Abstract
We determined abundance of Aedes aegypti mosquitoes and presence of dengue virus (DENV) in females collected from schools in Mérida, México, during 2008 and 2009. Backpack aspiration from 24 schools produced 468 females of Ae. aegypti and 1,676 females of another human biter, Culex quinquefasciatus. Ae. aegypti females were collected most commonly from classrooms followed by offices and bathrooms. Of these females, 24.7% were freshly fed. Examination of 118 pools of Ae. aegypti females (total of 415 females) for presence of DENV RNA produced 19 positive pools (16.1%). DENV-infected pools were detected from 11 (45.8%) of 24 schools and came from different room types, including classrooms, offices, and bathrooms. The overall rate of DENV infection per 100 Ae. aegypti females was 4.8. We conclude that schools in Mérida present a risk environment for students, teachers, and other personnel to be exposed to mosquitoes and bites of DENV-infected Ae. aegypti females.
Introduction
The arbovirus vector Aedes aegypti, which transmits viruses causing important human diseases including dengue, yellow fever, and chikungunya, is closely associated with indoor and peridomestic environments.1 Immatures can be found in a wide range of containers located indoors (vases, flower pots, etc.) or in backyards or other peridomestic settings (bottles, cans, buckets, tires, drums, water storage tanks, etc.).2 The female, which almost exclusively bites humans,3–5 most commonly feeds and rests indoors and if there are larval development sites available indoors, may not even venture outside to lay eggs.6
Indoor and peridomestic use patterns of the adult stage of Ae. aegypti have been examined in numerous studies (reviewed by García-Rejón and others7). Home environments have been shown to harbor large numbers of Ae. aegypti females.5,8–10 Furthermore, dengue virus (DENV)-infected Ae. aegypti females were reported in the home environment in Southeast Asia11–20 and the Americas.7,21–27 For example, we recently showed that DENV-infected Ae. aegypti females are commonly found in the homes of laboratory-confirmed dengue patients in Mérida, México, up to 4 weeks after onset of symptoms.7
Although it is well-understood that the home is an important risk environment for exposure to Ae. aegypti females and DENV, there is a lack of knowledge of the potential epidemiological significance of other indoor environments where people congregate, such as schools, work places, hospitals, and business areas. Schools have commonly been associated with presence and sometimes with high abundance of Ae. aegypti immatures,28–36 but few published studies have presented data on indoor infestation of schools with Ae. aegypti adults.21,34,37 In the Americas, Ae. aegypti females were collected from 63% of examined schools in Valle del Cauca State, Colombia, and abundances of Ae. aegypti adults on school premises in Iquitos, Peru, reached 6.1/ha in the winter.21,34 In contrast, a study from Thailand reported collection of very few Ae. aegypti adults from schools (total catch of eight Ae. aegypti females from 11 schools), with none of the females testing positive for DENV RNA.37
We present here a follow-up study to our previous work on DENV-infected Ae. aegypti females in homes in Mérida, and we now shift the focus to the potentially very important but poorly studied indoor environment of the school. The goals of the study were to (1) document infestation of schools by Ae. aegypti and other mosquitoes, (2) determine use patterns by key mosquito species of different room types in the schools, and (3) show the presence of DENV-infected Ae. aegypti females in schools.
Materials and Methods
Study environment.
Studies were conducted in the city of Mérida (population ∼ 800,000) in the Yucatan peninsula of southern México. The flat and low Yucatan peninsula (elevation range = 0–250 m above sea level) has a bedrock dominated by limestone and is characterized by a subtropical climate. Mean monthly maximum temperatures in Mérida range from 29°C in December to 34°C in July, and the majority of the rainfall occurs from May to October, with a peak from June to September (data from Comision Nacional del Agua weather station at the Mérida airport). Ae. aegypti adults and dengue cases may occur throughout the year in Mérida, but mosquito abundance and numbers of dengue cases typically peak from July to October.7,38
Mosquito collection.
Adult mosquitoes were collected from 24 schools in the southern part of Mérida from October 2008 to December 2009. Schools included 5 kindergartens, 14 elementary schools, 2 junior high schools, 2 high schools, and 1 college. Twenty-one schools were sampled during October 13–29, 2008, and 18 schools were sampled again during December 2–13, 2008. Three additional schools were sampled in early December of 2008. All 24 schools were then sampled again from November 25 to December 11, 2009. School locations were georeferenced using a global positioning system (GPS) receiver (Garmin, Salem, OR), and mosquito collection environments were classified as follows: bathroom, classroom (including computer rooms and laboratories), office, storage room, other room (including libraries, workshops, kitchens, etc), and outdoors.
Mosquitoes were collected from the schools during 0800–1500 hours using Centers for Disease Control and Prevention (CDC)-style backpack aspirators.8 Collected mosquitoes were separated by school, date, and collection environment. Indoor collection included aspiration from furniture, behind hanging clothes and curtains, and from dark and humid places where mosquitoes can be found resting. Outdoor collection focused primarily on areas directly adjacent to the school building. The length of time spent actively collecting per school varied with school size, but the overall time typically was in the range of 45 minutes to 1 hour. Mosquitoes were identified to species using stereo microscopes and published identification keys.39,40
Blood feeding status of females (Sella's stages) was determined by external examination of the abdomen.41 Sella's stages include I (unfed: collapsed abdomen and ovaries occupying one-third of the abdomen), II (freshly fed: bright red blood and ovaries occupying two to three segments ventrally and four segments dorsally), III–IV (half-gravid: dark red blood and ovaries occupying four to five segments ventrally and six segments dorsally), V (sub-gravid: blood greatly reduced and dark in color and ovaries occupying most of abdomen), and VI–VII (gravid: blood completely digested or present only as a black trace or line). Ae. aegypti females were pooled by school, date, and collection environment and stored at −70°C before processing for presence of DENV by reverse transcription polymerase chain reaction (RT-PCR).
DENV detection from Ae. aegypti female pools.
We processed 118 mosquito pools containing 415 Ae. aegypti females (range per pool = 1–13 females with the exception of four pools containing 14, 18, 18, and 31 females, respectively) for DENV identification by RT-PCR. Pooled females were triturated using sterile pestles and Eppendorf tubes in 0.6 mL cold minimum essential medium (MEM) containing 2% fetal bovine serum (FBS) (HyClone, Logan, UT) and antibacterial and antifungal antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B). The resulting suspension was added to QIAshredder columns (QIAGEN, Valencia, CA), and the columns were centrifuged at 14,000 rpm for 3 minutes at 4°C. Thereafter, 300 μL of each sample were transferred to Eppendorf tubes for RNA extraction, and the remaining suspensions were stored at −70°C.
