Abstract
African Burkitt lymphoma is an aggressive B-cell, non-Hodgkin lymphoma linked to Plasmodium falciparum malaria. Malaria biomarkers related to onset of African Burkitt lymphoma are unknown. We correlated age-specific patterns of 2,602 cases of African Burkitt lymphoma (60% male, mean ± SD age = 7.1 ± 2.9 years) from Uganda, Ghana, and Tanzania with malaria biomarkers published from these countries. Age-specific patterns of this disease and mean multiplicity of P. falciparum malaria parasites, defined as the average number of distinct genotypes per positive blood sample based on the merozoite surface protein-2 assessed by polymerase chain reaction, were correlated and both peaked between 5 and 9 years. This pattern, which was strong and consistent across regions, contrasted parasite prevalence, which peaked at 2 years and decreased slightly, and geometric mean parasite density, which peaked between 2 and 3 years and decreased sharply. Our findings suggest that concurrent infection with multiple malaria genotypes may be related to onset of African Burkitt lymphoma.
Introduction
Burkitt lymphoma (BL) is an aggressive, monoclonal B-cell, non-Hodgkin lymphoma that was first described in African children in 1958.1 Today, it is known to occur as three clinical-epidemiologic variants: African (aBL), sporadic (sBL), and acquired immunodeficiency syndrome (AIDS)–related BL.2 Epstein-Barr virus (EBV), discovered in aBL tumors in 19643 and subsequently shown to be ubiquitous,4 is linked to aBL and several other malignant and non-malignant conditions.5 EBV is detected as clonal episomal or integrated DNA or as RNA in 95% of aBL tumors.6 In areas with a high incidence of BL, almost all children are infected with EBV by 2–3 years of age,7 well before peak age at aBL onset. This suggests that increased risk for aBL may be influenced by chronic, not acute, EBV infection.6 EBV infection is demonstrable up to 60 months before the onset of aBL.6 However, EBV is not essential for aBL because most persons who are infected do not develop aBL, and it appears to play a less prominent role in sBL and AIDS-related BL because it is detected in at most 10–20% and 30–40% of cases, respectively.8
African BL occurs in the context of Plasmodium falciparum malaria, which is presumed to be geographical co-factor of aBL,9,10 based on parallel distribution of both diseases.10,11 The carriage of the sickle cell gene, a genetic marker for reduced risk of severe malaria, was significantly or marginally reduced in children with aBL12 compared with hospital-based controls without aBL in two studies,13,14 but not in the third study.15 These findings, based on studies conducted more than 40 years ago, provide some support for an etiologic role of malaria in aBL. Two case–control studies recently conducted in Uganda and Malawi demonstrated 5-fold and 12-fold odds ratio, respectively, of elevated antibodies against malaria among children with aBL compared with controls.16,17
Plasmodium falciparum malaria induces polyclonal expansion of B-cells, impairs EBV-specific T cell immune responses,18 and may preferentially stimulate the expansion of EBV-positive B cells expression by its cysteine-rich interdomain region 1α, a P. falciparum erythrocyte membrane protein 1 receptor,19 all of which suggest a biological basis for how malaria may influence the risk for aBL. The biomarkers of malaria infection e.g., parasite prevalence, density, or genotypes, that may be related to onset of aBL are unknown. We performed an ecologic correlation of the age-specific incidence in a historical aBL case-series from Uganda, Ghana, and Tanzania with various malaria biomarkers published in studies conducted in countries where cases were detected to generate hypotheses about malaria biomarkers that may be related to onset of aBL.
Materials and Methods
Cases of aBL.
