Abstract
Plague, caused by the bacteria Yersinia pestis, is a severe, often fatal disease. This study focuses on the plague-endemic West Nile region of Uganda, where limited information is available regarding environmental and behavioral risk factors associated with plague infection. We conducted observational surveys of 10 randomly selected huts within historically classified case and control villages (four each) two times during the dry season of 2006 (N = 78 case huts and N = 80 control huts), which immediately preceded a large plague outbreak. By coupling a previously published landscape-level statistical model of plague risk with this observational survey, we were able to identify potential residence-based risk factors for plague associated with huts within historic case or control villages (e.g., distance to neighboring homestead and presence of pigs near the home) and huts within areas previously predicted as elevated risk or low risk (e.g., corn and other annual crops grown near the home, water storage in the home, and processed commercial foods stored in the home). The identified variables are consistent with current ecologic theories on plague transmission dynamics. This preliminary study serves as a foundation for future case control studies in the area.
Introduction
Plague is a severe, rodent-associated bacterial zoonosis caused by Yersinia pestis.1 Plague bacteria are maintained by various sylvatic and commensal rodent species and their fleas in enzootic and epizootic cycles.2,3 The majority of human infections are associated with epizootics or periods of rapid transmission that often cause local die-offs of susceptible rodent hosts, which forces potentially infectious fleas to infest alternative hosts, including humans. The severity of human illness depends, in part, on the clinical presentation. Bubonic plague, typically associated with flea-borne transmission, is the most common and usually the least severe form of the disease. Septicemic and primary pneumonic infections are commonly associated with cutaneous exposure or inhalation, respectively. These infections are rare but often more severe.3–5 Without treatment, human fatality rates range from 50% to 60% for bubonic plague to almost 100% for pneumonic infections.6 However, if plague is diagnosed and treated early with appropriate antibiotic therapy, outcome improves considerably.1,3,7
Although improvements in sanitation and availability of antibiotics have decreased the epidemicity of plague, the disease remains a public health threat in many areas of the world.8 Over the last two decades, the majority of human plague cases has been reported from eastern and southern Africa and the neighboring island of Madagascar.3,9–11 Within plague-endemic regions, risk of exposure to Y. pestis is spatially heterogeneous.12 Several statistical models have been constructed using geographic information system (GIS)-based and remotely sensed (RS) data to evaluate landscape variables that are associated with plague risk and attempt to explain this spatial heterogeneity.10,12–17 For example, within the West Nile region of Uganda, which is the primary epidemiologic focus within that country,18 a fine resolution GIS-based model revealed that areas of higher elevation (above 1,300 m) that are wetter, with less vegetative growth and more bare soil during the dry month of January, pose an elevated risk of plague occurrence.12
Such models are useful for identifying areas of elevated risk based on landscape variables, which aids in targeting limited public health resources. However, even within areas that are considered ecologically conducive for plague activity, the likelihood of human exposure to the disease agent is influenced by environmental and behavioral factors that are not included in these landscape-level models. Factors influencing the critical contact between rodent reservoirs, flea vectors, and humans as well as human behaviors that may enhance or diminish this contact with infectious vectors or hosts are not well-understood, particularly within the West Nile plague focus in Uganda.19–21 This information is essential to establishing evidence-based recommendations on the prevention and control of plague in endemic regions, but few studies have sought to identify these potential risk factors. Within many of the world's plague foci, humans are most often infected in the domestic and peridomestic environment.8,13,14,21–23 Availability of harborage and food sources for rodents, often a result of human behaviors within the home environment, was shown to be associated with plague cases in North America.21 In addition, exposure to plague-infected companion animals has been previously identified as a risk factor.24–26 Other studies suggested that poor, unsanitary conditions are responsible for plague epidemics, because these conditions promote cohabitation of homes by humans and competent rodent hosts, which ultimately increases the potential of transmission.21,27,28
Our study focuses on the plague-endemic West Nile region of Uganda, where, from 1999 to 2007, a mean of 223 human plague cases was reported annually to the Ugandan Ministry of Health.20 During this time period, cases were not typically investigated to determine the location of exposure. Thus, in this study, we assume that cases acquired infection within the village of residence. The objectives of this study were (1) to identify landscape and residential risk factors associated with randomly selected huts within villages that historically reported human plague cases compared with randomly selected huts within villages that have not reported human plague cases from 1999 to 2004, (2) to identify residence-based variables associated with a previously published classification of elevated versus low risk based on RS variables,12 and (3) within areas that were classified as elevated risk based on RS variables, to identify residence-based predictors of huts within case versus control villages. This preliminary study, which couples a previously published fine-scale landscape model with an observational survey of environmental and behavioral variables, reveals risk factors for plague at the hut and village levels and serves as a foundation for future case control studies in this plague-endemic region of Uganda.
Materials and Methods
Study area.
This study was conducted in the plague-endemic West Nile region of northwestern Uganda (Figure 1). It focused primarily on the hut and village levels in Vurra and Okoro Counties, within Arua and Nebbi Districts, respectively. Previous plague-risk models identified elevation as a key predictor of plague risk in this region.12,16 Specifically, areas above 1,300 m represented greater risk for epizootic activity than areas below this elevation. In our study, all of the villages sampled were above 1,300 m (1,348–1,638 m).
Figure 1.
