Abstract
Context
A high ratio of plasma amyloid-β peptide 40 (Aβ40) toAβ42, determined by both high Aβ40 and low Aβ42 levels, increases the risk of Alzheimer disease. In a previous study, we reported that depression is also associated with low plasma Aβ42 levels in the elderly population.
Objective
To characterize plasma Aβ40:Aβ42 ratio and cognitive function in elderly individuals with and without depression.
Design
Cross-sectional study.
Setting
Homecare agencies.
Participants
A total of 995 homebound elderly individuals of whom 348 were defined as depressed by a Center for Epidemiological Studies Depression score of 16 or greater.
Main Outcome Measures
Cognitive domains of memory, language, executive, and visuospatial functions according to levels of plasma Aβ40 and Aβ42 peptides.
Results
Subjects with depression had lower plasma Aβ42 levels (median, 14.1 vs 19.2 pg/mL; P = .006) and a higher plasma Aβ40:Aβ42 ratio (median, 8.9 vs 6.4; P < .001) than did those without depression in the absence of cardiovascular disease and antidepressant use. The interaction between depression and plasma Aβ40:Aβ42 ratio was associated with lower memory score (β = −1.9, SE = 0.7, P = .006) after adjusting for potentially confounders. Relative to those without depression, “amyloid-associated depression,” defined by presence of depression and a high plasma Aβ40:Aβ42 ratio, was associated with greater impairment in memory, visuospatial ability, and executive function; in contrast, nonamyloid depression was not associated with memory impairment but with other cognitive disabilities.
Conclusion
Amyloid-associated depression may define a subtype of depression representing a prodromal manifestation of Alzheimer disease.
Two Large Population studies, the Rotterdam and the Mayo Clinic cohorts, have shown that a low concentration of amyloid-β peptide 42 (Aβ42) combined with a high concentration of Aβ40 in plasma increases the risk of developing Alzheimer disease (AD).1,2 Depression also increases the risk or is an early symptom of AD in the elderly population.3 We recently reported that depressed elderly individuals without cardiovascular disease (CVD) have a lower concentration of Aβ42, leading to a high Aβ40:Aβ42 ratio, in plasma compared with both depressed elderly individuals with CVD and those without depression in a homebound elderly population. 4 This result led us to hypothesize that depression characterized by a high Aβ40: Aβ42 ratio may represent a distinct depression subtype, which we have termed amyloid-associated depression.
Although plasma Aβ peptides cannot be used to diagnose AD,5 plasma Aβ42 levels decline significantly at a preclinical or early stage of AD,6 suggesting a biomarker for a prodrome of the disease. In amyloid precursor protein (APP) transgenic mice, plasma Aβ42 level declines significantly before the pathological changes of AD are formed in the brain.7,8 Elevated plasma Aβ40 level is correlated with cerebral microvascular pathological features, which are linked with both late-life depression and AD.9,10 Therefore, it is possible that the combination of high Aβ40 and low Aβ42 levels in plasma may be used as a biomarker of pathological change in the brain at a preclinical stage of AD.
Studies have demonstrated that there is an increased risk of the development of AD in some, but not all, individuals with late-life depression, suggesting a prodromal state of AD.11,12 We hypothesized that a high plasma Aβ40:Aβ42 ratio plus clinical symptoms of depression may represent a prodromal depression of AD and may lead to a more imminent cognitive deterioration than in those who have a high plasma Aβ40: Aβ42 ratio without depression. If our assumption is correct, at the cross-sectional level, patients with amyloid-associated depression should present with a pattern of cognitive impairment consistent with prodromal AD, ie, prominent memory dysfunction, compared with those with nonamyloid depression or those without depression. To test this hypothesis, we investigated the relationships among plasma Aβ peptide levels, depression, and cognitive function in a homebound elderly population.
METHODS
STUDY POPULATION AND RECRUITMENT
We studied a group of 995 subjects, all of whom had been tested for depression status and plasma Aβ peptide levels in the Nutrition, Aging, and Memory in the Elderly study, an ongoing, population-based study supported by the National Institute on Aging. Subjects included homebound elderly clients who were enrolled in 1 of 4 home care agencies in the Boston area between 2003 and 2006. Anyone receiving home care services was registered with 1 of these agencies if he or she lived in the city of Boston, had an annual income less than $18 890, and needed home care service. All of the homebound elderly subjects 60 years or older from each of the 4 agencies were invited to participate in the study.
Eligibility for enrollment required that the participants speak English, be physically able to participate in the study home visits, and have sufficient vision and hearing to read and hear the content of the neuropsychological tests. Of all 1803 eligible subjects, 1190 individuals (66.0%) enrolled and gave informed consent to participate in the study. The population was screened for significant cognitive impairment by means of the Mini-Mental State Examination (MMSE)13 and for estimated verbal IQ by means of the North American Adult Reading Test.14 Those with MMSE scores of 10 or less or verbal IQ less than 75 were not eligible to continue in the study, and eligible subjects were subsequently examined. Each subject engaged in 3 home visits conducted by a research assistant, who drew a fasting blood sample and collected data on depression and medical conditions. 15 Among them, 995 subjects had both blood samples available for plasma Aβ measurements and data on depression.
PLASMA Aβ40 AND Aβ42 MEASUREMENTS
The blood samples were centrifuged immediately after the blood was drawn. The sandwich Aβ enzyme-linked immunosorbent assay was used. Plates were coated with 2G3 (anti-Aβ40) and 21F12 (anti-Aβ42) antibodies overnight at 4°C. Samples were then loaded and incubated overnight at 4°C followed by incubation with a biotinylated monoclonal anti–N-terminus Aβ antibody (3D6B) for 2 hours. Finally, streptavidin-conjugated alkaline phosphatase (Promega Corp, Madison, Wisconsin) was added and incubated, and the signal was amplified by adding alkaline phosphatase fluorescent substrate (Promega Corp), which was then measured. The lowest detection for both Aβ peptides was 1.6 pg/mL in the standard curves, with percentage coefficient of variation between 1.1 and 7.2, and these were used as the cutoff points for comparison and regression if levels were below the cutoff point of detection (6 samples). The samples with higher levels than the standard curve were repeated with dilutions for measurement. The intracorrelations with 2 other laboratories that have published the results of their Aβ measurements16,17 showed r = 0.63 and 0.84 for Aβ40 and r = 0.90 and 0.96 for Aβ42.
DEFINITION OF DEPRESSION
Depressive symptoms were assessed by means of the Center for Epidemiological Studies Depression Scale (CES-D)18; a CES-D score of 16 or greater was used as the cutoff point for clinical depression.19 In 106 subjects in our study, this CES-D cutoff point had a sensitivity of 0.90 and a specificity of 0.83 for the DSM-IV diagnosis of major depression by a board-certified psychiatrist.