The cases (in persons 0–14 years of age) were from four large studies conducted in Uganda,20,21 Ghana,22 and Tanzania23 during 1964–2009. The data from Uganda came from two studies, one conducted in the southern region, where the incidence of aBL is low,11 and the other in the northern region, where the incidence is high.11,20 Data from southern Uganda was obtained from the Kampala Cancer Registry (KCR) for cases registered during 1990–2009. The KCR is a population-based registry that covers approximately 1.8 million persons in Kampala, the capital city, and its immediate environs.24 Cancer registrars actively search records at local hospitals to find new cases. Coverage for KCR was estimated to be approximately 90% complete for cases diagnosed during 1994–1996.25 Data from northern Uganda were obtained from St. Mary's Hospital in Lacor for cases from 10 districts in northern Uganda and they were diagnosed and treated at the hospital during 1997–2006.20 Because there were no other hospitals with capacity to diagnose and treat aBL in the region, these data were assumed to be almost complete for cases arising in the 10 neighboring districts served by the hospital (approximately a 100-mile radius). Data from Ghana were obtained from the National Cancer Institute (USA) Burkitt's Tumor Project for cases in persons who originated mostly from the southern half of Ghana and were enrolled at Korle Bu Hospital, Accra, during 1965–1989.22 Data from Tanzania were obtained from Shirati Hospital for cases in persons from the North Mara region who were diagnosed and treated during 1960–2009.23 Completeness of these series is uncertain, but they were thought to be representative of the cases from the regions served by the hospitals.
Malaria genotypes and other biomarkers.
We compiled data on P. falciparum malaria biomarkers from published literature of studies conducted in the countries where the cases arose identified from PUBMED. These biomarkers included parasitemia prevalence (percentage of persons with a positive blood slide for parasites among all persons tested), geometric mean parasite density (GMPD), and multiplicity of infection (MOI), defined as the average number of distinct genotypes, based on the merozoite surface protein-2 (MSP-2) per positive blood sample tested by polymerase chain reaction.26–28 The MSP-2 genotypes were based on two allelic variants (IC = 400–750 basepairs and F27 = 250–500 basepairs), which were determined by using polymerase chain reaction primers specific for highly conserved regions flanking polymorphic domains of the gene block 3 of the MSP-2 gene.26–28 Monthly rainfall data for the study regions, Gulu and Kampala, Uganda (2004–2008),29 Accra, Ghana (1952–1970),30 and Shirati Hospital, Tanzania (1980–2008 from local measurements provided by co-author), were correlated with monthly percentage of aBL cases lagged by 1–4 months by using Pearson's correlation coefficient to explore correlation between monthly rainfall and monthly aBL case incidence.
Descriptive analysis were performed by using frequency tables for categorical variables: sex, anatomic tumor site (face or head tumors only; abdominal for tumors involving abdominal visceral organs with or without face or head involvement; and other or unspecified anatomic sites, including the central nervous system), age group (0–14 years in 3-year intervals), and calendar-year period (1960–2009 in 10-year intervals) in SAS version 9.1 (SAS Institute, Cary, NC). Chi-square tests and Student's t-test were performed to assess associations between categorical and continuous variables, respectively, with aBL. Pearson's correlation coefficients were calculated to assess strength of association between age-specific patterns of aBL cases with malaria biomarkers. Age-specific patterns of aBL and of malaria biomarkers were graphed by using R under the terms of the Free Software Foundation GNU General Public License (version 2.11.1, May 2010, The R Foundation for Statistical Computing). P values < 0.05 were considered statistically significant.
Results
Based on 2,602 aBL cases included in the study, the male:female case ratio was 1.5:1 for cases in the four regions combined, ranging from 1.2:1 in southern Uganda to 1.8:1 in northern Uganda (Table 1). The mean ± SD age for cases in all regions was 7.1 ± 2.9 years and it was non-significantly lower in boys than girls (6.9 years versus 7.3 years; P = 0.07). By region, mean age at diagnosis was lowest in cases from northern Uganda and the highest in cases from Ghana (6.6 years versus 8.0 years; P = 0.09). Only 2.4% of cases were 0–2 years of age; most (69.1%) were 3–8 years of age and 28.4% were 9–14 years of age. Anatomic tumor site information that was complete for 69% of cases, of whom most (61.1%) had abdominal involvement, with or without involvement of face or head, and 33.0% had face or head involvement only. Compared with boys, girls appeared to have more abdominal involvement, but the result was not significant (68.6% versus 56.1%; P = 0.25). Children with tumors involving the face or head were younger than children with tumors involving abdominal and/or other or unspecified sites (mean age = 6.4 years versus 7.5 years; P = 0.02).
