Abstract
Serum tumor necrosis factor-α (TNF-α) was evaluated in Vibrio vulnificus-infected patients at admission. The median TNF-α concentration in the non-survivor group was determined to be 261.0 pg/mL, in contrast to 69.5 pg/mL in the survivor group (P = 0.001). Hence, serum TNF-α concentration may potentially be an early predictor of the mortality in patients with Vibrio septicemia.
Vibrio vulnificus (V. vulnificus), is a halophilic, gram-negative bacillus that has been associated with fulminant sepsis in immunocompromised patients, and those with chronic liver disease. V. vulnificus infection is thought to occur following the ingestion of Vibrio-contaminated fish/shellfish, or after exposure to contaminated sea and surface waters.1,2 Previous studies have shown that the cytokine profiles of tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) are associated with the prognosis and mortality of sepsis patients.3,4 Human TNF-α (hTNF-α), also called cachectin, is a non-glycosylated cytokine composed of 157 amino acids, and is primarily produced by activated macrophages.5,6 Recent reports showed significantly higher serum TNF-α concentrations in patients infected with V. vulnificus compared with normal healthy volunteers.6 However, the prognostic value of measuring serum TNF-α levels in septic patients is not known. Therefore, this study was sought to determine whether the concentration of serum TNF-α is associated with mortality in V. vulnificus-infected patients.
In this study, we enrolled patients from four different university hospitals (i.e., Chosun University Hospital, Chonnam National University Hospital, Chonbuk National University Hospital, and Pusan National University Hospital) between 2006 and 2008. The patients, aged ≥ 18 years, all exhibiting skin and soft tissue infections such as cellulites or necrotizing fasciitis were enrolled in our study. Blood and skin tissue samples were collected from each patient. Blood samples were first collected between 6 and 48 hours, and also 3 to 9 days after antibiotic administration. Blood sera was immediately separated and stored at −70°C.7 Patients were diagnosed with a V. vulnificus infection after the identification of bacteria in blood, skin, or soft tissue samples. The concentration of serum TNF-α was determined using a commercially available enzyme linked-immunosorbent assay (ELISA) kit (BioSource International Inc., Camarillo, CA) at the Green Cross Reference Laboratory in Seoul, Korea. The measurement was performed at one time with the specimens stored at −70°C. The detection limit of the ELISA assay was 2 pg/mL. Thirteen serum samples of previous study,8 which was performed to determine the seroprevalence of antibody titers against Orientia tsutsugamushi in healthy subjects who underwent a health screening program, were used as controls (control group) in the measurement of TNF-α concentrations.
Statistical analyses were conducted using the Statistical Package for the Social Science (SPSS) for Windows (version 17.0) software (SPSS Inc., Chicago, IL). Discrete variables were expressed as a percentage, and continuous variables were expressed as the median and interquartile range (IQR). The association between the biomarkers (TNF-α, C-reactive protein [CRP], and white blood cell [WBC]) and mortality was evaluated using the non-parametric Mann-Whitney U test. The correlations between TNF-α, CRP, WBC count, and the acute physiology and chronic health evaluation (APACHE II) score were examined using the Spearmann correlation coefficient. A P value of < 0.05 was considered statistically significant.
V. vulnificus was identified in blood or skin samples from 32 patients, of the 135 total enrolled patients. Blood samples were collected from 25 of the 32 patients at the aforementioned time points. The TNF-α levels were measured for each of these samples. Within this group of 25 patients, 11 were the non-survivor, and died of septicemia (mortality rate: 44%), whereas the rest of the 14 patients were the survivors. Additionally, 20 (80%) of the 25 patients were male with mean age of 55.1 years.
