Abstract
The binding of Plasmodium falciparum parasitized erythrocytes to uninfected erythrocytes (rosetting) is associated with severe malaria. The glycosaminoglycan heparan sulfate is an important receptor for rosetting. The related glycosaminoglycan heparin was previously used in treatment of severe malaria, although abandoned because of the occurrence of severe bleedings. Instead, low anticoagulant heparin (LAH) has been suggested for treatment. LAH has successfully been evaluated in safety studies and found to disrupt rosettes and cytoadherence in vitro and in vivo in animal models, but the effect of LAH on fresh parasite isolates has not been studied. Herein, we report that two different LAHs (DFX232 and Sevuparin) disrupt rosettes in the majority of fresh isolates from Cameroonian children with malaria. The rosette disruption effect was more pronounced in isolates from complicated cases than from mild cases. The data support LAH as adjunct therapy in severe malaria.
Introduction
Malaria is a major health problem in sub-Saharan Africa but also in parts of Asia and Latin America, and it is estimated that about 1 million children die every year because of Plasmodium falciparum malaria.1 Despite major efforts, there is still no vaccine for malaria, and there is a great need for new effective therapies, especially against severe malaria. A striking difference between P. falciparum and other human Plasmodium species is the capacity of the P. falciparum parasitized red blood cells (pRBCs) to adhere to host cells, both through binding to the endothelium and other cells in the microvasculature (cytoadherence) and through binding to non-pRBCs (rosetting).2 Cytoadherence and rosetting lead to sequestration of pRBCs and RBCs, cause obstruction of the blood flow in the microvasculature, and may result in severe disease symptoms if binding is pronounced.3,4 A number of previous studies have shown a correlation between rosetting and the severity of malaria5–10 and also between parasitemia and rosetting.11
The binding of pRBCs to host cells is mediated by the variant multidomain protein P. falciparum erythrocyte membrane protein 1 (PfEMP1) encoded by the var gene family (with around 60 copies per genome). The N-terminus of PfEMP1 has a number of cysteine-rich domains.8,12–15 One such domain is the Duffy binding-like domain-α (DBL1α), which has been shown to mediate rosetting and endothelial binding of pRBCs through heparan sulfate (HS), blood group A antigen, and complement receptor 1 (CR1).13,16–19 Immunization of rats and macaques with one DBL1α domain from a highly rosetting strain (FCR3S1.2) has been shown to diminish sequestration of pRBC in the circulation of these animals.20
HS as well as heparin belong to a family of glycosaminoglycans (GAGs), and heparin is a highly sulfated form of HS. GAGs are composed of the same building blocks, glucosamine and glucuronic or iduronic acid, and most are negatively charged based on sulfate modifications. It has been shown that both HS and heparin bind directly to DBL1α of PfEMP1,13,16 and avid interaction requires at least a 12-mer (3.6-kDa) fragment of heparin as well as N-sulfatation and 6-O- and 2-O-sulfations.21
Previous work has shown that both HS and heparin have the ability to disrupt rosettes.19,22–24 Importantly, binding of heparin and HS to pRBC is more pronounced in isolates from children with severe malaria, thus suggesting that HS is one of the receptors involved in the causation of the pathology of severe malaria.6 Heparin was previously used in the treatment of severe malaria, especially when the malaria was accompanied by disseminated intravascular coagulation,25–29 but it was withdrawn because of its strong anticoagulant action, with side effects such as intracranial bleeding.30 The binding of heparin to antithrombin III (AT), which accounts for its major anticoagulant activity, is mediated by a pentameric sequence.31,32 Heparin devoid of its anticoagulant activity can be obtained through oxidation of this pentameric sequence, leading to depolymerization of the GAG and generating a low anticoagulant heparin (LAH).23,31,33 The molecular weight distribution and chemical composition of LAH are similar to that of the low molecular weight heparin (LMWH) frequently used in the clinic as an anticoagulant. However, the anticoagulant activity of LAH is strongly reduced compared with standard heparin and LMWH. The pentamer responsible for anticoagulant activity has been shown to not be essential for binding to the DBL1α domain,21,23,34 and the oxidation does not affect the rosette disruptive capacity or the inhibition of endothelial binding mediated by DBL1α.23 Experiments with laboratory isolates and in vitro adapted isolates from malaria patients in Uganda have shown that LAH can disrupt rosettes and reduce cytoadherence both in vitro and in rat and macaque monkey models of severe malaria in vivo.23,35 An intravenous (i.v.) injection of LAH blocked up to 80% of infected erythrocytes from binding in the microvasculature of the rat and also released previously sequestered pRBCs into the circulation. In the corresponding monkey model, 55% of the sequestration was reduced after i.v. treatment of the animal.23 Furthermore, LAH inhibited merozoite invasion of erythrocytes in vitro.23
It is important to investigate rosettes of fresh clinical isolates, because var gene expression and therefore, rosetting may change early after in vitro adaptation of P. falciparum.36–38 Thus, to fortify the pre-clinical data, we have here studied the capacity of LAHs to disrupt rosettes formed by P. falciparum-infected erythrocytes obtained directly from children with malaria. We report that LAH disrupts rosettes of 42 of 47 fresh isolates obtained from Cameroonian children with mild or complicated malaria, and we state that rosettes from children with complicated malaria are more sensitive to the LAH than are rosettes of children with mild malaria.
