Abstract
Aging impairs function in the nonischemic heart and is associated with mechanical remodeling. This process includes accumulation of collagen (i.e., fibrosis) and dysregulation of active matrix metalloproteinases (MMPs). Exercise training (ET) improves cardiac function, but the pathways of protection remain poorly understood. Young (3 mo) and old (31 mo) FBNF1 rats were assigned into sedentary and exercise groups, with ET group rats training on a treadmill 45 min/d, 5 d/wk for 12 wk. Nonlinear optical microscopy (NLOM), histology, immunohistochemistry (IHC), and Western blot analyses were performed on the left ventricle and septum. NLOM, IHC, and histological imaging revealed that ET reduced age-associated elevation of collagen type I fibers. Active MMP-1, active MMP-2, and MMP-14 in the ECM fraction of the left ventricle were reduced by aging, an effect abrogated by ET. Tissue inhibitor of MMP (TIMP-1) was elevated with age but protected by ET. Transforming growth factor-β (TGF-β), upstream regulator of TIMP-1, increased with age but was attenuated by ET. Therefore, exercise training could protect the aging heart against dysregulation of MMPs and fibrosis by suppressing elevation of TIMP-1 and TGF-β.—Kwak, H.-B., Kim, J.-H., Joshi, K., Yeh, A., Martinez, D. A., Lawler, J. M. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart.
Keywords: TIMP-1, collagen, remodeling
Aging is characterized by progressive impairment of heart function, associated with significant mechanical remodeling that includes “fibrosis”, or accumulation of collagen (i.e., fibrosis) and other extracellular matrix (ECM) proteins (1). The mechanical consequences of age-induced fibrosis and ventricular remodeling include increased wall stress, decreased elasticity, impaired early diastolic filling, and reduced rate of ventricular shortening (2–5). Aging results in a hypertrophied, yet weaker, heart, with greater internal work to overcome, thus reducing ejection fraction and increasing susceptibility for arrhythmias (3, 6). Structural remodeling of the aging heart indeed resembles that observed with heart failure, and might in part be in response to increased overload (7–9).
The ECM in the heart is composed of connective tissue proteins including collagens, glycoproteins (e.g., fibronectins, laminins, etc.), and proteoglycans (10). The most abundant ECM fibrillar proteins in the heart are collagens, particularly the collagen type I and collagen type III phenotypes (11). Connective tissue binds to the cytoskeleton by transmembrane molecules, serving as a physical connection (10). Cardiac ECM serves 4 functions: providing a scaffold and support for myocytes, fibroblasts, and endothelial cells; transmitting mechanical stress in and out of myocardial cells; ensuring elasticity and compliance during the cardiac cycle; and mediating signaling for growth, survival, and remodeling (12). However, excessive myocardial fibrosis occurs in senescent hearts and impairs function (13). Indeed, type I collagen may accumulate 2- to 3-fold with aging (9, 14). Age-associated fibrosis and architectural remodeling in the heart are primarily a function of decreased collagen degradation and, to a lesser extent, increased collagen synthesis and proliferation of fibroblasts (9, 15). Aging also alters the geometry of connective tissue by increasing the diameter of collagen fibrils, decreasing linearity of myocyte sheaths, and increasing disorder (9, 16, 17).
Degradation of fibrillar collagen and other ECM proteins is catalyzed by a family of zinc-dependent enzymes called matrix metalloproteinases (MMPs), with >20 members, including MMP-1, MMP-2, MMP-3, MMP-9, and MMP-14 (12, 18). Activation of MMPs is accomplished by cleavage of their full-length proteins (18). MMPs and collagen turnover are suppressed by tissue inhibitors of metalloproteinases (TIMPs), which inhibit MMPs by binding to their active sites (18, 19). It is now recognized that dysregulation of MMPs and TIMPs is an important contributor to cardiac remodeling characteristic of both aging and heart failure (9, 20). Indeed, large increases in TIMPs are linked with heart failure (21, 22). Upstream regulatory signaling for TIMPs and fibrosis may include transforming growth factor-beta (TGF-β) and oxidative stress (13, 23–25).