At the time of hospital admission, the median TNF-α level was determined to be 101 pg/mL (range = 2–641 pg/mL; IQR = 57.5–212.5 pg/mL). In the non-survivor group, the median TNF-α level was 261.0 pg/mL (IQR = 101.0–376.0 pg/mL), whereas the survivor group possessed a median level of 69.5 pg/mL (IQR = 17.5–103.5 pg/mL). Statistical analysis showed this difference to be statistically significant (P = 0.001). In contrast, the median WBC count was 2,350/mm3 (IQR = 1,500–5,700/mm3) in the non-survivor group and 6,250/mm3 (IQR = 2887.5–126,000.0/mm3) in the survivor group. However, this difference was not statistically significant (P = 0.085). Moreover, the median CRP level for the non-survivor group was 14.5 mg/dL (IQR = 9–20.5 mg/dL) and 4.75 mg/dL (IQR = 3.0–9.5 mg/dL) in the survivor group, which represents a statistically significant difference (P = 0.005). Furthermore, using samples collected between 6 and 48 hours after antibiotic administration, the TNF-α level was found to be significantly higher in the non-survivor group (P = 0.044) (Figure 1).
Figure 1.
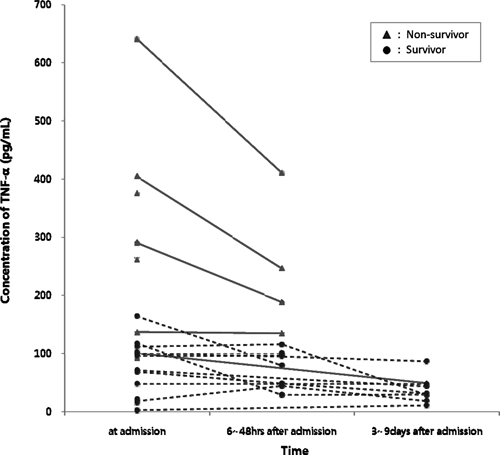
Concentration of tumor necrosis factor-α in septic Vibrio vulnificus-infected patients.
The receiver operating characteristics curve was used to determine the prognostic value of the aforementioned biomarkers. At the time of hospital admission, the area under the curve was 0.89 (95% confidence interval [CI] = 0.70–0.98) for TNF-α, 0.75 (95% CI = 0.56–0.89) for CRP, and 0.66 (95% CI = 0.47–0.82) for the WBC count. However, these differences were not statistically significant.
At the time of hospital admission, the median APACHE II score, which reflects clinical severity, was determined to be 19 (IQR = 13–23) in the non-survivor group and 9 (IQR = 6.75–14.0) in the survivor group showed statistically significant (P = 0.001). Additionally, the APACHE II score was found to significantly correlate with the TNF-α level (Spearmann's rank correlation: r = 0.597, P = 0.002). However, the APACHE II score did not significantly correlate with the WBC count (Spearmann's rank correlation: r = −0.218, P = 0.295).
At an optimal TNF-α cutoff value of 100, the sensitivity and specificity for mortality prediction were determined to be 91% (95% CI = 58.7–98.5%) and 71.4% (95% CI = 41.9–91.4%), respectively. The corresponding positive and negative likelihood ratios were 3.18 and 0.13, respectively. In addition, the median TNF-α level for the control group (N = 14) was 12.5 pg/mL (IQR = 8.9–14.9 pg/mL), which was statistically different than the TNF-α values observed for the non-survivor and survivor groups (P < 0.001).
The mortality rate of V. vulnificus sepsis often reaches ≥ 50% because of the rapid progression of sepsis by this bacterium. Therefore, the immediate administration of potent antibiotics is essential upon diagnosis of V. vulnificus9 Furthermore, both the prediction of disease severity and clinical outcome for patients with V. vulnificus sepsis are valuable and compulsory tools in the care for these infected individuals.