Materials and Methods
Sample collection in Cameroon.
The study was conducted in Buea, a city with 150 000 inhabitants in the southwestern part of Cameroon. Malaria transmission takes place throughout the year but peaks during the rainy season that lasts from April to October.39 The entomological inoculation rate (EIR) has been shown to be 0.56 infected Anopheles mosquito bites per person per night in this area during the rainy season.40 The fieldwork was performed between March and May 2007 at three different health centers and four different hospitals in the Buea area. The study included malaria-infected children between 6 months and 14 years of age. Informed consent was given by the guardian. The diagnosis was based on laboratory tests (Giemsa-stained blood smears) and clinical examination. Hemoglobin levels in whole blood were measured using HemoCue Hb 201+ (Hemocue, Ängelholm, Sweden). Complicated P. falciparum malaria was defined as a patient requiring hospital admission and quinine or artemether infusion because of anemia, hyperparasitemia (parasitemia > 5%), or severe symptoms including hyperpyrexia, seizures, prostration, and/or vomiting. Cases with a positive blood smear for P. falciparum without complicating manifestations were classified as mild malaria and treated as outpatients with treatment per os. Ethical permissions for the study were obtained both in Cameroon and Sweden (numbers G379/900 and 2006/1323-31, respectively).
P. falciparum clinical isolates used in the study.
Blood was withdrawn from patients with parasitemia above 10,000 pRBC/μL blood and collected in ethylene diamine tetraacetic acid (EDTA) tubes. The blood samples were depleted of leukocytes by treatment with polymorph preparation (Axis-Shield, Oslo, Norway) according to the manufacturer's instructions within 2 hours of collection. Briefly, 2–5 mL whole blood were carefully layered over 5 mL polymorph preparation and centrifuged at 500 × g for 30 min. Alternatively, if polymorph preparation could not be used because of the 2-hour time limit after collection, the fresh blood samples were centrifuged at 400 × g for 5 min to separate RBCs, leukocytes, and plasma. The plasma and leukocyte band were removed, and separated packed RBCs were washed three times with RPMI (Roswell Park Memorial Institute) 1640 medium (Sigma Aldrich, St. Louis, MO). A total of 200 μL packed RBCs was transferred to 4.0 mL malaria culture medium supplemented with 10% inactivated human AB+ non-immune Swedish serum and placed at 37°C for maturation of ring-stage parasites to trophozoites using standard methods.41,42 The parasitemia was counted, and the rosetting rate was determined by calculating the number of trophozoite pRBCs within rosettes relative to the total number of trophozoite pRBCs present in the culture. A rosette was defined as at least two uninfected RBCs bound to one pRBC.41
Glycosaminoglycans used in the study.
LAHs are heparin derivatives prepared from porcine intestinal heparin. LAH was chemically generated by periodate oxidation of the heparin at the antithrombin (AT) binding sequence.31 This process led to splitting of C2–C3 bonds of all non-sulfated hexuronic acid residues and subsequent cleavage of the heparin chain at these sites. Because the AT binding pentasaccharide sequence contains a non-sulfated glucuronic acid unit, this structure and hence, the anticoagulant activity were eliminated. A first LAH batch, DFX232, was prepared by oxidizing heparin followed by a reduction. The second LAH batch, Sevuparin, was prepared in the same manner but included an acidic hydrolysis step. The preparations were dried, and the anticoagulant activities of the two LAH batches were determined by measuring the anti-IIa and anti-Xa activities. The assays were performed according to the Ph Eur procedure for low molecular mass heparin (monograph 0828) in the European Pharmacopoeia.43 The anticoagulant activity of LAH was strongly reduced compared with heparin and LMWH. The final products DFX232 and Sevuparin had molecular weights of 10.1 and 7.4 kDa, respectively.
Rosette disruption assay.
Rosette disruption assays were performed on pRBC cultures harboring trophozoites 20–24 h post-invasion. LAH was diluted in RPMI, and samples were analyzed in triplicates as previously described.24 Briefly, parasite cultures were concentrated two times from a hematocrit of 5–10%, and 25 μL culture were added to 25 μL LAH in RPMI, giving final concentrations of 1,000 and 100 μg/mL. After incubation of samples at 37°C for 30 minutes, the parasites were stained with acridine orange, and the rosettes were counted. For each sample, 25 fields, equivalent to approximately 3,500–4,000 RBCs, were counted. In parallel, mock-treated samples were analyzed in the presence of RPMI alone.
Statistical analysis.
Before statistical analysis, the rosetting rates for the isolates were converted into relative rosetting rates by calculating the proportion of remaining rosettes after treatment compared with the mock-treated erythrocytes. The statistical analysis was carried out with Prism version 5.0 for Windows (Graphpad Software, San Diego, CA). Student paired t test or Wilcox signed rank test (when the samples were not normally distributed) was used. For the comparisons between groups with mild and complicated disease, unpaired t test and Mann–Whitney U test, respectively, were used. Pearson's χ2 test was used when the data were binary. As estimated in a previous study, rosette disruption of ≥ 15% was regarded as significant.24 Where applicable, data are presented as mean and standard error of mean (SEM).