Inactivity or sedentary lifestyle decreases cardiac output and stroke volume, and exacerbates heart disease risk (26). It is widely accepted that inactivity appears to accelerate cardiac aging (27). Regular exercise, particular endurance exercise, effectively improves heart function in both young and older populations (26–28). Exercise training improves maximal cardiovascular work capacity by increasing stroke volume and cardiac output (26). It is possible that exercise training in aging populations may reduce accumulation of connective tissue. Limited data indicate that exercise training might attenuate collagen content in the aging heart (29). Collagen cross-linking [hydroxylysyl pyridinoline (HP)] of left ventricle (LV) free wall was significantly lower in old trained rats, compared with their sedentary counterparts (6, 29). The ability of exercise training to attenuate diastolic dysfunction and collagen cross-linking was recently cited (30). However, potential pathways by which exercise training ameliorates fibrosis in the aging heart are not understood.
Therefore, our purpose was 2-fold: to identify potential protection by exercise training against geometric changes in collagen fibers in LV using imaging techniques, including nonlinear optical microscopy (NLOM); and to determine whether amelioration of age-associated dysregulation of MMPs is a prospective and novel mechanism of exercise protection. We hypothesize that 12 wk of exercise training will attenuate or prevent reduction in active MMPs, linked with reduction in upstream regulators TIMPs and TGF-β in the rat heart.
MATERIALS AND METHODS
Animals
The Fischer 344 × Brown Norway F1 (FBNF1) hybrid rat strain is a preferred U.S. National Institutes of Health (NIH) aging model. Pathogen-free FBNF1 rats were purchased from the National Institute on Aging colony (National Institute on Aging, Bethesda, MD, USA). The FBNF1 rat was chosen because it is free of cardiovascular disease, allowing better assessment of true aging effects. Young (3 mo) and old (31 mo) FBNF1 rats were used as our aging and exercise model. Treadmill training began at 31 mo of age, close to the mean life span of the strain (31), for old rats and at 3 mo for young adults. Rats were thus 6 and 34 mo of age at the conclusion of the study. Animals were housed on a 12-h light-dark diurnal cycle, and cared for in accordance with NIH and American Physiological Association guidelines. All protocols had been approved by the University Laboratory Animal Compliance Committee at Texas A&M University prior to commencement of the study.
Exercise training protocol and study design
To test exercise regulation of fibrosis and remodeling in the aging heart, FBNF1 rats were trained on a treadmill at an intensity designed to ∼75% of maximal aerobic capacity, based on our previous experience (32). Young (3 mo) and old (31 mo) rats were divided into the following groups: young sedentary (YS; n=10), young with exercise training (YE; n=10), old sedentary (OS; n=10), and old with exercise training (OE; n=10). Rats in the exercise training groups were acclimated during the first 7 d by walking on the motor-driven treadmill without incline at 10 m/min for 10 min. Rats were gradually conditioned to perform exercise on a 12° incline up to 45 min over the first 3-wk training program. The exercise group then ambulated on the treadmill for 45 min/d at a 12° incline, 5 d/wk for the remainder (9 wk) of the 12-wk protocol. Walking/running speeds were 10.5 m/min (old) and 22 m/min (young) to reach a similar relative intensity for each age group. This exercise regimen had been shown previously to elevate citrate synthase activity as a marker of oxidative mitochondrial capacity in skeletal muscle (33).
Rats in both the sedentary and exercise groups were now 6 and 34 mo of age and were sacrificed at 48 h after the last exercise bout with 120 mg/kg sodium pentobarbital. Hearts were extracted, weighed, and dissected into the LV, septum, and right ventricle (RV). Heart samples were snap-frozen in isopentane cooled in liquid nitrogen for histochemical analysis and in liquid nitrogen for protein analysis. Samples remained at −80°C until analysis. Septa used in NLOM experiments were fixed in 10% formalin.
