Abstract
Angiostrongylus cantonensis meningitis was first reported in Cuba in 1981, and it was recently reported in South America. The aim of this paper is to evaluate the intrathecal immunoglobulin synthesis patterns from Cuba's and Ecuador's patients with angiostrongyliasis; 8 Ecuadorian patients from two different outbreaks and 28 Cuban patients were studied. Simultaneous blood and cerebrospinal fluid simples were taken. Immunoglobulin (Ig) A, IgM, IgG, and albumin were quantified by radial immunodiffusion. Corresponding Reibergrams were applied. A three-Ig pattern was the most frequent in the two groups, but IgM was presented in all Ecuadorian young mature patients; however, in the Cuban children, only 12 of 28 patients had intrathecal IgM, but about 90% had an IgA and IgG synthesis at time of later puncture. This indicates that, with a larger amount of parasites ingested, clinical symptoms are more severe, and a higher frequency of intrathecal IgM synthesis could be observed. This is discussed as a similarity with the intrathecal IgM synthesis in African trypanosomiasis.
Introduction
Angiostrongylus cantonensis is a helminth parasite. The definitive hosts of this parasite are rats; several mollusk species constitute intermediary hosts. The mature worms live in the pulmonary arteries of rats. The human is a non-permissive, accidental host. Human infections have been known to be acquired by the ingestion of the infective third-stage larvae contained in raw or inadequately cooked food—either the intermediate mollusk hosts (snails and slugs) or animals acting as a paratenic host (planarians, crustaceans, frogs, monitor lizards, etc.)—and contaminated fresh vegetables, including raw vegetable juice.1
The term human angiostrongyliasis refers primarily to eosinophilic meningitis, the major clinical feature of A. cantonensis infection in human beings. It is considered an important and sometimes fatal human disease.2 The infection frequently appears in outbreaks, with a number of cases between 8 and 100 patients.3
Meningitis by A. cantonensis was first described in Southeastern Asia and reported in Asia, Africa, and the Caribbean.3–11 In 1981, Cuba was the first country to report this disease in the Americas,12 and recently, it was reported in Ecuador.11,13
Previously, the parasite had been found in naturally infected snails of various genera and species in Northeastern Brazil,14 which also has reported cases of eosinophilic meningitis in the same areas.
For the most sensitive detection of a brain-derived fraction besides the blood-derived fraction of immunoglobulins, it was necessary to characterize a discrimination line in the quotient diagrams between these both fractions. Reibergram is a valuable method to determine the intrathecal synthesis of immunoglobulins.15,16
The humoral immune response in the central nervous system (CNS) is different from the immune response observed in blood. As a main difference, in cerebrospinal fluid (CSF), there was no switch from immunoglobulin M (IgM) class response to a more specific IgG class response in the course of inflammatory neurological diseases. The pattern of intrathecal IgG/IgA/IgM synthesis remains rather constant and depends on the cause, pathophysiology, and localization of the disease process. From a diagnostic point of view, the lack of IgM to IgG switch in CNS is the chance to characterize disease-related instead of acuity-related patterns.17,18
The first pattern of intrathecal Ig synthesis in a parasite-induced CNS disease was reported in eosinophilic meningoencephalitis caused by A. cantonensis.19 Later Reibergrams were reported in African trypanosomiasis.20
The use of Reibergram or Reiber's CSF/serum quotient diagrams in epidemiological studies has been reported previously.21,22 Different immunological patterns in CSF could be described for viral meningoencephalitis outbreaks.22,23
The aim of this paper is to establish a comparison between Ecuadorian and Cuban patients suffering from angiostrongyliasis from the neuroimmunological point of view by using Reibergrams.
Materials and Methods
Eight young adult patients of an average age of 23 years with a diagnosis of eosinophilic meningitis caused by A. cantonensis were studied. This study was preceded by an epidemic outbreak reported recently in Ecuador.13 These are the first human cases of angiostrongyliasis in humans from Ecuador. All had been eating raw snails. These patients were admitted to the Neurology Service of Eugenio Espejo Hospital of the City of Quito in December 2008 and April 2009. The research was approved by the Committee on Bioethics Research of the Hospital Eugenio Espejo. All patients gave their informed consent to carry out a diagnostic lumbar puncture and allow use of their clinical data history.
