Abstract
Darier's disease (DD) is an inherited autosomal-dominant skin disorder characterized histologically by loss of adhesion between keratinocytes. DD is typically caused by mutations in sarcoendoplasmic reticulum Ca2+-ATPase isoform 2 (SERCA2), a major regulator of intracellular Ca2+ homeostasis in the skin. However, a defined role for SERCA2 in regulating intercellular adhesion remains poorly understood. We found that diminution of SERCA2 function by pharmacological inhibition or siRNA silencing in multiple human epidermal-derived cell lines was sufficient to disrupt desmosome assembly and weaken intercellular adhesive strength. Specifically, SERCA2-deficient cells exhibited up to a 60% reduction in border translocation of desmoplakin (DP), the desmosomal cytolinker protein necessary for intermediate filament (IF) anchorage to sites of robust cell-cell adhesion. In addition, loss of SERCA2 impaired the membrane translocation of protein kinase C α (PKCα), a known regulator of DP-IF association and desmosome assembly, to the plasma membrane by up to 70%. Exogenous activation of PKCα in SERCA2-deficient cells was sufficient to rescue the defective DP localization, desmosome assembly, and intercellular adhesive strength to levels comparable to controls. Our findings indicate that SERCA2-deficiency is sufficient to impede desmosome assembly and weaken intercellular adhesive strength via a PKCα-dependent mechanism, implicating SERCA2 as a novel regulator of PKCα signaling.—Hobbs, R. P., Amargo, E. V., Somasundaram, A., Simpson, C. L., Prakriya, M., Denning, M. F., Green, K. J. The calcium ATPase SERCA2 regulates desmoplakin dynamics and intercellular adhesive strength through modulation of PKCα signaling.
Keywords: desmosome, Darier's disease, intracellular Ca2+, cell-cell contact
Desmosomes and adherens junctions are intercellular junctions that tether the keratin intermediate filament (IF) and actin cytoskeletal networks, respectively, to sites of cadherin-based cell-cell adhesion. While these two junction types share some structural similarities, the specific anchorage of the keratin filaments to desmosomes plays a special role in providing the epidermis with tissue strength and resistance to mechanical stress (1–3). The adhesive core of the desmosome consists of desmosomal cadherins, which form Ca2+-mediated cell-cell contacts through their extracellular domains. Intracellularly, the cadherin tails associate with the Armadillo family members plakoglobin and plakophilins, which bind to the desmosomal cytoskeletal linking protein desmoplakin (DP) (4).
DP contains 3 major domains: the N terminus, which is required for DP's association with the plasma membrane-associated junctional plaque, a central coiled-coil rod domain, which facilitates dimerization, and the C terminus, which is essential for direct linkage to the keratin intermediate filament (IF) cytoskeleton (1, 5). Both the N and C termini of DP are required for proper desmosome function. ΔN-DP was unable to target to sites of cell-cell contact, and its expression induced a collapse of the keratin filament network (6), while expression of DP-ΔC exhibited weakened intercellular adhesive strength due to the lack of keratin association with the desmosome (7).
Despite being a metabolically stable protein with a half-life of ∼72 h (8), DP is able to rapidly accumulate (∼3–10 min) in assembling desmosomes on cell-cell contact (9–11). In epithelial cells, DP translocation to cell-cell borders and desmosomal maturation are regulated by DP's association with keratin filaments. For instance, abrogation of keratin binding by deletion of the C-terminal IF-binding domain impairs DP dynamics (11). Likewise, enhancement of the DP-IF interaction by mutation of a PKC consensus sequence in the IF-binding domain (DPS2849G) (12) inhibited DP translocation to sites of desmosome assembly and induced a “beads-on-a-string” alignment of cytoplasmic DP-containing particles on keratin IF (9, 11, 13). Correspondingly, pharmacological inhibition of PKC impaired desmosome assembly and induced DP alignment on IF, similar to that exhibited by the DPS2849G mutant (9, 14, 15). Furthermore, specific siRNA depletion of PKCα, which is the only Ca2+-dependent PKC isoform expressed in keratinocytes (16), also resulted in defective DP trafficking to intercellular borders (9, 14, 15). PKCα is also known to regulate epidermal differentiation (17) and cellular sensitivity to Ca2+ depletion (1, 18, 19).
Over 50 inherited mutations have been reported in the desmoplakin gene (DSP), which result in disorders of the skin and/or heart, some of which may be lethal (20, 21). These mutations interfere with the structural integrity of desmosomes, and in some cases, have been shown to impair recruitment of DP to cell-cell interfaces (22). However, desmosomal dysfunction may also arise indirectly from environmental toxins or autoimmune responses (23), as well as from inherited mutations in nondesmosomal proteins such as in Hailey-Hailey's disease and Darier's disease (DD) (24, 25). It has been well established that defects in desmosome structure are a hallmark histological feature of DD epidermis (26, 27). More recently, abnormalities in DP localization during junction assembly have been observed in DD keratinocytes (28).
DD is caused by mutations in the sarcoendoplasmic reticulum Ca2+ ATPase isoform 2 (SERCA2) protein (25, 29). The SERCA family of proteins consists of 10 known isoforms from 3 alternatively spliced genes (29), which function by pumping free cytosolic Ca2+ against the concentration gradient and into the endoplasmic reticulum (ER) lumen in an ATP-dependent manner (24, 29). The importance of SERCA2 in regulating intracellular Ca2+ homeostasis and Ca2+ flux events is well documented (24, 30–34). However, ascribing a specific role for SERCA2 in regulating desmosome assembly and adhesive strength is complicated by the fact that most of these studies have been conducted in DD, where the initial loss of intercellular adhesion could be influenced by a host of environmental cues, such as mechanical stress, heat, UV irradiation, and inflammatory cytokines (35–37).
Here, we have impaired SERCA2 in multiple human epithelial cell lines through pharmacological inhibition and siRNA silencing to determine whether the loss of SERCA2 function in the absence of confounding external factors is sufficient to perturb desmosome assembly and weaken intercellular strength. Using a combination of microscopy, biochemical techniques, and functional assays, we show that defective intercellular junction assembly caused by SERCA2-deficiency occurs through the failure to induce PKCα translocation and activation. The data suggest that PKCα may be a putative target for therapeutic intervention of DD progression.
MATERIALS AND METHODS
Cell line and culture conditions
The SCC9 human-derived oral squamous cell carcinoma cell line (a gift from J. Rheinwald, Harvard School of Medicine, Boston, MA, USA) was maintained in DME/F12, 10% FBS, and 1% penicillin/streptomycin. Stably infected cell lines were similarly maintained but with the addition of 1 μg/ml puromycin. Low-Ca2+ medium (0.05 mM) contains DME, 10% FBS, and 1% penicillin/streptomycin. Generation of the SCC9 cell line stably expressing the DPS2849G point mutation has been described previously (11). A431-inducible cell lines were cultured in DME, 10% FBS, 1% penicillin/streptomycin, 400 μg/ml G418, and 1 μg/ml puromycin. DP-GFP protein expression was induced by culturing the cells in 2 μg/ml DOX ≥16 h prior to imaging, as described previously (11). Normal human epidermal keratinocytes (NHEKs) were derived from foreskin and maintained in 0.07 mM Ca2+ M154 medium (Invitrogen, Carlsbad, CA, USA), as described previously (38).
siRNA transfections and retroviral infections
siGAPDH (catalog no. D-001140-01) and siSERCA2 [M-004082-01 (pool), MQ-004082-01 (set of 4 individual)] oligos were purchased from Dharmacon (Thermo Fisher Scientific, Lafayette, CO, USA) and transfected into SCC9 cells using Dharmafect1 (T-2001), also from Dharmacon, following manufacturer's protocol, for 48–96 h. NHEK transfections were conducted with Amaxa Nucleofector transfection kit (VCO-1001N; Amaxa Biosystems, Gaithersburg, MD, USA) using solution V on program X-001 and following manufacturer's instructions. Generation of shPKCα and constitutively active (CA) PKCα retrovirus has been previously described (9, 17).