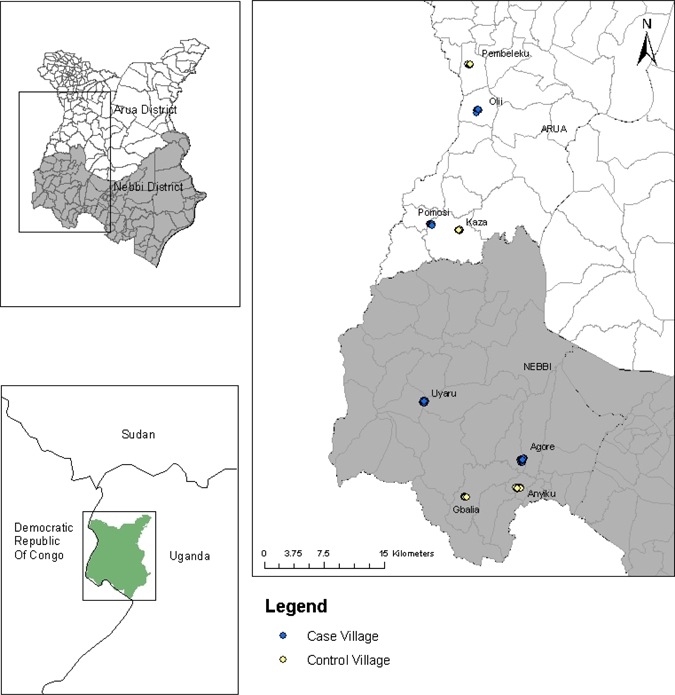
Distribution of case and control villages within the West Nile region of Uganda.
Plague case definition and selection of case and control sites.
In this area, plague was clinically diagnosed by symptomology alone: sudden onset of fever, chills, malaise, headache or prostration accompanied by painful regional lymphadenopathy (bubonic), hematemesis or hematochezia (septicemia), or cough with hemoptysis (pneumonic).12 The local health care delivery system did not include laboratory confirmation of provider diagnosis, and environmental investigations were not conducted to determine the exact location of exposure. We used a previously described database of suspected human plague cases reported from 1999 to 200416 to select villages with or without a history of human plague cases. Villages were categorized as case if a human plague case was reported in at least 3 of 6 years examined; these included Agore (N = 10 cases), Olii (N = 30 cases), Pomosi (N = 6 cases), and Upper Uyaru (N = 20 cases).19 Villages of similar estimated physical size and elevation that did not report any plague cases during the time period spanning 1999–2004 were selected as controls. These included Anyiku, Pembeleku, Kaza, and Gbalia (Figure 1).
Observational survey.
Observational surveys were conducted at the hut level within two case villages (Olii and Pomosi) and two control villages (Kaza and Pembeleku) in Arua district and two case villages (Agore and Upper Uyaru) and two control villages (Anyiku and Gbalia) in Nebbi district (Figure 1).
The observational survey was used to collect information from huts within case and control villages on potential residential risk factors related to plague infection. Surveys were conducted, and latitude and longitude coordinates were collected for 10 randomly selected huts within the villages during each visit. Villages were sampled two times during the time period of March to June 2006, with similar case and control villages being sampled within 1 day of each other. We focused on data collected during this seasonally dry period, because it preceded a large plague outbreak that occurred during the months of October through December of 2006.
The survey addressed environmental variables such as hut specifications, presence or absence of peridomestic animals, sources of rodent food in or around the hut, and basic human behaviors. Hut specifications included size of hut (perimeter measured in meters), number of rooms, type of floor (packed dirt, sand, cement, or other), inside wall cover (mud smear, brick, or other), painted walls (yes, no, or partially), type of roof cover (straw/grass, plastic, metal, or other), slope of roof (flat or sloped), distance to nearest toilet (measured in meters), and distance to nearest neighboring homestead (usually a cluster of three to seven huts; measured in meters). Sources of rodent food in or around the hut included observations about water availability to rodents within the residence (yes or no), type of crops grown or dried within 30 m of the residence (corn, coffee, cassava, melons, sugar cane, bananas, beans, or other; each coded as present or absent), and food stored within the residence (meat, sugar, fish, processed commercial foods, dry grain or dried corn, fresh vegetables, fresh fruit, animal feed, or processed grain; each coded as present or absent). Human behavior questions included the number of people who routinely slept in the hut and their age, insecticide or rodenticide used within the home in the last 30 days (yes or no), the type of bedding material used to sleep on (reed mats, usually made of papyrus, or other), and whether a dog or cat slept inside. Permission was granted by the residents and village leaders to collect this information, and the study was approved by the Centers for Disease Control and Preventions Institutional Review Board IRB protocol number 4696.
Landscape data.
The GIS-based and RS data used in this study have been previously described.12,16 The predictive variables included (1) administrative boundaries within Uganda depicting district, county, and parish (International Livestock Research Institute, 2006, unpublished data), (2) a 90-m digital elevation model (Shuttle Radar Topography Mission Elevation Dataset, 2008; http://seamless.usgs.gov/), and (3) Landsat 7 ETM+ images captured January 1, 2007 (row/path: 58/172) and January 10, 2007 (row/path: 58/173). These images were acquired through a cooperative agreement with the National Geospatial Intelligence Agency. In addition to the Landsat ETM+ band values, we used the normalized difference vegetation index (NDVI), brightness, wetness, and greenness. Derivations of each variable were previously described.16 We also included in our analyses a published model,12 which classified 30-m pixels in this area of Uganda as elevated risk or low risk for exposure to Y. pestis. Variables included in this model were 1,300-m elevation cut-off, Landsat ETM+ band 3, Landsat ETM+ band 6, and brightness. This model was extrapolated to the villages of interest in this study and used to control for the heterogeneity in landscape suitability of Y. pestis transmission observed within this plague-endemic region (Figure 2). Values for each of the variables described above were extracted for every spatially referenced hut using Gridspot in ArcGIS v9.3 (ESRI, Redlands, CA).
Figure 2.