The diagnosis of Ecuadorian patients was based on the history of ingesting raw snails, clinical symptoms, and CSF test characteristics and parasitological findings, like presence of L2 larvae in intermediate host and adult worm in lung's finite animal species like rats. Other parasites different from A. cantonensis were excluded according to their symptoms and previous epidemiological background and history.
The 28 Cuban patients studied were children of an average age of 5.8 years. The patients were admitted to the Pediatric Hospital of San Miguel Padrón of the City of Havana between 1998 and 2007 with typical symptoms. The diagnosis was based mainly on the epidemiology, clinical features, neuroimmunological response, and illness development, because, in Cuba, there is no other parasite that can cause eosinophilia in the CSF.3,24
The research project was approved by the Pediatric Hospital of San Miguel Padrón Ethical Committee, and written informed consent of the parent or guardian was obtained.
The serum and CSF samples were obtained simultaneously. The samples were collected at the time of admission at the onset of the symptoms in Cuban patients, and a second pair of samples was taken 8 days later; these samples were kept in small aliquots at 80°C until analysis. In Ecuadorian patients, the first samples of CSF and serum were taken only 2 weeks after the onset of the symptoms.
Serum and CSF analysis.
Major Ig levels in serum from Ecuadorian and Cuban patients were quantified using a radial immunodiffusion technique with NOR Partigen plates (Siemens, Marburg, Germany), and in CSF, levels were quantified using LC Partigen plates (Siemens).
To identify whether there was intrathecal synthesis of Igs and to determine its relationship to the blood–CSF barrier function, albumin in serum and CSF was quantified using the same procedures with NOR and LC Albumin Partigen plates (Siemens) .
Reibergrams.
Igs and albumin concentrations were plotted as CSF/serum quotients in a Reibergram or Reiber's quotient diagram for major Ig classes.16,17
Reibergrams are based on molecular diffusion/CSF flow theory, and the fundamental principle is that a decrease in the CSF flow rate is always accompanied by an increased concentration of blood-derived proteins that is followed secondarily by an increase in molecular diffusion from blood to CSF.25,26
To determine whether there is dysfunction of the blood–CSF barrier and intrathecal synthesis of IgA, IgM, and IgG, the Reibergrams16,17 have been used.