Table 1.
Characteristics of African Burkitt lymphoma cases in persons 0–14 years of age, by country
| Characteristic | All countries | Uganda (southern) | Uganda (northern) | Ghana | Tanzania | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | No. | (%) | No. | (%) | |
| All persons | 2,602 | 371 | 500 | 581 | 1,150 | |||||
| Sex* | ||||||||||
| Male | 1,551 | 59.7 | 206 | 55.5 | 324 | 64.8 | 363 | 62.5 | 658 | 57.5 |
| Female | 1,046 | 40.3 | 165 | 44.5 | 176 | 35.2 | 218 | 37.5 | 487 | 42.5 |
| Unknown | 5 | 0 | 0 | 0 | 5 | |||||
| Tumor site* | ||||||||||
| Face or Head | 594 | 33.0 | 0 | 0.0 | 177 | 37.9 | 173 | 29.8 | 244 | 32.5 |
| Abdominal | 1,099 | 61.1 | 0 | 0.0 | 280 | 60.0 | 321 | 55.2 | 498 | 66.4 |
| Other | 105 | 5.9 | 0 | 0.0 | 10 | 2.1 | 87 | 15.0 | 8 | 1.1 |
| Unknown | 804 | 371 | 33 | 0 | 400 | |||||
| Age group, years* | ||||||||||
| 0–2 | 63 | 2.4 | 13 | 3.5 | 8 | 1.6 | 6 | 1.0 | 36 | 3.2 |
| 3–5 | 791 | 30.6 | 118 | 32.0 | 206 | 41.2 | 110 | 18.9 | 357 | 31.4 |
| 6–8 | 998 | 38.6 | 133 | 36.1 | 179 | 35.8 | 238 | 41.0 | 448 | 39.5 |
| 9–11 | 464 | 18.0 | 68 | 18.4 | 66 | 13.2 | 140 | 24.1 | 190 | 16.7 |
| 12–14 | 269 | 10.4 | 37 | 10.0 | 41 | 8.2 | 87 | 15.0 | 104 | 9.2 |
| Unknown | 17 | 2 | 0 | 0 | 15 | |||||
| Mean (SD) | 7.1 (2.9) | 6.9 (3.0) | 6.6 (2.6) | 8.0 (2.8) | 6.9 (2.8) | |||||
| Diagnosis year* | ||||||||||
| 1960–1969 | 117 | 4.6 | 0 | 0.0 | 0 | 0.0 | 60 | 11.0 | 57 | 5.0 |
| 1970–1979 | 392 | 15.3 | 0 | 0.0 | 0 | 0.0 | 283 | 51.7 | 109 | 9.4 |
| 1980–1989 | 371 | 14.4 | 0 | 0.0 | 0 | 0.0 | 203 | 37.1 | 168 | 14.6 |
| 1990–1999 | 673 | 26.2 | 159 | 42.9 | 75 | 15.0 | 1 | 0.2 | 438 | 38.1 |
| 2000–2009 | 1,015 | 39.5 | 212 | 57.1 | 425 | 85.0 | 0 | 0.0 | 378 | 32.9 |
| Unknown | 34 | 0 | 0 | 34 | 0 | |||||
Percentage excludes unknown category.
