Abstract
Introduction
Incidence rates of bladder cancer are notably higher in men than women. While there is evidence that reproductive and hormonal risk factors may influence risk of bladder cancer, data are inconclusive.
Materials and Methods
We examined reproductive, menstrual and hormonal use history in our population-based case-control study of bladder cancer in New Hampshire (NH), USA (n=207 women cases and n=463 women controls). Additionally, we performed a meta-analysis of the published literature. We used unconditional logistic regression analysis to compute adjusted odds ratios associated with each risk factor in the NH study. We combined these estimates with those from the published literature using inverse variance effects models.
Results
In the NH study, a slightly decreased odds ratio was found among women who had ever had a birth compared to nulliparous women and an elevated odds ratio among women who underwent surgical menopause (bilateral oophorectomy), especially at an early age. No overall associations were found with oral contraceptive use or hormone replacement therapy. These findings were generally in agreement with the meta-analytic results for which the combined RR estimate was reduced among ever parous women (combined RR estimate for ever parous versus nulliparous =0.66, 95%CI 0.55-0.79) and elevated among those undergoing an early menopause (combined RR estimate for early versus late menopause =1.59, 95%CI 1.31–1.92). No consistent risk was observed for the other factors.
Discussion
Some reproductive and menstrual factors appear to be related to the incidence of bladder cancer among women; but whether effects are due to female hormones is uncertain.
Keywords: bladder cancer, reproductive hormones, menopause, case-control study, meta-analysis
1. Introduction
Bladder cancer is the most common urologic malignancy in many regions of the world. Several causes have been identified, including tobacco use and certain occupational exposures, and, in particular areas, infectious agents1. In general, men experience a two- to four-fold higher risk of bladder cancer than women2,3, although recent studies suggest decreasing mortality trends among men throughout Europe4. Prior studies of sex steroid-related factors (i.e., parity, exogenous hormone use, oral contraceptive use, age at menarche and menopause) and bladder cancer risk have yielded varying results. Some, but not all, of these studies suggest that parous women may be at a lower risk than nulliparous women2,5–11 and that early age at menopause may be related to an increased risk2,6,7,9,11,12. An association between oral contraceptives2,6,7,9 or hormone replacement therapy2,6–9,13,14 and bladder cancer risk also has been explored previously, but only to a limited extent.
Pregnancy and menopause may cause significant, long-lasting changes in sex steroid levels as well as alterations of bladder tissue and bladder function15. In light of the potential role of female sex steroids in bladder cancer etiology, we examined associations between bladder cancer and reproductive factors, oral contraceptive use, hormone replacement therapy, and menopause as part of an ongoing population-based case-control study of bladder cancer conducted in New Hampshire, USA. We further performed a meta-analysis of these factors encompassing the published literature.
2. Materials and methods
2.1 Study Group
Through the New Hampshire State Department of Health and Human Services' rapid reporting Cancer Registry, we identified newly diagnosed cases of bladder cancer among New Hampshire residents, aged 25–74 years from July 1, 1994 to December 31, 2001. For efficiency, we shared controls with a study of non-melanoma skin cancer covering a diagnostic period between July 1, 1993 and June 30, 1995 and July 1 1997 to March 30, 2000 frequency matched to cases by age (25–34, 35–44, 45–54, 55–64, 65–69, 70–74 years) and gender. Additional controls for the intervening period were selected frequency matched to the bladder cancer cases on age (25–34, 35–44, 45–54, 55–64, 65–69, 70–74 years) and gender16. Controls less than 65 years of age were randomly selected within age and gender strata using population lists obtained from the New Hampshire Department of Transportation comprised of those with a registered driver's license. Controls 65 years of age and older were randomly chosen in age and gender strata from data files provided by the Centers for Medicare & Medicaid Services (CMS) of New Hampshire residents enrolled in Medicare, a federal health plan provided to those 65 years of age and older. As described previously, these sources cover over 95% of the population in the age ranges selected for the study17.