Virus RNA was extracted using the RNeasy kit (QIAGEN). This was followed by RT-PCR–based DENV amplification using primers targeting the NS3 gene.42 A second round of semi-nested PCR including the upstream consensus primer and DENV 1–4 serotype-specific primers43 was used to determine DENV serotype. Amplification products were visualized on a 2% LE agarose gel (Promega Corp., Madison, WI) containing ethidium bromide.
Data analysis.
Statistical analyses were carried out using the JMP statistical package.44 Specific tests used are indicated in the text. Results were considered significant when P < 0.05.
Results
Summary of mosquito collections in the schools.
Collections of adult mosquitoes from Mérida schools from October 2008 to December 2009 produced a total of 7,964 specimens, including 6,033 Culex quinquefasciatus, 1,175 Ae. aegypti, 746 Ae. taeniorhynchus, 5 Ae. trivittatus, and 5 Cx. interrogator (Table 1). These collections included 468 females of Ae. aegypti and 1,676 females of another important human biter, Cx. quinquefasciatus (Table 1).
Table 1.
Summary of mosquito collections from schools in Mérida during 2008 and 2009
| Time period and species | Females | Males | ||||||
|---|---|---|---|---|---|---|---|---|
| No. collected | Percentage of total females for period | Range for individual schools | Percentage of schools with females | No. collected | Percentage of total males for period | Range for individual schools | Percentage of schools with males | |
| October 2008* | ||||||||
| Aedes aegypti | 217 | 32.6 | 1–32 | 100 | 322 | 28.1 | 1–39 | 100 |
| Aedes taeniorhynchus | 129 | 19.4 | 0–52 | 66.7 | 71 | 6.2 | 0–54 | 28.6 |
| Aedes trivittatus | 1 | 0.2 | 0–1 | 4.8 | 0 | 0 | 0 | 0 |
| Culex interrogator | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Culex quinquefasciatus | 318 | 47.8 | 0–62 | 90.4 | 751 | 65.6 | 0–212 | 90.4 |
| December 2008* | ||||||||
| Aedes aegypti | 66 | 12.3 | 0–15 | 85.7 | 82 | 5.1 | 0–43 | 61.9 |
| Aedes taeniorhynchus | 6 | 1.1 | 0–3 | 14.3 | 0 | 0 | 0 | 0 |
| Aedes trivittatus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Culex interrogator | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Culex quinquefasciatus | 466 | 86.6 | 1–144 | 100 | 1,515 | 94.9 | 0–566 | 90.5 |
| November to December 2009* | ||||||||
| Aedes aegypti | 185 | 12.0 | 0–39 | 87.5 | 303 | 12.2 | 0–136 | 95.8 |
| Aedes taeniorhynchus | 457 | 29.6 | 0–72 | 100 | 83 | 3.4 | 0–712 | 45.8 |
| Aedes trivittatus | 4 | 0.3 | 0–3 | 8.3 | 0 | 0 | 0 | 0 |
| Culex interrogator | 5 | 0.3 | 0–3 | 8.3 | 0 | 0 | 0 | 0 |
| Culex quinquefasciatus | 892 | 57.8 | 0–393 | 100 | 2,091 | 84.4 | 0–28 | 100 |
Numbers of schools sampled were 21 in October 2008, 21 in December 2008, and 24 in November and December 2009.
The percentage of schools from which Ae. aegypti females were collected was consistently high, ranging from 85.7% in December 2008 to 87.5% in November to December 2009 and 100% in October 2008 (Table 1). A similar pattern was seen for Cx. quinquefasciatus females, which were collected from 90.4% of schools in October 2008 and 100% of schools in December 2008 and November to December 2009. Most schools produced only a few Ae. aegypti females on a given sampling occasion (Table 2). However, 10 or more Ae. aegypti females were collected from a single school on 16 occasions (including from kindergartens, elementary schools, and junior high schools), with one school yielding as many as 39 females. With regards to the more abundant Cx. quinquefasciatus, 20 or more females were collected from a single school on 16 occasions (including from kindergartens, elementary schools, and junior high schools), and a single school produced as many as 393 females (Table 2).
Table 2.
Numbers of Ae. aegypti and Cx. quinquefasciatus collected from schools in Mérida during 2008 and 2009
| School* | October 2008 | December 2008 | November to December 2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ae. aegypti | Cx. quinquefasciatus | Ae. aegypti | Cx. quinquefasciatus | Ae. aegypti | Cx. quinquefasciatus | |||||||
| Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | Females | Males | |
| 1 (H) | 7 | 20 | 33 | 121 | 3 | 3 | 144 | 411 | 8 | 3 | 17 | 81 |
| 2 (K) | 10 | 22 | 4 | 15 | 1 | 2 | 6 | 20 | 1 | 2 | 2 | 10 |
| 3 (K) | 1 | 2 | 12 | 18 | 1 | 0 | 7 | 6 | 0 | 0 | 5 | 8 |
| 4 (E) | 2 | 17 | 9 | 6 | NS† | NS | NS | NS | 1 | 1 | 3 | 9 |
| 5 (E) | 9 | 10 | 53 | 212 | 0 | 0 | 4 | 8 | 6 | 9 | 13 | 83 |
| 6 (E) | 6 | 5 | 0 | 3 | 2 | 0 | 2 | 0 | 18 | 1 | 4 | 10 |
| 7 (K) | 15 | 28 | 2 | 3 | 2 | 5 | 2 | 8 | 0 | 4 | 2 | 4 |
| 8 (H) | 1 | 4 | 3 | 16 | NS | NS | NS | NS | 2 | 5 | 12 | 10 |
| 9 (E) | 2 | 2 | 4 | 17 | 0 | 0 | 4 | 7 | 2 | 6 | 23 | 133 |
| 10 (J) | 15 | 29 | 18 | 35 | 3 | 0 | 18 | 34 | 12 | 5 | 7 | 16 |
| 11 (E) | 28 | 39 | 7 | 3 | 10 | 43 | 10 | 33 | 7 | 1 | 17 | 43 |
| 12 (E) | 2 | 1 | 51 | 32 | 1 | 0 | 5 | 5 | 5 | 3 | 130 | 551 |
| 13 (K–J) | 22 | 33 | 26 | 52 | 1 | 6 | 18 | 51 | 39 | 55 | 34 | 48 |
| 14 (E) | 9 | 17 | 4 | 41 | 5 | 3 | 8 | 14 | 9 | 13 | 19 | 44 |
| 15 (C) | 2 | 7 | 62 | 108 | 5 | 1 | 118 | 566 | 21 | 13 | 393 | 712 |
| 16 (K) | 2 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 8 | 11 | 23 |
| 17 (E) | 8 | 13 | 11 | 36 | NS | NS | NS | NS | 1 | 3 | 15 | 12 |
| 18 (K) | 18 | 26 | 8 | 14 | 2 | 7 | 42 | 108 | 3 | 2 | 6 | 13 |
| 19 (E) | 32 | 22 | 1 | 0 | 5 | 5 | 2 | 8 | 5 | 10 | 14 | 60 |
| 20 (E) | 8 | 10 | 4 | 7 | 5 | 3 | 6 | 14 | 6 | 3 | 17 | 29 |
| 21 (J) | 18 | 12 | 6 | 12 | 15 | 1 | 16 | 60 | 12 | 15 | 28 | 89 |
| 22 (E) | NS | NS | NS | NS | 1 | 1 | 6 | 8 | 1 | 2 | 12 | 29 |
| 23 (E) | NS | NS | NS | NS | 3 | 2 | 22 | 17 | 22 | 136 | 91 | 52 |
| 24 (E) | NS | NS | NS | NS | 1 | 0 | 25 | 137 | 0 | 3 | 17 | 22 |
| Total | 217 | 322 | 318 | 751 | 66 | 82 | 466 | 1,515 | 185 | 303 | 892 | 2,091 |
School type denoted in parenthesis. K = kindergarten; E = elementary school; J = junior high school; H = high school; C = college.