The percentage of aBL cases peaked near five years and then decreased gradually thereafter. The pattern for mean MOI of P. falciparum resembled the age-specific pattern for aBL, including increasing gradually with age to a peak near five years and then decreasing gradually (Figure 1A and B for Ghana and Tanzania; not shown for Uganda). Strong correlations were demonstrated between age-specific patterns for aBL and MOI in all regions: southern Uganda (Pearson's correlation coefficient 0.91, P = 0.01), northern Uganda (Pearson's correlation coefficient 0.89, P = 0.02), Ghana (Pearson's correlation coefficient 0.90, P = 0.002), and Tanzania (Pearson's correlation coefficient 0.77, P = 0.01). This pattern contrasted that of prevalence of P. falciparum malaria parasitaemia and GMPD, which increased rapidly and peaked by two years of age and decreased only slightly thereafter for parasitaemia, but decreased rapidly to levels much below the peak by four years of age for GMPD (Figure 1C and D for Ghana and Tanzania; not shown for Uganda).
Figure 1.
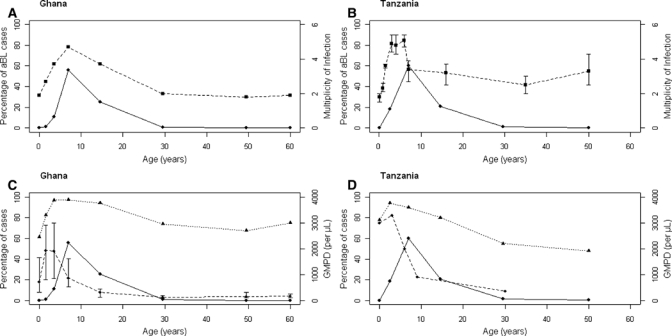
Characteristics of African Burkitt lymphoma cases correlated with Plasmodium falciparum malaria mean multiplicity of infection, prevalence, and geometric mean parasite density. A and B, Percentage of African Burkitt lymphoma cases by age and mean multiplicity of P. falciparum malaria parasites, defined as the average number of distinct genotypes per positive blood sample based on the merozoite surface protein-2 assessed by polymerase chain reaction, in Ghana27 and Tanzania.26 C and D, Percentage of African Burkitt lymphoma cases per age, prevalence of P. falciparum malaria, and geometric mean parasite density in the general population in Ghana27 and Tanzania.26,39 Age group intervals for the cases were plotted according to the age groups used in the malaria papers.
Monthly aBL cases lagged by four months were unrelated with monthly rainfall in all regions (northern Uganda: Pearson's correlation coefficient = 0.48, P = 0.12; Ghana: Pearson's correlation coefficient = –0.15, P = 0.65; Tanzania: Pearson's correlation coefficient = 0.10, P = 0.79, except in southern Uganda where the correlation was marginal (Pearson's correlation coefficient = 0.57, P = 0.05).
Discussion
The novel finding of our study is the strong and consistent correlation of age-specific patterns of aBL with age-specific patterns of concurrent malaria genotypes of P. falciparum malaria. This pattern contrasted sharply from that of asymptomatic parasitaemia prevalence and GMPD peaked approximately two years before the peak age for aBL and either remained elevated, as for parasitemia, or decreased rapidly, as for GMPD. Although ecologic, our findings support the hypothesis that genetic complexity of P. falciparum malaria infections and the associated immune response they trigger may be related to the onset of aBL. The prevalence and pattern of MOI with age is likely a function of the joint probability of exposure to diverse strains of P. falciparum malaria circulating in a given population and probability of developing cumulative immunity against any given strains circulating in the community.31 MOI is associated with elevated risk for clinical malaria among non-immune persons32. Conversely, it is associated with decreased risk from clinical malaria among persons with developed anti-malarial immunity,33 but its relationship with aBL has not hitherto been examined. MOI may be a surrogate for immunologic consequences of exposure to mixed genotype malaria infections and the greater propensity for aBL to be triggered in such persons.34
Few aBL cases were noted among children 0–2 years of age. This paucity sharply contrasts with the burden of malaria, which is typically high in this age group. The paucity of aBL cases in this age group argues against prenatal initiation of aBL accelerated by early childhood infections in the etiology of aBL. The sparseness of cases may be caused by competing mortality from acute severe malaria syndromes or to under-ascertainment of cases in infants and toddlers. Under-ascertainment is unlikely to account for major deficits because aBL in young children frequently involves the face or head anatomic sites and tumors at these sites are easily recognized by the parents, and would be recognized as aBL by clinicians practicing in regions where aBL is endemic.
