Abstract
Neurons incorporated into the adult main olfactory bulb (MOB) and accessory olfactory bulb (AOB) derive from the subventricular zone (SVZ). Despite some recent studies on the role of olfactory neurogenesis in sociosexual behaviors mediated by hormones, data on the implication of estrogens are still lacking. Taking advantage of female aromatase-knockout (ArKO) mice, which are unable to produce estradiol across their life span, we investigated the role of estradiol exposure during early postnatal and adult periods on adult neurogenesis in the MOB and AOB. We found that proliferation of progenitor cells in the adult female SVZ was not influenced by estradiol. However, whereas adult exposure to estradiol influences the turnover of MOB newborn neurons, the survival of those in the AOB depends on exposure to estradiol during the early postnatal period. Finally, based on their expression of Zif268, we showed that newborn neurons in the MOB responded to sociosexual odors, albeit to a lesser extent in ArKO females, suggesting a contribution of estradiol during the early postnatal period to this response. Together, these results suggest that the survival and functional integration of newborn neurons in the adult female MOB and AOB are differentially influenced by estrogens from the early postnatal period to adulthood.—Veyrac, A., Bakker, J. Postnatal and adult exposure to estradiol differentially influences adult neurogenesis in the main and accessory olfactory bulb of female mice.
Keywords: aromatase-knockout mice, neuronal survival, functional integration
Neurogenesis occurs continuously in some regions of the adult nervous system (1, 2), with the hippocampal dendate gyrus (DG) and the olfactory bulb (OB) representing the two best characterized networks that continuously integrate large numbers of newborn neurons during adulthood (3–5). In the olfactory system, newly generated cells originate from the subventricular zone (SVZ), migrate along the rostral migratory stream (RMS), and differentiate into local interneurons (granule and periglomerular cells) before integrating into functional circuitry in both the main olfactory bulb (MOB) and accessory olfactory bulb (AOB) (6–8). Adult neurogenesis is regulated by intrinsic programs and external factors at all levels, including the proliferation of progenitor cells and the survival and the integration of newborn neurons (9). Although sensory olfactory experience and learning play a crucial role in the survival and integration of newborn neurons in the MOB and AOB (8, 10–14), the physiological relevance of adult neurogenesis and subsequent plasticity (15) is still unknown. Some studies have suggested the involvement of olfactory neurogenesis in reproduction, such as mate selection (16), pregnancy (17), and maternal (18, 19) and paternal recognition behavior (20), which are all mediated by prolactin. However, little attention has been paid to a potential role for estrogens in modulating olfactory neurogenesis (21–23). This is surprising, since estradiol plays a key role in female olfactory reproductive behavior (24–26), and there is vast literature on the role of estrogens on neurogenesis in the adult DG (27, 28). Therefore, in the present study, we determined the role of estradiol in adult olfactory neurogenesis by analyzing cell proliferation and the survival and functional integration of newborn neurons in the adult MOB and AOB of female aromatase-knockout (ArKO) mice, which carry a targeted mutation in the Cyp19 gene and cannot convert androgens into estrogens. A great advantage of the ArKO model is that the mice fail to produce estradiol but they have functional estrogen receptors to respond to exogenous estradiol at any point during the life span (29, 30). To test the effects of estrogens in adult neurogenesis, we used wild-type (WT) and ArKO female mice that were either left gonadally intact or ovariectomized and were treated or not with estradiol at the beginning of adult life. Since the secretion of estrogens by the ovaries starts in the postnatal period around d 7 after birth in WT mice (31), the comparison between the different hormone-treated WT and ArKO female groups provides an unique opportunity to evaluate the contribution of estradiol exposure during the early postnatal (from wk 1–8) vs. the adult period (wk 8 and further) in the regulation of the adult olfactory neurogenesis and to determine whether any changes observed in ArKO mice could be reversed by adult treatment with estradiol.
MATERIALS AND METHODS
Animals
ArKO mice were generated by targeted disruption of exons 1 and 2 of the Cyp 19 gene (30). Heterozygous males and females for the ArKO mutation (strain C57Bl/6j/sv129) were bred to generate WT and homozygous-null (ArKO) offspring. Mice were genotyped by PCR analysis of tail DNA (32). All breeding and genotyping were performed at the department GIGA Neurosciences, University of Liège (Liège, Belgium). All experimental female mice (n=72) were group housed (6/cage) in climate-controlled housing units on a reversed 12-h light-dark cycle (lights on at 8:00 PM). Food and water were available ad libitum. All experiments were conducted in accordance with the guidelines set forth by the U.S. National Institutes of Health and were approved by the Ethical Committee for Animal Use of the University of Liège.
Surgery and hormonal treatment
Groups of adult WT and ArKO female mice were left gonadally intact (intact groups; n=24; 8 wk of age) and were grouped by genotype and housed 3 wk before the start of the experiment to promote synchronization of the estrous cycle in the WT females (33). Intact female mice did not receive any additional hormone treatment, and their estrous cycle was determined by cellular profile analysis of vaginal smears collected by a noninvasive approach of flushing with a saline solution using glass Pasteur pipettes. ArKO females do not show any estrous cycles due to their estrogen deficiency, and their vaginal smears look like permanent diestrus (34, 35).
Additional groups of adult WT and ArKO female mice were ovariectomized (OVX groups; n=24; 8 wk of age) under general anesthesia through an intraperitoneal injection of a mixture of ketamine (80 mg/kg/mouse) and medetomidine (Domitor; 1 mg/kg/mouse; Pfizer, Elsene, Belgium). Mice received atipamezole (Antisedan; 4 mg/kg/mouse, Pfizer) at the end of surgery to accelerate their recovery.
Finally, 2 groups of WT and ArKO female mice were ovariectomized (OVX-E2 groups; n=24; 8 wk of age) and implanted subcutaneously in the neck under general anesthesia with a 5-mm-long Silastic capsule containing 17-β-estradiol (diluted 1:1 with cholesterol), which produced circulating levels of estradiol similar to those observed during estrus in intact females (36).
Experimental procedures
Experiment 1: role of estradiol in cell proliferation in the adult SVZ
We evaluated the influence of estradiol on cell proliferation in the SVZ of WT (n=6/group) and ArKO (n=6/group) adult female mice under 3 different hormonal conditions (OVX, intact, and OVX-E2; Fig. 1A). OVX and OVX-E2 mice were ovariectomized or ovariectomized and implanted with E2 on the first day of the experiment and returned to their home cages for 3 wk. On d 21, all mice received 2 intraperitoneal injections, 2 h apart, of 5-bromo-2′deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO, USA; 50 mg/kg in physiological saline) and were killed 2 h after the last BrdU injection. Vaginal smears were performed in intact mice at the time of the first BrdU injection (Fig. 1A).
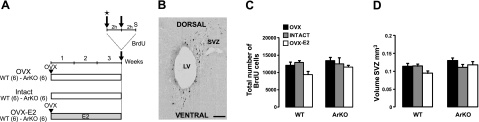
Figure 1.