Homogenization and nuclear and connective tissue fractionization
LV and septa samples were homogenized in lysis buffer (pH adjusted to 7.5) containing the following components: 20 mM HEPES, 350 mM NaCl, 20% glycerol, 1% Igepal-CA630, 1 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 0.1 mM DTT, and protease inhibitor cocktail (Roche, Indianapolis, IN, USA) at 4°C. Tissues were minced and homogenized (20:1 w/v) in lysis buffer, using a ground glass on ground glass homogenizer (Bellco Biotechnology, Vineland, NJ, USA) and then centrifuged twice at 10,000 g at 4°C. Soluble, nuclear, and ECM muscle fractions were isolated for analysis as adapted from Zheng et al. (34). Briefly, heart samples were homogenized (7:1 w/v) in buffer A, containing 20 mM HEPES free acid, 10 mM HEPES Na salt, 350 mM mannitol, 10% glycerol, 25 mM KCL, and 0.5 mM EDTA. Homogenates were centrifuged 10 min at 3000 g at 4°C, and the supernatant was removed. The pellet was resuspended (9:1 v/v) in buffer B, containing 20 mM HEPES free acid, 20 mM HEPES Na salt, 350 mM NaCl, 10% glycerol, 1 mM MgCl2, and 0.5 mM EDTA, and then centrifuged at 12,000 g for 30 min at 4°C. The supernatant nuclear fraction was then removed as the nuclear fraction. The “insoluble” or ECM (connective tissue) tissue pellet was boiled, and then resuspended in 50 mM Tris buffer with 2% sodium dodecyl sulfate (SDS), 0.1 mM dithiothreitol (DTT), and 0.6 mM EDTA. Poly(ADP-ribose)polymerase (PARP) in the first supernatant (cytosolic fraction) vs. the nucleosome fraction and collagen type-I in the resuspended second pellet were used as markers to ensure the efficacy of the procedure in separating the nuclear fraction from soluble.
Histology and immunohistochemistry (IHC)
For histology and IHC assays, LV cross sections were cut at a temperature of −20°C and dried for 30 min. Hematoxylin was used as a general stain for cardiomyocytes, nuclei, extracellular area, and geometry. Sections for IHCs were fixed in acetone (−20°C) for 60 min. We blocked LV with 10% serum of the secondary antibody host (e.g., goat) and 0.05% Tween20, in PBS (15 ml) for 30 min. The desired primary antibody (collagen type-I: 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was then applied in blocking buffer and placed on the section for 1 h. After washing, biotinylated secondary antibody (goat anti-rabbit) was applied to the sections (1:200 dilution in PBS buffer) and incubated for 30 min. Cross sections were then incubated at room temperature for 30 min with Vectastain elite ABC reagent and incubated in peroxidase substrate solution (Vector, Burlingame, CA, USA) for 10 min until a red positive stain appeared (BioQuant system; BioQuant, San Diego, CA, USA). Hematoxylin was used as a counterstain for visualization.
Masson's trichrome stain for collagen and myocytes
Colorimetric staining method for collagen used included an adaptation of the Masson's trichrome technique (35). In this technique, cardiomyocytes stain a bright red, collagen fibers blue, and nuclei black. Briefly, 10-μm frozen LV cross sections were cut at −15°C and placed on a slide. After a 20-min drying period, samples were fixed overnight at room temperature in Bouin's solution. Slides were rinsed in distilled water for 3 min, then running tap water for 5 min. Cross sections were stained in Weigert's hematoxylin for 15 min, washed in distilled water, then washed in running tap water for 5 min. Heart samples were then stained with 1% Biebrich scarlet-acid fuschin for 15 min, then washed in distilled water for 5 min. After differentiation in 2.5% phosphomolybdic-phosphotungstic acid solution for 15 min, sections were transferred directly into 2.5% aniline blue solution for 12 min. Heart samples were then differentiated in 1% acetic acid solution for 3 min, dehydrated in 95 and 100% ethanol, then cleared in xylene. Images were captured on a Zeiss Axioplot Vision-series microscope and software (Carl Zeiss, Oberkochen, Germany), and quantified using the NIH ImageJ analysis program (NIH, Bethesda, MD, USA). Serial sections in 6 rats/group were analyzed for percentage connective tissue area.
Custom, state-of-the-art NLOM system
Traditional 2-D histochemical imaging of collagen requires removal of tissue samples, embedding, fixation, and sectioning. A novel imaging technology, NLOM, was used that relies on nonlinear light matter interactions: 2-photon excited fluorescence (TPEF) and second-order harmonic generation (SHG). NLOM is designed to characterize intact cardiac microstructure, and is particular sensitive in the detection of insoluble proteins including fibrillar collagen with autofluorescent properties, via merging of TPEF and SHG signals. In our custom NLOM, we coupled sub-10-fs laser pulses (75-MHz repetition rate) into the epigalvanometer-driven motors mounted on an elevated breadboard (36). LV and septa samples were positioned in the sample chamber for series of 20-μm-thick wafer images.