School was not sampled for mosquitoes for this time period.
Indoor-use patterns of Ae. aegypti and Cx. quinquefasciatus.
Ae. aegypti females were collected most commonly from classrooms (N = 292) followed by offices (N = 65), bathrooms (N = 56), storage rooms (N = 36), and other rooms (N = 16) (Figure 1). Outdoor collections produced three Ae. aegypti females. A similar indoor-use pattern was observed for Ae. aegypti males, with the exception that males were more abundant in bathrooms (Figure 1). Cx. quinquefasciatus females were collected most commonly from classrooms (N = 647) followed by bathrooms (N = 559), offices (N = 157), other rooms (N = 127), and storage rooms (N = 113) (Figure 2). Outdoor collections produced 73 Cx. quinquefasciatus females. A similar indoor-use pattern was seen for Cx. quinquefasciatus males, with most specimens collected from bathrooms and classrooms (Figure 2).
Figure 1.
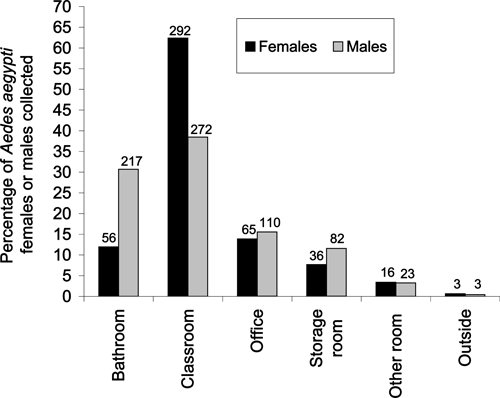
Percentages of Ae. aegypti females or males collected from different environments in Mérida schools during 2008 and 2009. Numbers above the bars indicate the total number collected by sex and environment.
Figure 2.
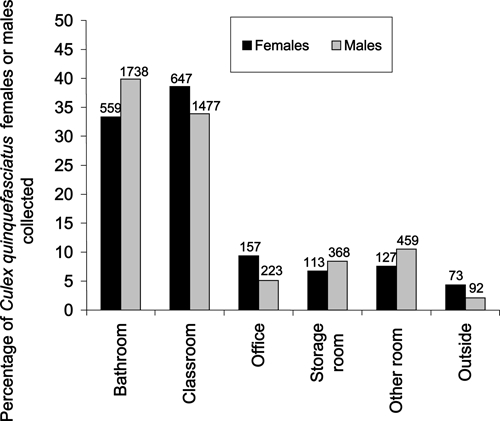
Percentages of Cx. quinquefasciatus females or males collected from different environments in Mérida schools during 2008 and 2009. Numbers above the bars indicate the total number collected by sex and environment.
The percentage of Ae. aegypti females collected by room type compared with Cx. quinquefasciatus females was higher for classrooms (62.4% versus 38.6%; contingency table analysis likelihood ratio: χ2 = 83.80, degrees of freedom [df] = 1, P < 0.001) and offices (13.9% versus 9.4%; χ2 = 7.57, df = 1, P = 0.006) but lower for bathrooms (12.0% versus 33.4%; χ2 = 92.96, df = 1, P < 0.001), other room types (3.4% versus 7.6%; χ2 = 11.70, df = 1, P < 0.001), and the outside environment (0.6% versus 4.4%; χ2 = 20.33, df = 1, P < 0.001). No significant difference was found for storage rooms (7.7% versus 6.7%; χ2 = 0.50, df = 1, P = 0.48).
Feeding status of Ae. aegypti and Cx. quinquefasciatus females.
Collections included females of Ae. aegypti and Cx. quinquefasciatus with different blood-feeding status. For Ae. aegypti, we recorded 137 unfed females (29.9%; Sella's stage I), 113 freshly fed females (24.7%; Sella's stage II), 93 half-gravid females (20.3%; Sella's stages III–IV), 52 sub-gravid females (11.3%; Sella's stage V), and 63 gravid females (13.8%; Sella's stages VI–VII). For Cx. quinquefasciatus, we collected 1,027 unfed females (70.5%; Sella's stage I), 64 freshly fed females (4.4%; Sella's stage II), 79 half-gravid females (5.4%; Sella's stages III–IV), 39 sub-gravid females (2.7%; Sella's stage V), and 247 gravid females (17.0%; Sella's stages VI–VII).
Notably, Ae. aegypti females collected from the schools were more than two times as likely to have fed compared with Cx. quinquefasciatus females (70.1% versus 29.5% of females classified as Sella's stages II–VII, respectively; χ2 = 238.82, df = 1, P < 0.001). Additional more detailed analyses showed that Ae. aegypti females compared with Cx. quinquefasciatus females were more likely to be freshly fed (24.7% versus 4.4%; χ2 = 143.04, df = 1, P < 0.001), half-gravid (20.3% versus 5.4%; χ2 = 80.64, df = 1, P < 0.001), or sub-gravid (11.3% versus 2.7%; χ2 = 48.57, df = 1, P < 0.001), whereas there was no significant difference for gravid females (13.8% versus 17.0%; χ2 = 2.83, df = 1, P = 0.09).
Dengue virus infection in Ae. aegypti females.
We examined a total of 118 pools of Ae. aegypti females comprising a total of 415 females for presence of DENV RNA (Table 3). This produced 19 positive pools: nine from October 2008, one from December 2008, and nine from November to December 2009. The percentage of DENV-positive pools ranged from 20.9% in November to December 2009 to 3.7% in December 2008 (Table 3). When comparing the 18 schools that were sampled during each time period, we found that the DENV infection rate in Ae. aegypti females was highest in November to December 2009 (6.1 per 100 females) followed by October 2008 (4.7 per 100 females) and December 2008 (1.9 per 100 females) (Table 4).