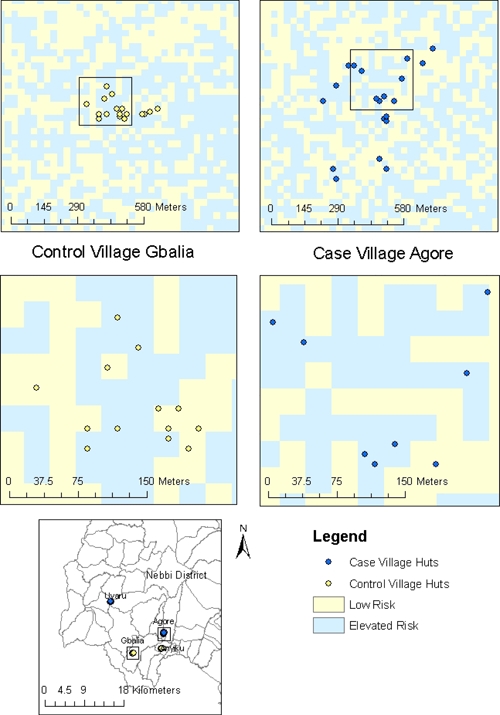
Predicted heterogeneity of pixels classified as elevated risk or low risk observed within case and control villages. The light blue-shaded regions represent pixels classified as elevated risk based on model extrapolation by Eisen and others.12 The regions shaded light yellow represent low risk.
Statistical analysis.
Univariate logistic regression analyses were conducted to identify (1) environmental variables associated with randomly selected huts within villages with (case) or without (control) a reported history of plague, (2) residence-based variables associated with a previously published RS classification of elevated versus low risk,12 and (3) residence-based predictors of huts that were situated in areas classified by the previously published model as elevated risk that were associated with case versus control villages. For each significant variable, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Each variable that was significant in univariate tests was included for selection in a multivariable logistic regression model; predictive variables included in the model were chosen by forward stepwise procedure, with a probability to enter of 0.25. An adjusted OR (aOR) and 95% CI were calculated for each variable within the model. A goodness of fit test was used to determine if the variables included in the model were adequate. The receiver-operating characteristic curve (ROC) was used to assess the overall discrimination of the model. The ROC curve is a graphical representation of the relationship between false-positive (one-specificity) and true-positive (sensitivity) rates.29 Overall model accuracy was determined based on the area under the ROC curve, which provides a threshold-independent measure of overall accuracy.30 Data were analyzed using JMP v8 statistical software (SAS Institute, Cary, NC). Comparisons were considered statistically significant when P < 0.05.
Results
Summary of landscape- and residence-based variables associated with case and control villages based on historic reports of human plague case occurrence.
During the period of March to June 2006, a total of 158 huts were surveyed. Among these huts, 78 were located in villages classified as case based on records of human plague case occurrence from 1999 to 2004, and 80 huts were in control villages that did not report any human plague cases during this time. Based on univariate logistic regression analyses, three residential variables (pigs observed in the peridomestic setting, bedding material, and distance to neighbor) and four landscape variables (elevation, wetness, brightness, and Landsat ETM+ band 6) were significantly associated with village classification (Table 1). Compared with huts within control villages, huts within case villages were more likely to have pigs observed in the peridomestic environment, less likely to have beds made of material other than reed, and more likely to be farther from neighboring homesteads than huts within control villages (case villages median [range] = 10 m [3–50 m], control villages = 8 m [2–45 m]). In addition, huts within case villages were more likely to be situated in pixels that were wetter with more bare soil (higher wetness values, and lower brightness and band 6 values) compared with huts in control villages. Although elevation was included in the model and had a statistically significant effect, the OR revealed little or no association with hut classification (OR = 1, 95% CI = 1–1.01). However, this may be because elevation is quantified in meters and changes in risk are not expected at such small increments of measurement. Furthermore, there was limited variation in elevation between huts in case and control villages, and all were situated above the previously described 1,300-m threshold.
Table 1.
Risk factors for plague in case and control villages
| Risk factor | Case huts (N = 78) | Control huts (N = 80) | OR | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|
| Present (N) | Percent* | Present (N) | Percent* | ||||
| Peridomestic pig | 41 (72) | 57 | 28 (73) | 38 | 2.31 | 1.09–4.13 | 0.025 |
| Bedding material (other) | 1 (64) | 2 | 9 (61) | 15 | 0.09 | 0.01–0.75 | 0.007 |
| Distance to neighboring homestead | 76 (76) | 77 (77) | 1.07 | 1.03–1.21 | 0.002 | ||
| Elevation | 78 (78) | 80 (80) | 1 | 1–1.01 | 0.014 | ||
| Wetness | 78 (78) | 80 (80) | 1.1 | 1.02–1.08 | 0.002 | ||
| Brightness | 78 (78) | 80 (80) | 0.98 | 0.96–1.0 | 0.031 | ||
| Landsat ETM+ band 6 | 78 (78) | 80 (80) | 0.95 | 0.92–0.98 | 0.0004 | ||
Percentages were calculated only for nominal variables.
Using the variables described above that were significantly different in univariate logistic regression analyses, we used a forward stepwise selection process to create multivariable logistic regression models predicting the odds of a hut being classified as belonging to a case village. The model included positive associations with presence of pigs in the peridomestic setting and distance to neighbor but negative associations with bedding material other than papyrus mats and Landsat ETM+ band 6 (Table 2). Based on the area under the ROC curve, this model had an overall accuracy of 78%.
Table 2.