A 1:5:1 beam expanding telescope consisted of two near-IR achromatic lenses housed within the epifluorescence port appropriately imaged a spot from between the galvanometer mirrors to the back focal aperture of the microscope objective by a short-pass diachronic mirror. The focusing objective collected nonlinear optical signals and directed them to a 2- or 16-channel multispectral detector unit mounted on accessory ports of the binocular head (nondescanned detection). The 2-channel detector unit housed diachronic mirrors, bandpass filters, focusing lenses, and a pair of photon-counting photomultiplier tubes (PMTs). For the multispectral detector, a multimode optical fiber delivered nonlinear optical signals to a line-ruled grating-based spectrometer and 16-channel PMT array. Each PMT (channel) was connected to a preamplifier/discriminator, which fed a counter card managed by maximum image acquisition rate of 1 Hz. The amount of dispersion introduced by the NLOM optical system changed with each microscope objective. We had characterized and minimized dispersion in our NLOM optical system for each of our microscope objectives (×20/0.75 NA, ×20/0.5 NA, ×40/0.8 NA, ×63/0.95 NA, ×63/1.2 NA, ×100/1.0 NA). We arranged dispersion compensating mirrors (DCMs) in a double-pass configuration, allowing a total of 32 bounces. This geometry gave simple dispersion tuning up to −6400 fs2 and −3800 fs3 in increments of −400 fs2 and −240 fs3, respectively. We used combinations of antireflection-coated BK7 windows at 2, 3, or 4 mm thickness and fused silica wedge pairs, each mounted on a linear translation stage providing an additional 400 μm to 3 mm of glass, for fine dispersion compensation adjustments. This corresponded to precise control of up to 518.2 fs2 and 62.5 fs3.
Western immunoblot
Protein expression was determined by Western immunoblot analysis similar to that outlined by Kwak et al. (37). LV homogenate (20 μg) was loaded on 10% polyacrylamide gels and electrophoresed using a Bio-Rad Protein III gel-box onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Briefly, separating gel (375 mM Tris-HCl, pH 8.8; 0.4% SDS; and 10% acrylamide) and stacking gel (125 mM Tris-HCl, pH 6.8; 0.4% SDS; and 10% acrylamide monomer) solutions were made, and polymerization was then initiated by tetramethylethylenediamine (TEMED) and ammonium persulfate. Separating and stacking gels were quickly poured into a Bio-Rad Protein III gel-box (Bio-Rad). LV samples in sample buffer (Tris, pH 6.8, with 2% SDS, 30 mM DTT, and 25% glycerol) were electrophoresed at 150 V. The gels were then transferred at 30 V overnight onto a nitrocellulose membrane (Bio-Rad). Membranes were blocked in 5% nonfat milk in PBS with 0.1% Tween-20 for 6 h. After blocking, membranes were incubated at room temperature in blocking buffer for 12 h with the appropriate primary antibodies: MMP-1 (1:1000; Calbiochem, San Diego, CA, USA), MMP-2 (1:2000, Santa Cruz Biotechnology), MMP-3 (1:5000; Chemicon, Billerica, MA, USA), MMP-9 (1:8000; Chemicon), MMP-14 (1:5000; Chemicon), TIMP-1 (1:1000; Chemicon), TIMP-2 (1:2000; Calbiochem), TIMP-3 (1:2000; Cedarlane, Burlington, ON, Canada), TIMP-4 (1:1000; Chemicon), TGF-β1 (1:250; R&D Systems, Minneapolis, MN, USA), and α-SMA (1:1000; Sigma, St. Louis, MO, USA). Following 3 washings with PBS with 0.1% Tween-20, horseradish peroxidase (HRP)-conjugated secondary antibodies and an enhanced chemiluminescence (ECL) detection system (Amersham, Piscataway, NJ, USA) were used for visualization. Densitometry and quantification were performed using NIH ImageJ. To ensure equal loading of protein, Ponceau-S staining was performed for each membrane as an internal control, which confirmed similar protein loading for each lane. In addition, the lane background reading was subtracted from each protein blot density reading. Further, additional samples from YS control rats served as an internal comparison. Membranes were stripped and reprobed for GAPDH or β-actin as “housekeeping” proteins and used as another layer of loading controls.