Table 3.
Detection of DENV RNA from Ae. aegypti females collected from schools in Mérida during 2008 and 2009
| School* | October 2008 | December 2008 | November and December 2009 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total females tested | No. of pools tested | No. of DENV-positive pools (%) | Total females tested | No. of pools tested | No. of DENV-positive pools (%) | Total females tested | No. of pools tested | No. of DENV-positive pools (%) | |
| 1 (H) | 7 | 3 | 2 (66.7) | 2 | 2 | 0 (0) | 8 | 3 | 0 (0) |
| 2 (K) | 10 | 2 | 2 (100) | 1 | 1 | 0 (0) | 1 | 1 | 0 (0) |
| 3 (K) | 1 | 1 | 0 (0) | 1 | 1 | 0 (0) | NT† | NT | NT |
| 4 (E) | 2 | 1 | 0 (0) | NS‡ | NS | NS | 1 | 1 | 0 (0) |
| 5 (E) | 9 | 2 | 2 (100) | NT | NT | NT | 6 | 2 | 0 (0) |
| 6 (E) | 6 | 1 | 0 (0) | 2 | 1 | 0 (0) | 18 | 1 | 0 (0) |
| 7 (K) | 15 | 2 | 2 (100) | 1 | 1 | 0 (0) | NT | NT | NT |
| 8 (H) | 1 | 1 | 0 (0) | NS | NS | NS | 2 | 1 | 0 (0) |
| 9 (E) | 2 | 2 | 0 (0) | NT | NT | NT | 2 | 1 | 0 (0) |
| 10 (J) | 15 | 3 | 0 (0) | 3 | 1 | 0 (0) | 12 | 5 | 3 (60.0) |
| 11 (E) | 28 | 4 | 0 (0) | 10 | 3 | 0 (0) | 7 | 3 | 1 (33.3) |
| 12 (E) | 2 | 2 | 0 (0) | 1 | 1 | 0 (0) | 5 | 2 | 1 (50.0) |
| 13 (K–J) | 22 | 4 | 1 (25.0) | 1 | 1 | 0 (0) | NT | NT | NT |
| 14 (E) | 9 | 3 | 0 (0) | 5 | 1 | 0 (0) | 9 | 2 | 0 (0) |
| 15 (C) | 2 | 2 | 0 (0) | 5 | 3 | 1 (33.3) | 21 | 6 | 0 (0) |
| 16 (K) | 2 | 2 | 0 (0) | NT | NT | NT | 4 | 2 | 0 (0) |
| 17 (E) | 8 | 3 | 0 (0) | NS | NS | NS | 1 | 1 | 0 (0) |
| 18 (K) | 18 | 2 | 0 (0) | 2 | 1 | 0 (0) | 3 | 2 | 0 (0) |
| 19 (E) | 32 | 2 | 0 (0) | 5 | 1 | 0 (0) | 5 | 3 | 2 (66.7) |
| 20 (E) | 8 | 3 | 0 (0) | 5 | 3 | 0 (0) | 6 | 2 | 0 (0) |
| 21 (J) | 18 | 3 | 0 (0) | 7 | 2 | 0 (0) | 8 | 0 | 0 (0) |
| 22 (E) | NS | NS | NS | 1 | 1 | 0 (0) | 1 | 1 | 0 (0) |
| 23 (E) | NS | NS | NS | 3 | 2 | 0 (0) | 22 | 3 | 2 (66.7) |
| 24 (E) | NS | NS | NS | 1 | 1 | 0 (0) | NT | NT | NT |
| Total | 217 | 48 | 9 (18.8) | 56 | 27 | 1 (3.7) | 142 | 43 | 9 (20.9) |
School type denoted in parenthesis. K = kindergarten; E = elementary school; J = junior high school; H = high school; C = college.
No Ae. aegypti females were tested from this school and time period.
School was not sampled for mosquitoes for this time period.
Table 4.
Minimum infection rates and maximum likelihood estimates for infection rates of DENV for Ae. aegypti females collected from schools in Mérida that were included for all three sampling periods during 2008 and 2009
| Time period | No. of females examined | No. of pools examined | No. of DENV-positive pools (%) | DENV infection rate per 100 females | |
|---|---|---|---|---|---|
| MIR* | MLE (95% CI)† | ||||
| October 2008 | 206 | 43 | 9 (20.9) | 4.4 | 4.7 (2.5–8.3) |
| December 2008 | 51 | 23 | 1 (4.3) | 2.0 | 1.9 (0.1–8.8) |
| November and December 2009 | 115 | 36 | 7 (19.4) | 6.1 | 6.1 (2.9–11.1) |
| Total for 2008 and 2009 | 372 | 102 | 17 (16.7) | 4.6 | 4.8 (3.0–7.3) |
Data were based on 18 schools that were examined for all three time periods during 2008–2009.
MIR = minimum infection rate per 100 females based on the assumption of a single infected female per infected pool.64
Determination of DENV serotypes present in positive pools indicated a shift from DENV-1 in 2008 to DENV-2 and DENV-3 in 2009. In 2008, 9 of 10 positive pools contained DENV-1 (the remaining pool contained DENV-4). In 2009, we commonly recorded multiple serotypes from a single pool. Of the nine positive pools from 2009, four contained DENV-1, whereas all nine pools contained DENV-2 and eight pools contained DENV-3.
DENV-infected pools were detected from 11 (45.8%) of the 24 examined schools, including two kindergartens, five elementary schools, one junior high school, one high school, one college, and one school including kindergarten to junior high (Table 3). Furthermore, DENV-infected pools came from a variety of different school room types, including classrooms (N = 6), offices (N = 5), storage rooms (N = 3), other room types (N = 3), and bathrooms (N = 2) (Figure 3). The percentages of DENV-positive pools for different room types were 8.3% for bathrooms, 10.7% for classrooms, 25.0% for storage rooms, 29.4% for offices, and 37.5% for other room types (including libraries, kitchens, etc). However, because of the limited sample sizes, there were no significant differences among these room types.
Figure 3.