V. vulnificus lipopolysaccharides are not a strong trigger for hTNF-α release, but it is known to induce septic shock syndrome by capsular polysaccharide-mediated cytokine release.10 A study by Powell and others10 showed the detection of TNF-α in mouse serum samples collected up to 12 hours after injection of V. vulnificus-encapsulated parent strain, MO 6-24. Additionally, capsular polysaccharide was shown to stimulate TNF-α release from human peripheral blood mononuclear cells in a dose-dependent manner, with maximal induction observed between 6 and 10 hours following antibiotic treatment. Furthermore, pre-treatment of cirrhotic mice with novel TNF receptor immune-adhesion agents resulted in a decrease in the mortality rate from 70% to 0%, thereby implicating a potential benefit of anti-cytokine therapy.11
The hTNF-α level of septic patients infected with V. vulnificus has been shown to be 224 times higher than normal healthy volunteers.6 However, few studies have compared hTNF-α concentrations between survivor and non-survivor groups. Therefore, in this study, we performed a comparative analysis of these two groups. In V. vulnificus septic patients, our results show a higher TNF-α level (261.0 pg/mL) in non-survivors relative to survivors (69.5 pg/mL). The TNF-α level was also found to be significantly higher in the non-survivor group following analysis of samples collected between 6 and 48 hours after antibiotic administration (Figure 1). The majority of V. vulnificus-related fatalities occurred within 48 hours after hospital admission caused by the rapid development of septic shock syndrome.6 Thus, we were unable to collect blood samples from these patients 7 days after admission, and could not compare the TNF-α levels between the survivor and non-survivor groups. At an optimal TNF-α cutoff value of 100, the sensitivity and specificity to predict mortality was 91% (95% CI = 58.7–98.5%) and 71.4% (95% CI = 41.9–91.4%), respectively. In 10 of the 11 non-survivors, TNF-α values were shown to be ≥ 100 pg/mL, whereas the eleventh patient exhibited a TNF-α value of 92 pg/mL.
Kumar and others12 reported a sequence of cytokine, hemodynamic, and lactate response in a murine model of Escherichia coli septic shock where TNF-α and IL-6 concentrations increased in serum 6 hours after septic insult. Then, lactate concentration started to decrease 12 hours later, and stroke volume and cardiac output decreased 18 hours later. This sequence of cytokine, hemodynamic, and lactate responses suggests that because the onset of septic shock with hypotension and lactate accumulation in serum is the critical inflection point associated with mortality, the mortality rate can be reduced by administering appropriate antibiotics before the critical point. Therefore, the measurement of serum cytokine levels at the initial septic insult may be important in the prediction of mortality for V. vulnificus septic patients.
In this study, we confirmed a significant association between hTNF-α concentration, and the severity or mortality of V. vulnificus sepsis. This association suggests that patients with dominant inflammatory cytokine responses during the early stage of sepsis may have poor clinical outcomes. Therefore, serum hTNF-α measurements might be helpful in the identification of patients who require more aggressive treatment when V. vulnificus sepsis is suspected. In conclusion, our study shows a significant increase of serum hTNF-α concentration in V. vulnificus septic patients. These results suggest that the determination of hTNF-α levels can be used as a mortality predictor in patients with V. vulnificus sepsis.
Disclaimer: The authors do not have any commercial interest or other association that might pose a conflict of interest.
Footnotes
Authors' addresses: Jun-Young Lee, Department of Orthopaedic Surgery, Chosun University College of Medicine, Dong-Gu, Gwangju, Republic of Korea, E-mail: leejy88@chosun.ac.kr. Dong-Min Kim, Na-Ra Yun, and Ganesh Prasad Neupane, Department of Internal Medicine, Chosun University College of Medicine, Dong-Gu, Gwangju, Republic of Korea, E-mails: drongkim@chosun.ac.kr, shinenara@gmail.com, and neupaneganesh@yahoo.com. Sook-In Jung, Kyung-Hwa Park, and Hee Chang Jang, Department of Infectious Diseases, Chonnam National University Hospital, Dong-gu, Gwangju, Republic of Korea, E-mails: sijung@jnu.ac.kr, medkid@dreamwiz.com, and haroc153@naver.com. Chang Seop Lee, Department of Internal Medicine, Chonbuk National University Medical School and Research Institute of Clinical Medicine, Geumam-dong, Jeonju, Republic of Korea, E-mail: lcsmd@jbnu.ac.kr. Sun Hee Lee, Department of Internal Medicine, College of Medicine, Pusan National University, Pusan, South Korea, E-mail: mdssampak@yahoo.co.kr.