Real-time qRT-PCR analysis
Total RNA was isolated from the heart tissues using an RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA) with the addition of a proteinase K step based on our previously published methodology for fibrous connective tissues (38). Total RNA quantity (A260 nm), purity (A260 nm/A280 nm, A260 nm/A230 nm) and integrity (RNA FA gels) were determined using a NanoDrop UV/Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA). Triplicate cDNA synthesis reactions were performed using 200 ng of Total RNA in 30-μl RT reactions using SuperScript II RNase H-reverse transcriptase (Invitrogen Corp., Carlsbad, CA, USA), according to the manufacturers protocol with some slight modifications (38). For the specific gene of interest (e.g., collagen type I), a 5′-TaqMan hydrolysis Q-PCR assay was performed using an MX-3005P real time Q-PCR machine (Stratagene Inc., La Jolla, CA, USA) as previously published (38). The rat Col 1a2 sequence (accession no. AF121217), forward (GTGCAGTCGGTGCTCCAG) and reverse (TTCTCCTTTGCCTCCAGGTATG) primers, and a TaqMan probe (CTCTGCTGGTGCCTCTGGTCCTGGT) containing a 5′-FAM fluorescent reporter and a 3′-BHQ-1 quencher (BioSearch Technologies, Novato, CA, USA) were generated using Beacon Designer 2 TaqMan multiplexing software, version 7.0 (Premier Biosoft, Palo Alto, CA, USA). Q-PCR standards were generated using synthetic-DNA (sDNA) amplicon oligonucleotides (Biosynthesis Inc., Lewisville, TX, USA) spanning the entire Q-PCR amplification region (95 nt). A standard curve methodology was performed to quantitate the specific gene targets of interest. Specific standard aliquots (108 to 101 copies of cDNA) were used to generate a linear curve (>R2=0.995) with a Q-PCR efficiency (slope) of −3.3 to determine the cDNA copy number of triplicate replicates. Each Q-PCR reaction employed a 3-min initial 95°C activation step of the Quantifast-Probe Taq polymerase (Qiagen) followed by 40 cycles of 2-step thermocycling (3 s at 95°C and 30 s at 60°C). The cDNA copy number of the collagen type I gene was normalized to the constitutively expressed rat cyclophilin A (accession no. M19533) gene (38). The cDNA copy number was derived for each sample based on triplicate measurements and a group mean for each treatment used in the statistical analyses.
Statistics
Two-way ANOVAs for repeated measures with Fisher's LSD post hoc test where appropriate were used to assess mean differences between the test groups for aging and exercise. Significance level was set at 0.05.
RESULTS
Young (3 mo) and old (31 mo) FBNF1 rats were divided into sedentary and exercise groups prior to the 12-wk exercise protocol. At 48 h following the training period, hearts in all groups were extracted; dissected into LV, RV, and septum; and snap frozen. Rats thus were 6 and 34 m, respectively, for the young and old age groups at the completion of the study. The first set of experiments was designed to test the hypothesis that exercise training reduced fibrosis in the aging heart and mitigated alterations in geometry that might increase internal work, impede diastolic filling, and impair contractility. To do this, we visualized the ECM, collagen, and collagen type-I accumulation.
Effect of exercise training on connective tissue and collagen in the aging heart
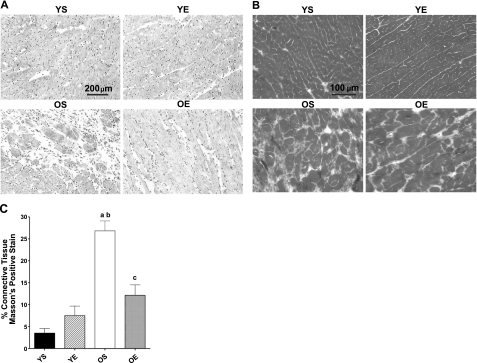
Hematoxylin staining revealed significant remodeling in the LVs from old rats. Extracellular space was greater in LVs from OS than the YS or exercise groups (Fig. 1A). The geometry of the LV in the OS group assumed a more weblike appearance in contrast with the more linear pattern of myocyte and extramyocyte space exhibited in young rats. In addition, the size of many of the cardiomyocytes was larger in the OE and OS groups compared to the YS and YE groups. However, substantially less remodeling was exhibited in LV samples from the OS and OE groups (Fig. 1A). A less alinear pattern with decreased extramyocyte space was seen. These observations with hematoxylin staining were similar to previous data quantified in a training study from our laboratory using the Fischer-344 strain of rat (37). Exercise had no discernable effect of remodeling in the young age group.