Antibodies and reagents
The following mouse monoclonal antibodies were used: 1G4 (anti-DP; gift from J. Wahl III, University of Nebraska, Omaha, NE, USA), HECD1 (anti-E-cadherin; gift from M. Takeichi and O. Abe, Riken Center for Developmental Biology, Kobe, Japan; ref. 39), 12G10 (anti-tubulin; provided by J. Frankel and E. M. Nelson, Development Hybridoma Studies Bank, under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA, USA), SERCA2 (anti-SERCA2; S1314; Sigma, St. Louis, MO, USA), KS-B17.2 (anti-keratin 18; C1399; Sigma), and 4B2 (anti-desmoglein1/2; ref. 38).
Polyclonal antibodies used included 1407 (chicken anti-PG; Aves Laboratories, Tigard, OR, USA), NW6 (rabbit anti-DP; ref. 40), C2206 (rabbit anti-β-catenin; C2206; Sigma), GAPDH (rabbit anti-GAPDH; G9545; Sigma), PKCα (rabbit anti-PKCα; SC-208; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and keratin 14 [rabbit anti-K14; gift from Julie Segre, U.S. National Institutes of Health (NIH), Bethesda MD, USA].
Secondary antibodies utilized for Western blot analysis included goat anti-mouse, rabbit, and chicken conjugated to peroxidase (Rockland, Gilbertsville, PA, USA). Secondary antibodies used for immunofluorescence included goat anti-mouse, rabbit, and chicken antibodies conjugated to fluorophores of 488 or 568 nm (Alexa Fluor; Invitrogen).
Thapsigargin (T9033; Sigma), ionomycin (I0634; Sigma), and PMA (phorbol myristate acetate; 79346; Sigma) were resuspended into DMSO and utilized at concentrations of 100 nM, 2 μM, and 1 μM, respectively.
Calcium switch
Cells were incubated overnight in low-Ca2+ medium (DME, 10% FBS, 1% penicillin/streptomycin, 0.05 mM CaCl2) and then switched to normal growth medium (1.8 mM Ca2+) to induce the assembly of intercellular junctions for time periods ranging from 15 to 240 min before being processed for Western blot analysis and/or immunofluorescence analysis.
Preparation of cell lysates for immunoblot analysis
Whole-cell lysates in urea sample buffer (8 M deionized urea; 1% SDS; 10% glycerol; 60 mM Tris, pH 6.8; 0.1% pyronin-Y; and 5% β-mercaptoethanol) were resolved by 7.5 or 10% SDS-PAGE and transferred to nitrocellulose for Western blot analysis. Immunoreactive proteins were visualized using enhanced chemiluminescence (ECL). Immunoblots were scanned (HP OfficeJet 5610; Hewlett-Packard, Palo Alto, CA, USA) and quantified densitometrically using ImageJ software (NIH). All images were cropped using Adobe Photoshop (CS3; Adobe Systems, San Jose, CA, USA) and formatted for optimal presentation using Adobe Illustrator (CS3).
Immunofluorescence analysis and image acquisition
Cells were seeded onto collagen I-coated (0.1 mg/ml; 354249; BD Biosciences, Bedford, MA, USA) coverslips ≥24 h prior to the addition of pharmacological agent, transfection reagent, or retrovirus. Prior to immunostaining, cells were fixed for 2 min in dehydrated ice-cold methanol. For staining with anti-PKCα antibody, cells were treated with 10% ice-cold trichloroacetic acid (TCA) in water for 15 min, followed by a 15-min extraction in ice-cold 0.2% Triton X-100 (41, 42). Cells were incubated with primary and secondary antibodies for a period of 45 min each in a 37°C humidified incubator. Coverslips were mounted using polyvinyl alcohol (Sigma). All samples within a given experiment were treated identically, and images were captured using the exact same parameters.
Fixed cells were imaged using a Leica microscope (model DMR) or an LSM 510 confocal microscope (Carl Zeiss Microimaging, Thornwood, NY, USA). The DMR microscope was fitted with a ×63 objective (PL APO, NA 1.32), an Orca 100 CCD camera (model C4742–95; Hamamatsu, Bridgewater, NJ, USA), and MetaMorph 7.6 imaging software (Molecular Devices, Sunnyvale, CA, USA). The LSM 510 confocal microscope images were taken using a ×100 objective (Plan Apochromat, NA 1.4; Zeiss) and Zen software version 5,5,0,375 (Zeiss). For the LSM 510 confocal microscope, the fluorescence was measured using single-track method. Linescan analysis was conducted using ImageJ software (43). All images were equally cropped, brightened, and contrasted for optimal presentation using Adobe Photoshop (CS3) and compiled using Adobe Illustrator (CS3).
Time-lapse imaging and retrospective immunofluorescence analysis
Cells transfected with siRNA were seeded onto a collagen I-coated (0.1 mg/ml) 2-well Lab-Tek chambered coverglass (Nunc, Roskilde, Denmark) and treated with 2 μg/ml doxycycline overnight to induce DP-GFP expression (11). Prior to time-lapse image acquisition, cell monolayers were wounded using a 26-gauge needle and incubated in imaging medium (Hanks balanced salt solution, 20 mM HEPES, 1% FBS, 2 mM l-glutamine, 4.5 g/L glucose, and 1× amino acids; recipe courtesy of G. Kreitzer, Weill Medical College of Cornell University, New York, NY, USA) at 37°C for 15 to 30 min. Time-lapse image recordings were obtained using mercury illumination with a Leica DMI 6000B inverted microscope equipped with a Hamamatsu ImagEM electron multiplier-CCD camera, Prior Pro Scan II high-speed motorized stage for collections of multiple fields, and ×100 (HCX PL APO, oil, NA 1.4) objective housed inside a temperature-controlled 37°C chamber. The time-lapse images were captured at 1-min intervals using Simple PCI 6 software (Hamamatsu).
For retrospective analysis, cells were fixed after 80 min of image acquisition, and immunofluorescence procedures were performed using anti-SERCA2 primary antibody (S1314; Sigma). Imaged cells were relocated, and images were captured using the Leica DMI 6000B imaging system mentioned above.
The acquired time-lapse images were processed using ImageJ for cropping and for normalizing image stacks using a bleach correction macro obtained from EAMNet (European Advanced Light Microscopy Network). MetaMorph 7.6 was then used for compiling the time-lapse images into AVI movies and for merging the dual-label images from the retrospective analysis. Adobe Photoshop CS3 was used for scaling image sizes and adjusting brightness and contrast. All images were equally adjusted.