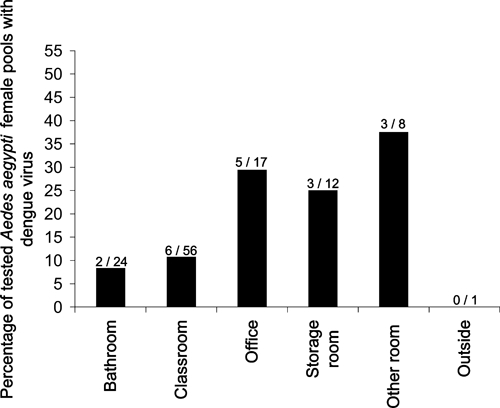
Percentages of tested pools of Ae. aegypti females with dengue virus RNA from different environments in Mérida schools during 2008 and 2009. Numbers above the bars indicate numbers of positive pools per total tested pools.
Discussion
The published literature contains minimal information about infestation of schools with adult Ae. aegypti and the risk for exposure to DENV-infected Ae. aegypti females in this important indoor environment where children congregate. To close this knowledge gap, we assessed entomological risk factors in schools in Mérida, México, to determine the potential epidemiological significance of schools for DENV transmission. Our study shows that students, teachers, and other personnel in Mérida schools are at risk for exposure to human-biting mosquitoes, especially Ae. aegypti and Cx. quinquefasciatus, as well as exposure to bites by DENV-infected Ae. aegypti females. Backpack aspiration of individual schools commonly produced > 10 Ae. aegypti females, and the overall rate of DENV infection per 100 Ae. aegypti females was as high as 4.8. Furthermore, DENV-infected pools of Ae. aegypti females came from a variety of different school room types, including classrooms, offices, storage rooms, and bathrooms. Thus, schools may serve as transmission nodes for DENV in Mérida, contributing to virus dispersal in the city during dengue outbreaks.
One weakness of the study was that Ae. aegypti females were examined for presence of DENV RNA in pooled samples. To determine rates of infected mosquitoes (those containing DENV) as well as potentially infectious mosquitoes (those with disseminated DENV infections), follow-up studies could assay individual mosquitoes and include assessment of virus dissemination to the head/salivary glands. Another issue that needs to be addressed in future studies is an abundance measure for adult mosquitoes in the schools. In the case of homes, which are readily sampled in toto with backpack aspirators, abundance of adults per examined home is a reasonable abundance measure. Schools, however, may differ dramatically in size and contain room types with distinct uses, such as classrooms, offices, and storage rooms. Based on the results of this study, perhaps the most appropriate abundance measure would be adults per examined classroom.
Schools in Mérida were infested with multiple species of human-biting mosquitoes that are capable of transmitting a wide range of pathogens. As expected from a previous study focusing on infestation of homes in Mérida by adult mosquitoes,7 the species most commonly collected from Mérida schools was Cx. quinquefasciatus followed by Ae. aegypti and Ae. taeniorhynchus. With regards to pathogen transmission, Ae. aegypti is the primary vector of DENV in Latin America and also is capable of transmitting the viruses causing yellow fever and chikungunya if these viruses emerge/reemerge in this part of the world.45–47 Cx. quinquefasciatus readily bites humans and is capable of transmitting several pathogens, including arboviruses (e.g., West Nile virus, St. Louis encephalitis virus, and Japanese encephalitis virus) and parasites (Wuchereria bancrofti that causes filariasis).48–51 In the specific case of Mérida, Cx. quinquefasciatus may, in addition to its role as a nuisance biter of humans,52 contribute as an enzootic vector in mosquito–bird West Nile virus transmission cycles and potentially, also as a bridging vector to humans of this virus. Furthermore, a novel flavivirus called T'Ho virus with as yet unknown pathogenicity to humans recently was detected from Cx. quinquefasciatus in Mérida.53 Ae. taeniorhynchus feeds on mammals, occasionally including humans, and is capable of transmitting West Nile virus and eastern equine encephalitis virus.50,54–56 As a side note, this mosquito also is considered an important vector of heartworm, Dirofilaria immitis, to dogs in Mérida.57
Both Ae. aegypti and Cx. quinquefasciatus females were collected from a variety of indoor environments in the schools, including classrooms, offices, bathrooms, and storage rooms. Perhaps the most notable result was that these two species appeared to exhibit different room type preferences. Ae. aegypti females were more prevalent, relative to the total numbers collected by species in the schools, in classrooms and offices, whereas Cx. quinquefasciatus females were more prevalent in bathrooms and the outdoor environment. Together with previous studies from home environments showing a preference of Ae. aegypti females for bedrooms,7,58–61 this underscores the importance of a detailed understanding of indoor-use patterns by key mosquito vector species in different indoor environments to most effectively implement indoor-targeted control measures.
Because Ae. aegypti females feed almost exclusively on humans,4,5 it was not surprising to find that most females collected from the schools, which provide an abundance of human hosts, had fed previously and that one-quarter of the females contained a fresh blood meal. In contrast, the majority of Cx. quinquefasciatus females were unfed, and very few (< 5%) contained a fresh blood meal. We speculate that Cx. quinquefasciatus may, in part, use the indoor school environment as a resting place when they are not actively seeking blood meals from their favored avian hosts. This is supported by a recent study from Mérida showing that Cx. quinquefasciatus females collected from peridomestic environments had fed predominantly on birds (accounting for 82% of blood meals) and only rarely on humans (7%).52
To the best of our knowledge, this is the first study to show that DENV-infected Ae. aegypti females can occur commonly in school environments. We found that (1) the overall rate of DENV infection per 100 Ae. aegypti females during the study period was as high as 4.8, (2) DENV-infected pools of Ae. aegypti females were detected from 11 (45.8%) of the 24 examined schools, and (3) DENV-infected females originated from a variety of different school environments, including classrooms, offices, storage rooms, and bathrooms. Our data on infected mosquitoes also indicated a shift in DENV serotypes circulating in the schools from DENV-1 during October–December 2008 to DENV-2 and DENV-3 during November–December 2009. Data for dengue patients in the Yucatan similarly showed an increase in the frequency of DENV-2 infections relative to DENV-1 from 2008 to 2009 (Loroño-Pino MA and Farfán-Ale JA, unpublished data).
Taken together, our results indicate that schools may play an important role in DENV transmission dynamics in Mérida. Schools may serve as DENV transmission nodes during dengue outbreaks, because both children and adults (teachers and other school staff) congregate in the schools on a regular basis and if they are infected with DENV in the schools, then can disperse the virus to their homes. The spatial range of this dispersal mechanism likely differs by school type. Kindergartens and elementary schools tend to have small recruitment areas and therefore will contribute primarily to local DENV dispersal by infected humans, whereas high schools and especially colleges have larger recruitment areas and thus, may contribute to DENV dispersal by infected humans at a larger spatial scale within a city.