Figure 1.
A, B) Examination of remodeling, extramyocyte space, and collagen location in the LV using hematoxylin (A) and Masson's trichrome staining (B) in young (6 mo) and old (34 mo) sedentary (YS, OS) and exercise-trained (YE, OE) FBNF1 rats. Unstained areas indicate extramyocyte space for hematoxylin staining (A); unstained areas are dark gray for Masson's trichrome imaging (B). C) Percentage collagen area. aP < 0.05 vs. YS; bP < 0.05 vs. YE; cP < 0.05 vs. OS.
To determine whether age- and exercise-related differences in extramyocyte space were related indeed to changes in the ECM structural proteins such as collagen, we used initially Masson's trichrome staining to visualize fibrillar collagen that might be located in the extramyocyte space. We found greater positive staining for total collagen (blue) in LV samples from the OS rats compared with LV s from young rats (Fig. 1B). Similar to the hematoxylin stains, a web-like geometric pattern was also evident in the LVs from OS rats for collagen. Collagen-positive staining in the OS group was in stark contrast with the clear linear or sheath-like patterns for myocyte “pods” observed in the YS and YE groups. Remarkably, exercise training substantially reduced or mitigated age-related elevation in collagen-positive staining (Fig. 1B). Clear protection against age-related alterations in collagen fiber network ultrastructure was observed.
We also quantified collagen area in the LV by analyzing Masson's trichrome staining using NIH ImageJ. OS rats displayed >5-fold higher collagen-positive staining in the LV compared with YS counterparts (Figs. 1B and 2A), or >25% of the LV. Exercise training did indeed significantly reduce the amount or prevent the accumulation of connective tissue in LVs from the OE group. Indeed, the amount of collagen-positive staining in OE samples was quantified as less than half that observed in the OS group, or 11% of the total area positive for collagen.
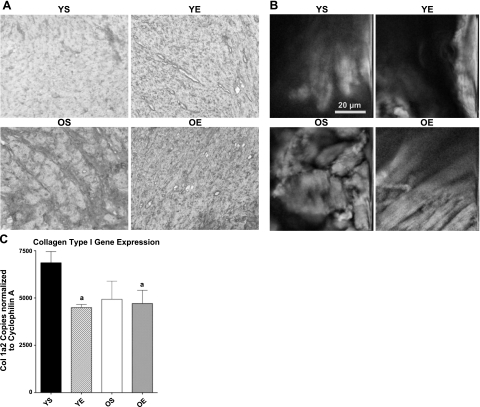
Figure 2.
A) Collagen type I-positive staining is visualized by IHC for YS, YE, OS, and OE FBNF1 rats. B) NLOM merging TPEF and SHG was used to identify collagen fibers. C) cDNA copy number of the collagen type I gene expression was measured by real-time qRT-PCR normalized to cyclophilin A. aP < 0.05 vs. YS.
To test the hypothesis that exercise training reduces accumulation of connective tissue and collagen by reducing fibrous collagen type I, we used IHC with an antibody specific for collagen type I. Very little collagen type I-positive staining was observed in LV samples for both the YS and YE groups (Fig. 2A). In contrast, significant collagen type I-positive staining was visualized in the LV samples from OS rats. However, 12 wk of exercise training resulted in limited collagen type I-positive staining (Fig. 2A), consistent with the notion that exercise protection against fibrosis and accumulation of collagen was related to a reduction in collagen type 1.