2.2 Interviews
We conducted standardized personal interviews with the study participants, usually in their home, to obtain information on demographic traits, use of tobacco (including frequency, duration and intensity of cigarette smoking) and other exposures. We requested the original paraffin-embedded tumour specimens for histopathology rereview by the study pathologist who classified tumours according to both WHO 1973 and WHO ISUP criteria18. Due to the high overall diagnosis concordance rates (>90%), we classified subjects based on the original pathologist's diagnosis. We obtained informed consent from each participant and all procedures and study materials were approved by the Committee for the Protection of Human Subjects at Dartmouth College.
2.3 Exposure Assessment
Participants were asked if they had ever taken oral contraceptives or exogenous hormones other than oral contraceptives (hormone replacement therapy (HRT)) prior to their reference date (for cases, their diagnosis dates and for controls, randomly assigned dates selected from the cases' diagnoses dates to which they were matched). Subjects were classified as ever users, if they had taken oral contraceptives or HRT for at least three months, otherwise they were classified as never users. Those who responded positively were asked the year of first use, age at first use, total duration of use and time since their last use of either oral contraceptives or HRT. In addition, participants were asked about their reproductive history including whether they ever had a birth, number of births, and age at first birth. Women also were asked their menopausal status, e.g., whether they were pre- or post-menopausal, age at menopause, and type of menopause, e.g., natural or surgical. For surgical menopause, we elicited whether they had a hysterectomy with or without oophorectomy and number of ovaries removed. To minimize potential reporting bias, we did not reveal the specific hypotheses of interest to either the interviewer or participant, and we did not inform the interviewers of the case-control status of participants.
2.4 Statistical Analysis
We computed odds ratios (OR) and their 95% confidence intervals (CI) for bladder cancer risk associated with oral contraceptives (ever versus never use, duration, and time since last use), parity (ever versus never, and number of births), menopause (pre- versus post-menopausal, type and age at menopause), and hormone replacement therapy (ever versus never use) using unconditional logistic regression, taking into account multiple confounding factors19. In addition to age, we adjusted for the potential confounding effects of smoking status (current, former, never). Parity was included as a covariate in the models for oral contraceptives, menopause and hormone replacement therapy. We examined all bladder cancers combined as well as invasive and noninvasive tumours separately.
2.5 Meta-Analysis
We further conducted a meta-analysis that included prior studies that examined parity (ever/never), use of oral contraceptives (ever/never), use of hormone replacement therapy (ever/never), menopausal status (pre-/post-), and early age at menopause (yes/no). We searched MEDLINE for observational, case-control studies and cohort studies using the following search terms: hormone replacement therapy, oral contraceptives, parity, menopause and bladder cancer. We did not use any language restrictions or other limits in our search, and we did not attempt to contact authors or editors about unpublished studies. Studies included in the meta-analysis were either cohort or case-control studies published in English that examined relationships between bladder cancer and at least one of the following: parity, oral contraceptive use, menopause status, age at menopause, or use of hormone replacement therapy. In the parity analysis, one study was excluded because it only presented data on parous women in the analysis (i.e., did not include nulliparous women as the referent group)9. A summary of the study characteristics that met the criteria for the meta-analysis is provided in Supplemental Table 1. Published, adjusted relative risk (RR) estimates from each study were combined using an inverse variance fixed effects model or maximum likelihood estimation procedure based on the random effects model if inter-study heterogeneity of estimates was detected20,21. To estimate whether individual study estimates were heterogeneous or homogeneous we computed a Q-statistic and applied the chi-square test22.
In studies that did not examine variables of interest dichotomously, the RR estimates and its CIs based on categorical data were converted to those for dichotomous variables (e.g., we converted estimates based on number of births into those for nulliparous versus parous). We then used the crude estimates to calculate combined risk estimates of the dichotomous variables. While different cut points were used for age at menopause across studies, they were similar. Therefore, we compared the youngest age group to the oldest age group evaluated in each study. Additionally, in Huang and colleagues11, the youngest age at menopause was compared to the second tertile; in order to keep the direction of comparisons uniform, we calculated the crude odds ratio comparing the youngest group to the oldest group for this study.
3. Results
A total of 207 cases and 463 control women were included in the study and asked about their number of offspring. As questions for women were added after the initiation of interviews in the parent study23, 207 cases and 364 controls were administered questions on oral contraceptive and hormone replacement therapy use, and menopause.