Results
Characteristics of the study sample.
In all, 1,079 children between 6 months and 14 years of age were screened for malaria, and 63% were found to be infected (682 of 1,079). When the parasite density was ≥ 10,000 pRBC/μL blood, a venous blood sample was collected, which resulted in a total of 144 samples (Table 1). Of the 144 samples, 140 samples were cultivated, and 112 isolates (80%) successfully grew into trophozoites. Forty-seven isolates showed a rosetting phenotype, with a rosetting rate that ranged from 2.1% to 79% (Figure 1 and Table 1). Of these, 20 samples were from children diagnosed with complicated malaria, and the other 27 samples were from children with mild malaria. There was a significant difference in the number of isolates that formed rosettes between the mild and complicated malaria groups (P = 0.0009) (Table 1), because rosettes were present in 22% of the samples in the mild group and 50% of the samples in the complicated group. There was a significant difference in parasitemia between the groups, with a mean parasitemia of 3.4% and 6.1% for the mild and complicated groups, respectively (P = 0.04). However, there were no significant differences in rosetting rates or hemoglobin levels between the mild and complicated groups.
Table 1.
Details of field isolates
| Field isolate | Measurement |
|---|---|
| No. of children tested for Plasmodium falciparum | 1,079 |
| No. of children infected with P. falciparum | 682 (63%) |
| No. of children included in the study (> 10,000 parasites/μL blood) | 144 (12%) |
| No. of cases of complicated malaria | 54 (38%) |
| No. of cases of mild malaria | 90 (62%) |
| No. of isolates successfully matured to trophozoites | 112 (80%)* |
| No. of rosetting isolates (mild/complicated) | 47; 42%† (27/20) |
| Percent rosetting isolates (mild/complicated) | 22%/50%‡ |
| Mean rosetting rate (range) | 20.42% (2–79%) |
| Mean rosetting rate (mild/complicated) | 20.9%/19.7% |
| Mean parasitemia (mild/complicated) | 3.6% (2.7%/5.3%)‡ |
| Mean parasitemia in rosetting isolates (mild/complicated) | 4.5% (3.4%/6.1%)‡ |
| Mean hemoglobin level (mild/complicated) | 90.2 g/L (95/82)§ |
| Mean hemoglobin level in rosetting isolates (mild/complicated) | 90.9 g/L (92/89) |
One hundred twelve of one hundred forty isolates grew to the mature trophozoites stage in culture.
Forty-seven of one hundred twelve isolates showed a rosetting phenotype.
P < 0.05.
P < 0.0001.
Figure 1.
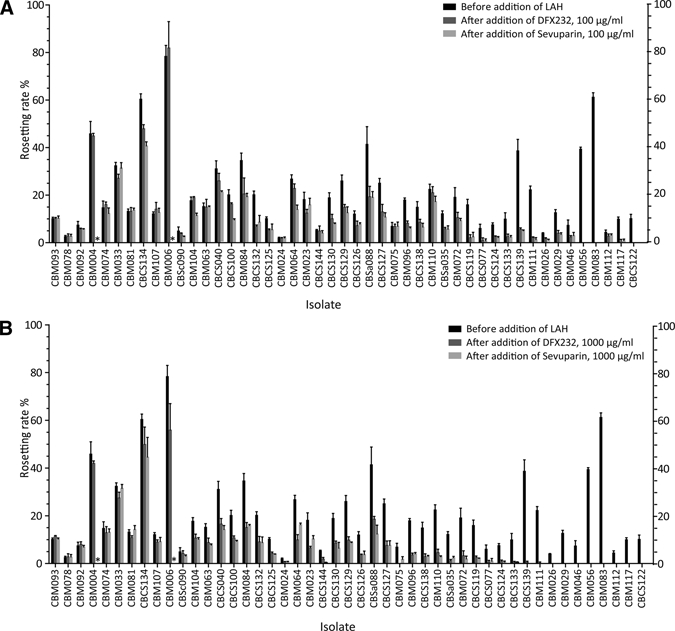
Rosetting rates before and after the addition of LAHs in the 47 isolates. The mean rosetting rate ± SEM is shown. Rosetting rate before addition of LAH (black bars), DFX232 (dark grey bars), or Sevuparin (light gray bars). *Not done. (A) DFX232 and Sevuparin at concentrations of 100 μg/mL. (B) DFX232 and Sevuparin at concentrations of 1,000 μg/mL.
LAH disrupt rosettes from fresh isolates.