References
- 1.Hlady WG, Klontz KC. The epidemiology of Vibrio infections in Florida, 1981–1993. J Infect Dis. 1996;173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 2.Bullen JJ, Spalding PB, Ward CG, Gutteridge JM. Hemochromatosis, iron, and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151:1606–1609. [PubMed] [Google Scholar]
- 3.Simpson AJ, Smith MD, Weverling GJ, Suputtamongkol Y, Angus BJ, Chaowagul W, White NJ, van Deventer SJ, Prins JM. Prognostic value of cytokine concentrations (tumor necrosis factor-alpha, interleukin-6, and interleukin-10) and clinical parameters in severe melioidosis. J Infect Dis. 2000;181:621–625. doi: 10.1086/315271. [DOI] [PubMed] [Google Scholar]
- 4.Waage A, Halstensen A, Espevik T. Association between tumor necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;14:355–57. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 5.Hor LI, Chang YK, Chang CC, Lei HY, Ou JT. Mechanism of high susceptibility of iron-overloaded mouse to Vibrio vulnificus infection. Microbiol Immunol. 2000;44:871–878. doi: 10.1111/j.1348-0421.2000.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 6.Shin SH, Shin DH, Ryu PY, Chung SS, Rhee JH. Proinflammatory cytokine profile in Vibrio vulnificus septicemic patients' sera. FEMS Immunol Med Microbiol. 2002;33:133–138. doi: 10.1111/j.1574-695X.2002.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Kim DM, Neupane GP, Lee YM, Yang NW, Jang SJ, Jung SI, Park KH, Park HR, Lee CS, Lee SH. Comparison of conventional, nested, and real-time PCR assays for rapid and accurate detection of Vibrio vulnificus. J Clin Microbiol. 2008;46:2992–2998. doi: 10.1128/JCM.00027-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim DM, Lee YM, Back JH, Yang TY, Lee JH, Song HJ, Shim SK, Park MY. A serosurvey of Orientia tsutsugamushi from patients with scrub typhus. Clin Microbiol Infect. 2010;16:447–451. doi: 10.1111/j.1469-0691.2009.02865.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim DM, Lym Y, Jang SJ, Han H, Kim YG, Chung CH, Hong SP. In vitro efficacy of the combination of ciprofloxacin and cefotaxime against Vibrio vulnificus. Antimicrob Agents Chemother. 2005;49:3489–3491. doi: 10.1128/AAC.49.8.3489-3491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell JL, Wright AC, Wasserman SS, Hone DM, Morris JG., Jr Release of tumor necrosis factor alpha in response to Vibrio vulnificus capsular polysaccharide in in vivo and in vitro models. Infect Immun. 1997;65:3713–3718. doi: 10.1128/iai.65.9.3713-3718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espat NJ, Auffenberg T, Abouhamze A, Baumhofer J, Moldawer LL, Howard RJ. A role for tumor necrosis factor-alpha in the increased mortality associated with Vibrio vulnificus infection in the presence of hepatic dysfunction. Ann Surg. 1996;223:428–433. doi: 10.1097/00000658-199604000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A, Haery C, Paladugu B, Kumar A, Symeoneides S, Taiberg L, Osman J, Trenholme G, Opal SM, Goldfarb R, Parrillo JE. The duration of hypotension before the initiation of antibiotic treatment is a critical determinant of survival in a murine model of Escherichia coli septic shock: association with serum lactate and inflammatory cytokine levels. J Infect Dis. 2006;193:251–258. doi: 10.1086/498909. [DOI] [PubMed] [Google Scholar]