Parameter estimates and adjusted odds ratios for the multivariable logistic regression model predicting risk factors for exposure to Y. pestis in case and control villages
| Model covariates | Parameter estimate | Likelihood ratio test | Odds ratios | ||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | χ2 | df | P value | aOR | 95% CI | |
| Intercept | 0.63 | 1.07 | 0.35 | 1 | 0.55 | ||
| Peridomestic pig | 0.34 | 0.22 | 2.40 | 1 | 0.12 | 1.99 | 0.84–4.88 |
| Bedding material (other) | −1.36 | 0.59 | 8.24 | 1 | 0.004 | 0.07 | 0.003–0.46 |
| Distance to neighboring homestead | 0.07 | 0.03 | 8.49 | 1 | 0.004 | 1.08 | 1.02–1.14 |
| Landsat ETM+ band 6 | −0.06 | 0.02 | 11.78 | 1 | 0.0006 | 0.94 | 0.90–0.97 |
SE = standard error; aOR = adjusted odds ratio; df = degrees of freedom.
Summary of residence-based variables associated with huts situated in areas classified as elevated or low risk.
As described in the subsection above, landscape-level RS variables that were used in a GIS-based risk assessment of plague in this region remained as significant indicators of risk. Although the previous study indicated that these variables were suggestive of certain agricultural practices posing a risk, the study did not include data to verify this assertion. Here, we sought to identify residence-based correlates of the previously published plague-risk model based on RS variables. Of the 158 huts surveyed and described above, 100 huts were located within pixels classified by the model as elevated risk, and 58 huts were located in pixels classified as low risk. When comparing huts within pixels classified as elevated risk versus low risk based on RS data, nine residence-based variables differed significantly between these categories (Table 3). Huts within pixels classified as elevated risk were less likely to have water available to rodents inside the hut than low-risk areas, and they were also less likely to have coffee growing within 30 m of the residence and processed commercial foods stored within the residence. Compared with huts located in pixels classified as low risk, those in areas classified as elevated risk were more likely to have sheep observed in the peridomestic environment, to have corn grown or dried within 30 m of the residence, and to grow melons on both vines and trees than low-risk areas. Melons growing on trees were defined as a melon vine growing up a tree. However, it should be noted that, compared with the other variables considered, the number of huts that grew melons was small (12 of 156) (Table 3). Lastly, huts within areas that were classified as elevated risk were more likely to have other crops grown or dried within 30 m of the residence. These crops were typically other row or seasonal crops.
Table 3.
Residence-based variables associated with RS classification of elevated versus low plague risk
| Risk factor | Elevated risk (N = 100) | Low risk (N = 58) | OR | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|
| Present (N) | Percent | Present (N) | Percent | ||||
| Water available to rodent | 45 (87) | 52 | 34 (48) | 71 | 0.44 | 0.21–0.94 | 0.031 |
| Peridomestic sheep | 33 (91) | 36 | 9 (54) | 17 | 2.84 | 1.24–6.55 | 0.012 |
| Corn grown or dried within 30 m | 60 (98) | 61 | 16 (58) | 28 | 4.14 | 2.05–8.39 | < 0.0001 |
| Coffee grown within 30 m | 44 (98) | 45 | 40 (58) | 69 | 0.37 | 0.19–0.73 | 0.004 |
| Melons (vine) grown or dried within 30 m | 11 (98) | 11 | 1 (58) | 2 | 7.2 | 0.91–57.35 | 0.031 |
| Melons (tree) grown or dried within 30 m | 12 (98) | 12 | 1 (58) | 2 | 7.95 | 1.01–62.86 | 0.022 |
| Other crops grown or dried within 30 m | 34 (98) | 35 | 11 (58) | 19 | 2.27 | 1.04–4.94 | 0.036 |
| Processed commercial foods stored within residence | 34 (88) | 35 | 37 (58) | 64 | 0.36 | 0.18–0.71 | 0.003 |
Using these significant variables, a multivariable regression model was created based on a forward stepwise selection process. The final model that predicted the odds of classification as elevated risk, which had an overall accuracy of 75%, was based on a positive association with corn and other crops grown or dried within 30 m of the residence and negative associations with water availability to rodents and processed commercial foods stored within the residence (Table 4).
Table 4.
Parameter estimates and adjusted odds ratios for the multivariable logistic regression model predicting residential risk factors for exposure to Y. pestis in areas classified as elevated and low risk
| Model covariates | Parameter estimate | Likelihood ratio test | Odds ratios | ||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | χ2 | df | P value | aOR | 95% CI | |
| Intercept | −1.23 | 0.54 | 5.17 | 1 | 0.02 | ||
| Water available to rodents | −0.26 | 0.44 | 0.36 | 1 | 0.55 | 0.77 | 0.32–1.83 |
| Corn grown or dried within 30 m | 1.13 | 0.46 | 6.14 | 1 | 0.01 | 3.1 | 1.28–7.73 |
| Other crops grown or dried within 30 m | 0.97 | 0.51 | 3.92 | 1 | 0.047 | 2.65 | 1.01–7.44 |
| Processed commercial foods stored within residence | −1.06 | 0.46 | 5.42 | 1 | 0.02 | 0.35 | 0.13–0.85 |
SE = standard error; aOR = adjusted odds ratio; df = degrees of freedom.
Summary of risk factors for plague in case and control villages within areas classified as posing an elevated risk for plague.
A total of 100 huts located within pixels classified as elevated risk for plague12 were included in this analysis, with 53 huts being in historic case villages and 47 huts in control villages. Of the variables considered in univariate logistic regression analyses, four residential variables were significantly associated with village classification. Compared with huts in control villages, huts within case villages were more likely to have pigs in the peridomestic environment, were more likely to have a greater number of males under the age of 12 years sleeping in the hut on a regular basis, and were more likely to be farther from their neighbors (case village median [range] = 15 m [3–40 m], control village median [range] = 10 m [6–32 m]). Huts within case villages were less likely to have bedding material other than reed mats compared with huts within control villages (Table 5). Bedding material within the category other included foam pads and stuffed mattresses.