Subjects with a CES-D score of 16 or greater and a plasma Aβ40:Aβ42 ratiogreater than the median (7.1) were defined as having amyloid-associated depression (n = 177). Those with a CES-D score of 16 or greater and a plasma Aβ40:Aβ42 ratio less than or equal to the median were defined as having nonamyloid depression (n = 171). The other subgroup included those without depression (CES-D score <16; n = 647).
MEASUREMENTS
Cognition
Research assistants, trained by a board-certified neuropsychologist, administered the following cognitive tests.
Digit Symbol: Nine different shapes were coded with the numbers 1 to 9. The subject was given 2 minutes to draw the appropriate shapes in the allotted space according to the code. The total number of correct shapes was recorded. This test was used to evaluate nonverbal general cognition.
Wechsler Adult Intelligence Scale III Block Design: The subject was asked to replicate pictures of colored designs by using a set of blocks. Total raw score based on the number of correct designs completed in the given amount of time was recorded to assess both visuospatial and executive functions.
Trails B: The subject was asked to perform alternations between numbers and letters while the time of the task was recorded. The cap time was 301 seconds. This test was used to measure executive function.
Verbal Fluency (Controlled Oral Word Association Test): The subject was given 1 minute to say as many words as possible that began with a certain letter, 3 separate times. The total number of correct responses to the 3 different letters was recorded. This test was used to measure language ability that is also related to executive function.
Wechsler Memory Scale III Logical Memory (LM): Two stories (A and B) were read aloud to the subject; the subject was then asked to repeat as many details from the story as possible after each reading for immediate recall. After 30 minutes, the subject was asked to repeat details from both stories, which were recorded as Delayed Recall. These tests measured a different aspect of memory from word list learning, which is described in the next paragraph.
Wechsler Memory Scale III Word List Learning: The subject was read a list of 12 words, 4 separate times. The subject was asked to recall the list after each time it was read. The recall total score was calculated for immediate recall. After 30 minutes, the subject was asked to recall the same list of words again, which was recorded as the Delayed Recall score. These tests were used to measure verbal learning and memory.
Other Measurements
Subjects were classified as having CVD according to whether they had been previously informed by a physician that they had congestive heart failure, coronary heart disease, angina pectoris, or a heart attack. Diabetes mellitus was defined as the use of antidiabetic medication or fasting glucose level greater than 126 mg/dL (to convert to millimoles per liter, multiply by 0.0555) (available on 96% of the samples). Stroke history was recorded. Current hypertension was defined as the average of systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg at 2 determinations or was considered present if the subject was taking antihypertensive medications.
A 244–base pair segment of the APOE gene (OMIM 107741), which included the 2 polymorphic sites, was amplified by polymerase chain reaction with a robotic thermal cycler (ABI 877; Applied Biosystems, Foster City, California). The polymerase chain reaction products were digested with 5 U of HhaI, and the fragments were separated by electrophoresis on 8% polyacrylamide nondenaturing gel. The specific allelic fragments were E2, E3, and E4. APOE4 was defined by E4/4, E3/4, or E2/4.20 Renal function, which is associated with plasma Aβ,21 was assessed through measurements of serum creatinine.
STATISTICAL ANALYSIS
Statistical analysis was performed with SAS (version 9.1; SAS Institute Inc, Cary, North Carolina). Mean with standard deviation and t test or analysis of variance were used for the variables with a normal distribution, and median (quartile 1–quartile 3 [Q1–Q3]) and Wilcoxon ranksumtest or Kruskal-Wallis test were used for the variables with a skewed distribution. The χ2 test was used to compare proportions for binary end points. Subjects’ data were also divided into plasma Aβ quartiles and stratified by depression. Linear regression was used to examine associations between different cognitive domains as an outcome and the interaction between depression and plasma Aβ40:Aβ42 ratio or amyloid-associated depression or nonamyloid depression while adjusting for potential confounders including age, race, sex, school, creatinine level, APOE4, vascular diseases, and antidepressant medication use. All Aβ40, Aβ42, and Aβ40:Aβ42 ratio values were transformed to log10 (logAβ). These analyses were also stratified by the cognitive scores (MMSE score ≤23 and >23; LM Delayed Recall score ≤14.6and >14.6) to observe the relationships in subjects with and without significant cognitive impairment. The correlations between logAβ42 and the different cognitive scores were analyzed by Spearman correlation in each quartile of plasma Aβ40 stratified by depression status. Because Bonferroni correction was applied for regression analyses, the 2-sided significance level of P<.0167 was used.
RESULTS
STUDY POPULATION
Nine hundred ninety-five subjects with depression status and plasma Aβ measurements from the Nutrition, Aging, and Memory in the Elderly study were included in this analysis. The average age of this sample was 75.3 (SD, 8.4) years, and 757 subjects (76.1%) were female. The sample was multiethnic, with 607 (61.0%) white, 375 (37.7%) African American, and 13 (1.3%) other ethnicities. Of 989 subjects who provided information on education, 641 (64.8%) had completed high school.
DEPRESSION AND PLASMA Aβ PEPTIDES
Depression, defined as a CES-D score of 16 or greater, was observed in 348 of the subjects (35.0%). Subjects with depression were younger (mean [SD] age, 73.8 [8.5] vs 76.0 [8.3]; P < .001) and tended to have less education than those without depression, whereas there were no differences in sex and ethnicities between the groups (Table 1). Medically, the depression subgroup had similar APOE4 frequencies, lipid profile, and rates of hypertension, stroke, and diabetes mellitus. Those with depression had higher rates of CVD (51.5% vs 38.6%; P < .001) and were more likely to be taking antidepressants (40.1% vs 21.8%; P < .001) than were those without depression. In addition, subjects with depression tended to have a slightly higher level of creatinine than did those without depression.
Table 1.