Results
The main symptoms in the Ecuadorian patients appeared 2 weeks after consumption of raw snails. These symptoms were characterized by fever, vomiting, neurological disorders, constipation, nausea, abdominal pain, headache, and severe radicular pain in all patients. Twelve days after the first symptoms, two patients had worsening symptoms, adding slightly stiff neck and alteration of consciousness. One of them fell into coma. Then, it was decided to perform lumbar puncture on the entire group.
The main symptoms in the Cuban children were fever, vomiting, headache, and some meningeal signs in 50% of the patients.
Intrathecal Ig synthesis patterns in the Ecuadorian patients and their frequency are shown in Figure 1. A three-class response was the most frequent, with five of eight cases. All eight of eight cases showed an intrathecal IgM response.
Figure 1.
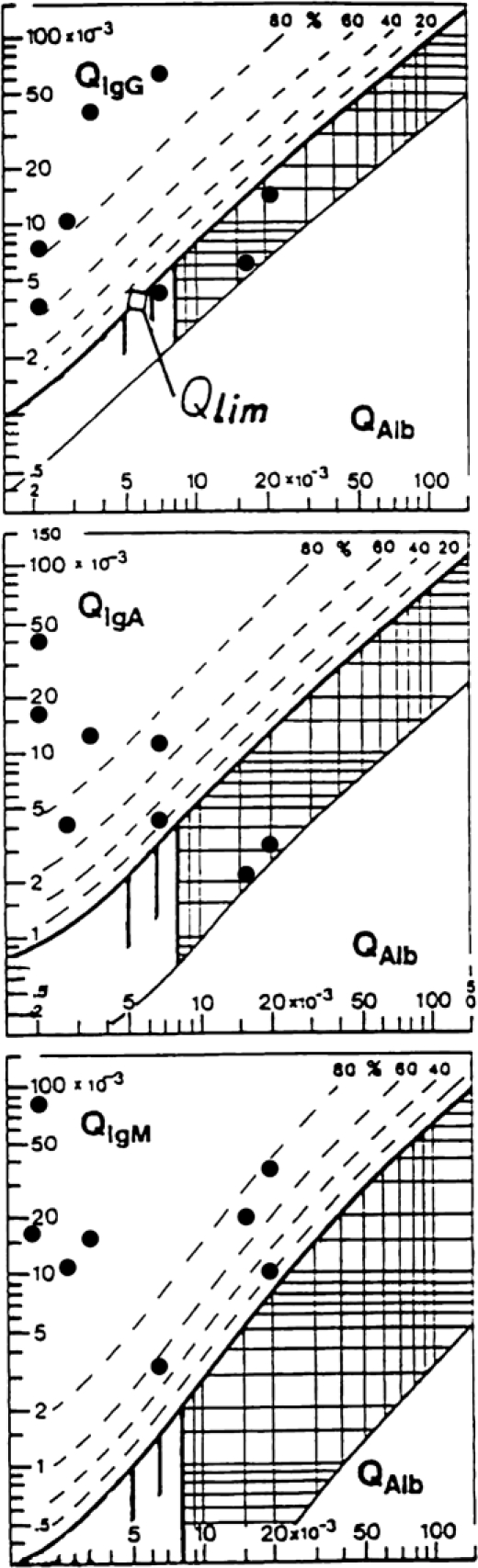
Reibergrams of Ecuadorian patients suffering from A. cantonensis meningitis. The upper hyperbolic curves (bold lines) represent the discrimination lines between brain-derived and blood-derived Ig fractions. Values above these upper discrimination lines represent intrathecal IgG, IgA, or IgM synthesis. The dashed lines indicate the extent of intrathecal synthesis as intrathecal fractions (IgGIF, IgAIF, or IgMIF) with 20%, 40%, 60%, and 80% of the measured total Ig concentration in CSF, with reference to the discrimination line as 0% intrathecal synthesis. The limit of the reference range for QAlb between normal and increased CSF protein concentrations because of blood–CSF barrier dysfunction is indicated by the age-dependent vertical lines at QAlb 5 × 10−3 (up to 15 years), QAlb 6.5 × 10−3 (up to 40 years), and QAlb 8 × 10−3 (up to 60 years). Values below the lower hyperbolic line in range 5 indicate a methodological fault.14,15
There was no intrathecal Ig synthesis at time of the onset of the symptoms in the Cuban patients according to the first early diagnostic lumbar puncture.19 The frequencies in the second lumbar puncture are shown in Figure 2. A three-class immune response was the most frequent in 20 of 28 cases. However, 8 of 28 cases did not develop an intrathecal IgM synthesis at this time, about 8 days after start of the symptoms.
Figure 2.
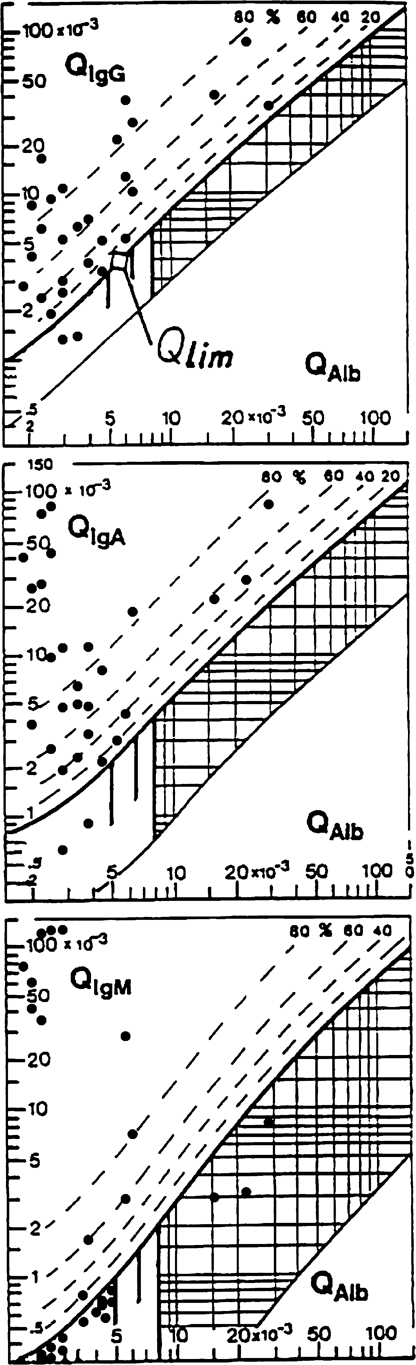
Reibergrams of Cuban patients suffering from A. cantonensis meningitis.