Overall, cases were more likely to be current or former smokers than controls, but were comparable with respect to age. Cases also were more likely to have a family history of bladder cancer and not have education beyond high school or technical college (Table 1).
Table 1.
- Selected Characteristics of Women Bladder Cancer Cases and Controls.
| Controlsa | Bladder Cancer Cases b | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total Subjects | 331 | 100 | 187 | 100 | |
| Age | |||||
| <55 | 98 | 30% | 51 | 27% | |
| 55–63 | 78 | 24% | 46 | 25% | |
| 64–68 | 64 | 19% | 34 | 18% | |
| ≥69 | 91 | 27% | 56 | 30% | |
| Smoking | |||||
| Never | 151 | 46% | 50 | 27% | |
| Former | 126 | 38% | 62 | 33% | |
| Current | 54 | 16% | 75 | 40% | |
| Pack- Years | |||||
| ≤32 | 129 | 73% | 66 | 49% | |
| >32 | 48 | 27% | 68 | 51% | |
| Education | |||||
| High School or Technical College | 173 | 53% | 135 | 73% | |
| College | 106 | 32% | 40 | 22% | |
| Graduate or Professional School | 49 | 15% | 10 | 5% | |
| Family History of Bladder Cancer | |||||
| No | 318 | 99% | 159 | 93% | |
| Yes | 4 | 1% | 12 | 7% | |
| Histology | |||||
| Transitional | - | - | 170 | 96% | |
| Non-Transitional | - | - | 8 | 4% | |
| Stage | |||||
| CIS | - | - | 1 | 1% | |
| Non-Invasive Low Grade | - | - | 112 | 69% | |
| Non-Invasive High Grade | - | - | 8 | 5% | |
| Invasive | - | - | 41 | 25% | |
3 controls missing data on education, 9 missing data on family history
2 cases missing data on education, 16 missing data on family history, 9 missing transitional case status, 25 missing bladder cancer stage
A reduced odds ratio was observed for women who had had a birth (OR=0.71, 95%CI 0.40–1.26) compared to women who had never had a birth (Table 2). However, the confidence intervals were wide, and the risk estimates did not change appreciably with an increasing number of births (Table 2). Further, these results did not appear to differ significantly between invasive (OR=0.78, 95%CI 0.27–2.23) and non-invasive (OR=0.67, 95%CI 0.35–1.28) tumours. Forty-two percent of cases and 43% of controls reported a history of oral contraceptive use. The adjusted odds ratio for ever use of oral contraceptives was modestly elevated (OR=1.55, 95%CI 0.83–2.89), and was elevated specifically among those who last used oral contraceptives 25 or more years ago (OR=2.13, 95%CI 1.08–4.20); however, there was no overall trend by duration of use (p for trend=0.259) (Table 2). Additionally, the association with ever use of oral contraceptives did not appear to differ between invasive (OR=1.34, 95%CI 0.45–3.94) and non-invasive (1.47, 95%CI 0.70–3.10) tumours.
Table 2.
- Adjusted Odds Ratios (95% Confidence Intervals) for Parity, Oral Contraceptive Use, Menopausal Status and Hormone Replacement Therapy Use Among Women Cases and Controls.