Demographic, Medical, and Plasma Aβ Data Among Subgroups With and Without Depression
| Covariables | Depression (n=348) |
No Depression (n=647) |
P Value |
|---|---|---|---|
| Age, mean (SD), y | 73.8 (8.5) | 76.0 (8.3) | <.001 |
| Sex, No. F/total (%) | 267/348 (76.7) | 490/647 (75.7) | .73 |
| Education, No. high school and above/total (%) | 209/347 (60.2) | 432/642 (67.3) | .07 |
| Race, No. African American/total (%) | 129/348 (37.1) | 246/647 (38.0) | .26 |
| No. with APOE4/total (%) | 73/346 (21.1) | 163/636 (25.6) | .12 |
| Total cholesterol, mean (SD), mg/dL | 184.5 (44.3) | 186.9 (42.7) | .33 |
| HDL cholesterol, mean (SD), mg/dL | 50.1 (14.6) | 51.4 (14.8) | .24 |
| Hypertension, No./total (%) | 300/341 (88.0) | 536/624 (85.9) | .56 |
| Stroke, No./total (%) | 64/337 (19.0) | 129/634 (20.3) | .61 |
| Diabetes, No./total (%) | 136/329 (41.3) | 214/622 (34.4) | .04 |
| CVD, No./total (%) | 174/338 (51.5) | 240/622 (38.6) | <.001 |
| Creatinine, mean (SD), mg/dL | 1.2 (1.1) | 1.1 (0.9) | .06 |
| Antidepressant use, No./total (%) | 137/342 (40.1) | 138/632 (21.8) | <.001 |
| Plasma Aβ peptides | |||
| Overall | |||
| Aβ40:Aβ42 ratio, median (Q1–Q3) | 7.2 (4.5–11.4) | 7.0 (4.0–10.4) | .16 |
| Aβ40, pg/mL, median (Q1–Q3) | 131.3 (92.2–175.7) | 133.0 (96.6–172.7) | .51 |
| Aβ42, pg/mL, median (Q1–Q3) | 17.1 (11.7–28.0) | 19.4 (12.8–29.5) | .02 |
| Among those without CVD | (n=164) | (n=382) | |
| Aβ40:Aβ42 ratio, median (Q1–Q3) | 7.3 (5.3–11.4) | 6.7 (3.9–9.6) | .05 |
| Aβ40, pg/mL, median (Q1–Q3) | 129.8 (89.9–172.2) | 126.6 (92.6–167.6) | .91 |
| Aβ42, pg/mL, median (Q1–Q3) | 15.7 (11.3–23.4) | 19.2 (12.7–30.4) | .003 |
| Among those without CVD who did not use antidepressants | (n=85) | (n=303) | |
| Aβ40:Aβ42 ratio, median (Q1–Q3) | 8.9 (5.9–12.6) | 6.4 (3.4–10.3) | <.001 |
| Aβ40, pg/mL, median (Q1–Q3) | 138.7 (98.5–184.7) | 125.9 (94.2–172.7) | .06 |
| Aβ42, pg/mL, median (Q1–Q3) | 14.1 (10.1–22.8) | 19.2 (13.1–33.3) | .006 |
Abbreviations: Aβ, amyloid-β; Aβ40, amyloid-β peptide 40; Aβ42, amyloid-β peptide 42; CVD, cardiovascular disease; HDL, high-density lipoprotein; Q, quartile. SI conversion factors: To convert total cholesterol and HDL cholesterol to millimoles per liter, multiply by 0.0259; creatinine to micromoles per liter, multiply by 88.4.
Distributions of plasma Aβ peptides were skewed, showing the following levels: Aβ40: median, 132.7 pg/mL; minimum, 1.6 pg/mL; maximum, 1324.9 pg/mL; Aβ42: median, 18.7 pg/mL; minimum, 1.6 pg/mL; maximum, 780.8 pg/mL; and Aβ40:Aβ42 ratio: median, 7.1; minimum, 0.04; maximum, 86.0. Subjects with depression had a lower concentration of plasma Aβ42 (median, 17.1 vs 19.4 pg/mL; P = .02) than did those without depression, but there were no differences in plasma Aβ40 level and Aβ40:Aβ42 ratio between those with and without depression (Table 1). When the subjects with CVD and antidepressant use were removed, those with depression had significantly higher plasma Aβ40:Aβ42 ratios (median, 8.9 vs 6.4; P < .001), lower concentrations of plasma Aβ42 (median, 14.1 vs 19.2 pg/mL; P = .006), and a tendency toward higher concentrations of plasma Aβ40 (median, 138.7 vs 125.9 pg/mL; P = .06) than did those without depression. This result was consistent with our previously published study.4,22
ASSOCIATION OF COMBINATION OF HIGH PLASMA Aβ40:Aβ42 RATIO AND DEPRESSION WITH COGNITIVE IMPAIRMENT
In this study sample, subjects with depression showed significantly lower scores in every cognitive domain than did those without depression (data not shown). With multivariate linear regression analysis after adjusting for potential confounders including age, education, the APOE4 allele, and medical conditions, the interaction between depression and a high plasma Aβ40:Aβ42 ratio was found to be associated with cognitive impairment (Table 2), especially with memory (LM Delayed Recall:β = −1.9, SE = 0.7, P = .006) and tended to be associated with language (Verbal Fluency: β = −1.8, SE = 0.9, P = .05) and executive function (Trails B: β = 9.0, SE = 5.7, P = .12). Because plasma Aβ is associated with the preclinical stage of AD1,2 but the association disappears once the disease occurs,5 we expected that these relationships should be different between those with and without significant cognitive impairment if these associations truly represent a prodromal stage of AD. As shown in Table 2, the interaction between depression and plasma Aβ40:Aβ42 ratio remained associated with cognitive impairment among those with an MMSE score greater than 23 or with an LM Delayed Recall score greater than 14.6 (the norm −1.5 SDs), cutoffs used to exclude cases with severe cognitive impairment including potential cases with dementia in the study sample23; however, the relationships disappeared among those with an MMSE score of 23 or less or with an LM Delayed Recall score of 14.6 or less. Adding cholesterol and high-density lipoprotein cholesterol levels into the regression models did not influence the relationships (data not shown).
Table 2.
Linear Multivariate-Adjusted Correlates of Each Cognitive Domain as an Outcomea
| LM Delayed Recall (n=885) |
Verbal Fluency (n=888) |
Trails B (n=873) |
||||
|---|---|---|---|---|---|---|
| β Estimate (SE) |
P Value |
β Estimate (SE) |
P Value |
β Estimate (SE) |
P Value |
|
| Age, y | −0.3 (0.04) | <.001 | −0.2 (0.1) | .001 | 3.3 (0.3) | <.001 |
| School, y | 0.9 (0.1) | <.001 | 1.1 (0.1) | <.001 | −7.9 (0.8) | <.001 |
| APOE4 | −1.1 (0.7) | .11 | 1.2 (0.9) | .19 | 4.2 (5.8) | .47 |
| Depression | 1.1 (1.4) | .43 | 0.2 (1.9) | .94 | 8.1 (12.2) | .50 |
| Antidepressant use | 1.0 (0.7) | .13 | 1.0 (0.9) | .28 | 11.2 (5.7) | .05 |
| LogAβ40:Aβ42 | 0.2 (0.4) | .62 | 0.5 (0.5) | .32 | −2.1 (3.3) | .53 |
| Depression × logAβ40:Aβ42 | −1.9 (0.7) | .006 | −1.8 (0.9) | .05 | 9.0 (5.7) | .12 |
| Depression × logAβ40:Aβ42 by subgroup | ||||||
| MMSE score > 23 (n=652)b | −1.3 (0.4) | <.001 | −1.6 (0.5) | <.001 | +11.4 (3.1) | <.001 |
| MMSE score ≤ 23 (n=233)b | −0.03 (0.5) | .95 | −1.0 (0.6) | .10 | +3.4 (3.1) | .24 |
| LM Delayed Recall score > 14.6 (n=575)b | −0.7 (0.3) | .02 | −1.6 (0.6) | .003 | +11.9 (3.6) | .001 |
| LM Delayed Recall score ≤ 14.6 (n=310)b | −0.4 (0.3) | .15 | −0.9 (0.6) | .13 | +7.1 (3.5) | .04 |
Abbreviations: Aβ40, amyloid-β peptide 40; Aβ42, amyloid-β peptide 42; LM, Logical Memory; MMSE, Mini-Mental State Examination.