Intracellular calcium imaging
To image intracellular Ca2+, SCC9 cells grown in low-Ca2+ medium (0.05 mM) were loaded with 4 μM Fura-2 AM with 0.02% pluronic acid for 30 min at 37°C. Medium was removed, and cells were washed with 0 mM Ca2+ Ringer solution (150 mM NaCl, 4.5 mM KCl, 10 mM d-glucose, 5 mM HEPES, 1 mM EGTA, and 3 mM MgCl2); 2 mM Ca2+ Ringer solution (150 mM NaCl, 4.5 mM KCl, 10 mM d-glucose, 5 mM HEPES, 1 mM EGTA, 1 mM MgCl2, and 2 mM CaCl2) was used to induce intracellular Ca2+ flux; 1 μM thapsigargin or 2 μM ionomycin was used in 0 mM Ca2+ Ringer solution to deplete ER Ca2+ stores. Images were acquired every 6 s using an IX71 inverted microscope (Olympus, Center Valley, PA, USA) equipped with a ×40 oil-immersion objective (Olympus), a 175-W xenon arc lamp (Sutter, Novatao, CA, USA), and excitation and emission filter wheels (Sutter). Images were captured on a cooled CCD camera (Hamamatsu). Image acquisition was performed with IPLab software (Scanalytics, Rockville, MD, USA). Regions of interest were drawn around single cells; after background subtraction, the ratio of intensities in the W1 (340 nm) and the W2 (380 nm) excitation channels was plotted as a function of time, averaged over all of the cells. Intracellular calcium concentration was determined by the following equation: [Ca2+] = KdQ[(R − Rmin)/(Rmax − R)], where Kd is the dissociation constant for Fura-2. The constants Q, Rmin, and Rmax were obtained using in vivo calibration. Cells were treated with 0 mM Ca2+ + 2 μM ionomycin to obtain Rmin and 20 mM Ca2+ + 2 μM ionomycin to obtain Rmax. Q was calculated as Fmin/Fmax, the intensities in the 380 channel obtained at minimum and maximum calcium concentrations.
PKCα membrane translocation assay
At 48 h after transfection with siRNA oligos, SCC9 cells were switched to serum-free medium. At 24 h after serum starvation, cells were washed twice with ice-cold PBS and then collected on ice using a rubber scraper in 30 μl of lysis buffer [20 mM Tris, pH 7.5; 5 mM EDTA; 1× protease inhibitor cocktail (P8340; Sigma); and 1× phosphatase inhibitor cocktail IV (524628; EMD Chemicals, Gibbstown, NJ, USA)]. Lysates were then sonicated (5 s) and subjected to ultracentrifugation at 100,000 g for 1 h at 4°C (Optima TLX, TLA 100.2 rotor; Beckman Coulter, Brea, CA, USA). The supernatant (S1) represents the soluble protein pool. The pellet was solubilized in resuspension buffer [1% Triton X-100; 20 mM Tris, pH 7.5; 5 mM EDTA; 1× protease inhibitor cocktail (P8340; Sigma); 1× phosphatase inhibitor cocktail IV (524628; EMD)], incubated on ice for 1 h, and subjected to ultracentrifugation at 100,000 g for 1 h at 4°C (Optima TLX, TLA 100.2 rotor). This supernatant (S2) represents the membrane protein pool. Laemmli sample buffer (10% glycerol; 1% SDS; 63 mM Tris, pH 6.8; 0.01% pyronin-Y; and 5% β-mercaptoethanol) was added to all samples prior to loading onto gels for electrophoresis. The amount of membrane protein loaded onto the gel was at a 3:1 volumetric ratio compared to the amount of soluble protein loaded.
Dispase mechanical dissociation assay
Cells were plated in triplicate in a 6-well plate and treated with siRNA as described above. At 48 h after transfection, Ca2+ concentration of medium was switched to 0.5 mM. At 24 h after reaching confluency, cells were rinsed twice with PBS and then incubated with 2 ml/well of dispase II (2.4 U/ml; 04942078001; Roche Diagnostics, Indianapolis, IN, USA) for 30 min at 37°C (44). Released monolayers were then subjected to orbital rotation (150 rpm) for 5 min prior to imaging. Fragments were counted using a dissecting microscope (Leica MZ6), and final images were generated using Adobe Photoshop (CS3) and Adobe Illustrator (CS3).
Statistical analysis
All statistical analysis was conducted using Microsoft Excel (Microsoft, Redmond, WA, USA). All error bars represent se, and statistical significance was determined by 2-tailed, 2-sample, equal variance Student's t test.
RESULTS
Loss of SERCA2 is sufficient to weaken intercellular adhesion and impair desmosome assembly
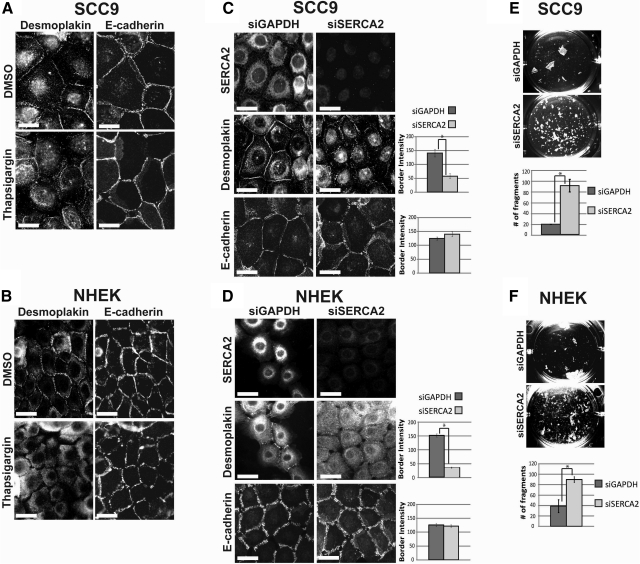
To address whether SERCA2 plays a role in the formation of intercellular junctions, we examined the translocation of desmosomal and adherens junction proteins to sites of cell-cell contact after either treatment with thapsigargin, a potent and irreversible inhibitor of SERCA2 (45), or transient transfection of siRNA oligos specifically silencing SERCA2. A 30-min treatment of SCC9 or NHEK cells with thapsigargin prior to a 3-h Ca2+ switch severely impaired DP border localization compared to DMSO-treated control cells. However, E-cadherin was localized to cell borders (Fig. 1A, B), consistent with previously reported work from simple epithelial MDCK cells (46). SCC9 or NHEK cells transiently transfected with a pool of 4 siSERCA2 oligos each exhibited an ∼60% reduction in the intensity of DP particles that accumulated at cell-cell interfaces, without any detectable alterations in E-cadherin border intensity (Fig. 1C, D). Each individual siRNA oligo targeting SERCA2 was also tested; results showed a comparable reduction in SERCA2 protein expression and similar impairment of DP border localization (Supplemental Fig. S1A, B). Notably, the total protein levels of DP and other junctional proteins examined (desmoglein 1/2, plakoglobin, E-cadherin) were not affected by any of the four independent oligos (Supplemental Fig. S1A, C). These data suggest that the failure of DP to accumulate at cell-cell interfaces is specifically due to loss of SERCA2 function, without an effect on total protein levels of the junctional molecules.
Figure 1.
Pharmacological or genetic inhibition of SERCA2 impairs desmoplakin localization to cell-cell borders and weakens intercellular adhesive strength. A, B) SCC9 (A) or NHEK (B) cells were double-immunostained using antibodies against desmoplakin (NW6) and E-cadherin (HECD-1) after being treated with 100 nM thapsigargin or DMSO control for 30 min prior to a 3-h calcium switch. C, D) SCC9 (C) or NHEK (D) cells were immunostained using antibodies against either SERCA2 (left panels), desmoplakin (middle panels), or E-cadherin (right panels) after a 72 h siRNA transfection followed by a 3-h calcium switch. Bar graphs depict quantitation of pixel intensity at cell-cell borders for DP and E-cadherin; n > 75 borders/condition. E, F) Confluent monolayers of SCC9 (E) or NHEK (F) cells after a 72-h siRNA transfection were subjected to a dispase mechanical dissociation assay in triplicate. Bar graphs depict quantitation of fragmentation; n = 3, in triplicate. *P < 0.01; Student's t test. Error bars = se. Scale bars = 20 μm.