Ig intrathecal synthesis in the Ecuadorian patients is shown in Figure 1. Three patients had a blood–CSF barrier dysfunction with a Q albumin value greater than 7 × 10−3. This situation corresponds with the time of sampling, which does not coincide with the acute face of disease. Five of eight patients show a normal blood–CSF barrier function and intrathecal synthesis of IgG + IgA, but all of them synthesized IgM. It is easy to notice, because their respective CSF/serum quotients were placed over the hyperbolic bold line in the IgM Reibergram.
In the first lumbar puncture, the Cuban patients showed a blood–CSF barrier dysfunction without an intrathecal IgG, IgA, and IgM synthesis, but in the second lumbar puncture, some of the Cuban patients (Figure 2) still showed a blood–CSF barrier dysfunction. In the second lumbar puncture, the Ecuadorian patients showed a blood–CSF barrier dysfunction. Samples from these Ecuadorian were not taken in the acute phase when the first symptoms started.
The most interesting result was that IgM presented in all of the Ecuadorian patients' patterns compared with the Cubans' patterns, where IgG was the major Ig always present.
Discussion
Helminth A. cantonensis transmission has never been reported in Ecuador until the middle of 2008 when a first outbreak occurred. In December 2008, a second outbreak was reported in the province of Santo Domingo de los Tsachilas. The patients included in this paper were part of two outbreaks localized in Guayas and Los Ríos that occurred in March and April 2009.
At the sites where the patients live,13 L3 larvae were observed in snails, and mature worms were observed in lungs of dissected rats.
At the beginning, health authorities denied that a species used for human consumption was involved, because only wild-caught snails were infected. Probably some infected imported snails or introduced rats produced the outbreaks, but one does not know exactly how it came into the country. It is most likely that infected snails were imported by the domestic industry. When improperly handled on farms, they were accessible to wild rats, which became infected for the first time.
The sources of the contamination were a yard near Santo Domingo, where the patients collected snails, which they ate raw with lemon and salt, and a store that sells snails supplied by the domestic industry. Some snails from these sources that remained at patients' home were contaminated with A. cantonensis larva. Life cycles were completed at the Parasitology Laboratory of the Instituto Nacional de Higiene Dr. Leopoldo Izquieta Perez, Guayaquil, Ecuador.11,13
In several regions from this country, one or two snail species constitute intermediate hosts, Pomacea lineada and Pomacea sp., and their infestation intensity is very high.
The first report of A. cantonensis in Cuba was published in 1981,12 but there is some unprinted evidence that the disease appeared some years before.
At the beginning, it was not easy to realize that a parasite that was reported in Southeastern Asia was settled in the New World. This parasite is endemic in Cuba and in the Caribbean basin, and the spread of the disease is by accidental uptake of larvae by children without proper hygienic habits like washing hands after contact with contaminated vegetables, soil, and snails. There is no tradition to eat raw snails in Cuba.27
The patients in this study came from the Municipality of San Miguel Padrón in the City of Havana. This consists of 150,000 inhabitants, is on the periphery of the Cuban capital, and has rural and semi-rural areas. All of the affected children lived in houses with earth patios, where it is common to also find terrestrial snails and rats, which are, respectively, the intermediate and finite hosts of the parasite.27,28
From 1981, the Pediatric Hospital San Miguel received all of the patients from the municipality—a total of 90 patients corresponding to an incidence of two to three cases per year.
Some of the Ecuadorian patients have neurological sequelae, but in the Cuban patients, no neurological sequelae were reported, probably because of the accidental intake and therefore, low quantity of swallowed larvae.25,26
Because the CSF and serum samples from the Ecuadorian patients were not taken at the beginning of the symptoms, only 37% of patients had a blood–CSF barrier dysfunction. These results are similar with the Cuban findings for the second lumbar puncture taken 8 days after the initial diagnostic lumbar puncture, where only 21.4% of Cuban patients showed a blood–CSF barrier dysfunction compared with almost 100% in the initial phase of the disease.