| Controlsc | Bladder Cancer Casesd | ||||
|---|---|---|---|---|---|
| n=379 | % | n=197 | % | ORa (95% CI) | |
| Number of Births | |||||
| None | 39 | 10% | 23 | 6% | 1.00 (reference) |
| Some | 340 | 90% | 174 | 46% | 0.71 (0.40–1.26) |
| 1–2 | 161 | 47% | 78 | 23% | 0.67 (0.36–1.24) |
| 3–4 | 132 | 39% | 71 | 21% | 0.75 (0.40–1.39) |
| ≥5 | 47 | 14% | 25 | 7% | 0.74 (0.35–1.57) |
| p for trend (categorical) | 0.632 | ||||
| Oral Contraceptive Use | |||||
| Never | 188 | 57% | 108 | 58% | 1.00 (reference) |
| Ever | 143 | 43% | 79 | 42% | 1.55 (0.83–2.89) |
| Total Duration of Use | |||||
| No Use | 188 | 57% | 108 | 59% | 1.00 (reference) |
| 3 months – 2 years | 43 | 13% | 29 | 16% | 1.70 (0.77–3.79) |
| 3–6 years | 46 | 14% | 13 | 7% | 0.87 (0.31–2.46) |
| 7 years or more | 52 | 16% | 33 | 18% | 1.73 (0.81–3.73) |
| p for trend | 0.259 | ||||
| Time Since Last Use | |||||
| No Use | 188 | 57% | 108 | 58% | 1.00 (reference) |
| 25 or more years | 41 | 12% | 40 | 21% | 2.13 (1.08–4.20) |
| 0–24 years | 102 | 31% | 39 | 21% | 0.82 (0.35–1.92) |
| p for trend | 0.91 | ||||
| Menopause Status | |||||
| Premenopausal | 68 | 21% | 26 | 14% | 1.00 (reference) |
| Postmenopausal | 262 | 79% | 160 | 86% | 1.30 (0.45–3.77) |
| Natural | 156 | 60% | 95 | 59% | 0.84 (0.21–3.39) |
| Surgical (Bilateral Oophorectomy) | 48 | 18% | 31 | 19% | 2.80 (0.65–12.05) |
| Age at Menopauseb | |||||
| ≥45 years | 158 | 65% | 99 | 63% | 1.00 (reference) |
| <45 years | 85 | 35% | 59 | 37% | 1.33 (0.72–2.47) |
| Age at Natural Menopauseb | |||||
| ≥45 years | 124 | 86% | 79 | 83% | 1.00 (reference) |
| <45 years | 20 | 14% | 16 | 17% | 0.73 (0.21–2.54) |
| Age at Bilateral Oophorectomyb | |||||
| ≥45 years | 23 | 50% | 10 | 33% | 1.00 (reference) |
| <45 years | 23 | 50% | 20 | 67% | 5.37 (1.20–23.93) |
| Hormone Replacement Therapy Useb | |||||
| Never | 151 | 58% | 101 | 64% | 1.00 (reference) |
| Ever | 110 | 42% | 58 | 36% | 1.08 (0.60–1.96) |
Adjusted for age and smoking status
Restricted to post-menopausal women only
48 controls missing data on oral contraceptive use, 2 missing data on duration of oral contraceptive use, 49 missing data on menopausal status, 19 missing data on age at menopause, 12 missing age of natural menopause, 2 missing age at bilateral oophorectomy, 1 post-menopausal women missing data on HRT use
10 cases missing data on oral contraceptive use, 4 missing data on duration of oral contraceptive use, 11 missing data on menopausal status, 2 missing data on age at menopause, 1 missing age of bilateral oophorectomy, 1 post-menopausal women missing data on HRT use
The odds ratio for bladder cancer was elevated among postmenopausal compared with premenopausal women, particularly among those who underwent a surgically-induced menopause (i.e., bilateral oophorectomy) (OR=2.80, 95%CI 0.65–12.05 (Table 2). We found an increased odds ratio associated with early age at menopause (<45 years versus ≥ 45 years) (OR=1.33, 95%CI 0.72–2.47), and this was most evident among those with an early surgical menopause (OR=5.37, 95%CI 1.20–23.93) (Table 2). Odds ratios for early surgery menopause were likewise similar between invasive (OR=4.00, 95%CI 0.28–56.65) and non-invasive (OR=4.69, 95%CI 0.59–37.29) tumours. When adjusted for smoking pack years, the estimate only increased (OR=8.25, 95%CI 1.15–59.24), however with more limited statistical precision. A similar percentage of cases and controls reported a history of hormone replacement therapy (OR=1.08, 95%CI 0.60–1.96), and associations did not differ by type of menopause (data not shown).