On the basis of ultrastructural studies of DD patient skin biopsies demonstrating the loss of DP at sites of acantholytic lesions (26, 27), it has been presumed that intercellular adhesive strength is compromised in DD keratinocytes. To test whether the observed loss of DP accumulation at cell-cell junctions following the knockdown of SERCA2 is accompanied by a loss of intercellular adhesive strength, we subjected the SERCA2-deficient cells to a mechanical dissociation assay. In this assay, a confluent monolayer of cells is enzymatically released from the Petri dish and subjected to mechanical stress to generate fragments of the epithelial sheet (7, 47). The loss of SERCA2 in either SCC9 or NHEK cells was found to weaken intercellular adhesive strength, as determined by the observance of a greater number of fragments in the SERCA2-deficient epithelial sheet (Fig. 1E, F). Altogether, these findings demonstrate that the loss of SERCA2 alone is sufficient to impair desmosome assembly and weaken intercellular adhesive strength in vitro without any additional modulating factors that have been hypothesized to contribute to DD lesions (36, 37).
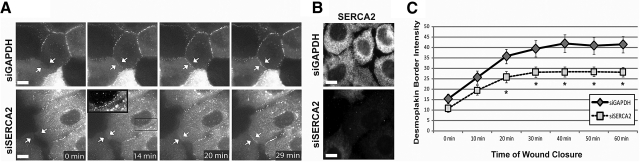
To directly evaluate the temporal sequence of DP border localization in SERCA2-deficient cells, we carried out time-lapse imaging of DP-GFP in single-planes of A431 (human vulvar epithelial) cells coming into contact at the edge of a scrape wound (9–11). In control cells, DP steadily accumulated at cell borders over the first 30 min after cell-cell contact before reaching a plateau of border intensity (Fig. 2A, top panels; C), consistent with prior determinations of DP dynamics during desmosome assembly (11). In contrast, SERCA2-deficient cells exhibited significantly reduced DP border intensity compared to control cells after only 20 min following the initiation of cell-cell contact (Fig. 2A, bottom panels; C). In addition, numerous cytoplasmic DP-containing particles remained in these cells (Fig. 2A, boxed inset). The absence of SERCA2 in the imaged cells was confirmed through retrospective analysis by fixing the sample after imaging and subsequently staining with an anti-SERCA2 antibody (Fig. 2B). These experiments confirm that SERCA2 is required for efficient DP translocation to cell borders. Furthermore, the data suggest that the aberrant DP trafficking observed in SERCA2-deficient cells is independent of the method of initiating cell contact, as similar results were observed for both Ca2+ switch (Fig. 1) and scratch-wound experiments (Fig. 2).
Figure 2.
Loss of SERCA2 impairs temporal sequence of desmoplakin border translocation. A) Time-lapse single-plane images of A431 cells expressing DP-GFP and transiently transfected for 72 h with siRNA oligos targeting either GAPDH (top panels) or SERCA2 (bottom panels) coming into contact following scratch wounding. Arrows indicate a newly forming cell-cell border. Inset: boxed region highlights cytoplasmic DP-GFP particles in a “beads-on-a-string” pattern that fail to incorporate into the assembling junction. B) Retrospective immunostaining of the imaged cells for SERCA2 to confirm SERCA2 expression in the siGAPDH-treated cells (top panel) and SERCA2-deficiency in the siSERCA2-treated cells (bottom panel). C) Quantitation of DP intensity at assembling cell-cell borders in cells treated with siGAPDH oligos (solid line with diamonds; n=12 movies) or siSERCA2 oligos (dashed line with squares; n=23 movies). *P < 0.05; Student's t test. Error bars = se. Scale bars = 10 μm.
Loss of SERCA2 results in retention of DP on keratin IF
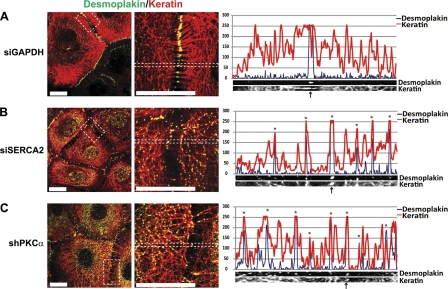
The observations that DP protein levels were unchanged by the loss of SERCA2 (Supplemental Fig. S1) and that DP-GFP particles appeared to be retained in the cytoplasm of SERCA2-deficient cells (Fig. 2) suggested that the observed defects in desmosome assembly may be due to a post-translational effect on DP trafficking behavior. During the course of normal desmosome maturation, DP-enriched precursor particles form in the cytoplasm in association with keratin IF, beginning ∼15–20 min following the initiation of nascent desmosome formation (11). These particles are then cleared from the cytoplasm to the plasma membrane over a 60- to 180-min time course (8, 11). To begin to determine the mechanism underlying the failure of DP to efficiently assemble into desmosomes, we examined DP distribution following a 180-min Ca2+ switch at high resolution using confocal immunofluorescence microscopy. In control cells, DP robustly localized to sites of cell-cell contact where keratin filaments are anchored, and cytoplasmic DP-containing particles were rarely observed at this time point (Fig. 3A). However, in cells deficient for SERCA2, DP border intensity appeared to be reduced compared to control cells, and numerous DP-containing particles were abnormally retained in the cytoplasm and aligned along keratin IF in a “beads-on-a-string” pattern (Fig. 3B). Analysis of a DP particle distribution relative to keratin IF in the SERCA2-deficient cells revealed a one-to-one correlation between particle location and the presence of a keratin IF tonofibril, suggesting a close association between DP and keratin IF (Fig. 3). While these IF-retained DP particles in the SERCA2-deficient cells resemble the DP-enriched precursor particles that form in control cells following the initiation of cell-cell contact (11), their ability to properly translocate into assembling desmosomes is impaired. Altogether, these findings suggest that SERCA2 is required for the normal dynamic behavior of DP precursor particles during their translocation into assembling desmosomes.
Figure 3.
Desmoplakin aligns along keratin filaments and is retained in the cytoplasm on loss of SERCA2 or PKCα. SCC9 cells transiently transfected for 72 h with siRNA targeting either GAPDH (A), SERCA2 (B), or PKCα (C) were subjected to a 3-h calcium switch, double-immunostained using antibodies against DP (green) and keratin-18 (red), and imaged at ×100 by confocal microscopy. All images are of a single confocal plane. Images in right panels are split-channel ×3 zoomed versions of areas outlined in white dots in left panels. Linescan analyses (graphs in right panels) are derived from the zoomed images and depict the pixel intensity of DP (blue trace) and keratin-18 (red trace) within that region. Arrows at bottom indicate sites of cell-cell contact. Asterisks above each point in graphs represent sites of one-to-one correlation of DP to keratin-18. Images and linescans shown are representative of >50 cell borders across ≥3 experiments. Scale bars = 20 μm.
The delay in DP translocation and aberrant alignment of DP on keratin IF exhibited in SERCA2-deficient cells has been previously observed in PKC-inhibited cells (9). Similar to cells depleted for SERCA2, DP-containing particles were also retained on keratin IF in the cytoplasm of cells silenced for PKCα (Fig. 3C). Given the striking similarities of impaired DP localization and aberrant DP-IF association in cells deficient for either SERCA2, a regulator of intracellular Ca2+, or PKCα, a Ca2+-dependent protein kinase, these findings raise the possibility that SERCA2 may regulate DP translocation through the modulation of PKCα signaling.