We found that the age at onset for aBL in Ghana was higher than that in northern Uganda. The regional disparities in mean age at aBL may be caused by differences in the intensity of malaria in these countries, differences in the specific malaria genotypes circulating in the countries, or to other factors correlated with malaria, such as helminthic parasites, which may be different between Ghana and northern Uganda. We found that males predominate in all regions and are more likely to be diagnosed with aBL at a young age and to have tumors that involve the face or head in cases from all regions. Male predominance is also observed in sBL and AIDS-related BL,35 suggesting that males may be genetically predisposed to BL independent of geographic origin of the cases and of antecedent illnesses, including EBV, malaria, or human immunodeficiency virus. Monthly rainfall was not correlated with aBL incidence, in accordance with some studies,36,37 but not all studies.38 The poor correlations between rainfall and aBL, although rainfall is associated with increase in malaria transmission, may be caused by a negative impact of heavy rainfall on medical-seeking behaviors of persons in rural areas or an influence of other factors modulated by rainfall, such as co-transmission of soil transmitted parasites and/or viral infections or nutrition, whose effects may distort and obscure correlations of rainfall with aBL incidence.
Our study has limitations. Our findings are based on an ecologic analysis using historical hospital-based case-series over many years and markers compiled from published malaria data. Both data sets are of uncertain accuracy in their case diagnosis and completeness. The cases and malaria data compiled were sometimes from different time periods and different areas, which should be considered when interpreting the correlations. Because our results for MOI are based only on one gene, our conclusions about genetic diversity of malaria and aBL must be interpreted cautiously. The malaria results are subject to publication and other biases, but such systematic bias would probably attenuate the patterns, correlations, and the consistency in diverse areas studied. The strengths of our study include its large numbers, wide geographic area covered, which gives some reassurance about generalizability of the patterns we demonstrated, and novelty of assessing age-specific patterns to develop clues about etiology.
In conclusion, we demonstrated the expected patterns of male predominance, the young age of onset of aBL, especially on the face and head among boys, and later age onset, especially for tumors in the abdomen among girls using historical data of aBL case series from Uganda, Ghana, and Tanzania. The novel findings were the strong and consistent correlations between age-specific patterns of aBL with those for MOI of P. falciparum malaria, which have not been previously shown. Our results support the hypotheses that mixed genotypic infections and, perhaps associated immunologic immune responses, may be related to onset of aBL.
ACKNOWLEDGMENTS
We thank Dr. Paul H. Levine (Department of Epidemiology, The George Washington University School of Public Health, Washington, DC) for his continuous advice and review through this study, and Dr. Ruth Parsons and Stella Munuo (Information Management System (Rockville, MD) and Sarah Nambooze (Kampala Cancer Registry (Kampala, Uganda) for preparing data analysis files.
Disclaimers: The content of this report is the responsibility of the authors alone and does not necessarily reflect the views or policies of the United States Department of Health and Human Services.
Footnotes
Financial support: The study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services (grants HHSN26120090068P, HHSN261200555004C, N02-CP-31003, and N01-CO-12400).