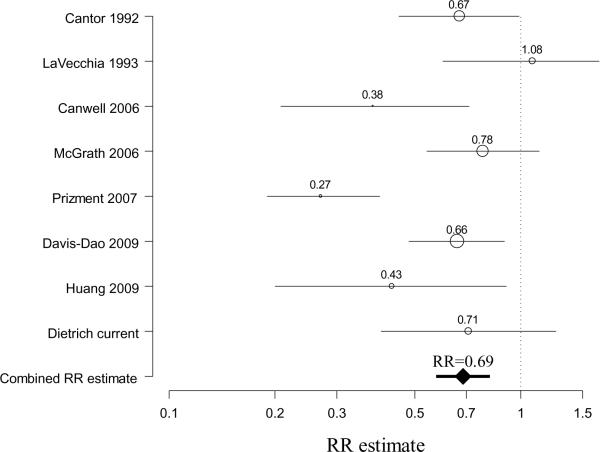
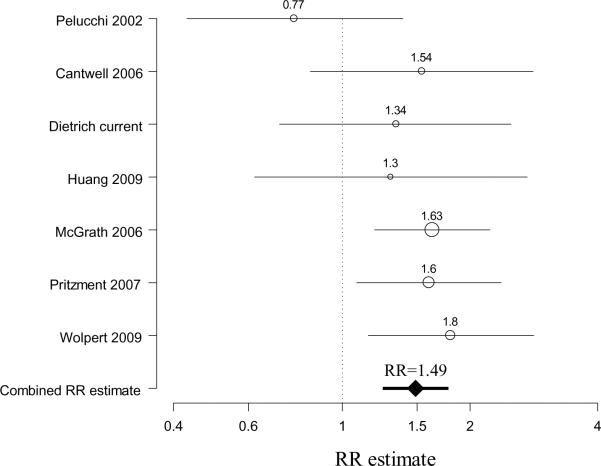
Meta-analysis results also indicated a decreased odds ratio with ever having had a birth (combined RR estimate =0.69, 95%CI 0.57–0.82, based on 8 studies2,5–8,10,11) without evidence of heterogeneity between studies (Chi-square for heterogeneity=7.12, p=0.42) (Figure 1). An increased odds ratio was observed with an early age at menopause (combined RR estimate =1.49, 95%CI 1.25–1.78, based on 7 studies2,6,7,9,11,12, again without evidence of inter-study heterogeneity (Chi-square for heterogeneity=6.23, p=0.40) (Figure 2). While the summary RR estimate was increased slightly for ever use of HRT (combined RR estimate =1.27, 95%CI 1.03–1.57, based on 7 studies2,6,7,9,13,14), there was evidence of heterogeneity (Chi-square for heterogeneity =12.30, p=0.06). The summary estimate for ever use of oral contraceptives was close to one (combined RR estimate =0.95, 95%CI 0.66–1.37, based on 5 studies2,6,7,9, Chi-square for heterogeneity=4.17, p=0.38) and was modestly elevated for menopausal status, but with wide confidence intervals (combined RR estimate =1.42, 95%CI 0.88–2.26, based on 5 studies2,6,9,11, Chi-square for heterogeneity=4.85, p=0.30).
Figure 1. Meta-analysis forest plot of parity and bladder cancer risk in women.
For individual studies, the center of the circle represents the individual study relative risk estimate and the radius of the circle being proportional to the weight of the study relative risk estimate in the summary relative risk (RR) computation (reciprocal of the variance)20–22. The line represents the confidence interval. The combined RR estimate derived from the meta-analysis is represented by a diamond, with the 95% confidence interval represented by the bold line.
Figure 2. Meta-analysis forest plot of early menopause and bladder cancer risk in women.
For individual studies, the center of the circle represents the individual study relative risk estimate and the radius of the circle being proportional to the weight of the study relative risk estimate in the summary relative risk (RR) computation (reciprocal of the variance)20–22. The line represents the confidence interval. The combined RR estimate derived from the meta-analysis is represented by a diamond, with the 95% confidence interval represented by the bold line.
4. Discussion
In our population-based case-control study parous women appeared to be at a lower risk of developing bladder cancer than nulliparous women. However, our individual study risk estimates were imprecise, and we did not observe a trend with number of births. We found evidence of an increased risk of bladder cancer for post-menopausal women compared to pre-menopausal women, particularly in women who underwent a bilateral oophorectomy at an early age (<45 years). We did not find any overall association between use of hormone replacement therapy or oral contraceptives.