Impaired intracellular calcium homeostasis caused by SERCA2 deficiency is accompanied by attenuated PKCα signaling
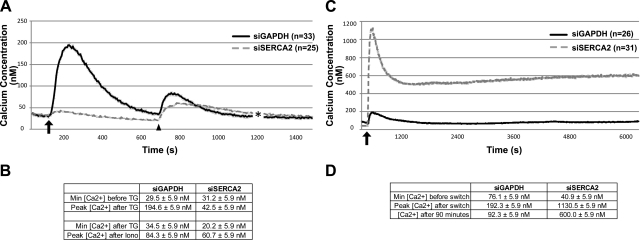
Previous studies have demonstrated that SERCA2 inhibition or deficiency leads to depleted ER Ca2+ stores, followed by constitutive Ca2+ entry through activation of store-operated Ca2+ channels (SOCCs) at the plasma membrane (30, 32, 48). We confirmed that ER Ca2+ stores were intact in control cells by determining that intracellular Ca2+ concentrations increase from 29.5 to 194.5 nM after the addition of thapsigargin to release ER Ca2+ stores (Fig. 4A, solid line). In the case of the SERCA2-deficient cells, the thapsigargin target in these cells (SERCA2) has been knocked down; thus, the intracellular Ca2+ concentration only marginally increased from 31.2 to 42.5 nM following thapsigargin treatment (Fig. 4A, dashed line). To ensure that ER Ca2+ stores were depleted in cells lacking SERCA2, we followed the thapsigargin treatment with an ionomycin treatment to release all intracellular Ca2+ stores. If SERCA2-deficient cells were harboring ER Ca2+, then the spike in intracellular Ca2+ concentration after the addition of ionomycin should be greater in the siSERCA2-treated cells than in control cells. However, the percentage increase in intracellular Ca2+ concentration from baseline to peak after ionomycin treatment was similar between control and SERCA2-deficient cells (59% for siGAPDH cells and 67% for siSERCA2 cells), which is consistent with the notion of ER Ca2+ stores being depleted in cells lacking SERCA2 (Fig. 4A, B). In addition, when subjected to Ca2+ switch, control cells exhibited a rapid increase in intracellular Ca2+ (76.1 to 192.3 nM) that plateaued at 92.3 nM after 100 min. SERCA2-deficient cells exhibited an influx of intracellular Ca2+ (40.9 to 1130.5 nM) that remained elevated over time (600.0 nM after 100 min; Fig. 4C, D), which is consistent with previous reports of Ca2+ influx in cells with defective SERCA2 activity (30, 32, 48). While we did not determine whether this change occurs solely through capacitive Ca2+ influx due to the depletion of ER Ca2+ stores, collectively, the data confirm that SCC9 cells lacking SERCA2 exhibit aberrant intracellular Ca2+ homeostasis through the depletion of ER Ca2+ stores and the inability to effectively store incoming Ca2+.
Figure 4.
Loss of SERCA2 depletes ER calcium stores and induces a sustained elevation of intracellular calcium following a calcium switch in SCC9 cells. Intracellular calcium concentrations were determined in SCC9 cells transiently transfected for 72 h with siRNA targeting either GAPDH (solid black line) or SERCA2 (dashed gray line). A) ER calcium levels were determined by treating cells cultured in low-calcium medium with 1 μM thapsigargin gray to induce the release of ER calcium stores; arrow indicates time of thapsigargin addition. To ensure ER stores were depleted in the SERCA2-deficient cells, cells were also treated with 2 μM ionomycin to release all calcium stores; arrowhead indicates time of ionomycin addition. B) Minimum and peak Ca2+ concentrations for imaging conducted in panel A. SERCA2-deficient cells exhibit minimal Ca2+ release following thapsigargin treatment. Ca2+ release following ionomycin treatment is similar between control and siSERCA2-treated cells, suggesting that ER stores are indeed depleted by SERCA2 knockdown. C) Measurement of intracellular calcium concentration in cells during a 100-min calcium switch revealed a massive influx and sustained elevation of intracellular calcium levels in siSERCA2-treated cells (n=31) compared to siGAPDH-treated controls (n=26). Arrow indicates time of calcium switch. D) Minimum and peak Ca2+ concentrations for imaging conducted in panel C. Plots represent an average of the number of cells indicated; Error bars = means ± se.
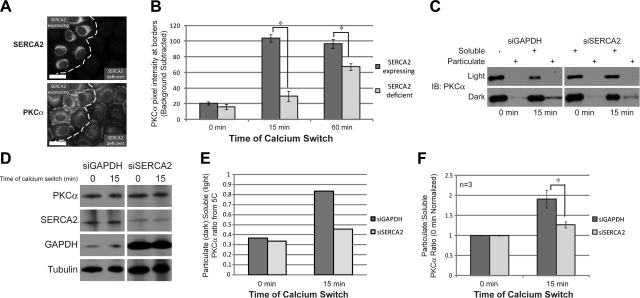
We next aimed to determine whether the aberrant intracellular Ca2+ homeostasis in SERCA2-deficient cells has an effect on PKCα translocation. To address this question, we analyzed PKCα localization in SERCA2-expressing and SERCA2-deficient cells following a 15- or 60-min Ca2+ switch to induce PKCα border translocation. SERCA2 deficiency significantly impaired the ability of PKCα to localize to the plasma membrane at both 15- and 60-min switch time points. The patches of cells that failed to be efficiently targeted by the siSERCA2 oligos, and therefore still expressed SERCA2, were used as an internal control (Fig. 5A, B). In addition, SERCA2-deficient cells were subjected to a membrane extraction assay 15 min after a Ca2+ switch to determine the levels of PKCα present in the soluble and membrane fractions. In control cells, the proportion of PKCα present in the membrane fraction is significantly greater at 15 min that at 0 min, indicating that membrane translocation has occurred. However, in SERCA2-deficient cells the induction of membrane-translocated PKCα was reduced compared to control cells (Fig. 5C, E). This finding was observed in 3 separate experiments (Fig. 5F). No changes in total PKCα levels were observed (Fig. 5D). Altogether, these findings indicate that SERCA2 regulates PKCα activity by limiting its ability to translocate to the plasma membrane during the induction of junction assembly.
Figure 5.
Loss of SERCA2 attenuates PKCα signaling. A) Double immunostaining for SERCA2 and PKCα in SCC9 cells transiently transfected for 72 h with siRNA targeting SERCA2 after a 15-min calcium switch. Images depict a field of cells containing both SERCA2-deficient cells and SERCA2-expressing cells (used as an internal control) that were not efficiently targeted by the siSERCA2 oligos. Dashed line separates the two cell populations. Scale bar = 20 μm. B) Quantitation of PKCα border intensity in SCC9 cells either expressing or deficient for SERCA2 at 15 and 60 min during a calcium switch; n > 100 borders for each condition. C) Membrane extraction assay was performed on SCC9 cells transiently transfected for 72 h with siRNA targeting either GAPDH or SERCA2. Blots were probed with anti-PKCα antibody. Representative light and dark exposures are shown to highlight changes in soluble and particulate band intensities, respectively. D) Total protein levels for membrane extraction assay. E) Quantitation of particulate:soluble PKCα from panel C. Particulate bands from the dark exposure and soluble bands from the light exposure were used to determine the ratio. F) Quantitation of 3 separate membrane extraction assays with values normalized to 0 min time point. Error bars = means ± se. *P < 0.05; Student's t test.