Effect of estradiol on cell proliferation in the SVZ. A) Adult WT and ArKO female mice were given 3 different hormonal treatments for 3 wk: ovariectomy (OVX), ovary-intact estrous cycle (intact), and ovariectomy + implantation of an estradiol capsule (OVX-E2). Mice were injected with BrdU (2 injections at 2 h interval) on d 21 and sacrificed (S) 2 h afterward for assessment of cell proliferation in the SVZ. Stage of the estrous cycle of intact mice was determined before the first BrdU injection by analysis of vaginal smears (star). B) Representative image showing BrdU+ cells in the SVZ, analyzed 2 h after BrdU injections. LV, lateral ventricle. Scale bar = 50 μm. C) Total number of BrdU+ cells in the SVZ of the 6 experimental groups. D) Volume of the SVZ in the experimental groups. OVX: WT, n = 6; ArKO, n = 6. Intact: WT, n = 6; ArKO, n = 4. OVX-E2: WT, n = 5; ArKO, n = 4. Values are expressed as means ± se.
Experiment 2: role of estradiol in newborn neuron survival in the MOB and AOB
In the adult MOB and AOB, the role of estradiol on the survival of newborn neurons independently of its observed effects on the proliferation of progenitor cells remains unknown. Therefore, to determine the influence of estradiol on only the survival rate of newborn neurons during their integration in the olfactory bulb, additional groups of female WT (n=6/group) and ArKO (n=6/group) mice (intact, OVX, and OVX-E2) were used (Fig. 2). Mice of the OVX and OVX-E2 groups were ovariectomized on the first day of the experiment and returned to their home cages for 3 wk without receiving any hormone treatment. On d 21, all mice received 4 intraperitoneal BrdU injections (50 mg/kg; Sigma-Aldrich) every 2 h, before being returned to their respective cages for the remaining 4 wk of the experiment. To exclude any effects of estrogens on cell proliferation, OVX-E2 mice were implanted with the E2 capsule under light anesthesia the day after the BrdU injections, and the capsule was left in place for the remaining 4 wk of the experiment. Vaginal smears were taken in intact mice at the time of the first BrdU injection on d 21 and again on the last day of the experiment, just before the mice were killed (Fig. 2).
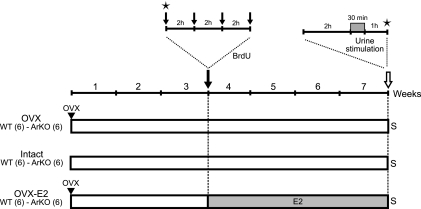
Figure 2.
Experimental design for assessing cell survival and the activation of newborn neurons in the MOB and AOB. Three additional groups of adult WT and ArKO female mice were submitted to 3 distinct hormonal treatments for 3 wk. OVX and OVX-E2 groups were ovariectomized on the first day and returned to their home cages for 3 wk. On d 21, all mice received 4 BrdU injections (at 2-h intervals) and were returned to their respective cages for 4 wk. OVX-E2 mice received implants the day after BrdU injections and kept the implants for the remaining 4 wk of the experiment. Vaginal smears (stars) were performed in intact mice at the time of the first BrdU injection on d 21 and again on the final day of the experiment, just before sacrifice (S). On the final day, all mice were separated into 2 subgroups, isolated for 2 h before stimulation, and exposed for 30 min to either deionized water or to male urinary odors. Mice were killed 60 min later to assess immediate Zif268 gene expression.
Experiment 3: role of estradiol in the functional integration of newborn neurons in the OB
Finally, we determined whether newly generated neurons that survived in the MOB and AOB were actually integrated into a functional circuitry by determining whether these cells were able to respond to a socially relevant odor stimulus for females, such as male urinary odors (11). Before death, we exposed groups of WT and ArKO females of all 3 different hormonal conditions (OVX, intact, and OVX-E2) to either male urine or water to serve as control and processed their brains for double-label immunocytochemistry for BrdU and Zif268. Zif268 is an immediate early gene with neuronal expression driven by activity-dependant plasticity (37) in newborn granular cells (20, 38–40).
Urine was collected from 10 C57Bl/6j males (Charles River Laboratories, L'Arbresles, France) by holding the mouse by the scruff off the neck over a funnel, taking care that no fecal contamination occurred. Urine samples were pooled and subsequently portioned into aliquots and stored at −80°C until use. One week before the end of the experiment, all mice of experiment 2 (cell survival; Fig. 2) were trained daily for 1 wk to the urine exposure procedure by applying deionized water onto the nose, as described previously (41). This method of urine exposure has been shown to activate both the main and the accessory olfactory system (42). On the day of urine exposure, all mice of the 3 experimental groups were housed alone 2 h before olfactory stimulation in clean cages without food and water and were then separated into 2 subgroups. Treatment-subgroup mice were exposed to 30 μl of male urine that was applied onto the nose and were additionally exposed to 100 μl of male urinary odors applied onto a cotton swab that was subsequently placed in a tea ball and suspended from the cage lid for 30 min (exposure to volatile urinary odors). Control-subgroup mice received 30 μl of deionized water on the nose and additionally 100 μl of water in the tea ball for 30 min. Mice were killed 60 min after the end of olfactory stimulation to measure the expression of Zif268 in newborn cells labeled by BrdU.
Tissue preparation and sectioning
Mice were deeply anesthetized with the same mixture of ketamine/medetomidine used for ovariectomy and killed by intracardiac perfusion of a solution containing 4% cold paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Brains were dissected out and fixed overnight in the same perfusion solution at 4°C, immersed for 96 h at 4°C in phosphate buffer containing 20% sucrose, and frozen in chilled isopentane (−50°C). The brains were sectioned in the coronal plane with a cryostat (Leica, Nussloch, Germany) into 14-μm-thick serial sections collected at the anatomical levels comprising the MOB, AOB, and SVZ. The sections were mounted onto superfrost plus sides (Carl Zeiss, Oberkochen, Germany).
Immunochemistry
All brain sections were processed for immunoreactivity as described previously (43). Brain sections were preincubated in Target Retrieval Solution (Dako, Heverlee, Belgium) for 20 min at 95°C. After cooling for 20 min, sections were treated with 0.5% Triton in PBS for 30 min and then for 3 min with pepsin (0.0125% in 0.1 N HCl; Sigma-Aldrich). Endogenous peroxidases were blocked with a solution of 3% H2O2 in 0.1 M PBS. Sections were then incubated for 90 min in 5% normal serum (Vector Laboratories, Burlingame, CA, USA) in 5% BSA (Sigma-Aldrich) and 0.125% Triton X-100 to block nonspecific binding, and incubated overnight at 4°C in a mouse anti-BrdU primary antibody (1:100; Chemicon, Temecula, CA, USA) to label newborn cells or 48 h at 4°C in a rabbit anti-caspase-3 (Casp3) antibody (1:500; Cell Signaling Technology, Danvers, MA, USA) to label apoptotic cells. Sections were then incubated in a horse biotinylated anti-mouse (1:200; Vector Laboratories) or in a goat anti-rabbit (1:200; Vector Laboratories) secondary antibody for 2 h at room temperature. Sections were then processed with avidin-biotin-peroxidase complex (ABC Elite Kit; Vector Laboratories) for 30 min, followed by 3 rinses of 5 min in PBS. Finally, sections were reacted in 0.05% 3,3-diaminobenzidine-tetrahydrochloride (Sigma-Aldrich), 0.03% NiCl2, and 0.03% H2O2 in Tris-HCl buffer (0.05 M, pH 7.6); dehydrated in graded ethanols; and coverslipped in DPX.