Authors' addresses: Benjamin Emmanuel, Kishor Bhatia, and Sam M. Mbulaiteye, Infections and Immunoepidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, E-mails: emmanuelb@mail.nih.gov, bhatiak@mail.nih.gov, and mbulaits@mail.nih.gov. Esther Kawira, Shirati Health, Education, and Development Foundation, Shirati, Tanzania, E-mail: elkawira@gmail.com. Martin D. Ogwang, Department of Surgery, St. Mary's Hospital, Lacor, Gulu, Uganda, E-mail: ogwang.martin@lacorhospital.org. Henry Wabinga, Kampala Cancer Registry, Department of Pathology, Makerere University, Kampala, Uganda, E-mail: hwabinga@med.mak.ac.ug. Josiah Magatti and Glen Brubaker, Interchurch Medical Assistance, New Windsor, MD, E-mails: jomagati2000@yahoo.com and grb176@comcast.net. Francis Nkrumah, Noguchi Memorial Institute, University of Ghana, Legon, Legon, Ghana, E-mail: fnkrumah@noguchi.mimcom.org. Janet Neequaye, Department of Child Health, Korle Bu University Teaching Hospital, Korle-Bu, Accra, Ghana, E-mail: janet.neequaye@yahoo.com. Robert J. Biggar, Department of Epidemiology Research, Staten Serum Institute, Copenhagen S, Denmark, E-mail: rjbiggar@gmail.com.
Reprint requests: Sam M. Mbulaiteye, 6120 Executive Boulevard, Executive Plaza South, Room 7080, Rockville, MD 20852-7248, E-mail: mbulaits@mail.nih.gov.
References
- 1.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–223. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 2.Leoncini L, Raphael M, Stein H, Harris NL, Jaffe ES, Kluin PM. In: WHO Classification of Tumors of Hematopoetic and Lymphoid Tissues. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. Lyon, France: International Agency for Research on Cancer; 2008. pp. 262–264. (Burkitt Lymphoma). [Google Scholar]
- 3.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 4.Henle G, Henle W, Clifford P, Diehl V, Kafuko GW, Kirya BG, Klein G, Morrow RH, Munube GM, Pike P, Tukei PM, Ziegler JL. Antibodies to Epstein-Barr virus in Burkitt's lymphoma and control groups. J Natl Cancer Inst. 1969;43:1147–1157. [PubMed] [Google Scholar]
- 5.Klein G. Burkitt lymphoma–a stalking horse for cancer research? Semin Cancer Biol. 2009;19:347–350. doi: 10.1016/j.semcancer.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.de-The G, Geser A, Day NE, Tukei PM, Williams EH, Beri DP, Smith PG, Dean AG, Bronkamm GW, Feorino P, Henle W. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature. 1978;274:756–761. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]
- 7.Biggar RJ, Henle G, Bocker J, Lennette ET, Fleisher G, Henle W. Primary Epstein-Barr virus infections in African infants. II. Clinical and serological observations during seroconversion. Int J Cancer. 1978;22:244–250. doi: 10.1002/ijc.2910220305. [DOI] [PubMed] [Google Scholar]
- 8.Bornkamm GW. Epstein-Barr virus and the pathogenesis of Burkitt's lymphoma: more questions than answers. Int J Cancer. 2009;124:1745–1755. doi: 10.1002/ijc.24223. [DOI] [PubMed] [Google Scholar]
- 9.Burkitt D. A children's cancer dependent on climatic factors. Nature. 1962;194:232–234. doi: 10.1038/194232a0. [DOI] [PubMed] [Google Scholar]
- 10.Rainey JJ, Omenah D, Sumba PO, Moormann AM, Rochford R, Wilson ML. Spatial clustering of endemic Burkitt's lymphoma in high-risk regions of Kenya. Int J Cancer. 2007;120:121–127. doi: 10.1002/ijc.22179. [DOI] [PubMed] [Google Scholar]
- 11.Morrow RH., Jr Epidemiological evidence for the role of falciparum malaria in the pathogenesis of Burkitt's lymphoma. IARC Sci Publ. 1985;60:177–186. [PubMed] [Google Scholar]
- 12.Hill AV. The immunogenetics of resistance to malaria. Proc Assoc Am Physicians. 1999;111:272–277. doi: 10.1046/j.1525-1381.1999.99234.x. [DOI] [PubMed] [Google Scholar]