PKCα stimulation can restore desmosome assembly and intercellular adhesive strength in SERCA2-deficient cells
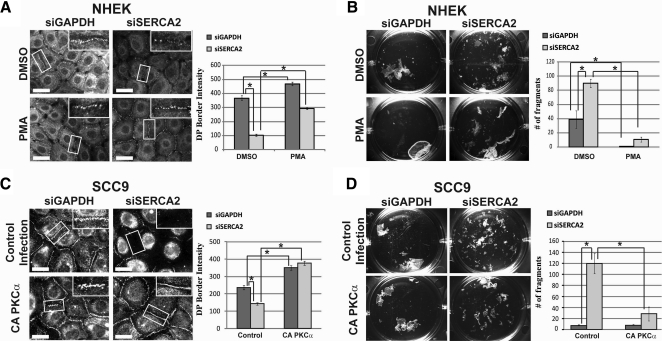
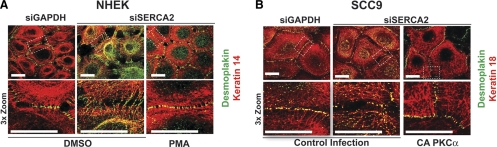
Given that PKCα is known to promote desmosome assembly (9, 14, 15), coupled with our findings that SERCA2 regulates PKCα localization, we hypothesized that PKCα stimulation may restore desmosome assembly and intercellular adhesive strength in SERCA2-deficient cells. Indeed, we found that when NHEK cells knocked down for SERCA2 were treated with PMA to stimulate PKC activity, DP border localization was no longer perturbed by the SERCA2 deficiency and resembled the DP pattern in control cells (Fig. 6A). In addition, the weakened intercellular adhesive strength in cells lacking SERCA2 could be restored by PMA treatment (Fig. 6B). Similar results were observed in SCC9 cells stably expressing CA PKCα that were treated with siSERCA2 oligos (Fig. 6C, D), suggesting that it is specifically PKCα that may be responsible for driving DP translocation and promoting the ability of cells to withstand mechanical stress. Furthermore, PKC stimulation via PMA treatment (in NHEKs) or CA PKCα expression (in SCC9s) resulted in the disappearance of keratin-associated DP and the clearing of DP from the cytoplasm. Instead, DP robustly localizes at sites of cell-cell contact in a manner comparable to control cells (Fig. 7A, B). Overall, these findings indicate that the effects of PKCα signaling on DP function and desmosome assembly are downstream of SERCA2 and suggest that PKCα stimulation is able to compensate for SERCA2 deficiency.
Figure 6.
PKCα stimulation can restore defects caused by SERCA2-deficiency. A, C) NHEK (A) and SCC9 (C) cells were transiently transfected for 72 h with siRNA targeting either GAPDH or SERCA2. NHEKs were treated with DMSO or 1 μM PMA for 30 min prior to a 3-h calcium switch. SCC9s were stably expressing an empty vector or CA PKCα and subjected to a 3-h calcium switch. Insets show zoomed versions of boxed areas. Graphs depict pixel intensity of DP at cell borders; n > 75/condition. Scale bars = 20 μm. B, D) NHEK (B) and SCC9 (D) cells under the same conditions as in A and C, respectively, were subjected to a dispase mechanical dissociation assay. Experiments were performed in triplicate; n = 3. Graphs depict quantitation of fragments. Error bars = means ± se. *P < 0.05; Student's t test.
Figure 7.
PKCα stimulation rescues the aberrant DP-IF alignment observed following SERCA2 knockdown. NHEK (A) or SCC9 (B) cells were transiently transfected for 72 h with siRNA targeting either GAPDH or SERCA2 and double-immunostained for DP (green) and keratin 18 (red) following a 3-h calcium switch. NHEKs were treated with DMSO or 1 μM PMA for 30 min prior to the calcium switch. SCC9s were stably expressing an empty vector or CA PKCα. Images in bottom panels are 3× zoomed versions of areas outlined in white dots in top panels. All images were collected at ×100 and are representative of >20 analyzed single-plane confocal borders for each condition. Scale bars = 10 μm.
DISCUSSION
Using RNAi technology to generate epithelial cells deficient for SERCA2, we have demonstrated that the loss of SERCA2 is sufficient to cause defects in desmosome assembly, similar to those observed in DD keratinocytes. In addition, we have determined that the diminution of SERCA2 expression induces a weakening of intercellular adhesive strength, a phenomenon that has long been suspected to contribute to DD pathogenesis. Such findings allow us to clearly demonstrate a role for SERCA2 in the regulation of desmosome function without any secondary influences from environmental factors that have been hypothesized to contribute to DD in patients (35–37). Altogether, our data suggest a model whereby SERCA2 regulates desmosome assembly and intercellular adhesion through modulation of the DP-keratin association in a PKCα-dependent manner (Fig. 8). In support of this linear model, we were able to reverse the effects of SERCA2 deficiency on desmosome assembly and intercellular adhesive strength by stimulating PKCα signaling in the absence of SERCA2 in both NHEK and SCC9 cell types (Figs. 6 and 7).
Figure 8.
Model of SERCA2-mediated desmosome assembly. SERCA2 is required for proper translocation and activation of PKCα (Fig. 5), which regulates the desmoplakin-keratin intermediate filament association (Fig. 3) to promote desmosome assembly (Figs. 1 and 2) and strengthen intercellular adhesion (Fig. 1). The data suggest this pathway to be linear in that stimulation of PKCα in the absence of SERCA2 is capable of rescuing the effects of SERCA2 deficiency on desmoplakin-keratin association, desmosome assembly, and intercellular adhesive strength (Figs. 6 and 7).
Under normal desmosome assembly conditions, DP-containing particles coalesce in the cytoplasm, where they are associated with keratin IF, and then translocate to the plasma membrane at sites of cell-cell contact (11). Following the loss of SERCA2 or PKCα expression, IF-bound DP failed to efficiently translocate into junctions, suggesting that a PKCα-regulated event may be required for DP translocation and desmosome assembly to proceed. One possibility is that the C terminus of DP is phosphorylated in a PKCα-dependent manner to modulate DP-IF binding and allow DP to translocate into desmosomes. Consistent with this hypothesis, we previously showed that a DP C-terminal point mutant within a PKC consensus sequence (DPS2849G) is associated more tightly with keratin IF, thus delaying its incorporation into assembling desmosomes (11, 13).
While PKC signaling has been previously associated with altering SERCA function in sensory neurons and cardiac tissue (49, 50), in this report we have identified SERCA2 as a regulator of PKCα localization and activity. Deficiency in SERCA2-gated Ca2+ stores has also been associated with alterations in keratinocyte cell cycle and differentiation pathways, including compromised up-regulation of cyclin-dependent kinase inhibitor p21(WAF1) and delayed exit from the cell cycle (48, 51). Recently, PKCα membrane localization and substrate phosphorylation were observed in the suprabasal cell layers of normal human epidermis (17). Further, activation of PKCα was sufficient to inhibit DNA synthesis through p21, and PKCα inhibition impaired differentiation in an organotypic model of epidermal differentiation. Taken together with the results that we present here, the possibility is raised that the observed impact of a deficiency in SERCA2-gated Ca2+ stores on cell cycle exit and differentiation may be due to its regulation of PKCα.
It has been suggested that the loss of SERCA2 may initiate ER stress pathways that can lead to increased transcriptional activity, accumulation of misfolded proteins, or an apoptotic response (48). In addition, the possibility that aberrant DP localization during desmosome assembly may be due to protein misfolding has been raised (28). While we have not extensively studied the possible role of ER stress, we did not observe any changes in DP expression, overall ER structure, or apoptosis/cell growth in SERCA2-deficient cells (Supplemental Fig. S1 and unpublished results). Moreover, the striking similarity of DP behavior and localization in SERCA2-deficient cells to that of PKCα-deficient cells, while not ruling out stress-induced changes in folding, suggests that aberrant PKCα signaling contributes importantly to DP's behavior in these cells. Our observation that PKCα activation restores adhesive strength in SERCA2-deficient cells further underscores the importance of this signaling pathway in regulating desmosome assembly and intercellular adhesion.
The primary treatment options for DD patients are oral and topical retinoids, which alleviate pathogenic symptoms in only a subset of patients, with the other portion exhibiting little or no response to treatment (52). By restoring desmosomal adhesion in SERCA2-deficient cells in culture through stimulation of PKCα signaling, treatment using known PKC activators may provide a novel therapeutic strategy for combatting DD. Interestingly, the active form of vitamin D has been used successfully as a treatment for Hailey-Hailey's disease (HHD) (53–55), which is a related genodermatosis caused by mutations in an intracellular Ca2+ pump from another protein family (24, 26). Although the molecular course of action for vitamin D in HHD keratinocytes has not been resolved, vitamin D has been shown to enhance PKCα activity in primary keratinocytes (56). Further clinical studies will be necessary to test the possible efficacy of PKCα activation in restoring defects in DD keratinocytes and in treating DD patients.