Furthermore, some aspects of dengue virus infection dynamics in humans and the feeding behavior of Ae. aegypti females conspire to make the school a potentially very effective DENV transmission environment. First, humans may be infectious to feeding mosquitoes before the onset of fever, which typically occurs 2–7 days after the DENV infection event.62 Second, Ae. aegypti females are nervous feeders that frequently take multiple blood meals from different human hosts to engorge sufficiently to produce an egg batch.63 This provides a scenario where infectious children or adults are present in schools and where the biting habits of Ae. aegypti increases the likelihood of these infectious individuals being bitten. In addition, the propensity of Ae. aegypti to take multiple blood meals increases the likelihood that an infected and infectious mosquito will transmit DENV to more than one person (for example, children are easy targets in a classroom).
In conclusion, our study underscores the critical need for additional studies on the potential role of schools for DENV transmission in dengue endemic areas and highlights the need for improved indoor control of Ae. aegypti in schools in Latin America.
ACKNOWLEDGMENTS
The authors thank Roger Arana, Rosa Cetina, Wilberth Chi, Carlos Estrella, Alex Ic, Jorge Morales, Maria Puc, Lourdes Talavera, Hugo Valenzuela, and Ivan Villanueva of Universidad Autónoma de Yucatán for technical assistance with field and laboratory work and the principals of the involved schools for granting us permission to collect mosquitoes.
Footnotes
Financial support: The study was funded by the Innovative Vector Control Consortium.
Authors' addresses: Julián E. García-Rejón, María Alba Loroño-Pino, José Arturo Farfán-Ale, Luis F. Flores-Flores, and Mildred P. López-Uribe, Laboratorio de Arbovirología, Centro de Investigaciones Regionales Dr. Hideyo Noguchi, Universidad Autónoma de Yucatán, Centro, Mérida, Yucatán, México, E-mails: grejon@tunku.uady.mx, maria.lorono@gmail.com, jafarfan@tunku.uady.mx, fflores@uady.mx, and mpaluo@hotmail.com. Maria del Rosario Najera-Vazquez and Guadalupe Nuñez-Ayala, Servicios de Salud de Yucatán, Centro, Mérida, Yucatán, México, E-mails: dengue.yucatan@ssy.gob.mx and guadalupe.nunez@ssy.gob.mx. Barry J. Beaty and Lars Eisen, Department of Microbiology, Immunology, and Pathology, Colorado State University, Fort Collins, CO, E-mails: Barry.Beaty@ColoState.EDU and lars.eisen@colostate.edu.
References
- 1.Halstead SB. Dengue virus—mosquito interactions. Annu Rev Entomol. 2008;53:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- 2.Focks DA, Alexander N. A Multi-Country Study on the Methodology for Surveys of Aedes Aegypti Pupal Productivity: Findings and Recommendations. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 3.Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–849. doi: 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- 4.Scott TW, Chow E, Strickman D, Kittayapong P, Wirtz RA, Lorenz LH, Edman JD. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J Med Entomol. 1993;30:922–927. doi: 10.1093/jmedent/30.5.922. [DOI] [PubMed] [Google Scholar]
- 5.Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- 6.Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee S, Kittayapong P, Sithiprasasna R. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 7.García-Rejón J, Loroño-Pino MA, Farfán-Ale JA, Flores-Flores L, Rosado-Paredes ED, Rivero-Cardenas N, Najera-Vazquez R, Gomez-Carro S, Lira-Zumbardo V, Gonzalez-Martinez P, Lozano-Fuentes S, Elizondo-Quiroga D, Beaty BJ, Eisen L. Dengue virus-infected Aedes aegypti in the home environment. Am J Trop Med Hyg. 2008;79:940–950. [PubMed] [Google Scholar]
- 8.Clark GG, Seda H, Gubler DJ. Use of the “CDC backpack aspirator” for surveillance of Aedes aegypti in San Juan, Puerto Rico. J Am Mosq Control Assoc. 1994;10:119–124. [PubMed] [Google Scholar]
- 9.Schultz GW. Seasonal abundance of dengue vectors in Manila, Republic of the Philippines. Southeast Asian J Trop Med Public Health. 1993;24:369–375. [PubMed] [Google Scholar]
- 10.Tidwell MA, Williams DC, Carvalho Tidwell T, Pena CJ, Gwinn TA, Focks DA, Zaglul A, Mercedes M. Baseline data on Aedes aegypti populations in Santo Domingo, Dominican Republic. J Am Mosq Control Assoc. 1990;6:514–522. [PubMed] [Google Scholar]
- 11.Chow VTK, Chan YC, Yong R, Lee KM, Lim LK, Chung YK, Lam-Phua SG, Tan BT. Monitoring of dengue viruses in field-caught Aedes aegypti and Aedes albopictus mosquitoes by a type-specific polymerase chain reaction and cycle sequencing. Am J Trop Med Hyg. 1998;58:578–586. doi: 10.4269/ajtmh.1998.58.578. [DOI] [PubMed] [Google Scholar]
- 12.Chung YK, Pang FY. Dengue virus infection rate in field populations of female Aedes aegypti and Aedes albopictus in Singapore. Trop Med Int Health. 2002;7:322–330. doi: 10.1046/j.1365-3156.2002.00873.x. [DOI] [PubMed] [Google Scholar]
- 13.Halstead SB, Scanlon JE, Umpaivit P, Udomsakdi S. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. IV. Epidemiologic studies in the Bangkok metropolitan area. Am J Trop Med Hyg. 1969;18:997–1021. doi: 10.4269/ajtmh.1969.18.997. [DOI] [PubMed] [Google Scholar]
- 14.Ilkal MA, Dhanda V, Hassan MM, Mavale M, Mahadev PVM, Shetty PS, Guttikar SN, Banerjee K. Entomological investigations during outbreaks of dengue fever in certain villages in Maharashtra state. Indian J Med Res. 1991;93:174–178. [PubMed] [Google Scholar]
- 15.Kabilan L, Velayutham T, Sundaram B, Tewari SC, Natarajan A, Rathnasamy R, Satyanarayana K. Field- and laboratory-based active dengue surveillance in Chennai, Tamil Nadu, India: observations before and during the 2001 dengue epidemic. Am J Infect Control. 2004;32:391–396. doi: 10.1016/j.ajic.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Kow CY, Koon LL, Yin PF. Detection of dengue viruses in field caught male Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Singapore by type-specific PCR. J Med Entomol. 2001;38:475–479. doi: 10.1603/0022-2585-38.4.475. [DOI] [PubMed] [Google Scholar]