Traditional histochemical imaging of connective tissue and collagen requires removal of tissue samples, slicing, embedding and sectioning. A novel imaging technology, NLOM, is designed to characterize intact cardiac microstructure and is particularly sensitive to insoluble proteins such as fibrillar collagen, via merging of TPEF and SHG signals. We used merged TPEF and SHG signaling to image and characterize collagenous, fibrous material in the septum of the heart, which also undergoes significant remodeling with aging (39). Consistent with collagen and ECM staining described above, an increased accumulation of fibrotic tissue was expressed in hearts from OS rats. Substantial alinearity of collagen fibers was also observed in OS hearts (Fig. 2B). In contrast, the OE rats exhibited more linear geometry of fibrous tissue and reduced abundance of collagen fibers (Fig. 2B). These data are consistent with the hypothesis that exercise training blunts or reverses remodeling, collagen accumulation, and fibrosis in the aging heart.
To determine whether lower levels of collagen and fibrous tissue in the aging heart were related to a reduced signal for synthesis, we tested the hypothesis that exercise training would reduce collagen type I mRNA. If habitual exercise reverses age-related increases in collagen production, then it would be expected to reduce gene expression of collagen type I in old rats. However, we found that collagen gene expression was in fact lower in hearts of old rats overall compared with young rats (Fig. 2C). In addition, both exercise groups expressed less collagen mRNA than the YS group (Fig. 2C) suggesting reduced signal for collagen synthesis in response to exercise.
Exercise training effects on MMP dysregulation in the aging heart
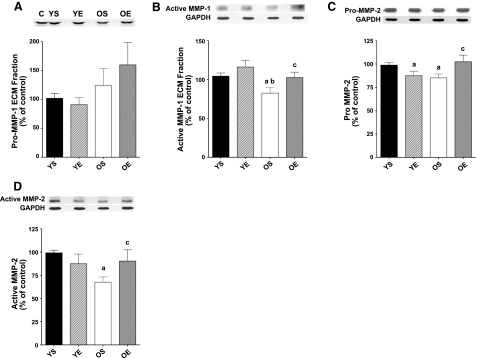
If exercise exhibits its protective effects in the hearts of old rats by modulating collagen degradation rather than synthesis, then an effect on MMP activation is expected. Therefore, we sought to determine whether the protective effect of exercise training against age-related collagen accumulation and fibrosis could be related to changes and regulation MMPs. Protein expression for MMP-1, MMP-2, MMP-3, MMP-9, and MMP-14 was assessed in LV samples. Pro-, cleaved (i.e., active) MMP forms were measured, in the soluble and ECM fractions. No significant changes in pro-MMP-1 were found in the ECM fraction (Fig. 3A). However, hearts from old, sedentary rats exhibited significantly lower (−21%) protein levels for active MMP-1 in the ECM fraction (Fig. 3B). Exercise training resulted in a significant higher (+24%) expression for active MMP-1 (ECM fraction) in the OE group compared with LVs from OS rats. Pro-MMP-2 protein levels in the LV were significantly lower with age (−14%) (Fig. 3C). Exercise training resulted in an increase in pro-MMP-2 (+20%) in the old age group, while MMP-2 levels were 11% lower in YE compared with YS rats. Active MMP-2 protein levels were substantially lower in OS vs. YS rats (−32%). Exercise training in the old group resulted in a significant elevation of active MMP-2 (+34%) (Fig. 3D). No exercise-induced alterations for active MMP-2 were found in young rats.
Figure 3.
Protein expression for MMPs pro-MMP-1 (A), active MMP-1 (B), pro-MMP-2 (C), and active MMP-2 (D) using Western immunoblotting in LV samples from YS, YE, OS, and OE FBNF1 rats. C, control. Data are expressed as means ± se. Matched controls or GAPDH blots are displayed with the protein blots. aP < 0.05 vs. YS; bP < 0.05 vs. YE; cP < 0.05 vs. OS.