These findings are interesting, because the Cuban patients are children and Ecuadorians are young adults. The median albumin quotient of the children group at 8 days after start of symptoms is QAlb = 3.8. In the Ecuadorian group, the median QAlb = 6.5. This difference reflects mainly the age-related decrease of CSF flow rate with increasing age (i.e., increase of QAlb).27,28
It is a remarkable finding that the intrathecal synthesis pattern of major Igs varies in Ecuadorian and Cuban patients. IgM is always present in the different patterns in the people from Ecuador suffering from this parasite neuroinfection. This typical feature is similar to the reported cases of African trypanosomiasis with involvement of the brain (stage II and III) that used Reibergrams also.20,29
In African trypanosomiasis, IgM is associated with a special type of B cell, called Mott cell, and it can be observed in other neuroinflammatory diseases like neurosyphilis.30
A predominant IgM response is also observed in Lyme neuroborreliosis and mumps meningoencephalitis. Isolated IgM synthesis is occasionally observed in non-Hodgkin's lymphoma involving the CNS.18 These examples, however, indicate that the Ig pattern can guide the choice of further analyses, such as detection of specific antibody synthesis, use of polymerase chain reaction, and detection of the causative microorganism.29–31
IgM and interleukin-10 (IL-10) in CSF are typical components of the neuroinflammatory response in patients with the brain stage of Trypanosoma brucei gambiense infection.32
The intrathecal synthesis patterns in Cuban adults do not vary compared with children, and it is a typical immune response that supports the diagnosis. IgG is always present in such patterns.19,21,22
Increment of intrathecal IgM synthesis may have a connection with the quantity of the larvae intake. Additionally, the differentiation in the neuroimmunological patterns found in Ecuadorians and Cubans may be explained by taking into account additional factors like age and sampling time.
In both groups, a three-class intrathecal synthesis pattern is the most frequent pattern. This IgG + IgA + IgM pattern is reported to be related with the special pathological mechanisms of the different diseases, the time of sampling, and the brain localization of the infection.19,22
Differentiation in the neuroimmunological patterns found in Ecuadorians and Cubans may be explained by taking into account the parasite–host interaction, survival strategies of the parasite in the environment, defense mechanisms of human hosts, like complement activation,1,33 and quantity of larvae intake.
Conclusions
The CSF data pattern in angiostrongylus infection depends on time of puncture in the course of the disease, age of the patients, and particularly, amount of parasites ingested. An immediate appearance of the blood–CSF barrier dysfunction is followed by an intrathecal immune response 1–2 weeks after start of clinical symptoms. Besides a frequent three-class immune response, the intrathecal IgM response seems to be more frequent with higher amounts of parasite ingested. This is a new aspect that adds to the earlier observation that, in African trypanosomiasis, the appearance of intrathecal IgM synthesis is the most sensitive diagnostic sign for involvement of the brain.
ACKNOWLEDGMENTS
The authors would like to thank Manuel Rodriguez for technical assistance and the reviewers for their helpful comments. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Authors' addresses: Bárbara Padilla-Docal, Alberto J. Dorta-Contreras, María Esther Magraner-Tarrau, and Raisa Bu-Coifiu-Fanego, Laboratorio Central de Líquido Cefalorraquídeo (LABCEL), Facultad de Ciencias Médicas Dr. Miguel Enríquez, Universidad de Ciencias Médicas de La Habana, Cuba, E-mails: barbara.padilla@infomed.sld.cu, adorta@infomed.sld.cu, mariae.magraner@infomed.sld.cu, and raisabu@infomed.sld.cu. Juan M. Moreira, Dirección de Control y Mejoramiento de la Salud Pública, Ministerio de Salud Pública, Quito, Ecuador, E-mail: jmoreira@msp.gov.ec. Luiggi Martini-Robles and Jenny Muzzio-Aroca, Departamento de Parasitología, Instituto Nacional de Higiene, Dr. Leopoldo Izquieta Perez, Guayaquil, Ecuador, E-mails: luiggimartini8@hotmail.com and jennymuzzio@yahoo.com. Fernando Alarcón, Servicio de Neurología, Hospital Eugenio Espejo, Quito, Ecuador, E-mail: falarcn2000@hotmail.com.
