Abstract
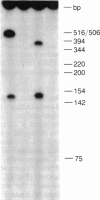
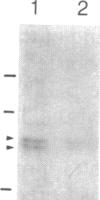
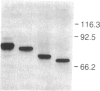
We report here the cDNA sequence for human placental beta-glucuronidase (beta-D-glucuronoside glucuronosohydrolase, EC 3.2.1.31) and demonstrate expression of the human enzyme in transfected COS cells. We also sequenced a partial cDNA clone from human fibroblasts that contained a 153-base-pair deletion within the coding sequence and found a second type of cDNA clone from placenta that contained the same deletion. Nuclease S1 mapping studies demonstrated two types of mRNAs in human placenta that corresponded to the two types of cDNA clones isolated. The NH2-terminal amino acid sequence determined for human spleen beta-glucuronidase agreed with that inferred from the DNA sequence of the two placental clones, beginning at amino acid 23, suggesting a cleaved signal sequence of 22 amino acids. When transfected into COS cells, plasmids containing either placental clone expressed an immunoprecipitable protein that contained N-linked oligosaccharides as evidenced by sensitivity to endoglycosidase F. However, only transfection with the clone containing the 153-base-pair segment led to expression of human beta-glucuronidase activity. These studies provide the sequence for the full-length cDNA for human beta-glucuronidase, demonstrate the existence of two populations of mRNA for beta-glucuronidase in human placenta, only one of which specifies a catalytically active enzyme, and illustrate the importance of expression studies in verifying that a cDNA is functionally full-length.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Bell C. E., Sly W. S. Human beta-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell. 1978 Sep;15(1):269–278. doi: 10.1016/0092-8674(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Achord D., Brot F., Gonzalez-Noriega A., Sly W., Stahl P. Human beta-glucuronidase. II. Fate of infused human placental beta-glucuronidase in the rat. Pediatr Res. 1977 Jul;11(7):816–822. doi: 10.1203/00006450-197707000-00008. [DOI] [PubMed] [Google Scholar]
- Bishop D. F., Calhoun D. H., Bernstein H. S., Hantzopoulos P., Quinn M., Desnick R. J. Human alpha-galactosidase A: nucleotide sequence of a cDNA clone encoding the mature enzyme. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4859–4863. doi: 10.1073/pnas.83.13.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot F. E., Bell C. E., Jr, Sly W. S. Purification and properties of beta-glucuronidase from human placenta. Biochemistry. 1978 Feb 7;17(3):385–391. doi: 10.1021/bi00596a001. [DOI] [PubMed] [Google Scholar]
- Catterall J. F., Leary S. L. Detection of early changes in androgen-induced mouse renal beta-glucuronidase messenger ribonucleic acid using cloned complementary deoxyribonucleic acid. Biochemistry. 1983 Dec 20;22(26):6049–6053. doi: 10.1021/bi00295a001. [DOI] [PubMed] [Google Scholar]
- Faust P. L., Kornfeld S., Chirgwin J. M. Cloning and sequence analysis of cDNA for human cathepsin D. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4910–4914. doi: 10.1073/pnas.82.15.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S. Beta-glucuronidase binding to human fibroblast membrane receptors. J Biol Chem. 1980 Jun 10;255(11):5069–5074. [PubMed] [Google Scholar]
- Frankel H. A., Glaser J. H., Sly W. S. Human beta-glucuronidase. I. Recognition and uptake by animal fibroblasts suggests animal models for enzyme replacement studies. Pediatr Res. 1977 Jul;11(7):811–816. doi: 10.1203/00006450-197707000-00007. [DOI] [PubMed] [Google Scholar]
- Fukushima H., de Wet J. R., O'Brien J. S. Molecular cloning of a cDNA for human alpha-L-fucosidase. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1262–1265. doi: 10.1073/pnas.82.4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J. H., Sly W. S. Beta-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med. 1973 Dec;82(6):969–977. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise K. S., Korneluk R. G., Waye J., Lamhonwah A. M., Quan F., Palmer R., Ganschow R. E., Sly W. S., Gravel R. A. Isolation and expression in Escherichia coli of a cDNA clone encoding human beta-glucuronidase. Gene. 1985;34(1):105–110. doi: 10.1016/0378-1119(85)90300-2. [DOI] [PubMed] [Google Scholar]
- Guo L. H., Yang R. C., Wu R. An improved strategy for rapid direct sequencing of both strands of long DNA molecules cloned in a plasmid. Nucleic Acids Res. 1983 Aug 25;11(16):5521–5540. doi: 10.1093/nar/11.16.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieber V. C. Cloning of a cDNA complementary to rat preputial gland beta-glucuronidase mRNA. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1271–1278. doi: 10.1016/0006-291x(82)91387-0. [DOI] [PubMed] [Google Scholar]