It is possible that our findings were due to chance, unmeasured confounding or other biases. For example, detection bias is possible in women who have undergone a bilateral oophorectomy if the reason was for cancer or another condition resulting in increased surveillance. An important limitation of our study is statistical power; while our study population was relatively large, we had a relatively small group of women. For this reason, we considered our findings along with others by conducting a meta-analysis.
Indeed, a number of prior studies have investigated reproductive history, menopause and sex steroid use in relation to bladder cancer among women. Consistent with ours, nearly all prior studies observed an inverse association between parity and bladder cancer risk5,8,11. Our meta-analysis showed a decrease in risk with parity irrespective of study design. Interestingly, prior studies have not found trends of decreasing risk with increasing number of births2,6,7,9,10 as opposed to findings for breast cancer, for example, in which risk is lower with each additional birth24.
In agreement with our study, prior studies indicated no overall association with ever use of oral contraceptives2,6,7,9. Cantwell and colleagues6 and McGrath and colleagues2 further examined the effect of duration of oral contraceptive use in relation to bladder cancer risk and found no association. Our data suggested an elevated risk among those who used oral contraceptives in the distant past, but to our knowledge no prior studies examined time since last use. Thus, this finding would need to be confirmed or refuted in future research.
Recent data indicate that risk of bladder cancer may be influenced by menopause. The US Nurses' Health Study reported a two-fold increase in risk for women who experienced a natural or surgical menopause compared to those who had not undergone menopause2, and others have observed similar trends6,9. In contrast, a recent study by Huang and colleagues11, found a reduced odds ratio among post-menopausal women, however with wide confidence intervals. Another study that specifically examined bilateral oophorectomy found an increased bladder cancer risk among these women compared to women who did not undergo a bilateral oophorectomy7. In at least three studies, younger menopause (<45 years old) was associated with an increased risk2,7,12; in two studies relative risk estimates tended to be elevated for women in the oldest age at menopause category7,10, but the results were not statistically significant, and in one, the association was inversed6,9,11. In the New Hampshire population, we observed an increased risk primarily with younger age at bilateral oophorectomy, and a more modest increase with a younger age at menopause overall. In our meta-analysis, the summary relative risk estimate was increased for early versus late age at menopause, and results appeared fairly consistent across studies. Hence, the underlying reasons for this observation may deserve further inquiry.
Studies investigating hormone replacement therapy use in relation to bladder cancer risk have been inconsistent. Two case-control studies (106 and 110 cases respectively) found two- to three-fold higher risks of bladder cancer among ever users of hormone replacement therapy compared to never users, as well as a pattern of increasing risk with duration of use9,13. In addition to the present New Hampshire population-based study, four published cohort studies reported no association with ever use of hormone replacement therapy2,6–8. Only two of these studies examined the potential difference between combined estrogen-progesterone treatments and estrogen alone, and neither found a difference between therapy types2,8. Further, one Swedish cohort study found no association among all women, but a decreased risk among smokers who had used hormone replacement therapy14. As a result of the various types of HRT regiments that were used in each study, we were not able to examine a homogeneous exposure. Thus, further research will need to resolve whether an association exists.
The reasons for higher incidence of bladder cancer among men than among women remain elusive, and whether sex steroids play a role in bladder cancer etiology is likewise unclear. Urothelial cells contain estrogen and progesterone receptors and are hormone responsive25–28. Moreover, estrogens inhibit and androgens increase the growth and development of bladder malignancies in animal models29–31. Decreasing levels of estrogen in post-menopausal women have been associated with bladder dysfunction (e.g., incontinence) and recurring urinary tract infections15,32, which in turn could increase bladder cancer risk in women2. Future research to elucidate the potential mechanism of sex steroids on bladder carcinogenesis may provide insights as to why factors that affect endogenous sex steroids (e.g., parity and timing of menopause) have been repeatedly associated with risk of bladder cancer in epidemiologic studies. Finally, in light of the small number of women in individual studies, larger efforts will be required to understand whether sex-steroid related factors play a role in the development of bladder malignancies.
Supplementary Material
Acknowledgements
We thank the physicians, pathology laboratories, staff members and many participants of the New Hampshire Health Study for making this study possible.