For BrdU double-labeling experiments, sections treated as described before were first incubated overnight at 4°C in mouse anti-NeuN (1:500; Chemicon) or rabbit anti-Zif268 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies and incubated for 2 h at room temperature in biotinylated horse anti-mouse or goat anti-rabbit secondary antibodies followed by streptavidin Alexa 488 (1:1000; Molecular Probes, Eugene, OR, USA). Sections were then processed for BrdU detection (mouse anti-BrdU, 1:100; Chemicon; or rat anti-BrdU, 1:100; Harlan Sera Laboratory, Loughborough, UK) overnight at 4°C. Immunoreactivities were revealed 2 h at room temperature with goat antirat or horse anti-mouse conjugated to Alexa 546 (1:200; Molecular Probes).
Quantification and image analysis
BrdU and Casp3-stained nuclei quantification
All cell counts were conducted in a masked fashion with regard to the experimental condition. Labeled profiles were counted with a Zeiss microscope coupled with mapping software (Mercator Pro; Explora Nova, La Rochelle, France). All BrdU nuclei were counted in the granular cell layer (GrCL) of the MOB (5 sections/animal spaced by 336 μm), in the GrCL of the AOB (4 sections/animal spaced by 84 μm), and in the SVZ (4 sections/animal spaced by 336 μm; from bregma 1.54 mm to bregma 0.14 mm) as described previously (43). Concerning the Casp3+ cells, all nuclei were counted in the GrCL of the MOB (3 sections/animal, intersection intervals of 504 μm) and in the GrCL of the AOB (3 sections/animal, intersection intervals of 84 μm). The volume of the region of interest was calculated according to a conventional stereological equation (44), the profile density (number of labeled cell outlines/μm2 on sections) was derived from these data, and the total number of cells was calculated as described previously (43, 45).
Double labeling analysis and quantification
All the sections were mounted in Vectashield medium with DAPI (Vector Laboratories) and analyzed using the Olympus Fluoview FV1000 confocal system equipped with the Olympus IX81 inverted microscope (Olympus Europa, Hamburg, Germany). To establish the percentage of BrdU cells double-labeled for NeuN or Zif268, serial sections from all mice were examined throughout the MOB and AOB. Colocalizations were evaluated in 20–40 BrdU-labeled cells from each animal by performing z-stack acquisitions and 3-dimensional reconstructions using Fluoview 10-ASW 1.7.
Statistical analysis
The data were averaged across animals within each experimental group and are presented as means ± se. Statistical comparisons were conducted by 2-way ANOVA followed by Fisher's least significant difference post hoc test. Only effects detected by the ANOVA tests with a value of P < 0.05 are mentioned as significant in the results.
RESULTS
Postnatal and adult exposure to estradiol does not influence progenitor cell proliferation in the adult SVZ
The rate of cell proliferation was measured in WT and ArKO female mice of the 3 experimental groups (OVX, intact, and OVX-E2) 2 h after BrdU administration, and the labeled cells were counted in the SVZ (Fig. 1A, B). Overall, no differences in the number of BrdU-labeled cells and SVZ volume were observed between ArKO and WT mice, suggesting that cell proliferation is not affected by estradiol in the hormonal conditions tested. Indeed, the lack of estrogens during the postnatal period in ArKO mice did not affect progenitor cell proliferation or the volume of the adult SVZ [Fig. 1C, D; no significant effect of genotype: total number of cells, F(1,12)=1.862, P=0.197; SVZ volume, F(1,12)=4.710, P=0.061]. Moreover, in comparison to OVX mice, the number of BrdU-labeled proliferating cells and the volume of the SVZ in WT and ArKO mice were not affected in the intact condition (5 of 6 WT mice were in proestrus) and the OVX-E2 condition, which mimics circulating levels of estradiol observed during estrus (36) [Fig. 1C, D; no effect of hormonal condition: F(2,12)=2.432, P=0.130; F(2,12)=1.350, P=0.296].
Adult estradiol negatively regulates the survival of newly generated neurons in the MOB
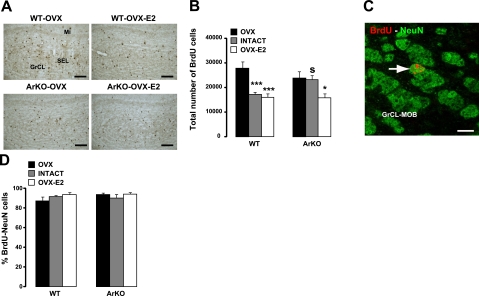
The rate of surviving newborn neurons in the MOB was evaluated 4 wk after BrdU injections in intact, OVX, and OVX-E2 WT and ArKO adult mice (Figs. 2 and 3A). Overall, adult estradiol decreased the survival of newborn cells in the GrCL, which is the main target of newly generated neurons in the MOB [Fig. 3A, B; significant effect of hormonal condition: F(2,11)= 6.840, P=0.012]. In WT mice, the number of BrdU+ cells was reduced by 38% in the intact group and by 43% in the OVX-E2 group, compared with the OVX group (P=0.0023, WT-OVX vs. WT-intact; P=0.001, WT-OVX vs. WT-OVX-E2). Likewise, a similar decrease in number of BrdU-labeled cells was observed in ArKO mice treated with estradiol, whereas intact ArKO females, which are deprived of estrogens, exhibited the same number of surviving BrdU cells as OVX females, further confirming that adult estradiol decreased cell survival (P=0.0210, ArKO-OVX vs. ArKO-OVX-E2; P=0.8631, ArKO-OVX vs. ArKO-intact). By contrast, the absence of estrogens during the early postnatal period (i.e., before being actually ovariectomized or treated with estradiol in adulthood) did not seem to have any effect on the survival of newborn cells in the MOB, since numbers of surviving BrdU cells were similar in WT-OVX vs. ArKO-OVX and WT-OVX-E2 vs. ArKO-OVX-E2 mice [Fig. 3A, B; no significant effect of genotype: F(1,11)=0.058, P=0.814). Finally, the decreased survival of newborn cells by adult rather than postnatal estrogens is supported by the increased number of BrdU cells in intact ArKO compared with intact WT mice (P=0.0162).