To examine the activity of LAH against a large sample size of fresh patient samples collected from malaria-infected children, 47 rosetting isolates were tested for sensitivity to two different batches of LAH (DFX232 and Sevuparin). Two concentrations, 100 and 1,000 μg/mL, were used, and results were compared with mock-treated samples prepared in RPMI. Rosette disruption of ≥ 15% (defined as significant24) was shown in 42 of 47 (89%) samples with DFX232 at a concentration of 1,000 μg/mL, 39 of 47 (83%) samples with Sevuparin at a concentration of 1,000 μg/mL, 34 of 47 samples with DFX232 at 100 μg/mL, and 36 of 47 samples with Sevuparin at 100 μg/mL (Figures 1 and 2). Both DFX232 and Sevuparin caused disruption of ≥ 50% of the rosettes in 32 of 47 (68%) samples at the higher concentration. At the lower concentration, the number of highly reacting samples was 18 of 47 for DFX232 and 25 of 47 for Sevuparin (Figure 2A and B). Only three samples showed no significant effect with either of the two substrates used at any of the concentrations. Relative rosetting rates (RR; the proportion of remaining rosettes after treatment compared with the control-treated erythrocytes) ranged from 36% (Sevuparin, higher concentration) to 61% (DFX232, lower concentration) (Figure 3A). Statistically significant rosette disruption compared with the control was seen in all groups, with a P < 0.0001 (Figure 3A). There was no significant difference in rosette disruption effect between the two batches.
Figure 2.
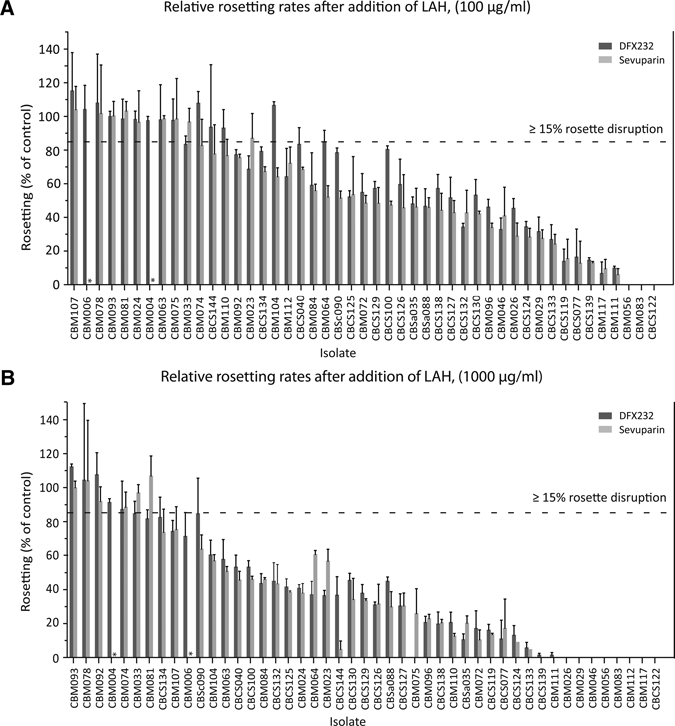
Relative rosetting rate after the addition of LAH compared with control. The mean relative rosetting rate (the proportion of remaining rosettes after treatment compared with the control-treated erythrocytes) ± SEM is shown. Significant rosette disruption ≥ 15% is indicated by the horizontal line. Dark grey bars = DFX232; light grey bars = Sevuparin. *Not done. (A) DFX232 and Sevuparin at concentrations of 100 μg/mL. Significant disruption was shown in 42 of 47 samples with DFX232 and 39 of 47 samples with Sevuparin. (B) DFX232 and Sevuparin at concentrations of 1,000 μg/mL. Significant disruption was shown in 34 of 47 samples with DFX232 and 36 of 47 samples with Sevuparin.
Figure 3.
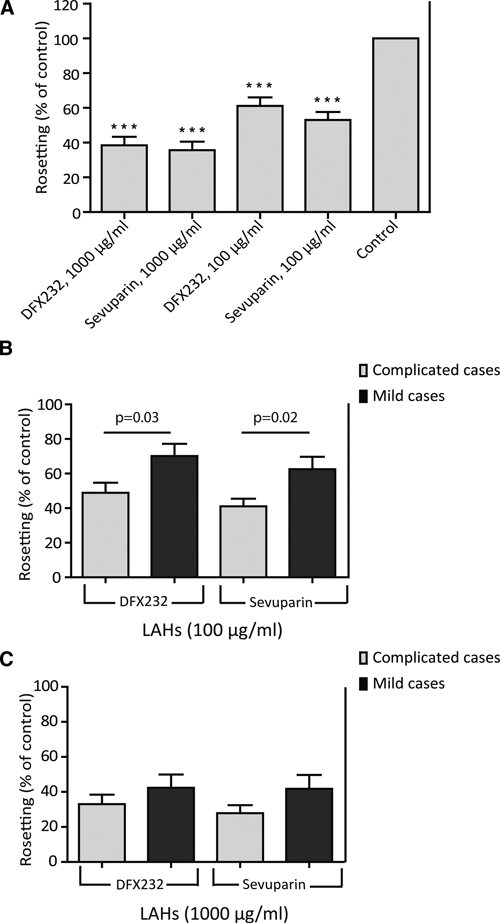
(A) Overall rosette disruption effect of the two different LAHs. The mean relative rosetting rate (the proportion of remaining rosettes after treatment compared with the control-treated erythrocytes) ± SEM is shown. There was a significant disruption effect for DFX232 and Sevuparin at both concentrations compared with the mock-treated samples. There was no statistically significant difference between the two LAH preparations. ***P < 0.001. (B and C) Rosette disruption effect in isolates from mild and complicate malaria cases. The mean relative rosetting rate (the proportion of remaining rosettes after treatment compared with the control-treated erythrocytes) ± SEM is shown. Dark grey bars = mild malaria; light grey bars = complicated malaria. The mean relative rosetting rate is shown. (B) DFX232 and Sevuparin at concentrations of 100 μg/mL. (C) DFX232 and Sevuparin at concentrations of 1,000 μg/mL.