Additional variables adjusting for sex, race, creatinine level, diabetes mellitus, stroke, and cardiovascular disease are also included. Depression × logAβ40:Aβ42 is the interaction between depression (defined as Center for Epidemiological Studies Depression Scale score ≥ 16) and log10 of plasma Aβ40:Aβ42 ratio. The same regression models were applied to subgroups according to cognitive scores after adjusting for age, school, APOE4, depression, antidepressant use, and logAβ40:Aβ42 in addition to sex, race, creatinine level, diabetes mellitus, stroke, and cardiovascular disease, and only the data on depression × logAβ40:Aβ42 are shown. Because Bonferroni correction was applied, P<.0167 is considered significant.
Includes all of the above adjusting variables.
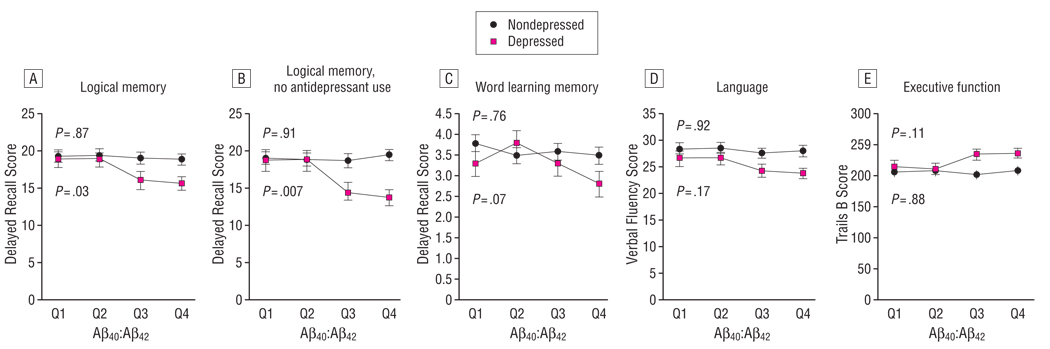
These relationships were further visualized by dividing subjects into 4 quartiles by plasma Aβ40:Aβ42 ratio (Figure). Among subjects who had current depression, LM Delayed Recall scores decreased with every increase of plasma Aβ40:Aβ42 quartile, with a P value of .03 (Figure, A), and this relationship became more significant (mean [SE]: Q1, 18.6 [1.4]; Q2, 18.6 [1.3]; Q3, 14.3 [1.4]; Q4, 13.7 [1.0]; P = .007) when the subjects who were using antidepressants were removed (Figure, B). Word Learning List Delayed Recall, which assessed not only memory but also verbal learning, and the other cognitive scores tended to decrease with the increase in each Aβ40:Aβ42 quartile but did not reach statistical significance among subjects with depression (Figure, C–E). In contrast, at the cross-sectional level, mean cognitive scores were similar across all quartiles of Aβ40:Aβ42 in those without depression (Figure).
Figure.
Quartiles (Q1–Q4) of the ratio of plasma amyloid-β peptide 40 to amyloid-β peptide 42 (Aβ40:Aβ42) and cognitive function among those with and without depression. Different cognitive domains are shown in each Aβ40:Aβ42 quartile among those with and without depression. Values are shown as mean[SE]. P values are for comparisons among the 4 quartiles in those with and without depression.
ASSOCIATION OF DECREASED PLASMA Aβ42 IN DEPRESSION WITH COGNITIVE IMPAIRMENT ONLY AMONG THOSE WITH THE HIGHEST CONCENTRATION OF PLASMA Aβ40
To investigate the interplaying role of both peptides, the relationship between plasma Aβ42 and cognition was further analyzed within each Aβ40 quartile. Among the depressed subjects in the highest plasma Aβ40 quartile (Table 3), plasma Aβ42 concentration was positively correlated with LM Delayed Recall scores (r = 0.29, P = .005) and tended to correlate with MMSE scores (r = 0.22, P = .04) and inversely with Trails B scores (r = −0.23, P = .03), but was not correlated with verbal fluency. In contrast, all of these correlations between plasma Aβ42 and cognitive functions were not observed among nondepressed subjects, even though they also had the highest quartile of plasma Aβ40 (Table 3). There was no correlation between plasma Aβ42 levels and cognitive function among those in the lower quartiles of plasma Aβ40 regardless of depression status (data not shown).
Table 3.
Univariate Correlation Between Plasma Aβ42 and Cognitive Function in Those in the Highest Quartile of Plasma Aβ40 and Comparisons Between Those With and Without Depressiona
| LogAβ42 in Patients in Highest Aβ40 Quartile |
||
|---|---|---|
| Depression (n=91) |
No Depression (n=159) |
|
| MMSE score | ||
| r | 0.22 | 0.046 |
| P value | .04 | .046 |
| LM Delayed Recall | ||
| r | 0.29 | 0.10 |
| P value | .005 | .24 |
| Verbal Fluency | ||
| r | 0.06 | 0.04 |
| P value | .59 | .58 |
| Trails B | ||
| r | −0.23 | −0.02 |
| P value | .03 | .76 |
Abbreviations: Aβ40, amyloid-β peptide 40; Aβ42, amyloid-β peptide 42; LM, Logical Memory; MMSE, Mini-Mental State Examination.
Because Bonferroni correction was applied, P < .0167 is considered significant.
COGNITIVE CHARACTERIZATION OF AMYLOID-ASSOCIATED DEPRESSION
While subjects in the fourth quartile had an Aβ40:Aβ42 ratio with a median equal to 14.2, which is near the levels (> 12 to 16) shown to increase the risk of AD in the 2 cohorts,1,2 both the fourth and third quartiles showed significantly lower LM Delayed Recall scores than the first or second quartile of Aβ40:Aβ42 among subjects with depression (Figure). We therefore used the Aβ40:Aβ42 median of the whole sample to define amyloid-associated depression as the following: (1) no depression: CES-D score less than 16; (2) amyloid-associated depression: CES-D score of 16 or greater and Aβ40:Aβ42 greater than the median; and (3) nonamyloid depression: CES-D score of 16 or greater and Aβ40:Aβ42 ratio less than or equal to the median.