Figure 3.
Influence of estradiol on the survival of newborn neurons in the adult MOB. A) Representative images showing newly generated BrdU cells in the GrCL of the MOB, analyzed 4 wk after BrdU injections. Number of BrdU cells was decreased in WT-OVX-E2 and ArKO-OVX-E2 compared with WT-OVX and ArKO-OVX female mice. SEL, subependymal layer; Mi, mitral cell layer. B) Total number of BrdU+ cells in the MOB GrCL from the 6 experimental groups. C) Representative image of BrdU-NeuN double-labeled cell (arrow) under confocal microscopy. D) Percentage of BrdU-NeuN double-labeled cells counted in the GrCL of the MOB. OVX: WT, n = 6; ArKO, n = 6. Intact: WT, n = 4; ArKO, n = 4. OVX-E2: WT, n = 6; ArKO, n = 5. Values are expressed as means ± se. Scale bars = 60 μm (A); 6 μm (C). *P < 0.05, ***P < 0.005 vs. OVX; SP < 0.05 vs. WT; 2-way ANOVA followed by Fisher's post hoc test.
The reduced survival of newborn cells observed in the different experimental groups was correlated with a decrease in the GrCL volume, which is generally considered as a sign of cell loss (45, 46) [data not shown; effect of hormonal condition: F(2,10)=10.753, P=0.003; but no effect of genotype: F(1,10)=0.228, P=0.643].
BrdU+ cells in the MOB were identified as mature granule cells by their localization in the MOB (Fig. 3A) and by their expression of the neuronal marker NeuN (Fig. 3C). Confocal microscopy analysis of double-labeled cells revealed that the mean percentage of BrdU cells expressing NeuN was statistically similar between groups [Fig. 3D; hormonal condition: F(2,13)=1.137, P=0.351; genotype: F(1,13)=0.649, P=0.435]. This result suggests that the reduced numbers of BrdU+ cells seen in Fig. 3B resulted from a decrease in the number of newborn neurons in the experimental MOB and not from a change in the neuronal differentiation rate of newborn cells. Since WT-OVX-E2 vs. WT-OVX and ArKO-OVX-E2 vs. ArKO-OVX mice showed the greatest decrease in GrCL volume and number of BrdU-labeled cells (Fig. 3B), we correlated the reduced cell survival observed in these groups with an increase in the number of Casp3 apoptotic cells (47) [effect of hormonal condition: F(1,8)=15.299, P = 0.004; but no effect of genotype: F(1,8)=2.921, P=0.126]. Indeed, estradiol treatment induced a significant increase in the number of Casp3+ dying cells, and this effect did not differ between genotypes [WT-OVX-E2 (1040±340) and ArKO-OVX-E2 (665±141) in comparison to WT-OVX (188±39; P=0.001) and ArKO-OVX (222±54; P=0.038), respectively].
Together, these results demonstrate that estrogen exposure in adulthood, but not earlier in life, somehow reduces the incorporation of newborn granule neurons in the adult MOB and thereby affects their survival.
Survival of newborn neurons in the adult AOB depends on postnatal exposure to estradiol
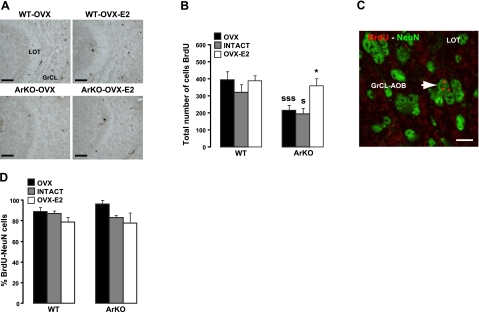
In contrast with the MOB, ArKO mice had a significantly lower number of newborn granule cells in the AOB compared with WT mice [Fig. 4A, B; significant effect of genotype: F(1,8)= 9.650, P=0.015]. Indeed, 4 wk after being born in the SVZ, BrdU cells were found in lower numbers in the GrCL of the AOB of OVX and intact ArKO mice, which are completely deprived of estrogens starting from the postnatal period, compared with WT mice (P=0.003, WT-OVX vs. ArKO-OVX; P=0.050, WT-intact vs. ArKO-intact). However, this effect was completely reversed by adult treatment with estradiol, since OVX-E2 ArKO mice had very similar numbers of BrdU-labeled neurons as WT mice (P=0.018, ArKO-OVX-E2 vs. ArKO-OVX; P=0.011, ArKO-OVX-E2 vs. ArKO-intact). Contrary to what was observed in the MOB, estradiol exposure in adulthood did not have any effect on cell survival of newborn cells in the AOB of WT mice [no significant effect of hormonal condition: F(2,8)=2.832, P=0.117]. Four weeks after BrdU injections, most of the BrdU+ cells in the AOB GrCL expressed NeuN (Fig. 4C), indicating that they were mature neurons. There were neither genotype differences nor an effect of hormonal condition on the proportion of BrdU/NeuN double-labeled cells in the AOB, suggesting that estradiol did not influence the rate of neuronal differentiation of newly generated cells in the AOB [Fig. 4D; hormonal condition: F(2,12)=4.510, P=0.055; genotype: F(1,12)=1.422, P=0.256]. Despite that the number of 4-wk-old neurons in the AOB was reduced in ArKO-OVX mice (Fig. 3B), the number of Casp3+ cells did not vary significantly in function of genotype or hormonal treatment [not shown; hormonal condition: F(1,7)= 2.118, P=0.189; genotype: F(1,7)=1.584, P=0.249]. This result is in agreement with a recent study (8) reporting that newborn granule cells in the AOB are predominantly sensitive to cell apoptosis between 7 and 15 d after birth.
Figure 4.
Influence of estradiol on the survival of newborn neurons in the adult AOB. A) Representative images showing newly generated BrdU cells in the GrCL of the AOB, analyzed 4 wk after BrdU injections. Number of BrdU cells was decreased in ArKO-OVX compared with WT-OVX, WT-OVX-E2, or ArKO-OVX-E2 mice. LOT, lateral olfactory tract. B) Total number of BrdU+ cells in the AOB GrCL. C) Representative image of a BrdU-NeuN double-labeled cell (arrow) under confocal microscopy. D) Percentage of BrdU-NeuN double-labeled cells counted in the GrCL of the AOB. OVX: WT, n = 6; ArKO, n = 5. Intact: WT, n = 5; ArKO, n = 4. OVX-E2: WT, n = 5; ArKO, n = 5. Values are means ± se. Scale bars = 50 μm (A); 6 μm (C). *P < 0.05 vs. OVX; SP < 0.05, SSSP < 0.005 vs. WT; 2-way ANOVA followed by Fisher's post hoc test.
All these results suggest that postnatal exposure to estrogens is somehow required to present a normal (WT) rate of neurogenesis that occurs in the adult female AOB. However, adult treatment with estradiol can reverse this reduction in cell survival.