Rosettes in complicated malaria isolates are more sensitive to Lah than rosettes in mild malaria isolates.
When comparing samples from mild and complicated malaria, a difference in rosette disruption effect by the LAHs was revealed (Figure 3B and C). Both DFX232 and Sevuparin had a greater rosette disruption effect on the isolates from the complicated malaria cases compared with the mild cases, with an LAH concentration of 100 μg/mL. Thus, after treatment with 100 μg/mL DFX232, the remaining relative RR was 70% (range = 0–115%) in the mild malaria isolates and 49% (range = 14–94%) in the complicated malaria isolates (P = 0.03) (Figure 3B). With Sevuparin at 100 μg/mL, the figures were 62% (range = 0–104%) in the mild cases and 41% (range = 13–78%) in the complicated cases (P = 0.02) (Figure 3B). A similar trend but no statistically significant difference was seen between the groups when the higher concentrations of both compounds were used (Figure 3C). No correlation was found between sensitivity to the LAH and parasitemia or RR (data not shown).
Discussion
The present investigation shows that LAH effectively disrupts rosettes in fresh clinical isolates. We compared two different preparations of LAH (DFX232 and Sevuparin) at two different concentrations, 100 and 1000 μg/mL, on 47 fresh isolates from Cameroonian children with mild or complicated malaria. The concentrations were selected based on previously reported studies performed in vitro.13,16,22,24 The chosen concentrations are in the range of the doses of LAH evaluated in a finalized Phase I study. In the Phase I study, no severe adverse events were reported, and the drug candidate was proven to be safe and well-tolerated (unpublished data).
A majority, 68% (32/47), of the samples showed a rosette disruption effect ≥ 50% when the compounds were analyzed at the higher concentration. In all, 89% (42/47) of the isolates were sensitive (≥ 15%) to rosette disruption by any of the LAH studied (Figures 1 and 2). We further found a difference in rosette disruption effect by LAH depending on the severity of the malaria infection. Both LAH compounds, when analyzed at the lower concentration, had a significantly stronger rosette disruption effect (P < 0.05) in samples from complicated malaria cases than in samples from mild cases (Figure 3B). The difference in rosette disruption effect was about 40% for both compounds between the mild and complicated isolates (Figure 3B). For the higher concentration, the effect was also more pronounced in the complicated cases than in the mild cases, although not significant (Figure 3C). These results are in line with previous findings showing that binding of soluble heparin to the surface of pRBC is significantly associated with severe disease.6 The parasites from the mild and complicated groups may use different rosetting receptors. One can speculate that the use of CR1 or blood group A as a rosetting receptor may be more frequent in the mild compared with the complicated group, making these rosettes less sensitive to LAH treatment.
Rosette disruption by heparin, HS, and LAH and the binding of GAGs to the DBL1α domain of PfEMP1 are well-described in several studies.13,16,17,21–23 When comparing rosette disruption data for heparin or HS19,24 with the data for LAH, the present study might indicate a stronger rosette disruption effect by DFX232 and Sevuparin. Carlson and others24 reported that 30% (16/54) of the samples collected in Gambia showed ≥ 50% rosetting disruption effect of heparin, and 50% (27/54) of the samples showed ≥ 15% disruption at a concentration of 650 μg/mL.24 In the present study, we found ≥ 50% rosetting disruption effect in 53% (25/47) of the samples and ≥ 15% disruption effect in 77% (36/47) of the samples at the concentration 100 μg/mL. Three rosetting isolates (6%) in our study showed no sensitivity to either of the two LAH preparations tested. This finding is not surprising but in concordance with the results of previous studies where it has been shown that some isolates are insensitive to heparin, heparin derivatives, and other rosette-disrupting sulfated glycoconjugates, even at very high concentrations.23,24,44
In the present study, there was a statistically significant difference (P < 0.05) between the complicated and mild groups, with a higher parasitemia and more isolates forming rosettes in the complicated malaria cases than in the mild malaria cases. Another observation is that the isolates that showed a rosetting phenotype in the mild group were of higher parasitemia and lower hemoglobin levels compared with the isolates in the same group that did not show any rosetting. There was a trend but no statistical difference in hemoglobin levels between the mild and complicated groups, which may be because the complicated group includes children with malaria with diverse symptoms such as hyperpyrexia, anemia, hyperparasitemia, seizures, prostration, and vomiting. The children with severe anemia, therefore, belong to a subgroup within the complicated group. The rosetting rate did not significantly differ between the mild and complicated groups, although a significantly higher number of isolates formed rosettes in the complicated group than in the mild group. The similarity in rosetting rates in the mild versus complicated group may be a consequence of a different prevalence of hemoglobinopathies, such as sickle cell anemia or thalassemia, in the mild and complicated groups, because these diseases have been shown to impair the ability to form rosettes instead creating small and weak rosettes.10 Indeed, both sickle cell anemia and thalassemias are common in Cameroon and surrounding areas.45
In this report, we only used fresh parasitized blood samples collected from malaria-infected children. The samples were directly analyzed without freezing and thawing. The aim was to obtain parasite samples as close to in vivo conditions as possible. In some rosette disruption studies, frozen and thawed parasite samples or laboratory strains have been used.19,22,23,44 In a recent study, it was found that the dominant var gene coding for PfEMP1 changed before and after cryopreservation in a number of isolates, which was analyzed by quantitative polymerase chain reaction (Q-PCR) using strain and stage-specific primers.36 By using fresh isolates, the risk of changes in antigens presented on the erythrocyte surface, including expression of PfEMP1, is limited. Hence, the use of entirely fresh blood samples may present a more correct picture of the infected cells' surfaces in vivo than frozen and thawed blood samples.