- 17.Pankhong P, Siriprasertkul W, Patpoparn S, Srisuphanunt M, Rojanapremsuk J, Sithiprasasna R, Coleman RE, Nisalak A, Endy TP, Attatippaholkun MK, Attatippaholkun WH. Molecular serotyping of dengue viruses in field-caught Aedes mosquitoes by in-house RNA extraction/RT-PCR reagent kits. Southeast Asian J Trop Med Public Health. 2002;33((Suppl 3)):139–144. [PubMed] [Google Scholar]
- 18.Sithiprasasna R, Patpoparn S, Attatippaholkun W, Suvannadabba S, Srisuphanunt M. The geographic information system as an epidemiological tool in the surveillance of dengue virus-infected Aedes mosquitoes. Southeast Asian J Trop Med Public Health. 2004;35:918–926. [PubMed] [Google Scholar]
- 19.Tewari SC, Thenmozhi V, Katholi CR, Manavalan R, Munirathinam A, Gajanana A. Dengue vector prevalence and virus infection in a rural area in south India. Trop Med Int Health. 2004;9:499–507. doi: 10.1111/j.1365-3156.2004.01103.x. [DOI] [PubMed] [Google Scholar]
- 20.Angel B, Joshi V. Distribution of dengue virus types in Aedes aegypti in dengue endemic districts of Rajasthan, India. Indian J Med Res. 2009;129:665–668. [PubMed] [Google Scholar]
- 21.Mendez F, Barreto M, Arias JF, Rengifo G, Munoz J, Burbano ME, Parra B. Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am J Trop Med Hyg. 2006;74:678–683. [PubMed] [Google Scholar]
- 22.Pinheiro VCS, Tadei WP, Barros PMSS, Vasconcelos PFC, Cruz ACR. Detection of dengue virus serotype 3 by reverse transcription-polymerase chain reaction in Aedes aegypti (Diptera, Culicidae) captured in Manaus, Amazonas. Mem Inst Oswaldo Cruz. 2005;100:833–839. doi: 10.1590/s0074-02762005000800003. [DOI] [PubMed] [Google Scholar]
- 23.Urdaneta L, Herrera F, Pernalete M, Zoghbi N, Rubio-Palis Y, Barrios R, Rivero J, Comach G, Jimenez M, Salcedo M. Detection of dengue viruses in field-caught Aedes aegypti (Diptera: Culicidae) in Maracay, Aragua state, Venezuela by type-specific polymerase chain reaction. Infect Genet Evol. 2005;5:177–184. doi: 10.1016/j.meegid.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 24.de la Mora-Covarrubias A, Jimenez-Vega F, Trevino-Aguilar SM. Geospatial distribution and detection of dengue virus in Aedes (Stegomyia) aegypti mosquitoes in Ciudad Juarez, Chihuahua, Mexico. Salud Publica Mex. 2010;52:127–133. [PubMed] [Google Scholar]
- 25.Guedes DRD, Cordeiro MT, Melo-Santos MAV, Magalhaes T, Marques E, Regis L, Furtado AF, Ayres CFJ. Patient-based dengue virus surveillance in Aedes aegypti from Recife, Brazil. J Vector Borne Dis. 2010;47:67–75. [PubMed] [Google Scholar]
- 26.Vilela APP, Figueiredo LB, dos Santos JR, Eiras AE, Bonjardim CA, Ferreira PCP, Kroon EG. Dengue virus 3 genotype I in Aedes aegypti mosquitoes and eggs, Brazil, 2005–2006. Emerg Infect Dis. 2010;16:989–992. doi: 10.3201/eid1606.091000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lourenco-de-Oliveira R, Honorio NA, Castro MG, Schatzmayr HG, Miagostovich MP, Alves JC, Silva WC, Leite PJ, Nogueira RM. Dengue virus type 3 isolation from Aedes aegypti in the municipality of Nova Iguacu, State of Rio de Janeiro. Mem Inst Oswaldo Cruz. 2002;97:799–800. doi: 10.1590/s0074-02762002000600009. [DOI] [PubMed] [Google Scholar]
- 28.Hwang J-S, Hsu E-L. Investigations on the distribution and breeding habitats of dengue vectors in Kaohsiung City. Chin J Entomol. 1994;14:233–244. [Google Scholar]
- 29.Avila-Montes GA, Martinez M, Sherman C, Cerna EF. Evaluation of an educational module on dengue and Aedes aegypti for schoolchildren in Honduras. Rev Panam Salud Publica. 2004;16:84–94. doi: 10.1590/s1020-49892004000800003. [DOI] [PubMed] [Google Scholar]
- 30.Sharma RS, Panigrahi N, Kaul SM. Aedes aegypti prevalence in hospitals and schools, the priority sites for DHF transmission in Delhi, India. Dengue Bull. 2001;25:107–108. [Google Scholar]
- 31.Strickman D, Kittayapong P. Dengue and its vectors in Thailand: introduction to the study and seasonal distribution of Aedes larvae. Am J Trop Med Hyg. 2002;67:247–259. doi: 10.4269/ajtmh.2002.67.247. [DOI] [PubMed] [Google Scholar]
- 32.Strickman D, Sithiprasasna R, Kittayapong P, Innis BL. Distribution of dengue and Japanese encephalitis among children in rural and suburban Thai villages. Am J Trop Med Hyg. 2000;63:27–35. doi: 10.4269/ajtmh.2000.63.27. [DOI] [PubMed] [Google Scholar]
- 33.da Silva VC, Scherer PO, Falcao SS, Alencar J, Cunha SP, Rodrigues IM, Pinheiro NL. Diversity of oviposition containers and buildings where Aedes albopictus and Aedes aegypti can be found. Rev Saude Publica. 2006;40:1106–1111. doi: 10.1590/s0034-89102006000700021. [DOI] [PubMed] [Google Scholar]
- 34.Morrison AC, Sihuincha M, Stancil JD, Zamora E, Astete H, Olson JG, Vidal-Ore C, Scott TW. Aedes aegypti (Diptera: Culicidae) production from non-residential sites in the Amazonian city of Iquitos, Peru. Ann Trop Med Parasitol. 2006;100:S73–S86. doi: 10.1179/136485906X105534. [DOI] [PubMed] [Google Scholar]
- 35.Troyo A, Calderon-Arguedas O, Fuller DO, Solano ME, Avendano A, Arheart KL, Chadee DD, Beier JC. Seasonal profiles of Aedes aegypti (Diptera: Culicidae) larval habitats in an urban area of Costa Rica with a history of mosquito control. J Vector Ecol. 2008;33:76–88. doi: 10.3376/1081-1710(2008)33[76:spoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoedojo ST. Aedes aegypti, control through source reduction by community efforts in Pekalongan, Indonesia. Mosquito-Borne Dis Bull. 1990;7:59–62. [Google Scholar]