While no differences were found in any of the other variables between the 2 depression subgroups, subjects with amyloid-associated depression were older (mean [SD] age, 74.9 [8.5] vs 72.7 [8.3] years; P = .01), had a slightly higher level of creatinine (mean [SD], 1.3 [1.2] vs 1.1 [1.0] mg/dL; P = .01) (to convert creatinine to micromoles per liter, multiply by 88.4), and were less likely to take antidepressants (32.4% vs 46.2%; P = .009) than were those with nonamyloid depression (Table 4).
Table 4.
Comparison of Cognitive Domains Between Subjects With Amyloid-Associated Depression and Those With Nonamyloid Depression
| Amyloid-Associated Depression (n=177) |
Nonamyloid Depression (n=171) |
df |
t Value |
P Valuea |
|
|---|---|---|---|---|---|
| Age, mean (SD), y | 74.9 (8.5) | 72.7 (8.3) | 346 | −2.46 | .01 |
| Antidepressant use, No./total (%) | 56/173 (32.4) | 78/169 (46.2) | 1 | 6.82 | .009 |
| CES-D score, mean (SD) | 24.4 (7.3) | 24.9 (8.3) | 346 | 0.63 | .67 |
| APOE4, No./total (%) | 39/175 (22.3) | 34/171 (19.9) | 1 | 0.30 | .58 |
| General cognition | |||||
| MMSE score, mean (SD) | 24.2 (3.9) | 24.9 (3.4) | 346 | 1.82 | .15 |
| Digit Symbol, mean (SD) | 31.0 (13.6) | 32.6 (14.1) | 334 | 1.04 | .32 |
| Visuospatial function | |||||
| Block Design, mean (SD) | 18.6 (8.6) | 18.8 (8.8) | 324 | 0.65 | .59 |
| Executive function | |||||
| Trails B, mean (SD) | 236.0 (77.8) | 214.1 (84.3) | 334 | −2.48 | .01 |
| Language | |||||
| Verbal Fluency, mean (SD) | 23.9 (10.9) | 26.5 (11.7) | 342 | 2.15 | .03 |
| Memory | |||||
| LM Delayed Recall, mean (SD) | 15.9 (9.6) | 18.9 (9.2) | 340 | 2.99 | .004 |
| WLL Delayed Recall, mean (SD) | 3.0 (2.7) | 3.6 (2.6) | 342 | 1.94 | .03 |
Abbreviations: CES-D, Center for Epidemiological Studies Depression Scale; LM, Logical Memory; MMSE, Mini-Mental State Examination; WLL, Word List Learning.
By t test for means and χ2 test for numbers and percentages.
Although there was no difference in CES-D scores between the 2 subgroups, those with amyloid-associated depression had poorer memory scores in the LM Delayed Recall (mean [SD], 15.9 [9.6] vs 18.9 [9.2]; P = .004) and tended to have lower Word Learning List Delayed Recall scores (mean [SD], 3.0 [2.7] vs 3.6 [2.6]; P = .03), lower language ability (Verbal Fluency mean [SD], 23.9 [10.9] vs 26.5 [11.7]; P = .03), and lower executive function (Trails B test mean [SD], 236.0 [77.8] vs 214.1 [84.3]; P = .01) than did those with nonamyloid depression (Table 4). In contrast, scores evaluating general cognition measured by either the MMSE or Digit Symbol and scores on visuospatial function measured by Block Design were similar between those with amyloid-associated depression and those with nonamyloid depression.
To further test the existence of the foregoing depression subtypes, multivariate linear regression analysis was applied to investigate the relationship between each cognitive domain and amyloid-associated depression vs nonamyloid depression relative to those without depression, after adjusting for potential confounders. Among the subjects with amyloid-associated depression and those without depression combined, amyloid-associated depression was associated with poor memory (LM Delayed Recall: β estimate = −1.9, SE = 0.4; P < .001), visuospatial dysfunction (Block Design: β estimate = −1.4, SE = 0.4; P < .001) (Table 5), and executive dysfunction (Trails B: β estimate = 16.0, SE = 3.3; P < .001) after adjusting for the confounders including antidepressant use and others. Although subjects with nonamyloid depression had a significantly higher level of plasma Aβ42 (median, 30.7 vs 19.2 pg/mL; P < .001), but not Aβ40, than those without depression, nonamyloid depression was not associated with memory performance (LM Delayed Recall: β estimate = −0.9, SE = 0.8; P = .30) but was related to visuospatial (Block Design: β estimate = −3.0, SE = 0.8; P < .001) (Table 5) and executive (Trails B: β estimate = 21.2, SE = 6.8; P = .002) dysfunction after adjusting for these confounders. Adding covariables of lipids and statins did not affect these relationships (data not shown).
Table 5.
Multivariate-Adjusted Correlates of Each Cognitive Domain as an Outcome in Subjects Without Depression Combined With Those With Either Amyloid-Associated Depression or Nonamyloid Depressiona
| Subjects With Amyloid-Associated Depression + Without Depression |
Subjects With Nonamyloid Depression + Without Depression |
|||||||
|---|---|---|---|---|---|---|---|---|
| LM Delayed Recall (n=735) |
Block Design (n=710) |
LM Delayed Recall (n=730) |
Block Design (n=704) |
|||||
| β Estimate (SE) |
P Value |
β Estimate (SE) |
P Value |
β Estimate (SE) |
P Value |
β Estimate (SE) |
P Value |
|
| Age, y | −0.3 (0.04) | <.001 | −0.2 (0.04) | <.001 | −0.3 (0.04) | <.001 | −0.2 (0.04) | <.001 |
| School, y | 0.9 (0.1) | <.001 | 0.8 (0.1) | <.001 | 0.9 (0.1) | <.001 | 0.9 (0.1) | <.001 |
| APOE4 | −1.5 (0.7) | .04 | −2.0 (0.7) | .005 | −1.1 (0.8) | .16 | −1.1 (0.7) | .14 |
| Antidepressant use | 1.3 (0.8) | .09 | 0.1 (0.7) | .87 | 0.4 (0.8) | .65 | 0.5 (0.7) | .46 |
| Amyloid-associated depression | −1.9 (0.4) | <.001 | −1.4 (0.4) | <.001 | ||||
| Nonamyloid depression | −0.9 (0.8) | .30 | .30 | <.001 | ||||
Abbreviation: LM, Logical Memory.
All of the listed variables were included in the regression model after adjusting for sex, race, creatinine level, diabetes mellitus, stroke, and cardiovascular disease. Because Bonferroni correction was applied, P<.0167 is considered significant.