Another sulfated glycoconjugate that has been shown to be effective in rosette disruption and inhibition of parasite invasion of RBCs is curdlan sulfate (CRDS).44,46 This compound has been reported to be effective in vitro at the concentration 50 μg/mL in the majority of 18 P. falciparum isolates from Kenyan children. The mechanism of rosette disruption by CRDS is unclear; hence, it is not known if the mechanism of action of CRDS is a specific binding to the PfEMP1 protein. Two clinical trials with a small number of severe malaria patients have shown that fever clearance was shortened in the group receiving CRDS, but the study was unable to show any difference in mortality, parasitemia, or coma resolution after treatment with CRDS.47
LAH is being documented as adjunct treatment of severe malaria. This includes a recent clinical phase I safety study conducted in healthy male volunteers (unpublished data). No severe adverse effects were reported, and the candidate drug (Sevuparin) was proven to be safe and well-tolerated (unpublished data). Here, we show that LAH affects the majority of fresh rosetting clinical isolates analyzed without any manipulation of the parasites. In addition, LAHs have earlier been shown to inhibit cytoadhesion and merozoite invasion in vitro and block and reverse sequestration of pRBCs in vivo in rats and monkeys.23 By inhibiting the three processes rosetting, cytoadherence, and merozoite invasion with a receptor analogue like LAH, a change in the sequestered parasite load and parasitemia may also be achieved in humans. One can speculate that this may reestablish the microcirculation and release bound pRBCs into the circulation; this renders the latter more available for an immune attack on passing through the spleen. We conclude that drugs reducing the sequestration of pRBCs, such as LAH, have a potential as adjunct treatment of severe malaria.
ACKNOWLEDGMENTS
The authors thank the children and their parents who participated in the study. They are grateful to the staff at the University of Buea. This work was supported by Dilafor AB, Erik and Edith Fernströms stiftelse, and the Swedish National Board of Health and Welfare.
Disclaimer: The authors declare competing financial interest. A.M.L. and M.W. hold stock and/or stock options in Dilafor AB, which owns the intellectual property of the substances generated in this manuscript. A.M.L. has received research funding from Dilafor AB. The remaining authors have no known conflict of interest.
Footnotes
Authors' addresses: Anna M. Leitgeb, Dilafor AB, Solna, Sweden, E-mail: anna.leitgeb@dilafor.com. Karin Blomqvist and Mats Wahlgren, Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet (MTC), Stockholm, Sweden, E-mails: karin.blomqvist@ki.se and mats.wahlgren@ki.se. Fidelis Cho-Ngwa, Moses Samje, Peter Nde, and Vincent Titanji, University of Buea, Biotechnology Unit, Faculty of Science, Buea, Cameroon, E-mails: chongwa_ub@yahoo.co.uk, msamje@yahoo.com, ndepf@yahoo.com, and vpk.titanji@yahoo.com.