Estradiol differentially influences the activation of newborn neurons by male urinary odors in the adult female MOB and AOB
Finally, we determined the influence of estrogens on the functional integration of newly generated neurons by determining their expression of Zif268 in response to a socially relevant odor stimulus for females, such as male urinary odors. We observed in WT mice that after a 4 wk survival time, in absence of any odor stimulation (i.e., water-exposed groups), a significant subpopulation of MOB (Fig. 5A) and AOB (Fig. 5B) newly generated cells expressed Zif268 (20.6±4.4 and 20.65±6%, respectively). We observed that this basal expression of Zif268 in BrdU cells was not affected by either the hormonal condition or the genotype [data not shown; MOB: F(1,4)=0.613, P=0.478 and F(1,4)=4.390, P=0.104; AOB: F(1,4)=0.008, P=0.933 and F(1,4)= 0.405, P=0.559]. Therefore, the proportion of BrdU-Zif268 cells in mice stimulated with water was pooled as “control water” groups for each genotype (Fig. 5C, D; red bars).
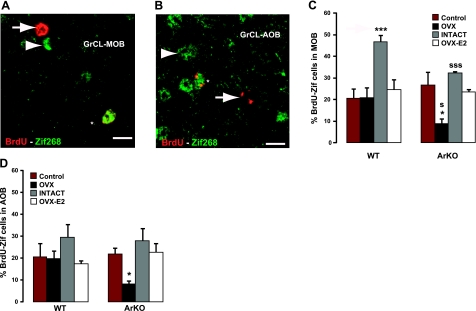
Figure 5.
Coexpression of BrdU and Zif268 in the MOB and AOB granular cell layers after exposure to male urinary odors. A) Representative image of a BrdU+ Zif268+ double-labeled cell (asterisk), a BrdU+ Zif268− cell (arrow), and a Zif268+ BrdU− cell (arrowhead) in the GrCL of the MOB after stimulation to male urinary odors. B) High magnification showing a BrdU+ Zif268+ double-labeled cell (asterisk), a BrdU+ Zif268− cell (arrow) and a Zif268+ BrdU− cell (arrowhead) in the GrCL of the AOB after stimulation to male urinary odors. C) Percentage of BrdU-Zif268 cells after exposure to water (control) or to male urinary odors counted in the GrCL of the MOB. D) Percentage of BrdU-Zif268 cells after exposure to water (control) or to male urinary odors counted in the GrCL of the AOB. OVX: WT, n = 6; ArKO, n = 6. Intact: WT, n = 6; ArKO, n = 4. OVX-E2: WT, n = 6; ArKO, n = 6. Values are means ± se. Scale bars = 6 μm. *P < 0.05, ***P < 0.005 vs. control; SP < 0.05, SSSP < 0.005 vs. WT; 2-way ANOVA followed by Fisher's post hoc test.
In the MOB, male urinary odors differentially activated BrdU cells in WT and ArKO mice as well as in OVX vs. intact vs. OVX-E2 mice [Fig. 5C; significant effect of hormonal condition: F(3,12)=6.169, P=0.009; significant effect of genotype: F(1,12)=30.332, P=0.0001]. An increase of 126% of BrdU/Zif268-expressing cells was observed in intact WT mice exposed to male urine in comparison to the water-exposed control group or ArKO mice (P=0.0001, WT-intact control water vs. urine stimulation; P=0.0001, WT-intact vs. ArKO-intact). This same increase was, however, not observed in WT or ArKO mice treated with estradiol in adulthood (P=0.501, WT-OVX-E2 control water vs. urine stimulation; P=0.680, ArKO-OVX-E2 control water vs. urine stimulation). These results suggest that some other hormone may be involved in the responsiveness of these newly born cells to urinary odors. Since levels of progesterone in intact ArKO mice are close to levels measured in estrus/proestrus stages in WT mice (5 of 6 intact mice were in estrus/ proestrus before the olfactory stimulation; ref. 29), it seems unlikely that progesterone influenced directly the responsiveness of MOB newborn cells to male urinary odors in intact WT vs. ArKO mice. However, it should be noted that the expression of progesterone receptors is not normal in intact ArKO mice (data not shown) since progesterone receptors are induced by estradiol (48, 49). Thus, we cannot rule out the possibility that progesterone in conjunction or not with fluctuating estrogen levels influences the responsiveness of MOB newborn cells to male urinary odors. Interestingly, OVX ArKO mice exposed to male urine had significantly lower numbers of double-labeled BrdU/Zif268 cells than ArKO mice exposed to water (P=0.027). This decrease in the responsiveness of BrdU-labeled cells to male urinary odors was reversed in intact and OVX-E2 ArKO mice; however, they never reached higher levels than those observed in the water-exposed groups (P=0.409, ArKO-intact control water vs. urine stimulation; P=0.680, ArKO-OVX-E2 control water vs. urine stimulation). These results suggest that postnatal exposure to estrogens is required to significantly activate newborn granule cells in response to male urinary odors.
In the AOB, a decreased responsiveness of newborn cells to male odors was also observed in OVX ArKO mice [Fig. 5D; hormonal condition: F(3,12)= 5.162, P=0.016; Fisher's post hoc test: P=0.050 control water vs. urinary odors], which was reversed in intact and OVX-E2 ArKO mice (P=0.402, ArKO-intact control water vs. urine stimulation; P=0.909, ArKO-OVX-E2 control water vs. urine stimulation). By contrast, we did not observe any significant increase in the proportion of BrdU-Zif268-labeled cells in the AOB of intact WT mice as was observed in the MOB. Moreover, no significant differences between groups [genotype: F(1,12)= 0.540, P=0.476] were observed. Taken together, these results suggest that only newborn MOB neurons are responsive to male urinary odors under postnatal and adult cycling estrogen influences.
DISCUSSION
Three main results have been obtained in this study. First, the proliferation of progenitor cells produced in the adult female SVZ is not influenced by estradiol regardless of whether it is present during the postnatal period or in adulthood or whether the brain is exposed to estradiol in a chronic fashion or a cyclical one as a function of the estrous cycle. Second, opposite effects of estradiol have been observed on the survival of newborn cells in the MOB vs. the AOB. Overall, adult estrogens influence the neuronal turnover of the adult MOB by negatively regulating the survival of newly generated neurons. By contrast, the incorporation of surviving newborn neurons in the adult AOB depends on a normal exposure to estrogens during the postnatal period. Finally, the responsiveness of newborn neurons of the MOB to male odors is modulated by both early postnatal and adult exposure to estradiol.
Taken together, these results demonstrate that the turnover and functional integration of newborn neurons in the adult female MOB and AOB are differentially influenced by estradiol from the early postnatal period to adulthood, opening new insights into the dual role of the MOB and AOB neurogenesis in the olfactory processing of sociosexual cues regulated by estrogens.