Funding: This publication was funded in part by grant numbers 5 P42 ES007373 from the National Institute of Environmental Health Sciences, NIH and CA57494 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NCI and NIH.
Sources of Support This publication was funded in part by grant numbers 5 P42 ES007373 from the National Institute of Environmental Health Sciences, NIH and CA57494 from the National Cancer Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: None declared.
References
- 1.Kogevinas M, Trichopoulos D. Urinary Bladder Cancer. In: Adami H-O, Hunter D, Trichopoulos D, editors. Textbook of Cancer Epidemiology. Oxford University Press; New York: 2008. pp. 446–466. [Google Scholar]
- 2.McGrath M, Michaud D, Vivo ID. Hormonal and Reproductive Factors and the Risk of Bladder Cancer in Women. Am J Epidemiol. 2006;163(3):236–244. doi: 10.1093/aje/kwj028. [DOI] [PubMed] [Google Scholar]
- 3.Scosyrev E, Noyes K, Feng C, Messing E. Sex and Racial Differences in Bladder Cancer Presentation and Mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 4.Karim-Kos H, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh J. Recent trends of cancer in Europe: A combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–1389. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Cantor K, Lynch C, Johnson D. Bladder cancer, parity, and age at first birth. Cancer Causes Control. 1992;3:57–62. doi: 10.1007/BF00051913. [DOI] [PubMed] [Google Scholar]
- 6.Cantwell M, Jr JL, Schairer C, Schatzkin A, Michaud D. Reproductive factors, exogenous hormone use and bladder cancer risk in a prospective study. Int J Cancer. 2006;119:2398–2401. doi: 10.1002/ijc.22175. [DOI] [PubMed] [Google Scholar]
- 7.Prizment A, Anderson K, Harlow B, Rolsom A. Reproductive risk factors for incident bladder cancer: Iowa Women's Health Study. Int J Cancer. 2006;120:1093–1098. doi: 10.1002/ijc.22418. [DOI] [PubMed] [Google Scholar]
- 8.Davis-Dao C, Henderson K, Sullivan-Halley J, Ma H, Chang E, Horn-Ross P, West D, Anton-Culver H, Cortessis V, Bernstein L. Hormonal and reproductive factors and risk of bladder cancer: The California Teachers Study. Am J Epidemiol. 2009;169(Suppl):S1–S137. [Google Scholar]
- 9.Pelucchi C, Vecchia CL, Negri E, Maso LD, Franceschi S. Smoking and Oher Risk Factors for Bladder Cancer in Women. Prev Med. 2002;35:114–120. doi: 10.1006/pmed.2002.1061. [DOI] [PubMed] [Google Scholar]
- 10.LaVecchia C, Negri E, Franceschi S, Parazzini F. Long-term impact of reproductive factors on cancer risk. Int J Cancer. 1993;53:215–219. doi: 10.1002/ijc.2910530207. [DOI] [PubMed] [Google Scholar]
- 11.Huang A, Kogevinas M, Silverman D, Malats N, Rothman N, Tardon A, Serra C, Garcia-Closas R, Carrato A, Cantor K. Bladder cancer and reproductive factors aong women in Spain. Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolpert B, Amr S, Zheng Y-L, Zhan M, Squibb K, Loffredo C. Estrogen exposure and bladder cancer risk in Egyptian Women. Am J Epidemiol. 2009;169(Suppl):S1–S137. [Google Scholar]
- 13.Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, Vecchia CL. Hormone Replacement Therapy and Cancer Risk: A Systematic Analysis From a Network of Case-Control Studies. Int J Cancer. 2003;105:408–412. doi: 10.1002/ijc.11083. [DOI] [PubMed] [Google Scholar]
- 14.Olsson H, Bladstrom A, Ingvar C. Are Smoking-Associated Cancers Prevented or Postponed in Women Using Hormone Replacement Therapy? Obstet Gynecol. 2003;102:565–570. doi: 10.1016/s0029-7844(03)00564-7. [DOI] [PubMed] [Google Scholar]
- 15.Aikawa K, Sugino T, Matsumoto S, Chichester P, Whitbeck C, Levin R. The effect of ovariectomy and estradiol on rabbit bladder smooth muscle contraciton and morphology. J Urol. 2003;170:634–637. doi: 10.1097/01.ju.0000068723.05004.ca. [DOI] [PubMed] [Google Scholar]