References
- 1.World Health Organization . World Malaria Report. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 2.Udomsangpetch R, Wåhlin B, Carlson J, Berzins K, Torii M, Aikawa M, Perlmann P, Wahlgren M. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989;169:1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaul DK, Roth EF, Jr, Nagel RL, Howard RJ, Handunnetti SM. Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood. 1991;78:812–819. [PubMed] [Google Scholar]
- 4.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 5.Treutiger CJ, Hedlund I, Helmby H, Carlson J, Jepson A, Twumasi P, Kwiatkowski D, Greenwood BM, Wahlgren M. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. 1992;46:503–510. doi: 10.4269/ajtmh.1992.46.503. [DOI] [PubMed] [Google Scholar]
- 6.Heddini A, Pettersson F, Kai O, Shafi J, Obiero J, Chen Q, Barragan A, Wahlgren M, Marsh K. Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infect Immun. 2001;69:5849–5856. doi: 10.1128/IAI.69.9.5849-5856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts DJ, Pain A, Kai O, Kortok M, Marsh K. Autoagglutination of malaria-infected red blood cells and malaria severity. Lancet. 2000;355:1427–1428. doi: 10.1016/S0140-6736(00)02143-7. [DOI] [PubMed] [Google Scholar]
- 8.Rowe A, Obeiro J, Newbold CI, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 10.Carlson J, Nash GB, Gabutti V, al-Yaman F, Wahlgren M. Natural protection against severe Plasmodium falciparum malaria due to impaired rosette formation. Blood. 1994;84:3909–3914. [PubMed] [Google Scholar]
- 11.Rowe JA, Obiero J, Marsh K, Raza A. Short report: positive correlation between rosetting and parasitemia in Plasmodium falciparum clinical isolates. Am J Trop Med Hyg. 2002;66:458–460. doi: 10.4269/ajtmh.2002.66.458. [DOI] [PubMed] [Google Scholar]
- 12.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Barragan A, Fernandez V, Sundstrom A, Schlichtherle M, Sahlen A, Carlson J, Datta S, Wahlgren M. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum. J Exp Med. 1998;187:15–23. doi: 10.1084/jem.187.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su XZ, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JA, Peterson DS, Ravetch JA, Wellems TE. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 16.Vogt AM, Barragan A, Chen Q, Kironde F, Spillmann D, Wahlgren M. Heparan sulfate on endothelial cells mediates the binding of Plasmodium falciparum-infected erythrocytes via the DBL1alpha domain of PfEMP1. Blood. 2003;101:2405–2411. doi: 10.1182/blood-2002-07-2016. [DOI] [PubMed] [Google Scholar]
- 17.Vogt AM, Winter G, Wahlgren M, Spillmann D. Heparan sulphate identified on human erythrocytes: a Plasmodium falciparum receptor. Biochem J. 2004;381:593–597. doi: 10.1042/BJ20040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson J, Wahlgren M. Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. J Exp Med. 1992;176:1311–1317. doi: 10.1084/jem.176.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe A, Berendt AR, Marsh K, Newbold CI. Plasmodium falciparum: a family of sulphated glycoconjugates disrupts erythrocyte rosettes. Exp Parasitol. 1994;79:506–516. doi: 10.1006/expr.1994.1111. [DOI] [PubMed] [Google Scholar]
- 20.Moll K, Pettersson F, Vogt AM, Jonsson C, Rasti N, Ahuja S, Spangberg M, Mercereau-Puijalon O, Arnot DE, Wahlgren M, Chen Q. Generation of cross-protective antibodies against Plasmodium falciparum sequestration by immunization with an erythrocyte membrane protein 1-duffy binding-like 1 alpha domain. Infect Immun. 2007;75:211–219. doi: 10.1128/IAI.00749-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barragan A, Fernandez V, Chen Q, von Euler A, Wahlgren M, Spillmann D. The duffy-binding-like domain 1 of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a heparan sulfate ligand that requires 12 mers for binding. Blood. 2000;95:3594–3599. [PubMed] [Google Scholar]
- 22.Barragan A, Spillmann D, Kremsner PG, Wahlgren M, Carlson J. Plasmodium falciparum: molecular background to strain-specific rosette disruption by glycosaminoglycans and sulfated glycoconjugates. Exp Parasitol. 1999;91:133–143. doi: 10.1006/expr.1998.4349. [DOI] [PubMed] [Google Scholar]
- 23.Vogt AM, Pettersson F, Moll K, Jonsson C, Normark J, Ribacke U, Egwang TG, Ekre HP, Spillmann D, Chen Q, Wahlgren M. Release of sequestered malaria parasites upon injection of a glycosaminoglycan. PLoS Pathog. 2006;2:e100. doi: 10.1371/journal.ppat.0020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson J, Ekre HP, Helmby H, Gysin J, Greenwood BM, Wahlgren M. Disruption of Plasmodium falciparum erythrocyte rosettes by standard heparin and heparin devoid of anticoagulant activity. Am J Trop Med Hyg. 1992;46:595–602. doi: 10.4269/ajtmh.1992.46.595. [DOI] [PubMed] [Google Scholar]
- 25.Jaroonvesama N. Intravascular coagulation in falciparum malaria. Lancet. 1972;1:221–223. doi: 10.1016/s0140-6736(72)90621-6. [DOI] [PubMed] [Google Scholar]
- 26.Munir M, Tjandra H, Rampengan TH, Mustadjab I, Wulur FH. Heparin in the treatment of cerebral malaria. Paediatr Indones. 1980;20:47–50. [PubMed] [Google Scholar]