Estrogens do not affect the proliferation of progenitor cells in the adult female SVZ
Numerous studies (28) have demonstrated a role of estradiol in cell proliferation of progenitor cells in the DG of the hippocampus. Indeed, several studies (50, 51) have shown in different species positive correlations between high levels of estradiol during the estrous cycle (proestrus) and high rates of progenitor cell proliferation in the adult DG of females. As it was previously shown (51, 52), we did not observe in the present study any effect of estradiol on the proliferation of progenitor cells in the intact or in the OVX-E2 adult female SVZ, suggesting that estradiol differentially controls the birth of newly generated cells in the adult SVZ and the DG of the hippocampus (28). Moreover, our present results partly differ from what we previously observed with a short 2-d estradiol treatment (23), suggesting that, as has been reported for the DG of hippocampus, estradiol influences SVZ cell proliferation in a dose- and time-dependent manner (51, 53–56). Even if we cannot exclude that estradiol may influence proliferation of SVZ progenitor cells under some physiological conditions (21), the present data demonstrate that estradiol deficiency over a prolonged period of time as is the case in ArKO mice did not affect cell proliferation in the adult SVZ.
Estrogens have opposite effects on the survival of newborn neurons in MOB and AOB
In the adult female DG as well as in the MOB and AOB, the role of estradiol on cell survival independently of its effects observed on cell proliferation remains unknown, since all previous studies analyzed the effect of estradiol treatment only at the time of cell proliferation (22, 57). In the present study, we observed that exposure to estradiol during adulthood decreases the survival of newborn granule neurons in the adult MOB. After arriving in the MOB, only half of the newborn cells survive during a critical period that occurs between 15 and 45 d after birth (45, 58). Since our female mice were implanted with an estradiol capsule after receiving BrdU injections and since we did not obtain any effect of estradiol on cell proliferation in the SVZ, we assume that the decreased number of newborn neurons observed in the MOB is likely due to a decrease of survival of newly generated cells that mature and integrate into the MOB. Because this decrease in newborn cell survival was also observed in intact WT female mice, we can rule out any deleterious effect of a continuous exposure to estradiol on cell survival. Thus there seems to be a physiological role of estradiol in neuronal elimination of newborn cells that will or will not survive during this particular period. Future experiments are required to better understand the behavioral consequences of this regulatory effect of estradiol on newborn neuron turnover in the MOB. Interestingly, many studies have shown that the regulation of newborn neuron survival during this short critical period depends on sensory experience (1) and that turnover and active elimination processes of newborn neurons optimize olfaction by playing a crucial role in optimal odorant exploration, discrimination, and olfactory learning (13, 59).
In contrast to the MOB, the survival of newborn neurons in the AOB depends on a normal (WT) exposure to estradiol during the postnatal period. Even if the present data did not identify the precise postnatal stages at which exposure to estradiol is required, since basal secretion of estradiol by the ovaries starts around d 7 after birth, the decreased rates of surviving newborn neurons observed in the AOB of adult OVX and intact ArKO mice are obviously due to them being deprived from estrogens from P7 to P56 (time of ovariectomy in WT mice). This postnatal interval comprises prepubertal and pubertal periods during which increasing levels of estradiol can facilitate the later capacity to display female sexual behavior (26). However, this “organizational” effect of estrogens is not restricted to the postnatal period, since adult treatment with estradiol completely restored this neurogenic defect in ArKO females, whereas sexual behavior was not restored (26). Interestingly, it has also been shown that neurons generated during the postnatal period substantially differ in terms of turnover and function in olfaction from neurons that are born during adulthood (60), suggesting that postnatal events such as estradiol exposure and its influence on early neurogenesis could play an essential role in shaping the architecture of the AOB, thereby allowing neurons generated later in life to optimally survive and to be functionally integrated into the adult network.
Estrogens positively modulate the responsiveness of newborn neurons to urinary odors in the adult MOB only
We analyzed the expression of Zif268 (egr-1) in BrdU+ granule neurons in response to male urinary odors, which has been shown to be effective in activating newborn cells in both the MOB and AOB (11). We observed that exposure to male urinary odors only increased Zif268 expression in the MOB BrdU+ cells of intact WT female mice and not in any of the other WT and ArKO experimental groups, suggesting that both postnatal and adult fluctuating levels of estrogens significantly influence the responsiveness of newborn neurons. Even though levels of progesterone in intact ArKO mice are close to levels measured in intact WT females (29), the expression of progesterone receptors is not normal in ArKO females since it needs to be induced by estradiol (48, 49), and thus a synergistic action between estradiol and progesterone cannot be excluded (61). In addition to a complete absence of responsiveness of newborn neurons in ArKO female mice to male urinary odors, we obtained a clear down-regulation of Zif268 expression in newborn cells in OVX ArKO mice. Although surprising, this result suggests that the total absence of hormones in ArKO mice can negatively modulate Zif268 expression (62). Even if precise mechanisms by which estrogens regulate Zif268/egr-1 expression in MOB newborn cells remain unclear, it has already been observed in the anterior pituitary that cyclical levels of estrogens during the reproductive cycle can positively regulate egr-1 expression via an estrogen-responsive element in the egr-1 promoter (63) and later mediate GnRH-stimulated LHβ subunit gene expression (64–66) that controls various aspects of olfactory reproductive behaviors (67, 68). Furthermore, since it was notably demonstrated that Zif268 governs molecular events involved in the maintenance of long-term potentiation in the DG of the hippocampus (69), estrogen-induced Zif268 expression in newborn neurons in response to urinary odors could reflect an index of functional integration into the MOB network and also a particular sensitivity of these cells to synaptic plasticity (15) implicated in olfactory discrimination learning (40).
Estrogens differently modulate AOB and MOB neurogenesis
Our data show a strong difference in the implication of postnatal vs. adult exposure to estrogens in the survival and functional integration of newborn neurons in the AOB and MOB. In summary, postnatal exposure to estradiol strongly acts on the survival of AOB newborn neurons,whereas both survival and functional integration of MOB newborn neurons are predominantly controlled by adult estrogens. These results reinforce not only previous data on the complementary role of the accessory and main olfactory systems in the control of sociosexual behaviors (24) but also may explain the disturbed phenotype of ArKO female mice with regard to their olfactory investigation of volatile and nonvolatile sociosexual cues and their reproductive behaviors (26). Taken together, our data open new insights into the complementary roles of estradiol-regulated adult neurogenesis in the AOB and MOB and may lead to new perspective studies on their respective roles in estradiol-controlled sociosexual behaviors.
Acknowledgments
The authors thank Drs. Michael Baum, Serge Laroche, and Silvia DeMarchis for comments on an earlier version of the manuscript; Marie Tsampalas for technical assistance; and Frederic Lévy and Matthieu Keller for using the Mercator software.
This work was supported by U.S. National Institutes of Health grant HD-044897 and grants from the University of Liège (C-06/89 to J.B. and A.V.). J.B. is a research associate at the Fonds National de la Recherche Scientifique.