Table 5.
Risk factors for plague in case and control villages within ecologically conducive areas
| Risk factor | Case (N = 53) | Control (N = 47) | OR | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|
| Present (N) | Percent* | Present (N) | Percent* | ||||
| Peridomestic pig | 28 (48) | 58 | 10 (43) | 23 | 4.62 | 1.86–11.49 | 0.0007 |
| Bedding material (other) | 1 (46) | 2 | 5 (33) | 15 | 0.12 | 0.01–1.12 | 0.032 |
| Number of males ≤ 12 years old sleeping in hut | 49 (49) | 42 (42) | 1.58 | 1.03–2.65 | 0.05 | ||
| Distance to neighboring homestead | 52 (52) | 47 (47) | 1.07 | 1.02–1.13 | 0.01 | ||
Percentages were calculated only for nominal variables.
Of the variables that were significant in univariate tests, only two were chosen by forward stepwise regression to be included in the multivariable regression model. As in the model presented above that did not constrain the analysis to areas of elevated risk, pigs observed in the peridomestic environment and distance to neighbor were positively associated with classification of case villages (Table 6). Although the ORs were similar between the models for distance to water, when restricting the model to include only huts within elevated risk areas, the OR associated with pigs in the peridomestic environment notably increased (Tables 2 and 6). The overall accuracy of this multivariable logistic regression model was 74%.
Table 6.
Parameter estimates and adjusted odds ratios for the multivariable logistic regression model predicting residential risk factors for exposure to Y. pestis within ecologically conducive areas in case and control villages
| Model covariates | Parameter estimate | Likelihood ratio test | Odds ratios | ||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | χ2 | df | P value | aOR | 95% CI | |
| Intercept | −0.70 | 0.45 | 2.44 | 1 | 0.12 | ||
| Peridomestic pig | 0.79 | 0.24 | 11.49 | 1 | 0.0007 | 4.85 | 1.92–13.07 |
| Distance to neighboring homestead | 0.07 | 0.03 | 6.53 | 1 | 0.01 | 1.08 | 1.02–1.15 |
SE = standard error; aOR = adjusted odds ratio; df = degrees of freedom.
Discussion
Few studies have focused on environmental and behavioral variables associated with plague infection, especially in the plague-endemic West Nile region of Uganda. Information gleaned from such studies may aid in tailoring evidence-based recommendations for the prevention and control of plague in this region. By coupling a previously published landscape-level statistical model of plague risk12 with this observational survey, we were able to identify potential residence-based variables associated with huts within historic case or control villages (e.g., distance to neighboring homestead and presence of pigs near the home) and huts within areas previously predicted as elevated or low risk (e.g., corn and other annual crops grown near the home, water storage in the home, and processed commercial foods stored in the home).
Although additional studies are required to fully understand why these variables emerged as predictors, we believe that they are consistent with current ecological theories on plague host population dynamics. Plague epizootics, the periods when humans are at greatest risk of being exposed to infected fleas, are dependent on critical thresholds of rodent hosts and vector fleas. That is, as host abundance and flea infestation rates increase, so does the probability of epizootic activity.1,2,19,20,31–36 Human risk of exposure has been associated with behaviors that increase the probability of contact with infectious fleas or practices that increase the availability of food or harborage for rodents.21,25 Rodent movement is often driven by food availability and appropriate habitat.34,37 Therefore, behaviors that attract flea-infested rodents into peridomestic settings may increase the probability of human exposure to plague-infested fleas or rodent hosts.38
In an attempt to identify areas of elevated risk of human exposure to plague bacteria in the West Nile region, a fine-resolution model was recently created based primarily on RS variables.12 As previously mentioned, plague risk was higher at elevations greater than 1,300 m than below,16 and RS variables included in the model implied that risk was elevated in wetter areas with less vegetative growth and more bare soil during the dry month of January when fields of seasonal crops are typically fallow.12 The reasons for why these variables were indicative of elevated risk were not evaluated in the previous study, but it was hypothesized that these spectral signatures were associated with areas of more intensive agriculture.12 Presumably, agricultural crops would increase the food supply for rodents and may result in higher densities of rodents. Our residence-based observations showed that some seasonal crops, such as corn and other grains, were associated with elevated risk areas and may be more attractive to rodents than perennial plantation crops such as bananas or coffee beans, which were either similar between elevated- and low-risk areas or more frequently associated with low-risk areas. Indeed, previous studies rarely observed rodent damage to banana and coffee crops compared with other seasonal crops,37 suggesting that, perhaps, rodents are less attracted to banana and coffee crops. The differentiation between these crop types is consistent with the spectral signatures used to differentiate elevated- and low-risk areas. Specifically, corn and other grain fields are often fallow during the dry month of January and would be expected to yield high brightness values indicative of bare soil and little vegetative growth.
The RS model also identified a positive association between wetness and the probability of an area being classified as elevated risk. Our multivariable model indicated a negative association with availability of water for rodents (e.g., presence of water in containers) within huts situated in pixels classified as elevated risk. We believe that water storage in huts may be more common in drier areas that are more distant from water sources. Finally, storage of processed commercial foods within the hut was more frequently associated with areas of low risk. Again, this variable may relate back to agricultural practices, such that in areas of elevated risk, more subsistence crops such as corn are grown, and thus, there is less need for purchasing commercial foods. Alternatively, it is possible that commercially processed foods are more commonly purchased in areas that engage in growing cash crops, such as coffee, which is found in areas classified as low risk, as stated above. In addition, the packaging of this processed food could be a deterrent for the rodents if enough easily accessible food is available. Because our study did not quantify the abundance of such foods but rather surveyed simple presence or absence of these food types, it is difficult to draw conclusions about this variable.