Supplementary Material
Acknowledgments
The authors thank Lisa Godsel and Spiro Getsios (Northwestern University) for their valued advice, support, and comments on the paper.
This work was supported by NIH grants to K.J.G. (RO1 AR043380 and RO1 AR041836), M.P. (RO1 NS057499), and C.L.S. (F30 ES14990), as well as an American Heart Association fellowship to R.P.H (0810061Z). The authors declare no conflicts of interest.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Garrod D., Chidgey M. (2008) Desmosome structure, composition and function. Biochim. Biophys. Acta 1778, 572–587 [DOI] [PubMed] [Google Scholar]
- 2. Green K. J., Simpson C. L. (2007) Desmosomes: new perspectives on a classic. J. Invest. Dermatol. 127, 2499–2515 [DOI] [PubMed] [Google Scholar]
- 3. Niessen C. M. (2007) Tight junctions/adherens junctions: basic structure and function. J. Invest. Dermatol. 127, 2525–2532 [DOI] [PubMed] [Google Scholar]
- 4. Stokes D. L. (2007) Desmosomes from a structural perspective. Curr. Opin. Cell Biol. 19, 565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sonnenberg A., Liem R. K. (2007) Plakins in development and disease. Exp. Cell Res. 313, 2189–2203 [DOI] [PubMed] [Google Scholar]
- 6. Stappenbeck T. S., Green K. J. (1992) The desmoplakin carboxyl terminus coaligns with and specifically disrupts intermediate filament networks when expressed in cultured cells. J. Cell Biol. 116, 1197–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huen A. C., Park J. K., Godsel L. M., Chen X., Bannon L. J., Amargo E. V., Hudson T. Y., Mongiu A. K., Leigh I. M., Kelsell D. P., Gumbiner B. M., Green K. J. (2002) Intermediate filament-membrane attachments function synergistically with actin-dependent contacts to regulate intercellular adhesive strength. J. Cell Biol. 159, 1005–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pasdar M., Nelson W. J. (1988) Kinetics of desmosome assembly in Madin-Darby canine kidney epithelial cells: temporal and spatial regulation of desmoplakin organization and stabilization upon cell-cell contact. I. Biochemical analysis. J. Cell Biol. 106, 677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bass-Zubek A. E., Hobbs R. P., Amargo E. V., Garcia N. J., Hsieh S. N., Chen X., Wahl J. K., 3rd, Denning M. F., Green K. J. (2008) Plakophilin 2: a critical scaffold for PKC alpha that regulates intercellular junction assembly. J. Cell Biol. 181, 605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Godsel L. M., Dubash A. D., Bass-Zubek A. E., Amargo E. V., Klessner J. L., Hobbs R. P., Chen X., Green K. J. (2010) Plakophilin 2 couples actomyosin remodeling to desmosomal plaque assembly via RhoA. Mol. Biol. Cell 21, 2844–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Godsel L. M., Hsieh S. N., Amargo E. V., Bass A. E., Pascoe-McGillicuddy L. T., Huen A. C., Thorne M. E., Gaudry C. A., Park J. K., Myung K., Goldman R. D., Chew T. L., Green K. J. (2005) Desmoplakin assembly dynamics in four dimensions: multiple phases differentially regulated by intermediate filaments and actin. J. Cell Biol. 171, 1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meng J. J., Bornslaeger E. A., Green K. J., Steinert P. M., Ip W. (1997) Two-hybrid analysis reveals fundamental differences in direct interactions between desmoplakin and cell type-specific intermediate filaments. J. Biol. Chem. 272, 21495–21503 [DOI] [PubMed] [Google Scholar]
- 13. Stappenbeck T. S., Lamb J. A., Corcoran C. M., Green K. J. (1994) Phosphorylation of the desmoplakin COOH terminus negatively regulates its interaction with keratin intermediate filament networks. J. Biol. Chem. 269, 29351–29354 [PubMed] [Google Scholar]
- 14. Pasdar M., Li Z., Chan H. (1995) Desmosome assembly and disassembly are regulated by reversible protein phosphorylation in cultured epithelial cells. Cell Motil. Cytoskeleton 30, 108–121 [DOI] [PubMed] [Google Scholar]
- 15. Sheu H. M., Kitajima Y., Yaoita H. (1989) Involvement of protein kinase C in translocation of desmoplakins from cytosol to plasma membrane during desmosome formation in human squamous cell carcinoma cells grown in low to normal calcium concentration. Exp. Cell Res. 185, 176–190 [DOI] [PubMed] [Google Scholar]
- 16. Denning M. F. (2004) Epidermal keratinocytes: regulation of multiple cell phenotypes by multiple protein kinase C isoforms. Int. J. Biochem. Cell Biol. 36, 1141–1146 [DOI] [PubMed] [Google Scholar]
- 17. Jerome-Morais A., Rahn H. R., Tibudan S. S., Denning M. F. (2009) Role for protein kinase C-alpha in keratinocyte growth arrest. J. Invest. Dermatol. 129, 2365–2375 [DOI] [PubMed] [Google Scholar]
- 18. Garrod D. R., Berika M. Y., Bardsley W. F., Holmes D., Tabernero L. (2005) Hyper-adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. J. Cell Sci. 118, 5743–5754 [DOI] [PubMed] [Google Scholar]
- 19. Wallis S., Lloyd S., Wise I., Ireland G., Fleming T. P., Garrod D. (2000) The alpha isoform of protein kinase C is involved in signaling the response of desmosomes to wounding in cultured epithelial cells. Mol. Biol. Cell 11, 1077–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lai-Cheong J. E., Arita K., McGrath J. A. (2007) Genetic diseases of junctions. J. Invest. Dermatol. 127, 2713–2725 [DOI] [PubMed] [Google Scholar]
- 21. Van der Zwaag P., Jongbloed J., van Tintelen P. (2010) ARVC Database. Department of Genetics, University Medical Center Groningen, Groningen, The Netherlands [Google Scholar]
- 22. Yang Z., Bowles N. E., Scherer S. E., Taylor M. D., Kearney D. L., Ge S., Nadvoretskiy V. V., DeFreitas G., Carabello B., Brandon L. I., Godsel L. M., Green K. J., Saffitz J. E., Li H., Danieli G. A., Calkins H., Marcus F., Towbin J. A. (2006) Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ. Res. 99, 646–655 [DOI] [PubMed] [Google Scholar]
- 23. Amagai M. (2003) Desmoglein as a target in autoimmunity and infection. J. Am. Acad. Dermatol. 48, 244–252 [DOI] [PubMed] [Google Scholar]
- 24. Dhitavat J., Fairclough R. J., Hovnanian A., Burge S. M. (2004) Calcium pumps and keratinocytes: lessons from Darier's disease and Hailey-Hailey disease. Br. J. Dermatol. 150, 821–828 [DOI] [PubMed] [Google Scholar]
- 25. Foggia L., Hovnanian A. (2004) Calcium pump disorders of the skin. Am. J. Med. Genet. C. Semin. Med. Genet. 131C, 20–31 [DOI] [PubMed] [Google Scholar]
- 26. Burge S. M., Garrod D. R. (1991) An immunohistological study of desmosomes in Darier's disease and Hailey-Hailey disease. Br. J. Dermatol. 124, 242–251 [DOI] [PubMed] [Google Scholar]
- 27. de Dobbeleer G., Achten G. (1979) Disrupted desmosomes in induced lesions of familial benign chronic pemphigus. J. Cutan. Pathol. 6, 418–424 [DOI] [PubMed] [Google Scholar]