REFERENCES
- 1. Lledo P. M., Alonso M., Grubb M. S. (2006) Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 7, 179–193 [DOI] [PubMed] [Google Scholar]
- 2. Ming G. L., Song H. (2005) Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250 [DOI] [PubMed] [Google Scholar]
- 3. Kempermann G., Wiskott L., Gage F. H. (2004) Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 14, 186–191 [DOI] [PubMed] [Google Scholar]
- 4. Alvarez-Buylla A., Garcia-Verdugo J. M. (2002) Neurogenesis in adult subventricular zone. J. Neurosci. 22, 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imayoshi I., Sakamoto M., Ohtsuka T., Takao K., Miyakawa T., Yamaguchi M., Mori K., Ikeda T., Itohara S., Kageyama R. (2008) Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 11, 1153–1161 [DOI] [PubMed] [Google Scholar]
- 6. Carleton A., Petreanu L. T., Lansford R., Alvarez-Buylla A., Lledo P. M. (2003) Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 6, 507–518 [DOI] [PubMed] [Google Scholar]
- 7. Belluzzi O., Benedusi M., Ackman J., LoTurco J. J. (2003) Electrophysiological differentiation of new neurons in the olfactory bulb. J. Neurosci. 23, 10411–10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oboti L., Savalli G., Giachino C., De Marchis S., Panzica G. C., Fasolo A., Peretto P. (2009) Integration and sensory experience-dependent survival of newly-generated neurons in the accessory olfactory bulb of female mice. Eur. J. Neurosci. 29, 679–692 [DOI] [PubMed] [Google Scholar]
- 9. Zhao C., Deng W., Gage F. H. (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 [DOI] [PubMed] [Google Scholar]
- 10. Rochefort C., Gheusi G., Vincent J. D., Lledo P. M. (2002) Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 22, 2679–2689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang L., Bittman E. L. (2002) Olfactory bulb cells generated in adult male golden hamsters are specifically activated by exposure to estrous females. Horm. Behav. 41, 343–350 [DOI] [PubMed] [Google Scholar]
- 12. Yamaguchi M., Mori K. (2005) Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc. Natl. Acad. Sci. U. S. A. 102, 9697–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mouret A., Gheusi G., Gabellec M. M., de Chaumont F., Olivo-Marin J. C., Lledo P. M. (2008) Learning and survival of newly generated neurons: when time matters. J. Neurosci. 28, 11511–11516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sultan S., Mandairon N., Kermen F., Garcia S., Sacquet J., Didier A. Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J. 24, 2355–2363 [DOI] [PubMed] [Google Scholar]
- 15. Nissant A., Bardy C., Katagiri H., Murray K., Lledo P. M. (2009) Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat. Neurosci. 12, 728–730 [DOI] [PubMed] [Google Scholar]
- 16. Mak G. K., Enwere E. K., Gregg C., Pakarainen T., Poutanen M., Huhtaniemi I., Weiss S. (2007) Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat. Neurosci. 10, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 17. Shingo T., Gregg C., Enwere E., Fujikawa H., Hassam R., Geary C., Cross J. C., Weiss S. (2003) Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299, 117–120 [DOI] [PubMed] [Google Scholar]
- 18. Larsen C. M., Kokay I. C., Grattan D. R. (2008) Male pheromones initiate prolactin-induced neurogenesis and advance maternal behavior in female mice. Horm. Behav. 53, 509–517 [DOI] [PubMed] [Google Scholar]
- 19. Larsen C. M., Grattan D. R. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology 151, 3805–3814 [DOI] [PubMed] [Google Scholar]
- 20. Mak G. K., Weiss S. (2010) Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat. Neurosci. 13, 753–758 [DOI] [PubMed] [Google Scholar]
- 21. Smith M. T., Pencea V., Wang Z., Luskin M. B., Insel T. R. (2001) Increased number of BrdU-labeled neurons in the rostral migratory stream of the estrous prairie vole. Horm. Behav. 39, 11–21 [DOI] [PubMed] [Google Scholar]
- 22. Hoyk Z., Varga C., Parducz A. (2006) Estrogen-induced region specific decrease in the density of 5-bromo-2-deoxyuridine-labeled cells in the olfactory bulb of adult female rats. Neuroscience 141, 1919–1924 [DOI] [PubMed] [Google Scholar]
- 23. Brock O., Keller M., Veyrac A., Douhard Q., Bakker J. (2010) Short term treatment with estradiol decreases the rate of newly generated cells in the subventricular zone and main olfactory bulb of adult female mice. Neuroscience 166, 368–376 [DOI] [PubMed] [Google Scholar]
- 24. Keller M., Baum M. J., Brock O., Brennan P. A., Bakker J. (2009) The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav. Brain Res. 200, 268–276 [DOI] [PubMed] [Google Scholar]
- 25. Bakker J. (2003) Sexual differentiation of the neuroendocrine mechanisms regulating mate recognition in mammals. J. Neuroendocrinol. 15, 615–621 [DOI] [PubMed] [Google Scholar]
- 26. Bakker J., Baum M. J. (2008) Role for estradiol in female-typical brain and behavioral sexual differentiation. Front. Neuroendocrinol. 29, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spencer J. L., Waters E. M., Romeo R. D., Wood G. E., Milner T. A., McEwen B. S. (2008) Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocrinol. 29, 219–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galea L. A. (2008) Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res. Rev. 57, 332–341 [DOI] [PubMed] [Google Scholar]
- 29. Fisher C. R., Graves K. H., Parlow A. F., Simpson E. R. (1998) Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc. Natl. Acad. Sci. U. S. A. 95, 6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Honda S., Harada N., Ito S., Takagi Y., Maeda S. (1998) Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem. Biophys. Res. Commun. 252, 445–449 [DOI] [PubMed] [Google Scholar]
- 31. Mannan M. A., O'Shaughnessy P. J. (1991) Steroidogenesis during postnatal development in the mouse ovary. J. Endocrinol. 130, 101–106 [DOI] [PubMed] [Google Scholar]
- 32. Bakker J., Honda S., Harada N., Balthazart J. (2002) Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Horm. Behav. 42, 158–171 [DOI] [PubMed] [Google Scholar]
- 33. Maguire J. L., Stell B. M., Rafizadeh M., Mody I. (2005) Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 8, 797–804 [DOI] [PubMed] [Google Scholar]
- 34. Toda K., Takeda K., Okada T., Akira S., Saibara T., Kaname T., Yamamura K., Onishi S., Shizuta Y. (2001) Targeted disruption of the aromatase P450 gene (Cyp19) in mice and their ovarian and uterine responses to 17beta-oestradiol. J. Endocrinol. 170, 99–111 [DOI] [PubMed] [Google Scholar]
- 35. Britt K. L., Drummond A. E., Dyson M., Wreford N. G., Jones M. E., Simpson E. R., Findlay J. K. (2001) The ovarian phenotype of the aromatase knockout (ArKO) mouse. J. Steroid Biochem. Mol. Biol. 79, 181–185 [DOI] [PubMed] [Google Scholar]