Our observational survey was conducted before the identification of RS landscape variables associated with elevated plague risk in this region,12 and thus, we did not control for many of these variables in our village selection. Confirming and reemphasizing the importance of these RS variables in assessing the likelihood of human plague case occurrence, our univariate analyses (objective 1) (Table 1) showed that three of four variables included in the previous model (e.g., elevation, brightness, and Landsat ETM+ band 6) remained significant predictors of case or control designation. The multivariable analyses revealed that huts in case villages typically had lower Landsat ETM+ band 6 values compared with huts in control villages; this is indicative of wetter conditions in case areas compared with control areas and shows consistency with the model predictions.
In addition to these landscape variables, we identified three residence-based variables that were predictive of case or control village designation. Specifically, when huts in case villages were compared with huts in control villages, pigs were more commonly observed in the peridomestic setting, and huts were spaced farther from their neighbors. We speculate that peridomestic pigs were observed more frequently in historic case villages because of a similar dietary pattern between rodent species and pigs. Commensal and sylvatic rodents that are susceptible to plague infection could be attracted to food provided to the pigs. These food sources could serve to increase the local carrying capacity for these rodents. Regarding hut spacing, local observations suggest that the spaces between neighboring homesteads are often occupied either by agricultural plots that could serve as food sources for rodents (e.g., corn and other seasonally grown grains) or brush that could serve as rodent harborage. Land converted to agriculture increases the habitat and food availability for sylvatic rodents and potential interactions with peridomestic rodents.28,38 It is also believed that areas with increased primary production of food crops, which result from elevated precipitation rates and wetter conditions, may increase the carrying capacity of rodents.1,2,28,33,35,39,40 After repeating the analysis using only huts that occurred within pixels recently classified as elevated risk (objective 3) (Tables 5 and 6), the association with pigs in the peridomestic setting and distance to neighbor remained as significant predictors of case or control designation.
In addition to identifying variables that may increase the risk for plague infection, other factors were more commonly found within control villages, suggesting that these behaviors or environmental conditions may be protective against exposure to plague bacteria. Beds made of material other than reed mats (e.g., foam pad or stuffed mattress) were observed more frequently within huts in control villages compared with case villages. We speculate that reed mats may be more likely to be infested with fleas than other bedding, because the organic content or dust media in these mats provides a suitable place for fleas to breed.41
Although our models yielded overall accuracies of 74–78%, several additional studies are required to improve our understanding of plague risk factors. Because case investigations were not conducted to determine the location of exposure, our study was based on randomly selected huts within case and control villages. Within villages, huts are not homogenous with respect to many of the surveyed variables. Focusing future surveys on the residence of laboratory-confirmed plague patients and appropriate controls may improve model accuracy. As suggested before, targeting areas classified as elevated risk that continue to not report plague cases may provide insight into subtle differences in housing, crops, or behaviors that prove protective.12 In addition to focusing future efforts within ecologically conducive areas and in conjunction with laboratory-confirmed plague case investigations, it would be beneficial to conduct the surveys during plague outbreaks or during the wet season, when most plague cases have been reported. Many of the variables that we surveyed related to crop production and storage. These practices change seasonally and may be associated with observed differences over time and space in the distribution of zoonotic hosts and their vectors.12,19,20,37 Future studies could also include making adjustments to the environmental and behavioral questions on the observational survey. Questions should include type and frequency of mud smearing, quantity of seasonal crops growing around huts, quantity of foods being stored in huts, and frequency and type of interactions between peridomestic animals and humans. Lastly, further investigation of the variables that presented as significant in this study should be conducted to test our hypotheses for why they may enhance or diminish plague risk. Understanding the critical link between fleas, their reservoir hosts, and humans is essential to prevention and control of plague within this endemic region of Uganda.23 We believe that this study provides important preliminary information about environmental and behavioral factors that might be contributing to plague risk.
ACKNOWLEDGMENTS
We wish to acknowledge the tremendous contributions of many individuals who made this study possible. Our Uganda Virus Research Institute field team consisted of Simon Wakaalo, David Drajole Andabati, Jackson Olweny, the late Wilfred Cwinyaai, and Nicholos Owor (in addition to authors N.B., G.A.M., and L.A.A.). Key field assistance was also provided by Ali Sebbi, Environmental Health Officer for Nebbi District, Santos Angualia, Assistant Environmental Health Officer for Vurra Subcounty, and Appolo Midra, Assistant Environmental Health Officer for Logiri Subcounty. We wish to especially recognize the lifetime contributions of author Asaph Ogen-Odoi, who died in December 2006 while conducting field work to control the large plague outbreak mentioned in this paper that immediately followed this study. His contributions to this study and to the understanding and control of plague in Uganda can never be overestimated.
Footnotes
Authors' addresses: Katherine MacMillan, Russell E. Enscore, Jeff N. Borchert, Paul S. Mead, Kenneth L. Gage, and Rebecca J. Eisen, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mails: iky4@cdc.gov, rce0@cdc.gov, gqx1@cdc.gov, pfm0@cdc.gov, klg0@cdc.gov, and dyn2@cdc.gov. Nackson Babi, Gerald Amatre, and Linda A. Atiku, Uganda Virus Research Institute, Entebbe, Uganda, E-mails: nacksonbabi@yahoo.com, gamatrea@yahoo.com, and lindadawnpinky@yahoo.com. Nackson Babi and Gerald Amatre, Department of Zoology, Makerere University, Kampala, Uganda. Gerald Amatre, Department of Biological Sciences, Kyambogo University, Kampala, Uganda.