References
- 1.Padilla-Docal B, Dorta-Contreras AJ, Bu-Coifiú-fanego R. Activación y biosíntesis intratecal de C3c en niños con meningoencefalitis eosinofílica por Angiostrongylus cantonensis. Rev Neurol. 2009;48:632–635. [PubMed] [Google Scholar]
- 2.Qiao PW, De HL, Xing QZ, Xiao GC, Zhao RL. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- 3.Dorta-Contreras AJ. Meningoencefalitis eosinofílica en Cuba. Rev Neurol. 2001;32:999–1000. [PubMed] [Google Scholar]
- 4.Raccurt C, Balaise J, Durette DMC. Présence d'Angiostrongylus cantonensis en Haïti. Trop Med Int Health. 2003;8:423–426. doi: 10.1046/j.1365-3156.2003.01035.x. [DOI] [PubMed] [Google Scholar]
- 5.Anderson E, Gubler DJ, Sorensen K, Beddard J, Ash LR. First report of Angiostrongylus cantonensis in Puerto Rico. Am J Trop Med Hyg. 1986;35:319–322. doi: 10.4269/ajtmh.1986.35.319. [DOI] [PubMed] [Google Scholar]
- 6.Lindo JF, Waugh C, Hall J, Cunningham MC, Ashley D, Eberhard ML. Enzootic Angiostrongylus cantonensis in rat and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg Infect Dis. 2002;8:324–326. doi: 10.3201/eid0803.010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vargas M, Gómez PJD, Malek EA. First record of A. cantonensis (Chen, 1935). Nematode: metastrongyloidae in the Dominican Republic. Trop Med Parasitol. 1992;43:253–255. [PubMed] [Google Scholar]
- 8.Chikweto A, Bhaiyat MI, Macpherson CNL, DeAllie C, Pinckney RD, Richards C, Sharma RN. Existence of Angiostrongylus cantonensis in rats (Rattus norvegicus) in Grenada, West Indies. Vet Parasitol. 2009;162:160–162. doi: 10.1016/j.vetpar.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BG, Little MD. The finding of Angiostrongylus cantonensis in rats in New Orleans. Am J Trop Med Hyg. 1998;38:568–573. doi: 10.4269/ajtmh.1988.38.568. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg NS, Park SY, Blackburn BG, Sejvar JJ, Gaynor K, Cheng H. Distribution of eosinophilic meningitis cases attributable to Angiostrongylus cantonensis, Hawaii. Emerg Infect Dis. 2007;13:1675–1680. doi: 10.3201/eid1311.070367. [DOI] [PubMed] [Google Scholar]
- 11.Pincay T, García L, Narváez E, Decker O, Martini L, Moreira JM. Angiostrongiliasis due to Parastrongylus (Angiostrongylus) cantonensis in Ecuador. First report in South America. Trop Med Int Health. 2009;14((Suppl 2)):37. [Google Scholar]
- 12.Aguiar PH, Morera P, Pascual J. First record of Angiostrongylus cantonensis in Cuba. Am J Trop Med Hyg. 1981;30:963–965. doi: 10.4269/ajtmh.1981.30.963. [DOI] [PubMed] [Google Scholar]
- 13.Pincay T, García L, Narváez E, Decker O, Martini L, Moreira JM. Angiostrongiliasis por Parastrongylus (Angiostrongylus) cantonensis en Ecuador. Bol Epidemiol (Ecuador) 2009;52:25–32. [Google Scholar]
- 14.Graeff TC. Expansion of Achatina fulica in Brazil and potential increased risk for angiostrongyliasis. Trans R Soc Trop Med Hyg. 2007;8:743–744. doi: 10.1016/j.trstmh.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Reiber H. Flow rate of cerebrospinal fluid (CSF): a concept common to normal blood–CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci. 1994;122:189–203. doi: 10.1016/0022-510x(94)90298-4. [DOI] [PubMed] [Google Scholar]
- 16.Reiber H. The hyperbolic function: a mathematical solution of the protein flux/CSF flow model for blood–CSF barrier function. J Neurol Sci. 1994;126:243–245. [Google Scholar]
- 17.Reiber H. Cerebrospinal fluid—physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult Scler. 1998;4:99–107. doi: 10.1177/135245859800400302. [DOI] [PubMed] [Google Scholar]