COMMENT
The relationships between plasmaAβ level, cognitive function, and depression remain complex. The present study suggests that there may be at least 2 depression subtypes from the cognitive perspective: (1) amyloid-associated depression, which is associated with poor memory and other cognitive dysfunction (Table 2 and Figure), and (2) nonamyloid depression, which is associated with only visuospatial and executive dysfunction (Tables 4 and 5). Because a high plasma Aβ40:Aβ42 ratio increases the risk of AD prospectively,1,2 we hypothesize that amyloid-associated depression is more likely to be a prodromal stage of AD than is a high Aβ40:Aβ42 ratio without depression. Antidepressants, specifically selective serotonin reuptake inhibitors, have been shown to be associated with lower plasma Aβ40 levels.22 Consistently, this study showed that fewer subjects with amyloid-associated depression took antidepressants than did those with nonamyloid depression (Table 4).
There is growing evidence in the literature that depression may be either a risk factor for AD3,24–27 or an early symptom of AD.12,28–32 A neuropathology study has shown that history of depression is associated with increased amyloid plaques and neurofibrillary tangles, which are the neuropathological hallmarks of AD.33 The pattern of cognitive impairment, prominently poor memory, in amyloid-associated depression (Table 4 and 5) was consistent with mild cognitive impairment (MCI),23 which is presumed to be a prodromal stage of AD.27,34 Although MCI is associated with depression, depression is also found to increase the risk of developing MCI.32,35,36
The severity of depressive symptoms was correlated with lower concentration of plasma Aβ42, a determining factor of Aβ40:Aβ42 ratio, only when CVD cases were excluded from the study sample.4 Most likely there are different subtypes of depression in the elderly population, including (1) early-onset depression, (2) poststroke depression, 37 (3) vascular depression related to CVD and other vascular risk factors that lead to executive dysfunction, 38 (4) preclinical depression of AD, and (5) comorbid depression in AD. Because these depression subtypes have different underlying pathological characteristics and prognoses, not all should be related to plasma Aβ levels, or they may be related to plasma Aβ levels differently. In contrast to amyloid-associated depression, non-amyloid depression was not found to be associated with memory (Table 5) even though these subjects had higher levels of plasma Aβ42 than did those without depression. Although the rates of CVD (92 of 171 [53.8%] vs 82 of 177 [46.3%]; P = .32) and stroke (31 of 171 [18.1%] vs 35 of 177 [19.8%]; P = .89) were similar between subjects with nonamyloid depression and those with amyloid-associated depression, cerebral microvascular pathological data in these cases is unknown. Regardless, nonamyloid depression was strongly associated with a cognitive pattern of vascular depression and visuospatial and executive dysfunction (Table 5). This and other factors may explain the seemingly conflicting result in another study in which depressed patients had a higher, not lower, level of plasma Aβ42 than the controls.39,40 In fact, our own study has also shown that vascular depression related to CVD is not associated with lower plasma Aβ42 levels.4
It is intriguing to find that the combination of 2 peptides in plasma, a high level of Aβ40 and a low level of Aβ42, was associated with poor memory, whereas each peptide alone was not found to have this relationship in the regression analysis (Table 3). The peptides Aβ42, a major component of AD pathological findings in the brain,41 and Aβ40, a component of cerebral amyloid angiopathy, 42 are produced by the processing of APP. Although plasma Aβs reflect dynamic levels governed by both peripheral21,43–46 and central47 nervous system origins, these 2 peptides in plasma have been linked to pathological conditions in the brain. First, high plasma Aβ40 level is associated with cerebral microvascular pathological changes, white matter hyperintensities, and lacunar infarct.9,10 Both white matter hyperintensities and lacunar infarcts are linked to cognitive impairment,48,49 dementia incidence,50 and depression49 in the elderly population. Second, studies using APP transgenic mice have demonstrated that plasma Aβ42 level declines significantly before Aβ42 is deposited in the brain to form the AD changes.7,8 Therefore, a high Aβ40:Aβ42 ratio in plasma may be a biomarker to indicate cerebral microvascular pathological changes, which are associated with high plasma Aβ40 level, coexisting with the AD pathological features, which may be linked to plasma Aβ42 decline at the preclinical stage. These 2 pathological conditions in the brain additively or synergistically result in more severe cognitive dysfunction than either condition alone.51,52
Plasma Aβ level correlates with cerebrospinal fluid Aβ concentration in APP transgenic mice when they are young and pathological changes of AD have not yet formed. However, this correlation disappears as the animals age and Aβ is deposited in the brain.47 Similarly in humans, although plasma Aβ level cannot be used for the diagnosis of AD,5,18,53,54 it is still possible that plasma Aβ level can be used as a screening tool for the risk of AD. In this study, the relationship between poor memory and a high plasma Aβ40:Aβ42 ratio in depression was observed among subjects with normal cognition or MCI, but the relationship disappeared among those with severe cognitive impairment (Table 2). Two large population studies, mainly containing patients with late-onset AD, have shown that a high plasma Aβ40:Aβ42 ratio, determined by both low Aβ42 and high Aβ40 levels, increases the risk of AD prospectively.1,2 Although another population study reports that high plasma Aβ42 level is associated with the risk of AD,55 plasma Aβ42 level declines in these subjects before the onset of the disease, leading to low levels of plasma Aβ42 at the prodromal stage. Unlike late-onset AD, both early-onset AD56 and Down syndrome57–59 present with high plasma Aβ42 level at the preclinical stage. Another study shows that patients with MCI have higher levels of plasma Aβ42 than do controls but only in women.60 It is not yet known whether plasma Aβ status is different at the preclinical stage of AD with and without depression. Because only a subset of the patients with late-onset AD present with depression at the start of the disease, our data suggest that the combination of depressive symptoms and plasma Aβ level may more specifically inform the prodromal stage of AD than does either one status alone.
Although our study found that amyloid-associated depression presented with poor memory, and others hypothesized that elevated plasma Aβ42 level in depression may contribute to AD,61 without a longitudinal study we cannot yet conclude that amyloid-associated depression vs nonamyloid depression is a precursor of AD. We have used different analytical methods and applied Bonferroni correction to prevent against type I error, but the cross-sectional design is still the major limitation of our study. Other limitations include the following: (1) depression was based on the CES-D score rather than the DSM-IV criteria, and we had no information about the onset and the course; (2) some variables, such as CVD and stroke, were self-reported; (3) the complexity of plasma Aβ62,63 requires brain investigation to validate amyloid-associated depression as a unique depression subtype; (4) although our assays in measurements of Aβ40 vs Aβ42 in plasma had high sensitivity and specificity, the field lacks standard assays for interlaboratory comparisons; and (5) the correlation coefficients shown in Table 3 were significant, but probably not large enough to be applied in clinical practice. Nevertheless, our discoveries have found the relationship between depression severity and low plasma Aβ42 level4 and further have linked a high plasma Aβ40:Aβ42 ratio in depression with poor memory, especially among those with normal or mildly impaired cognition. Our findings warrant prospective studies to examine whether amyloid-associated depression is related to pathological changes in the brain and predict the onset of cognitive decline and AD in this and other populations.