References
- 1.Gage KL, Kosoy MY. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- 2.Eisen RJ, Gage KL. Adaptive strategies of Yersinia pestis to persist during inter-epizootic and epizootic periods. Vet Res. 2009;40:1. doi: 10.1051/vetres:2008039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dennis DT, Gage KL. In: Infectious Diseases. Cohen J, Powderly WG, editors. London, United Kingdom: Mosby; 2004. pp. 1641–1648. (Plague). [Google Scholar]
- 4.Begier EM, Asiki G, Anywaine Z, Yockey B, Schriefer ME, Aleti P, Ogden-Odoi A, Staples JE, Sexton C, Bearden SW, Kool JL. Pneumonic plague cluster, Uganda, 2004. Emerg Infect Dis. 2006;12:460–467. doi: 10.3201/eid1203.051051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Bubonic and pneumonia plague—Uganda, 2006. MMWR Morb Mortal Wkly Rep. 2009;58:778–781. [PubMed] [Google Scholar]
- 6.Poland JD, Dennis DT. Plague Manual: Epidemiology, Distribution, Surveillance and Control. Geneva, Switzerland: World Health Organization; 1999. pp. 43–54. (Diagnosis and clinical manifestations). [Google Scholar]
- 7.Crook LD, Tempest B. Plague. A clinical review of 27 cases. Arch Intern Med. 1992;152:1253–1256. doi: 10.1001/archinte.152.6.1253. [DOI] [PubMed] [Google Scholar]
- 8.Tikhomirov E. In: Plague Manual: Epidemiology, Distribution, Surveillance and Control. Dennis DT, Gage KL, Gratz N, Poland JD, Tikhomirov E, editors. Geneva, Switzerland: World Health Organization; 1999. pp. 11–37. (Epidemiology and distribution of plague). [Google Scholar]
- 9.Neerinckx S, Bertherat E, Leirs H. Human plague occurrences in Africa: an overview from 1877 to 2008. Trans R Soc Trop Med Hyg. 2010;104:97–103. doi: 10.1016/j.trstmh.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 10.Neerinckx SB, Peterson AT, Gulinck H, Deckers J, Leirs H. Geographic distribution and ecological niche of plague in sub-Saharan Africa. Int J Health Geogr. 2008;7:54. doi: 10.1186/1476-072X-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization 2005Outbreak news index Wkly Epidemiol Rec 80433, –44016379146 [Google Scholar]
- 12.Eisen RJ, Griffith KS, Borchert JN, MacMillan K, Apangu T, Owor N, Acayo S, Acidri R, Zielinski-Gutierrez E, Winters AM, Enscore RE, Schriefer ME, Beard CB, Gage KL, Mead PS. Assessing human risk of exposure to plague bacteria in northwestern Uganda based on remotely sensed predictors. Am J Trop Med Hyg. 2010;82:904–911. doi: 10.4269/ajtmh.2010.09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisen RJ, Enscore RE, Biggerstaff BJ, Reynolds PJ, Ettestad P, Brown T, Pape J, Tanda D, Levy CE, Engelthaler DM, Cheek J, Bueno R, Jr, Targhetta J, Montenieri JA, Gage KL. Human plague in the southwestern United States, 1957–2004: spatial models of elevated risk of human exposure to Yersinia pestis. J Med Entomol. 2007;44:530–537. doi: 10.1603/0022-2585(2007)44[530:hpitsu]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Eisen RJ, Reynolds PJ, Ettestad P, Brown T, Enscore RE, Biggerstaff BJ, Cheek J, Bueno R, Targhetta J, Montenieri JA, Gage KL. Residence-linked human plague in New Mexico: a habitat-suitability model. Am J Trop Med Hyg. 2007;77:121–125. [PubMed] [Google Scholar]
- 15.Holt AC, Salkeld DJ, Fritz CL, Tucker JR, Gong P. Spatial analysis of plague in California: niche modeling predictions of the current distribution and potential response to climate change. Int J Health Geogr. 2009;8:38. doi: 10.1186/1476-072X-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winters AM, Staples JE, Ogen-Odoi A, Mead PS, Griffith K, Owor N, Babi N, Enscore RE, Eisen L, Gage KL, Eisen RJ. Spatial risk models for human plague in the West Nile region of Uganda. Am J Trop Med Hyg. 2009;80:1014–1022. [PubMed] [Google Scholar]
- 17.Neerinckx S, Peterson AT, Gulinck H, Deckers J, Kimaro D, Leirs H. Predicting potential risk areas of human plague for the Western Usambara Mountains, Lushoto District, Tanzania. Am J Trop Med Hyg. 2010;82:492–500. doi: 10.4269/ajtmh.2010.09-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilonzo BS. Plague epidemiology and control in eastern and southern Africa during the period 1978 to 1997. Cent Afr J Med. 1999;45:70–76. [PubMed] [Google Scholar]
- 19.Amatre G, Babi N, Enscore RE, Ogen-Odoi A, Atiku LA, Akol A, Gage KL, Eisen RJ. Flea diversity and infestation prevalence on rodents in a plague-endemic region of Uganda. Am J Trop Med Hyg. 2009;81:718–724. doi: 10.4269/ajtmh.2009.09-0104. [DOI] [PubMed] [Google Scholar]
- 20.Eisen RJ, Borchert JN, Holmes JL, Amatre G, Van Wyk K, Enscore RE, Babi N, Atiku LA, Wilder AP, Vetter SM, Bearden SW, Montenieri JA, Gage KL. Early-phase transmission of Yersinia pestis by cat fleas (Ctenocephalides felis) and their potential role as vectors in a plague-endemic region of Uganda. Am J Trop Med Hyg. 2008;78:949–956. [PubMed] [Google Scholar]