- 13.Pike MC. Burkitt's lymphoma and sickle-cell trait in Uganda. Br J Prev Soc Med. 1970;24:63. doi: 10.1136/jech.24.1.63-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams AO. Haemoglobin genotypes, ABO blood groups, and Burkitt's tumour. J Med Genet. 1966;3:177–179. doi: 10.1136/jmg.3.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nkrumah FK, Perkins IV. Sickle cell trait, hemoglobin C trait, and Burkitt's lymphoma. Am J Trop Med Hyg. 1976;25:633–636. doi: 10.4269/ajtmh.1976.25.633. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter LM, Newton R, Casabonne D, Ziegler J, Mbulaiteye S, Mbidde E, Wabinga H, Jaffe H, Beral V. Antibodies against malaria and Epstein-Barr virus in childhood Burkitt lymphoma: a case-control study in Uganda. Int J Cancer. 2008;122:1319–1323. doi: 10.1002/ijc.23254. [DOI] [PubMed] [Google Scholar]
- 17.Mutalima N, Molyneux E, Jaffe H, Kamiza S, Borgstein E, Mkandawire N, Liomba G, Batumba M, Lagos D, Gratrix F, Boshoff C, Casabonne D, Carpenter LM, Newton R. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PLoS ONE. 2008;3:e2505. doi: 10.1371/journal.pone.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam KM, Syed N, Whittle H, Crawford DH. Circulating Epstein-Barr virus-carrying B cells in acute malaria. Lancet. 1991;337:876–878. doi: 10.1016/0140-6736(91)90203-2. [DOI] [PubMed] [Google Scholar]
- 19.Chene A, Donati D, Guerreiro-Cacais AO, Levitsky V, Chen Q, Falk KI, Orem J, Kironde F, Wahlgren M, Bejarano MT. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog. 2007;3:e80. doi: 10.1371/journal.ppat.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogwang MD, Bhatia K, Biggar RJ, Mbulaiteye SM. Incidence and geographic distribution of endemic Burkitt lymphoma in northern Uganda revisited. Int J Cancer. 2008;123:2658–2663. doi: 10.1002/ijc.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkin DM, Nambooze S, Wabwire-Mangen F, Wabinga HR. Changing cancer incidence in Kampala, Uganda, 1991–2006. Int J Cancer. 2010;126:1187–1195. doi: 10.1002/ijc.24838. [DOI] [PubMed] [Google Scholar]
- 22.Nkrumah FK, Olweny CL. Clinical features of Burkitt's lymphoma: the African experience. IARC Sci Publ. 1985;60:87–95. [PubMed] [Google Scholar]
- 23.Geser A, Brubaker G, Draper CC. Effect of a malaria suppression program on the incidence of African Burkitt's lymphoma. Am J Epidemiol. 1989;129:740–752. doi: 10.1093/oxfordjournals.aje.a115189. [DOI] [PubMed] [Google Scholar]
- 24.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82:1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkin DM, Wabinga H, Nambooze S. Completeness in an African cancer registry. Cancer Causes Control. 2001;12:147–152. doi: 10.1023/a:1008966225984. [DOI] [PubMed] [Google Scholar]
- 26.Smith T, Beck HP, Kitua A, Mwankusye S, Felger I, Fraser-Hurt N, Irion A, Alonso P, Teuscher T, Tanner M. Age dependence of the multiplicity of Plasmodium falciparum infections and of other malariological indices in an area of high endemicity. Trans R Soc Trop Med Hyg. 1999;93((Suppl 1):):15–20. doi: 10.1016/s0035-9203(99)90322-x. [DOI] [PubMed] [Google Scholar]
- 27.Owusu-Agyei S, Smith T, Beck HP, Amenga-Etego L, Felger I. Molecular epidemiology of Plasmodium falciparum infections among asymptomatic inhabitants of a holoendemic malarious area in northern Ghana. Trop Med Int Health. 2002;7:421–428. doi: 10.1046/j.1365-3156.2002.00881.x. [DOI] [PubMed] [Google Scholar]
- 28.Peyerl-Hoffmann G, Jelinek T, Kilian A, Kabagambe G, Metzger WG, von Sonnenburg F. Genetic diversity of Plasmodium falciparum and its relationship to parasite density in an area with different malaria endemicities in West Uganda. Trop Med Int Health. 2001;6:607–613. doi: 10.1046/j.1365-3156.2001.00761.x. [DOI] [PubMed] [Google Scholar]
- 29.Uganda Bureau of Statistics 2009 Statistical Abstract. 2009. http://www.ubos.org/onlinefiles/uploads/ubos/pdf%20documents/2009Statistical_%20Abstract.pdf Available at. Accessed March 29, 2010.