- 27.Rampengan TH. Cerebral malaria in children. Comparative study between heparin, dexamethasone and placebo. Paediatr Indones. 1991;31:59–66. [PubMed] [Google Scholar]
- 28.Sheehy TW, Reba RC. Complications of falciparum malaria and their treatment. Ann Intern Med. 1967;66:807–809. doi: 10.7326/0003-4819-66-4-807. [DOI] [PubMed] [Google Scholar]
- 29.Smitskamp H, Wolthuis FH. New concepts in treatment of malignant tertian malaria with cerebral involvement. BMJ. 1971;1:714–716. doi: 10.1136/bmj.1.5751.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization Severe and complicated malaria. World Health Organization Malaria Action Programme. Trans R Soc Trop Med Hyg. 1986;80((Suppl)):3–50. [PubMed] [Google Scholar]
- 31.Lindahl U, Backstrom G, Hook M, Thunberg L, Fransson LA, Linker A. Structure of the antithrombin-binding site in heparin. Proc Natl Acad Sci USA. 1979;76:3198–3202. doi: 10.1073/pnas.76.7.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petitou M, Lormeau JC, Choay J. Interaction of heparin and antithrombin III. The role of O-sulfate groups. Eur J Biochem. 1988;176:637–640. doi: 10.1111/j.1432-1033.1988.tb14324.x. [DOI] [PubMed] [Google Scholar]
- 33.Fransson LA. Periodate oxidation of D-glucuronic acid residues in heparan sulfate and heparin. Carbohydr Res. 1978;62:235–244. [Google Scholar]
- 34.Skidmore MA, Dumax-Vorzet AF, Guimond SE, Rudd TR, Edwards EA, Turnbull JE, Craig AG, Yates EA. Disruption of rosetting in Plasmodium falciparum malaria with chemically modified heparin and low molecular weight derivatives possessing reduced anticoagulant and other serine protease inhibition activities. J Med Chem. 2008;51:1453–1458. doi: 10.1021/jm701337t. [DOI] [PubMed] [Google Scholar]
- 35.Pettersson F, Vogt AM, Jonsson C, Mok BW, Shamaei-Tousi A, Bergstrom S, Chen Q, Wahlgren M. Whole-body imaging of sequestration of Plasmodium falciparum in the rat. Infect Immun. 2005;73:7736–7746. doi: 10.1128/IAI.73.11.7736-7746.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blomqvist K, Normark J, Nilsson D, Ribacke U, Orikiriza J, Trillkott P, Byarugaba J, Egwang TG, Kironde F, Andersson B, Wahlgren M. var gene transcription dynamics in Plasmodium falciparum patient isolates. Mol Biochem Parasitol. 2010;170:74–83. doi: 10.1016/j.molbiopara.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Peters J, Fowler E, Gatton M, Chen N, Saul A, Cheng Q. High diversity and rapid changeover of expressed var genes during the acute phase of Plasmodium falciparum infections in human volunteers. Proc Natl Acad Sci USA. 2002;99:10689–10694. doi: 10.1073/pnas.162349899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters JM, Fowler EV, Krause DR, Cheng Q, Gatton ML. Differential changes in Plasmodium falciparum var transcription during adaptation to culture. J Infect Dis. 2007;195:748–755. doi: 10.1086/511436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimbi HK, Tetteh KK, Polley SD, Conway DJ. Cross-sectional study of specific antibodies to a polymorphic Plasmodium falciparum antigen and of parasite antigen genotypes in school children on the slope of Mount Cameroon. Trans R Soc Trop Med Hyg. 2004;98:284–289. doi: 10.1016/S0035-9203(03)00068-3. [DOI] [PubMed] [Google Scholar]
- 40.Wanji S, Tanke T, Atanga SN, Ajonina C, Nicholas T, Fontenille D. Anopheles species of the mount Cameroon region: biting habits, feeding behaviour and entomological inoculation rates. Trop Med Int Health. 2003;8:643–649. doi: 10.1046/j.1365-3156.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- 41.Moll K, Ljungström I, Perlmann H, Scherf A, Wahlgren M. Methods in Malaria Research. Manassas, Virginia. Paris, France: BioMalPar; 2008. MR4/ATCC. [Google Scholar]
- 42.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 43.European Pharmacopoeia . Heparins Low-Molecular-Mass, Monograph 0828. Strasbourg, France: European Directorate for the Quality of Medicines and Health Care; 2003. [Google Scholar]
- 44.Kyriacou HM, Steen KE, Raza A, Arman M, Warimwe G, Bull PC, Havlik I, Rowe JA. In vitro inhibition of Plasmodium falciparum rosette formation by Curdlan sulfate. Antimicrob Agents Chemother. 2007;51:1321–1326. doi: 10.1128/AAC.01216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billa RF, Biwole MS, Juimo AG, Bejanga BI, Blackett K. Gall stone disease in African patients with sickle cell anaemia: a preliminary report from Yaounde, Cameroon. Gut. 1991;32:539–541. doi: 10.1136/gut.32.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Havlik I, Rovelli S, Kaneko Y. The effect of curdlan sulphate on in vitro growth of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1994;88:686–687. doi: 10.1016/0035-9203(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 47.Havlik I, Looareesuwan S, Vannaphan S, Wilairatana P, Krudsood S, Thuma PE, Kozbor D, Watanabe N, Kaneko Y. Curdlan sulphate in human severe/cerebral Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2005;99:333–340. doi: 10.1016/j.trstmh.2004.05.005. [DOI] [PubMed] [Google Scholar]


