1999. WPS edition. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Pennington BF. The development of psychopathology: Nature and nurture. New York: Guilford Press; 2002. [Google Scholar]
- Riggs NR, Jahromi LB, Razza RP, Dillworth-Bart JE, Mueller U. Executive function and the promotion of social-emotional competence. Journal of Applied Developmental Psychology. 2006;27:300–309. [Google Scholar]
- Siegel DJ, Minshew NJ, Goldstein G. Wechsler IQ profiles in diagnosis of high-functioning autism. Journal of Autism and Developmental Disorders. 1996;26:389–406. doi: 10.1007/BF02172825. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. Vineland adaptive behavior scales (Interview edition, survey form) Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Sparrow S, Cicchetti D, Balla D. Vineland adaptive behavior scales (Survey Interview Form) 2. Minneapolis, MN: NCS Pearson, Inc; 2005. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children-third edition (WISC-III) San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Intelligence Scale (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children-fourth edition (WISC-IV) San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]