- 18.Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. 2001;184:101–122. doi: 10.1016/s0022-510x(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 19.Dorta Contreras AJ, Reiber H. Intrathecal synthesis of immunoglobulins in eosinophilic meningoencephalitis due to Angiostrongylus cantonensis. Clin Diagn Lab Immunol. 1998;5:452–455. doi: 10.1128/cdli.5.4.452-455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lejon V, Reiber H, Legros D, Djé N, Magnus E, Woulters I, Sindic CJM, Büscher P. Intrathecal immune response pattern for improved diagnosis of central nervous system involvement in trypanosomiasis. J Infect Dis. 2003;187:1475–1483. doi: 10.1086/374645. [DOI] [PubMed] [Google Scholar]
- 21.Dorta-Contreras AJ. Reibergrama como herramienta epidemiológica: nuevo enfoque. Rev Neurol. 2001;33:36–40. [PubMed] [Google Scholar]
- 22.Dorta-Contreras AJ. Pattern of intrathecal immunoglobulin synthesis in pediatric patients with infectious meningoencephalitis. Biotecnol Apl. 2006;4:382–386. [Google Scholar]
- 23.Dorta Contreras AJ, Agüero Valdés E, Escobar Pérez X, Noris García E, Ferrá Valdés M. Respuesta inmune humoral intratecal en pacientes pediátricos con meningoencefalitis por Coxsackie B 5. Rev Neurol. 1999;28:739–741. [PubMed] [Google Scholar]
- 24.Dorta-Contreras AJ. Ausencia de anticuerpos anticisticercos en líquido cefalorraquídeo de pacientes pediátricos cubanos con convulsiones. Rev Neurol. 2001;32:597–600. [PubMed] [Google Scholar]
- 25.Reiber H. Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci. 2003;21:79–96. [PubMed] [Google Scholar]
- 26.Dorta-Contreras AJ, Reiber H. Teoría de la difusión molecular/flujo del líquido cefalorraquídeo. Rev Neurol. 2004;39:564–569. [PubMed] [Google Scholar]
- 27.Dorta-Contreras AJ, Núñez-Fernández FA, Pérez MO, Lastre GM, Magraner-Tarrau ME, Bu-Coifiú-Fanego R, Noris-García E, Padilla-Docal B. Peculiaridades de la meningoencefalitis por Angiostrongylus cantonensis en América. Rev Neurol. 2007;12:755–763. [PubMed] [Google Scholar]
- 28.Dorta-Contreras AJ, Noris-García E, Padilla-Docal B, Rodríguez-Rey A, González-Hernández M, Magraner-Tarrau ME, Bu-Coifiu-Fanego R, Reiber H, Interián-Morales MT, Sánchez-Zulueta E, Martínez-Delgado JF, Plana-Bouly R, Aguiar-Prieto PH, Núñez-Fernández FA, Pérez-Martín O, Lastre-González M, Alfonso-Manzanet E. Aportes cubanos al estudio del Angiostrongylus cantonensis. Ciudad de la Habana, Cuba; Academia: 2006. pp. 1–76. [Google Scholar]
- 29.Bisser S, Lejon V, Proux PH, Bouteille B, Stanghellini A, Jauberteau MO, Buscher P, Dumas M. Blood–cerebrospinal fluid barrier and intrathecal immunoglobulin compared to field diagnosis of control nervous system involvement in sleeping sickness. J Neurol Sci. 2002;193:127–135. doi: 10.1016/s0022-510x(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 30.Cortioux B, Bisser S. Neuroinmunología Clínica. Havana, Cuba: Editorial Academia; 2009. pp. 93–104. (Patrones de la neuroinflamación en la tripanosomiasis Africana). [Google Scholar]
- 31.Lejon V, Jo R, Francois X, N'siesi DM, Hoogstoel A, Bisser S, Reiber H, Boelaert M, Buscher P. Treatment failure related to intrathecal immunoglobulin M (IgM) synthesis, cerebrospinal fluid IgM, and interleukin-10 in patients with hemolymphatic-stage sleeping sickness. Clin Vaccine Immunol. 2007;14:732–737. doi: 10.1128/CVI.00103-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert PH, Berney M, Kazyumba G. Immune complexes in serum and in cerebrospinal fluid in African trypanosomiasis. J Clin Invest. 1981;67:77–85. doi: 10.1172/JCI110035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padilla-Docal B, Dorta-Contreras AJ, Bu-Coifiú-Fanego R, Rodríguez-Rey A, Gutiérrez-Hernández JC, de Paula-Almeida OS. Reibergram of intrathecal synthesis of C4 in patients with eosinophilic meningitis caused by Angiostrongylus cantonensis. Am J Trop Med Hyg. 2010;6:1094–1098. doi: 10.4269/ajtmh.2010.09-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]