- 16.Wallace K, Kelsey K, Schned A, Morris J, Andrew A, Karagas M. Selenium and Risk of Bladder Cancer: A Population-Based Case-Control Study. Cancer Prev Res. 2009;2(1):70–73. doi: 10.1158/1940-6207.CAPR-08-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortuny J, Kogevinas M, Zens M, Schned A, Andrew A, Heaney J, Kelsey K, Karagas M. Analgesic and anti-inflammatory drug use and risk of bladder cancer: a population based case control study. BMC Urol. 2007;7(13) doi: 10.1186/1471-2490-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schned A, Andrew A, Marsit C, Zens M, Kelsey K, Karagas M. Survival Following the Diagnosis of Noninvasive Bladder Cancer: WHO/International Society of Urological Pathology Versus WHO Classification Systems. J Urol. 2007;178:1196–1200. doi: 10.1016/j.juro.2007.05.126. [DOI] [PubMed] [Google Scholar]
- 19.Breslow N, Day N. Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Sci Publ. 1980;32:338. [PubMed] [Google Scholar]
- 20.Berkey C, Hoaglin D. A random-effects regression model for meta-analysis. Stat Med. 1995;14(4):395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- 21.Demidenko E. Mixed Models: Theory and Applications. 2005 [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Applebaum K, Nelson H, Zens M, Stukel T, Spencer S, Karagas M. Oral Contraceptives: A Risk Factor for Squamous Cell Carcinoma? J Invest Dermatol. 2009;129(12):2760–5. doi: 10.1038/jid.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colditz G, Baer H, Tamimi R. Breast Cancer. In: Schottenfeld D, JF Fraumeni J, editors. Cancer Epidemiology and Prevention. Oxford University Press; Oxford: 2006. [Google Scholar]
- 25.Iosif C, Batra S, Ek A, Astedt B. Estrogen receptors in the human female lower urinary tract. Am J Obstet Gynecol. 1981;141(7):817–820. doi: 10.1016/0002-9378(81)90710-9. [DOI] [PubMed] [Google Scholar]
- 26.Pacchioni D, Revelli A, Casetta G, Cassoni P, Piana P, Tizzani A, Bussolati G, Massobrio M. Immunohistochemical detection of estrogen and progesteron receptors in the normal urinary bladder and in pseudomembranous trigonitis. J Endocrinol Invest. 1992;15(10):719–725. doi: 10.1007/BF03347639. [DOI] [PubMed] [Google Scholar]
- 27.Blakeman P, Hilton P, Bulmer J. Oestrogen and progesterone receptor expression in the female lower urinary tract, with reference to oestrogen status. BJU Int. 2000;86(1):32–38. doi: 10.1046/j.1464-410x.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- 28.Rizk D, Raaschou T, Mason N, Berg B. Evidence of progesterone receptors in the mucosa of the urinary bladder. Scand J Urol Nephrol. 2001;35(4):305–309. doi: 10.1080/003655901750425891. [DOI] [PubMed] [Google Scholar]
- 29.Bertram J, Craig A. Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur J Cancer Prev. 1972;8(6):587–594. doi: 10.1016/0014-2964(72)90137-5. [DOI] [PubMed] [Google Scholar]
- 30.Shirai T, Tsuda H, Ogiso T, Hirose M, Ito N. Organ specific modifying potential of ethinyl estradiol on carcinogenesis initiated with different carcinogens. Carcinogenesis. 1987;8(1):115–119. doi: 10.1093/carcin/8.1.115. [DOI] [PubMed] [Google Scholar]
- 31.Reid L, Leav I, Kwan P, Russell P, Merk F. Characterization of a human, sex steroid-responsive transitional cell carcinoma maintained as a tumor line (R198) in athymic nude mice. Cancer Research. 1984;44(10):4560–73. [PubMed] [Google Scholar]
- 32.Hextall A. Oestrogens and lower urinary tract function. Maturitas. 2000;36:83–92. doi: 10.1016/s0378-5122(00)00143-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.