- 28. Dhitavat J., Cobbold C., Leslie N., Burge S., Hovnanian A. (2003) Impaired trafficking of the desmoplakins in cultured Darier's disease keratinocytes. J. Invest. Dermatol. 121, 1349–1355 [DOI] [PubMed] [Google Scholar]
- 29. Hovnanian A. (2007) SERCA pumps and human diseases. Subcell. Biochem. 45, 337–363 [DOI] [PubMed] [Google Scholar]
- 30. Foggia L., Aronchik I., Aberg K., Brown B., Hovnanian A., Mauro T. M. (2006) Activity of the hSPCA1 Golgi Ca2+ pump is essential for Ca2+-mediated Ca2+ response and cell viability in Darier disease. J. Cell Sci. 119, 671–679 [DOI] [PubMed] [Google Scholar]
- 31. Leinonen P. T., Hagg P. M., Peltonen S., Jouhilahti E. M., Melkko J., Korkiamaki T., Oikarinen A., Peltonen J. (2009) Reevaluation of the normal epidermal calcium gradient, and analysis of calcium levels and ATP receptors in Hailey-Hailey and Darier epidermis. J. Invest. Dermatol. 129, 1379–1387 [DOI] [PubMed] [Google Scholar]
- 32. Pani B., Cornatzer E., Cornatzer W., Shin D. M., Pittelkow M. R., Hovnanian A., Ambudkar I. S., Singh B. B. (2006) Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier's disease. Mol. Biol. Cell 17, 4446–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prasad V., Okunade G. W., Miller M. L., Shull G. E. (2004) Phenotypes of SERCA and PMCA knockout mice. Biochem. Biophys. Res. Commun. 322, 1192–1203 [DOI] [PubMed] [Google Scholar]
- 34. Shull G. E., Okunade G., Liu L. H., Kozel P., Periasamy M., Lorenz J. N., Prasad V. (2003) Physiological functions of plasma membrane and intracellular Ca2+ pumps revealed by analysis of null mutants. Ann. N. Y. Acad. Sci. 986, 453–460 [DOI] [PubMed] [Google Scholar]
- 35. Hedblad M. A., Nakatani T., Beitner H. (1991) Ultrastructural changes in Darier's disease induced by ultraviolet irradiation. Acta Derm. Venereol. 71, 108–112 [PubMed] [Google Scholar]
- 36. Jegasothy B. V., Humeniuk J. M. (1981) Darier's disease: a partially immunodeficient state. J. Invest. Dermatol. 76, 129–132 [DOI] [PubMed] [Google Scholar]
- 37. Mayuzumi N., Ikeda S., Kawada H., Ogawa H. (2005) Effects of drugs and anticytokine antibodies on expression of ATP2A2 and ATP2C1 in cultured normal human keratinocytes. Br. J. Dermatol. 152, 920–924 [DOI] [PubMed] [Google Scholar]
- 38. Getsios S., Simpson C. L., Kojima S., Harmon R., Sheu L. J., Dusek R. L., Cornwell M., Green K. J. (2009) Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J. Cell Biol. 185, 1243–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shimoyama Y., Hirohashi S., Hirano S., Noguchi M., Shimosato Y., Takeichi M., Abe O. (1989) Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 49, 2128–2133 [PubMed] [Google Scholar]
- 40. Angst B. D., Nilles L. A., Green K. J. (1990) Desmoplakin II expression is not restricted to stratified epithelia. J. Cell Sci. 97, 247–257 [DOI] [PubMed] [Google Scholar]
- 41. Hayashi K., Yonemura S., Matsui T., Tsukita S. (1999) Immunofluorescence detection of ezrin/radixin/moesin (ERM) proteins with their carboxyl-terminal threonine phosphorylated in cultured cells and tissues. J. Cell Sci. 112, 1149–1158 [DOI] [PubMed] [Google Scholar]
- 42. Yonemura S., Hirao-Minakuchi K., Nishimura Y. (2004) Rho localization in cells and tissues. Exp. Cell Res. 295, 300–314 [DOI] [PubMed] [Google Scholar]
- 43. Rasband W. S. (1997–2010) ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA [Google Scholar]
- 44. Hudson T. Y., Fontao L., Godsel L. M., Choi H. J., Huen A. C., Borradori L., Weis W. I., Green K. J. (2004) In vitro methods for investigating desmoplakin-intermediate filament interactions and their role in adhesive strength. Methods. Cell Biol. 78, 757–786 [DOI] [PubMed] [Google Scholar]
- 45. Sabala P., Czarny M., Woronczak J. P., Baranska J. (1993) Thapsigargin: potent inhibitor of Ca2+ transport ATP-ases of endoplasmic and sarcoplasmic reticulum. Acta Biochim. Pol. 40, 309–319 [PubMed] [Google Scholar]
- 46. Stuart R. O., Sun A., Bush K. T., Nigam S. K. (1996) Dependence of epithelial intercellular junction biogenesis on thapsigargin-sensitive intracellular calcium stores. J. Biol. Chem. 271, 13636–13641 [DOI] [PubMed] [Google Scholar]
- 47. Ishii K., Harada R., Matsuo I., Shirakata Y., Hashimoto K., Amagai M. (2005) In vitro keratinocyte dissociation assay for evaluation of the pathogenicity of anti-desmoglein 3 IgG autoantibodies in pemphigus vulgaris. J. Invest. Dermatol. 124, 939–946 [DOI] [PubMed] [Google Scholar]
- 48. Pani B., Singh B. B. (2008) Darier's disease: a calcium-signaling perspective. Cell. Mol. Life Sci. 65, 205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Braz J. C., Gregory K., Pathak A., Zhao W., Sahin B., Klevitsky R., Kimball T. F., Lorenz J. N., Nairn A. C., Liggett S. B., Bodi I., Wang S., Schwartz A., Lakatta E. G., DePaoli-Roach A. A., Robbins J., Hewett T. E., Bibb J. A., Westfall M. V., Kranias E. G., Molkentin J. D. (2004) PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat. Med. 10, 248–254 [DOI] [PubMed] [Google Scholar]
- 50. Usachev Y. M., Marsh A. J., Johanns T. M., Lemke M. M., Thayer S. A. (2006) Activation of protein kinase C in sensory neurons accelerates Ca2+ uptake into the endoplasmic reticulum. J. Neurosci. 26, 311–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muller E. J., Caldelari R., Kolly C., Williamson L., Baumann D., Richard G., Jensen P., Girling P., Delprincipe F., Wyder M., Balmer V., Suter M. M. (2006) Consequences of depleted SERCA2-gated calcium stores in the skin. J. Invest. Dermatol. 126, 721–731 [DOI] [PubMed] [Google Scholar]
- 52. Cooper S. M., Burge S. M. (2003) Darier's disease: epidemiology, pathophysiology, and management. Am. J. Clin. Dermatol. 4, 97–105 [DOI] [PubMed] [Google Scholar]
- 53. Aoki T., Hashimoto H., Koseki S., Hozumi Y., Kondo S. (1998) 1alpha,24-dihydroxyvitamin D3 (tacalcitol) is effective against Hailey-Hailey disease both in vivo and in vitro. Br. J. Dermatol. 139, 897–901 [DOI] [PubMed] [Google Scholar]
- 54. Bianchi L., Chimenti M. S., Giunta A. (2004) Treatment of Hailey-Hailey disease with topical calcitriol. J. Am. Acad. Dermatol. 51, 475–476 [DOI] [PubMed] [Google Scholar]
- 55. Rajpara S. M., King C. M. (2005) Hailey-Hailey disease responsive to topical calcitriol. Br. J. Dermatol. 152, 816–817 [DOI] [PubMed] [Google Scholar]
- 56. Hanafin N. M., Persons K. S., Holick M. F. (1995) Increased PKC activity in cultured human keratinocytes and fibroblasts after treatment with 1 alpha, 25-dihydroxyvitamin D3. J. Cell. Biochem. 57, 362–370 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.