- 21.Mann JM, Martone WJ, Boyce JM, Kaufmann AF, Barnes AM, Weber NS. Endemic human plague in New Mexico: risk factors associated with infection. J Infect Dis. 1979;140:397–401. doi: 10.1093/infdis/140.3.397. [DOI] [PubMed] [Google Scholar]
- 22.Akiev AK. Epidemiology and incidence of plague in the world, 1958–79. Bull World Health Organ. 1982;60:165–169. [PMC free article] [PubMed] [Google Scholar]
- 23.Gratz N. Plague Manual: Epidemiology, Distribution, Surveillance and Control. Geneva, Switzerland: World Health Organization; 1999. pp. 97–134. (Control of plague transmission). [Google Scholar]
- 24.Gage KL, Dennis DT, Orloski KA, Ettestad P, Brown TL, Reynolds PJ, Pape WJ, Fritz CL, Carter LG, Stein JD. Cases of cat-associated human plague in the Western US, 1977–1998. Clin Infect Dis. 2000;30:893–900. doi: 10.1086/313804. [DOI] [PubMed] [Google Scholar]
- 25.Gould LH, Pape J, Ettestad P, Griffith KS, Mead PS. Dog-associated risk factors for human plague. Zoonoses Public Health. 2008;55:448–454. doi: 10.1111/j.1863-2378.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- 26.Rust JH, Jr, Miller BE, Bahmanyar M, Marshall JD, Jr, Purnaveja S, Cavanaugh DC, Hla US. The role of domestic animals in the epidemiology of plague. II. Antibody to Yersinia pestis in sera of dogs and cats. J Infect Dis. 1971;124:527–531. doi: 10.1093/infdis/124.5.527. [DOI] [PubMed] [Google Scholar]
- 27.Schotthoefer AM, Eisen RJ, Reynolds PJ, Ettestad P, Brown T, Enscore RE, Biggerstaff BJ, Cheek J, Bueno R, Targhetta J, Montenieri JA, Gage KL. Socio-Economic Risk Factors Associated with Human Plague Cases in New Mexico. Fort Collins, CO: The Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 28.Duplantier JM, Duchemin JB, Chanteau S, Carniel E. From the recent lessons of the Malagasy foci towards a global understanding of the factors involved in plague reemergence. Vet Res. 2005;36:437–453. doi: 10.1051/vetres:2005007. [DOI] [PubMed] [Google Scholar]
- 29.SAS . JMP. Cary, NC: SAS; 2008. [Google Scholar]
- 30.Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv. 1997;24:38–49. [Google Scholar]
- 31.Davis S, Begon M, De Bruyn L, Ageyev VS, Klassovskiy NL, Pole SB, Viljugrein H, Stenseth NC, Leirs H. Predictive thresholds for plague in Kazakhstan. Science. 2004;304:736–738. doi: 10.1126/science.1095854. [DOI] [PubMed] [Google Scholar]
- 32.Davis S, Trapman P, Leirs H, Begon M, Heesterbeek JA. The abundance threshold for plague as a critical percolation phenomenon. Nature. 2008;454:634–637. doi: 10.1038/nature07053. [DOI] [PubMed] [Google Scholar]
- 33.Hirst LF. The Conquest of Plague. Oxford, United Kingdom: Claredon Press; 1953. p. 478. [Google Scholar]
- 34.Rahelinirina S, Duplantier JM, Ratovonjato J, Ramilijaona O, Ratsimba M, Rahalison L. Study on the movement of Rattus rattus and evaluation of the plague dispersion in Madagascar. Vector Borne Zoonotic Dis. 2010;10:77–84. doi: 10.1089/vbz.2009.0019. [DOI] [PubMed] [Google Scholar]
- 35.Krasnov BR, Khokhlova IS. The effect of behavioural interactions on the transfer of fleas (Siphonaptera) between two rodent species. J Vector Ecol. 2001;26:181–190. [PubMed] [Google Scholar]
- 36.Krasnov BR, Shenbrot GI, Mouillot D, Khokhlova IS, Poulin R. Ecological characteristics of flea species relate to their suitability as plague vectors. Oecologia. 2006;149:474–481. doi: 10.1007/s00442-006-0455-7. [DOI] [PubMed] [Google Scholar]
- 37.Duplantier JM, Duchemin JB, Chanteau S, Carniel E. Ecologically Based Rodent Management. Canberra, Australia: Australian Centre for International Agricultural Research; 1999. pp. 441–459. (The rodent problem in Madagascar: agricultural pest and threat to human health). [Google Scholar]
- 38.Makundi RH, Oguge NO, Mwanjabe PS. Ecologically Based Rodent Management. Canberra, Australia: Australian Centre for International Agricultural Research; 1999. (Rodent pest management in East Africa—an ecological approach). [Google Scholar]
- 39.Collinge SK, Johnson WC, Ray C, Matchett R, Grensten J, Cully JF, Gage KL, Kosoy M, Loye JE, Martin A. Testing the generality of the tropic-cascade model for plague. EcoHealth. 2005;2:102–112. [Google Scholar]
- 40.Enscore RE, Biggerstaff BJ, Brown TL, Fulgham RE, Reynolds PJ, Engelthaler DM, Levy CE, Parmenter RR, Montenieri JA, Cheek JE, Grinnell RK, Ettestad PJ, Gage KL. Modeling relationships between climate and the frequency of human plague cases in the southwestern United States, 1960–1997. Am J Trop Med Hyg. 2002;66:186–196. doi: 10.4269/ajtmh.2002.66.186. [DOI] [PubMed] [Google Scholar]
- 41.Hopkins GHE. Annotated and illustrated keys to the known fleas of East Africa. Uganda J. 1947;11:133–191. [Google Scholar]