- 37.Mammen MP, Pimgate C, Koenraadt CJM, Rothman AL, Aldstadt J, Nisalak A, Jarman RG, Jones JW, Srikiatkhachorn A, Ypil-Butac CA, Getis A, Thammapalo S, Morrison AC, Libraty DH, Green S, Scott TW. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5:e205. doi: 10.1371/journal.pmed.0050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loroño-Pino MA, Farfan-Ale JA, Rosado-Paredes EP, Kuno G, Gubler DJ. Epidemic dengue 4 in the Yucatan, Mexico, 1984. Rev Inst Med Trop Sao Paulo. 1993;35:449–455. doi: 10.1590/s0036-46651993000500011. [DOI] [PubMed] [Google Scholar]
- 39.Carpenter SJ, LaCasse WJ. Mosquitoes of North America (North of Mexico) Berkeley, CA: University of California Press; 1955. [Google Scholar]
- 40.Darsie RF, Jr, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida; 2005. [Google Scholar]
- 41.World Health Organization . Manual on Practical Entomology in Malaria, Part II. Geneva, Switzerland: World Health Organization; 1975. [Google Scholar]
- 42.Seah CLK, Chow VTK, Chan YC. Semi-nested PCR using NS3 primers for the detection and typing of dengue viruses in clinical serum specimens. Clin Diagn Virol. 1995;4:113–120. doi: 10.1016/0928-0197(94)00063-z. [DOI] [PubMed] [Google Scholar]
- 43.Seah CLK, Chow VTK, Tan HC, Chan YC. Rapid, single-step RT-PCR typing of dengue viruses using five NS3 gene primers. J Virol Methods. 1995;51:193–200. doi: 10.1016/0166-0934(94)00104-o. [DOI] [PubMed] [Google Scholar]
- 44.Sall J, Creighton L, Lehman A. JMP Start Statistics. 3rd ed. Belmont, CA: Brooks/Cole; 2005. [Google Scholar]
- 45.Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol. 2007;52:209–229. doi: 10.1146/annurev.ento.52.110405.091454. [DOI] [PubMed] [Google Scholar]
- 46.Gratz NG. Emerging and resurging vector-borne diseases. Annu Rev Entomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- 47.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 48.Bockarie MJ, Pedersen EM, White GB, Michael E. Role of vector control in the global program to eliminate lymphatic filariasis. Annu Rev Entomol. 2009;54:469–487. doi: 10.1146/annurev.ento.54.110807.090626. [DOI] [PubMed] [Google Scholar]
- 49.Day JF. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol. 2001;46:111–138. doi: 10.1146/annurev.ento.46.1.111. [DOI] [PubMed] [Google Scholar]
- 50.Turell MJ, Dohm DJ, Sardelis MR, O'Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- 51.van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 52.García-Rejón JE, Blitvich BJ, Farfán-Ale JA, Loroño-Pino MA, Chim WAC, Flores-Flores LF, Rosado-Paredes E, Baak-Baak C, Perez-Mutul J, Suarez-Solis V, Fernandez-Salas I, Beaty BJ. Host-feeding preference of the mosquito, Culex quinquefasciatus, in Yucatan State, Mexico. J Insect Sci. 2010;10:32. doi: 10.1673/031.010.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farfán-Ale JA, Loroño-Pino MA, García-Rejón JE, Hovav E, Powers AM, Lin M, Dorman KS, Platt KB, Bartholomay LC, Soto V, Beaty BJ, Lanciotti RS, Blitvich BJ. Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2009;80:85–95. [PMC free article] [PubMed] [Google Scholar]
- 54.Christensen HA, de Vasquez AM, Boreham MM. Host-feeding patterns of mosquitoes (Diptera: Culicidae) from central Panama. Am J Trop Med Hyg. 1996;55:202–208. doi: 10.4269/ajtmh.1996.55.202. [DOI] [PubMed] [Google Scholar]
- 55.Edman JD. Host-feeding patterns of Florida mosquitoes. I. Aedes, Anopheles, Coquilletidia, Mansonia and Psorophora. J Med Entomol. 1971;8:687–695. doi: 10.1093/jmedent/8.6.687. [DOI] [PubMed] [Google Scholar]
- 56.Turell MJ, Beaman JR, Neely GW. Experimental transmission of eastern equine encephalitis virus by strains of Aedes albopictus and A. taeniorhynchus (Diptera: Culicidae) J Med Entomol. 1994;31:287–290. doi: 10.1093/jmedent/31.2.287. [DOI] [PubMed] [Google Scholar]
- 57.Manrique-Saide P, Escobedo-Ortegon M, Bolio-Gonzalez M, Sauri-Arceo C, Dzib-Florez S, Guillermo-May G, Ceh-Pavia E, Lenhart A. Incrimination of the mosquito, Aedes taeniorhynchus, as the primary vector of heartworm, Dirofilaria immitis, in coastal Yucatan, Mexico. Med Vet Entomol. 2010;24:456–460. doi: 10.1111/j.1365-2915.2010.00884.x. [DOI] [PubMed] [Google Scholar]
- 58.Davila G, Nelson MJ, Turner A, Garcia A.1991Resting sites for Aedes aegypti in Panama J Am Mosq Control Assoc 7633–634.1686277 [Google Scholar]
- 59.Macdonald WW. Aedes aegypti in Malaya. I. Distribution and dispersal. Ann Trop Med Parasitol. 1956;50:385–398. [PubMed] [Google Scholar]
- 60.Perich MJ, Davila G, Turner A, Garcia A, Nelson M. Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama City, Panama. J Med Entomol. 2000;37:541–546. doi: 10.1603/0022-2585-37.4.541. [DOI] [PubMed] [Google Scholar]
- 61.Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol. 2008;22:62–69. doi: 10.1111/j.1365-2915.2008.00720.x. [DOI] [PubMed] [Google Scholar]
- 62.Nishiura H, Halstead SB. Natural history of dengue virus (DENV)-1 and DENV-4 infections: reanalysis of classic studies. J Infect Dis. 2007;195:1007–1013. doi: 10.1086/511825. [DOI] [PubMed] [Google Scholar]
- 63.de Benedictis J, Chow-Shaffer E, Costero A, Clark GG, Edman JD, Scott TW. Identification of the people from whom engorged Aedes aegypti took blood meals in Florida, Puerto Rico, using polymerase chain reaction-based DNA profiling. Am J Trop Med Hyg. 2003;68:437–446. [PubMed] [Google Scholar]
- 64.Gu W, Lampman R, Novak RJ. Assessment of arbovirus vector infection rates using variable size pooling. Med Vet Entomol. 2004;18:200–204. doi: 10.1111/j.0269-283X.2004.00482.x. [DOI] [PubMed] [Google Scholar]
- 65.Biggerstaff BJ. PooledInfRate, Version 3.0: A Microsoft Excel Add-In to Compute Prevalence Estimates from Pooled Samples. Fort Collins, CO: Centers for Disease Control and Prevention; 2006. [Google Scholar]