- Himeno M., Hashiguchi Y., Kato K. Beta-glucuronidase of bovine liver. Purification, properties, carbohydrate composition. J Biochem. 1974 Dec;76(6):1243–1252. doi: 10.1093/oxfordjournals.jbchem.a130677. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneluk R. G., Mahuran D. J., Neote K., Klavins M. H., O'Dowd B. F., Tropak M., Willard H. F., Anderson M. J., Lowden J. A., Gravel R. A. Isolation of cDNA clones coding for the alpha-subunit of human beta-hexosaminidase. Extensive homology between the alpha- and beta-subunits and studies on Tay-Sachs disease. J Biol Chem. 1986 Jun 25;261(18):8407–8413. [PubMed] [Google Scholar]
- Korneluk R. G., Quan F., Gravel R. A. Rapid and reliable dideoxy sequencing of double-stranded DNA. Gene. 1985;40(2-3):317–323. doi: 10.1016/0378-1119(85)90055-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Leighton P. H., Fisher W. K., Moon K. E., Thompson E. O. Amino acid sequences containing cysteine or half-cystine residues in beta-glucuronidase. Aust J Biol Sci. 1980 Oct;33(5):513–520. doi: 10.1071/bi9800513. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis A. J., Paigen K. The large scale isolation of mouse beta-glucuronidase and comparison of allozymes. J Biol Chem. 1978 Oct 25;253(20):7336–7345. [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie P. I. Rat liver UDP-glucuronosyltransferase. Sequence and expression of a cDNA encoding a phenobarbital-inducible form. J Biol Chem. 1986 May 5;261(13):6119–6125. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Myerowitz R., Piekarz R., Neufeld E. F., Shows T. B., Suzuki K. Human beta-hexosaminidase alpha chain: coding sequence and homology with the beta chain. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7830–7834. doi: 10.1073/pnas.82.23.7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natowicz M. R., Chi M. M., Lowry O. H., Sly W. S. Enzymatic identification of mannose 6-phosphate on the recognition marker for receptor-mediated pinocytosis of beta-glucuronidase by human fibroblasts. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4322–4326. doi: 10.1073/pnas.76.9.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Rosenfeld M. G., Kreibich G., Gubler U., Sabatini D. D., Adesnik M., Andy R. Nucleotide sequence of rat preputial gland beta-glucuronidase cDNA and in vitro insertion of its encoded polypeptide into microsomal membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7292–7296. doi: 10.1073/pnas.83.19.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R., Gallagher P. M., Boyko W. L., Ganschow R. E. Genetic control of levels of murine kidney glucuronidase mRNA in response to androgen. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7596–7600. doi: 10.1073/pnas.80.24.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Quinton B. A., McAlister W. H., Rimoin D. L. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973 Feb;82(2):249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- Sundelin J., Melhus H., Das S., Eriksson U., Lind P., Trägårdh L., Peterson P. A., Rask L. The primary structure of rabbit and rat prealbumin and a comparison with the tertiary structure of human prealbumin. J Biol Chem. 1985 May 25;260(10):6481–6487. [PubMed] [Google Scholar]
- Tomino S., Paigen K., Tulsiani D. R., Touster O. Purification and chemical properities of mouse liver lysosomal (L form) beta-glucuronidase. J Biol Chem. 1975 Nov 10;250(21):8503–8509. [PubMed] [Google Scholar]
- Tsuji S., Choudary P. V., Martin B. M., Winfield S., Barranger J. A., Ginns E. I. Nucleotide sequence of cDNA containing the complete coding sequence for human lysosomal glucocerebrosidase. J Biol Chem. 1986 Jan 5;261(1):50–53. [PubMed] [Google Scholar]
- Tulsiani D. R., Six H., Touster O. Rat liver microsomal and lysosomal beta-glucuronidases differ in both carbohydrate and amino acid compositions. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3080–3084. doi: 10.1073/pnas.75.7.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson G., Felder M., Rabinow L., Moore K., Labarca C., Tietze C., Vander Molen G., Bracey L., Brabant M., Cai J. D. Properties of rat and mouse beta-glucuronidase mRNA and cDNA, including evidence for sequence polymorphism and genetic regulation of mRNA levels. Gene. 1985;36(1-2):15–25. doi: 10.1016/0378-1119(85)90065-4. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]