Acknowledgments
Funding/Support: This study was supported by grants AG-022476 (Dr Qiu) and AG-21790 (Dr Folstein) from the National Institute on Aging. Support was also provided through the General Clinical Research Center funded by the National Center for Research Resources of the National Institutes of Health under grant MO1-RR00054. Dr Steffens was supported by grant MH70027 from the National Institutes of Mental Health.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: We thank Dennis J. Selkoe, MD, for his support and collaboration and the Nutrition, Aging, and Memory in Elders study staff and the Boston home care agencies for their hard work and acquisition of subjects.
REFERENCES
- 1.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Aβ1–40 and Aβ1–40 and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5(8):655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 2.Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of low plasma Aβ42/Aβ40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease [published correction appears in Arch Neurol. 2007;64(9):1246] Arch Neurol. 2007;64(3):354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 3.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu WQ, Sun X, Selkoe DJ, Mwamburi DM, Huang T, Bhadela R, Bergethon P, Scott TM, Summergrad P, Wang L, Rosenberg I, Folstein M. Depression is associated with low plasma Aβ42 independently of cardiovascular disease in the homebound elderly. Int J Geriatr Psychiatry. 2007;22(6):536–542. doi: 10.1002/gps.1710. [DOI] [PubMed] [Google Scholar]
- 5.Iwatsubo T. Amyloid beta protein in plasma as a diagnostic marker for Alzheimer’s disease. Neurobiol Aging. 1998;19(2):161–163. doi: 10.1016/s0197-4580(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 6.Pomara N, Willoughby LM, Sidtis JJ, Mehta PD. Selective reductions in plasma Aβ 1–42 in healthy elderly subjects during longitudinal follow-up: a preliminary report. Am J Geriatr Psychiatry. 2005;13(10):914–917. doi: 10.1176/appi.ajgp.13.10.914. [DOI] [PubMed] [Google Scholar]
- 7.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21(2):372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeMattos RB, Bales KR, Parsadanian M, O’Dell MA, Foss EM, Paul SM, Holtz-man DM. Plaque-associated disruption of CSF and plasma amyloid-beta (Aβ) equilibrium in a mouse model of Alzheimer’s disease. J Neurochem. 2002;81(2):229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 9.van Dijk EJ, Prins ND, Vermeer SE, Hofman A, van Duijn CM, Koudstaal PJ, Breteler MM. Plasma amyloid beta, apolipoprotein E, lacunar infarcts, and white matter lesions. Ann Neurol. 2004;55(4):570–575. doi: 10.1002/ana.20050. [DOI] [PubMed] [Google Scholar]
- 10.Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, Rosand J, Growdon JH, Greenberg SM. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66(1):23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 11.Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. 2005;57(3):381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 12.Steffens DC, Plassman BL, Helms MJ, Welsh-Bohmer KA, Saunders AM, Breitner JC. A twin study of late-onset depression and apolipoprotein E ε4 as risk factors for Alzheimer’s disease. Biol Psychiatry. 1997;41(8):851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8(6):847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- 15.Scott TM, Peter I, Tucker KL, Arsenault L, Bergethon P, Bhadelia R, Buell J, Collins L, Dashe JF, Griffith J, Hibberd P, Leins D, Liu T, Ordovas JM, Patz S, Price LL, Qiu WQ, Sarnak M, Selhub J, Smaldone L, Wagner C, Wang L, Weiner D, Yee J, Rosenberg I, Folstein M. The Nutrition, Aging, and Memory in Elders (NAME) study: design and methods for a study of micronutrients and cognitive function in a homebound elderly population. Int J Geriatr Psychiatry. 2006;21(6):519–528. doi: 10.1002/gps.1503. [DOI] [PubMed] [Google Scholar]
- 16.Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60(7):958–964. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- 17.Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Aβ42. J Biol Chem. 1999;274(27):18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- 18.Radloff L. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 19.Parikh RM, Eden DT, Price TR, Robinson RG. The sensitivity and specificity of the Center for Epidemiologic Studies Depression Scale in screening for post-stroke depression. Int J Psychiatry Med. 1988;18(2):169–181. doi: 10.2190/bh75-euya-4fm1-j7qa. [DOI] [PubMed] [Google Scholar]
- 20.Lahoz C, Osgood D, Wilson PW, Schaefer EJ, Ordovas JM. Frequency of phenotype-genotype discrepancies at the apolipoprotein E locus in a large population study. Clin Chem. 1996;42(11):1817–1823. [PubMed] [Google Scholar]
- 21.Arvanitakis Z, Lucas JA, Younkin LH, Younkin SG, Graff-Radford NR. Serum creatinine levels correlate with plasma amyloid beta protein. Alzheimer Dis Assoc Disord. 2002;16(3):187–190. doi: 10.1097/00002093-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Mwamburi DM, Bungay K, Prasad J, Yee J, Lin YM, Liu TC, Summergrad P, Folstein M, Qiu WQ. Depression, antidepressants, and plasma amyloid beta (beta) peptides in those elderly who do not have cardiovascular disease. Biol Psychiatry. 2007;62(12):1413–1417. doi: 10.1016/j.biopsych.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 24.Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Stern Y, Mayeux R. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53(2):175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 25.Geerlings MI, Schoevers RA, Beekman AT, Jonker C, Deeg DJ, Schmand B, Adèr HJ, Bouter LM, Van Tilburg W. Depression and risk of cognitive decline and Alzheimer’s disease: results of two prospective community-based studies in the Netherlands. Br J Psychiatry. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- 26.Paterniti S, Verdier-Taillefer MH, Dufouil C, Alperovitch A. Depressive symptoms and cognitive decline in elderly people: longitudinal study. Br J Psychiatry. 2002;181:406–410. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- 27.Kumar R, Parslow RA, Jorm AF, Rosenman SJ, Maller J, Meslin C, Anstey KJ, Christensen H, Sachdev PS. Clinical and neuroimaging correlates of mild cognitive impairment in a middle-aged community sample: the Personality and Total Health Through Life 60+ Study. Dement Geriatr Cogn Disord. 2006;21(1):44–50. doi: 10.1159/000089251. [DOI] [PubMed] [Google Scholar]
- 28.Berger AK, Fratiglioni L, Forsell Y, Winblad B, Backman L. The occurrence of depressive symptoms in the preclinical phase of AD: a population-based study. Neurology. 1999;53(9):1998–2002. doi: 10.1212/wnl.53.9.1998. [DOI] [PubMed] [Google Scholar]
- 29.Ritchie K, Ledesert B, Touchon J. Subclinical cognitive impairment: epidemiology and clinical characteristics. Compr Psychiatry. 2000;41(2) suppl 1:61–65. doi: 10.1016/s0010-440x(00)80010-3. [DOI] [PubMed] [Google Scholar]