- 30.Acheampong PK. Rainfall anomaly along the coast of Ghana. Its nature and causes. Geogr Ann, Ser A. 1982;64:199–211. [Google Scholar]
- 31.Mackinnon MJ, Marsh K. The selection landscape of malaria parasites. Science. 2010;328:866–871. doi: 10.1126/science.1185410. [DOI] [PubMed] [Google Scholar]
- 32.Ofosu-Okyere A, Mackinnon MJ, Sowa MP, Koram KA, Nkrumah F, Osei YD, Hill WG, Wilson MD, Arnot DE. Novel Plasmodium falciparum clones and rising clone multiplicities are associated with the increase in malaria morbidity in Ghanaian children during the transition into the high transmission season. Parasitology. 2001;123:113–123. doi: 10.1017/s0031182001008162. [DOI] [PubMed] [Google Scholar]
- 33.Farnert A, Rooth I, Svensson , Snounou G, Bjorkman A. Complexity of Plasmodium falciparum infections is consistent over time and protects against clinical disease in Tanzanian children. J Infect Dis. 1999;179:989–995. doi: 10.1086/314652. [DOI] [PubMed] [Google Scholar]
- 34.Gupta S, Trenholme K, Anderson RM, Day KP. Antigenic diversity and the transmission dynamics of Plasmodium falciparum. Science. 1994;263:961–963. doi: 10.1126/science.8310293. [DOI] [PubMed] [Google Scholar]
- 35.Mbulaiteye SM, Anderson WF, Bhatia K, Rosenberg PS, Linet MS, Devesa SS. Trimodal age-specific incidence patterns for Burkitt lymphoma in the United States, 1973–2005. Int J Cancer. 2010;126:1732–1739. doi: 10.1002/ijc.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biggar RJ, Nkrumah FK. Burkitt's lymphoma in Ghana: urban-rural distribution, time-space clustering and seasonality. Int J Cancer. 1979;23:330–336. doi: 10.1002/ijc.2910230310. [DOI] [PubMed] [Google Scholar]
- 37.Morrow RH, Pike MC, Smith PG. Further studies of space-time clustering of Burkitt's lymphoma in Uganda. Br J Cancer. 1977;35:668–673. doi: 10.1038/bjc.1977.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams EH, Day NE, Geser AG. Seasonal variation in onset of Burkitt's lymphoma in the West Nile District of Uganda. Lancet. 1974;2:19–22. doi: 10.1016/s0140-6736(74)91350-6. [DOI] [PubMed] [Google Scholar]
- 39.Smith T, Charlwood JD, Kihonda J, Mwankusye S, Billingsley P, Meuwissen J, Lyimo E, Takken W, Teuscher T, Tanner M. Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop. 1993;54:55–72. doi: 10.1016/0001-706x(93)90068-m. [DOI] [PubMed] [Google Scholar]