- 36. Bakker J., Honda S., Harada N., Balthazart J. (2002) The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J. Neurosci. 22, 9104–9112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Knapska E., Kaczmarek L. (2004) A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 74, 183–211 [DOI] [PubMed] [Google Scholar]
- 38. Mandairon N., Sacquet J., Garcia S., Ravel N., Jourdan F., Didier A. (2006) Neurogenic correlates of an olfactory discrimination task in the adult olfactory bulb. Eur. J. Neurosci. 24, 3578–3588 [DOI] [PubMed] [Google Scholar]
- 39. Magavi S. S., Mitchell B. D., Szentirmai O., Carter B. S., Macklis J. D. (2005) Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J. Neurosci. 25, 10729–10739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alonso M., Viollet C., Gabellec M. M., Meas-Yedid V., Olivo-Marin J. C., Lledo P. M. (2006) Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J. Neurosci. 26, 10508–10513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pierman S., Douhard Q., Bakker J. (2008) Evidence for a role of early oestrogens in the central processing of sexually relevant olfactory cues in female mice. Eur. J. Neurosci. 27, 423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martel K. L., Baum M. J. (2007) Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur. J. Neurosci. 26, 463–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Veyrac A., Sacquet J., Nguyen V., Marien M., Jourdan F., Didier A. (2009) Novelty determines the effects of olfactory enrichment on memory and neurogenesis through noradrenergic mechanisms. Neuropsychopharmacology 34, 786–795 [DOI] [PubMed] [Google Scholar]
- 44. Howard C., Reed M. eds. (1998) Unbiased Stereology: Three-Dimensional Measurement in Microscopy p. 41, Bios Scientific, Oxford, UK [Google Scholar]
- 45. Petreanu L., Alvarez-Buylla A. (2002) Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 22, 6106–6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guerin D., Sacquet J., Mandairon N., Jourdan F., Didier A. (2009) Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol. Aging 30, 272–283 [DOI] [PubMed] [Google Scholar]
- 47. Giachino C., De Marchis S., Giampietro C., Parlato R., Perroteau I., Schutz G., Fasolo A., Peretto P. (2005) cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J. Neurosci. 25, 10105–10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quadros P. S., Lopez V., De Vries G. J., Chung W. C., Wagner C. K. (2002) Progesterone receptors and the sexual differentiation of the medial preoptic nucleus. J. Neurobiol. 51, 24–32 [DOI] [PubMed] [Google Scholar]
- 49. Quadros P. S., Pfau J. L., Goldstein A. Y., De Vries G. J., Wagner C. K. (2002) Sex differences in progesterone receptor expression: a potential mechanism for estradiol-mediated sexual differentiation. Endocrinology 143, 3727–3739 [DOI] [PubMed] [Google Scholar]
- 50. Galea L. A., McEwen B. S. (1999) Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience 89, 955–964 [DOI] [PubMed] [Google Scholar]
- 51. Tanapat P., Hastings N. B., Reeves A. J., Gould E. (1999) Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 19, 5792–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Suzuki S., Gerhold L. M., Bottner M., Rau S. W., Dela Cruz C., Yang E., Zhu H., Yu J., Cashion A. B., Kindy M. S., Merchenthaler I., Gage F. H., Wise P. M. (2007) Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J. Comp. Neurol. 500, 1064–1075 [DOI] [PubMed] [Google Scholar]
- 53. Tanapat P., Hastings N. B., Gould E. (2005) Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J. Comp. Neurol. 481, 252–265 [DOI] [PubMed] [Google Scholar]
- 54. Ormerod B. K., Galea L. A. (2001) Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience 102, 369–379 [DOI] [PubMed] [Google Scholar]
- 55. Ormerod B. K., Lee T. T., Galea L. A. (2003) Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J. Neurobiol. 55, 247–260 [DOI] [PubMed] [Google Scholar]
- 56. Banasr M., Hery M., Brezun J. M., Daszuta A. (2001) Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur. J. Neurosci. 14, 1417–1424 [DOI] [PubMed] [Google Scholar]
- 57. Ormerod B. K., Lee T. T., Galea L. A. (2004) Estradiol enhances neurogenesis in the dentate gyri of adult male meadow voles by increasing the survival of young granule neurons. Neuroscience 128, 645–654 [DOI] [PubMed] [Google Scholar]
- 58. Winner B., Cooper-Kuhn C. M., Aigner R., Winkler J., Kuhn H. G. (2002) Long-term survival and cell death of newly generated neurons in the adult rat olfactory bulb. Eur. J. Neurosci. 16, 1681–1689 [DOI] [PubMed] [Google Scholar]
- 59. Mouret A., Lepousez G., Gras J., Gabellec M. M., Lledo P. M. (2009) Turnover of newborn olfactory bulb neurons optimizes olfaction. J. Neurosci. 29, 12302–12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lemasson M., Saghatelyan A., Olivo-Marin J. C., Lledo P. M. (2005) Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb. J. Neurosci. 25, 6816–6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Keller M., Pierman S., Douhard Q., Baum M. J., Bakker J. (2006) The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur. J. Neurosci. 23, 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Svaren J., Sevetson B. R., Apel E. D., Zimonjic D. B., Popescu N. C., Milbrandt J. (1996) NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol. Cell. Biol. 16, 3545–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Slade J. P., Carter D. A. (2000) Cyclical expression of egr-1/NGFI-A in the rat anterior pituitary: a molecular signal for ovulation? J. Neuroendocrinology 12, 671–676 [DOI] [PubMed] [Google Scholar]
- 64. Lawson M. A., Tsutsumi R., Zhang H., Talukdar I., Butler B. K., Santos S. J., Mellon P. L., Webster N. J. (2007) Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol. Endocrinol. 21, 1175–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sevetson B. R., Svaren J., Milbrandt J. (2000) A novel activation function for NAB proteins in EGR-dependent transcription of the luteinizing hormone beta gene. J. Biol. Chem. 275, 9749–9757 [DOI] [PubMed] [Google Scholar]
- 66. Kowase T., Walsh H. E., Darling D. S., Shupnik M. A. (2007) Estrogen enhances gonadotropin-releasing hormone-stimulated transcription of the luteinizing hormone subunit promoters via altered expression of stimulatory and suppressive transcription factors. Endocrinology 148, 6083–6091 [DOI] [PubMed] [Google Scholar]
- 67. Boehm U., Zou Z., Buck L. B. (2005) Feedback loops link odor and pheromone signaling with reproduction. Cell 123, 683–695 [DOI] [PubMed] [Google Scholar]
- 68. Yoon H., Enquist L. W., Dulac C. (2005) Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell 123, 669–682 [DOI] [PubMed] [Google Scholar]
- 69. Jones M. W., Errington M. L., French P. J., Fine A., Bliss T. V., Garel S., Charnay P., Bozon B., Laroche S., Davis S. (2001) A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 4, 289–296 [DOI] [PubMed] [Google Scholar]