- 30.Cervilla JA, Prince M, Joels S, Mann A. Does depression predict cognitive outcome 9 to 12 years later? evidence from a prospective study of elderly hypertensives. Psychol Med. 2000;30(5):1017–1023. doi: 10.1017/s0033291799002779. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 32.Lopez OL, Becker JT, Sweet RA, Klunk W, Kaufer DI, Saxton J, Habeych M, De-Kosky ST. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003;15(3):346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- 33.Rapp MA, Schnaider-Beeri M, Grossman HT, Sano M, Perl DP, Purohit DP, Gorman JM, Haroutunian V. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63(2):161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 34.Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. JAMA. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 35.Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Pankratz VS, Rocca WA. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63(3):435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 36.Elderkin-Thompson V, Thomas MA, Binesh N, Mintz J, Haroon E, Dunkin JJ, Kumar A. Brain metabolites and cognitive function among older depressed and healthy individuals using 2D MR spectroscopy. Neuropsychopharmacology. 2004;29(12):2251–2257. doi: 10.1038/sj.npp.1300553. [DOI] [PubMed] [Google Scholar]
- 37.Robinson RG, Bolduc PL, Price TR. Two-year longitudinal study of poststroke mood disorders: diagnosis and outcome at one and two years. Stroke. 1987;18(5):837–843. doi: 10.1161/01.str.18.5.837. [DOI] [PubMed] [Google Scholar]
- 38.Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. Am J Psychiatry. 1997;154(4):562–565. doi: 10.1176/ajp.154.4.562. [DOI] [PubMed] [Google Scholar]
- 39.Pomara N, Doraiswamy PM, Willoughby LM, Roth AE, Mulsant BH, Sidtis JJ, Mehta PD, Reynolds CF, III, Pollock BG. Elevation in plasma Aβ42 in geriatric depression: a pilot study. Neurochem Res. 2006;31(3):341–349. doi: 10.1007/s11064-005-9029-z. [DOI] [PubMed] [Google Scholar]
- 40.Qiu WQ, Summergrad P, Folstein M. Plasma Aβ42 levels and depression in the elderly. Int J Geriatr Psychiatry. 2007;22(9):930. doi: 10.1002/gps.1710. [DOI] [PubMed] [Google Scholar]
- 41.Selkoe DJ. The ups and downs of Aβ. Nat Med. 2006;12(7):758–759. doi: 10.1038/nm0706-758. [DOI] [PubMed] [Google Scholar]
- 42.Zhang-Nunes SX, Maat-Schieman ML, van Duinen SG, Roos RA, Frosch MP, Greenberg SM. The cerebral beta-amyloid angiopathies: hereditary and sporadic. Brain Pathol. 2006;16(1):30–39. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M, Inestrosa NC, Ross GS, Fernandez HL. Platelets are the primary source of amyloid β-peptide in human blood. Biochem Biophys Res Commun. 1995;213(1):96–103. doi: 10.1006/bbrc.1995.2103. [DOI] [PubMed] [Google Scholar]
- 44.Casoli T, Di Stefano G, Giorgetti B, Grossi Y, Balietti M, Fattoretti P, Bertoni-Freddari C. Release of β-amyloid from high-density platelets: implications for Alzheimer’s disease pathology. Ann N Y Acad Sci. 2007;1096:170–178. doi: 10.1196/annals.1397.082. [DOI] [PubMed] [Google Scholar]
- 45.Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid β-protein by degradation. J Biol Chem. 1998;273(49):32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 46.Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292(5521):1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 47.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295(5563):2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 48.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 49.de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57(11):1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 50.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 51.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease: the Nun Study. JAMA. 1997;277(10):813–817. [PubMed] [Google Scholar]
- 52.Heyman A, Fillenbaum GG, Welsh-Bohmer KA, Gearing M, Mirra SS, Mohs RC, Peterson BL, Pieper CF. Cerebral infarcts in patients with autopsy-proven Alzheimer’s disease: CERAD, part XVIII: Consortium to Establish a Registry for Alzheimer’s Disease. Neurology. 1998;51(1):159–162. doi: 10.1212/wnl.51.1.159. [DOI] [PubMed] [Google Scholar]
- 53.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid β proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57(1):100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 54.Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, Mehta PD. Plasma Aβ40 and Aβ42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61(9):1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 55.Mayeux R, Tang MX, Jacobs DM, Manly J, Bell K, Merchant C, Small SA, Stern Y, Wisniewski HM, Mehta PD. Plasma amyloid beta-peptide 1–42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46(3):412–416. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 56.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 57.Mehta PD, Mehta SP, Fedor B, Patrick BA, Emmerling M, Dalton AJ. Plasma amyloid β protein 1–42 levels are increased in old Down syndrome but not in young Down syndrome. Neurosci Lett. 2003;342(3):155–158. doi: 10.1016/s0304-3940(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 58.Mehta PD, Capone G, Jewell A, Freedland RL. Increased amyloid β protein levels in children and adolescents with Down syndrome. J Neurol Sci. 2007;254(1–2):22–27. doi: 10.1016/j.jns.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Schupf N, Patel B, Pang D, Zigman WB, Silverman W, Mehta PD, Mayeux R. Elevated plasma β-amyloid peptide Aβ42 levels, incident dementia, and mortality in Down syndrome. Arch Neurol. 2007;64(7):1007–1013. doi: 10.1001/archneur.64.7.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Assini A, Cammarata S, Vitali A, Colucci M, Giliberto L, Borghi R, Inglese ML, Volpe S, Ratto S, Dagna-Bricarelli F, Baldo C, Argusti A, Odetti P, Piccini A, Tabaton M. Plasma levels of amyloid β-protein 42 are increased in women with mild cognitive impairment. Neurology. 2004;63(5):828–831. doi: 10.1212/01.wnl.0000137040.64252.ed. [DOI] [PubMed] [Google Scholar]
- 61.Pomara N, Murali Doraiswamy P. Does increased platelet release of Aβ peptide contribute to brain abnormalities in individuals with depression? Med Hypotheses. 2003;60(5):640–643. doi: 10.1016/s0306-9877(02)00380-8. [DOI] [PubMed] [Google Scholar]
- 62.Zetterberg H, Blennow K. Plasma Aβ in Alzheimer’s disease: up or down? Lancet Neurol. 2006;5(8):638–639. doi: 10.1016/S1474-4422(06)70503-8. [DOI] [PubMed] [Google Scholar]
- 63.Oprisiu R, Serot JM, Godefroy O, Black SE, Fournier A. Plasma amyloid-β concentrations in Alzheimer’s disease: an alternative hypothesis. Lancet Neurol. 2006;5(12):1001–1002. doi: 10.1016/S1474-4422(06)70612-3. [DOI] [PubMed] [Google Scholar]