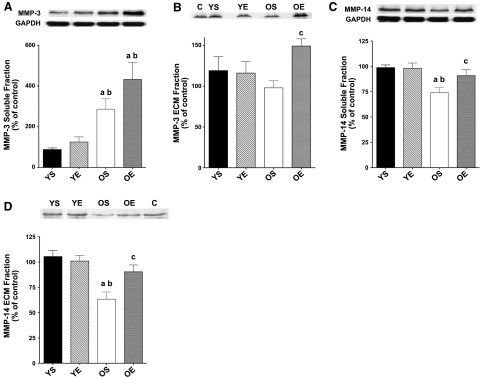
MMP-3 protein expression in the soluble fraction was elevated (+225%) in the LV of old rats (Fig. 4A). However, no significant changes with exercise were found compared with the sedentary groups. Active MMP-3 in the ECM fraction trended lower with aging (−18%), but this trend did not reach statistical significance. Exercise training resulted in a significant up-regulation of active MMP-3 protein expression (+52%) in the ECM fraction for the OE group (Fig. 4B). Again, exercise had no significant effect on active MMP-3 in the YE group. No significant alterations with age or exercise training were found for MMP-9 (data not shown). Soluble MMP-14 protein expression was significantly lower (−25%) in LVs from OS rats compared with YS counterparts (Fig. 4C). Exercise training significantly ameliorated age-associated diminishment of MMP-14. Indeed, soluble MMP-14 was 23% greater in OE compared with OS hearts (Fig. 4C). MMP-14 in the connective tissue fraction was also 40% lower in LV samples from OS rats. However, exercise training resulted in a 43% elevation of MMP-14 protein expression in the ECM fraction in the OE group (Fig. 4D). In contrast, exercise training had no effect on ECM MMP-14 in the YE group.
Figure 4.
Protein expression for soluble fraction MMP-3 (A), ECM fraction MMP-3 (B), soluble fraction MMP-14 (C), and ECM fraction MMP-14 (D) in LV samples from YS, YE, OS, and OE FBNF1 rats. C, control. Data are expressed as means ± se. aP < 0.05 vs. YS; bP < 0.05 vs. YE; cP < 0.05 vs. OS.
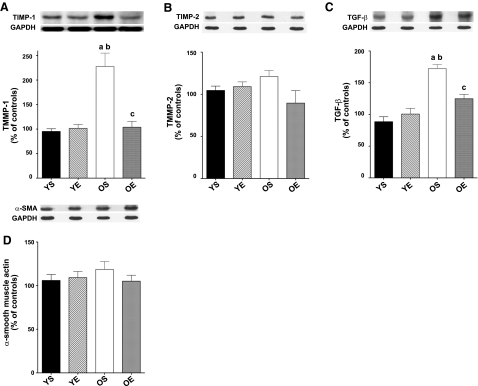
To determine whether exercise training might modulate an up-regulation of MMPs in LVs from OE rats, we tested the hypothesis that exercise training would down-regulate TIMPs. Specifically we measured potential changes in TIMP-1, TIMP-2, TIMP-3, and TIMP-4 as a function of age and exercise training in LV. We found that TIMP-1 protein expression was markedly elevated (+139%) in the LV of OS rats (Fig. 5A). Consistent with our hypothesis, 12 wk of exercise training significantly reduced TIMP-1 protein levels by 54% in the OE group. No significant effect of exercise was observed in the YE group. While a trend was observed toward a reduction (−26%) in TIMP-2 protein expression (Fig. 5B) in response to exercise training in LV samples from old FBNF1 rats, neither age nor exercise effects reached statistic significance. No significant differences in TIMP-3 and TIMP-4 protein expression were noted as a result of age and exercise training (data not shown).
Figure 5.
A, B) Protein expression for TIMPs TIMP-1 (A) and TIMP-2 (B) in LV samples from YS, YE, OS, and OE FBNF1 rats. C) Protein levels for TGF-β1. D) α-SMA protein expression. Data are expressed as means ± se. aP < 0.05 vs. YS; bP < 0.05 vs. YE; cP < 0.05 vs. OS.
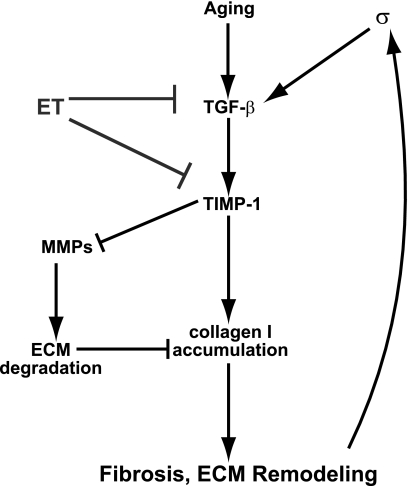
Given that the TIMP-1 appears to be a target of aging and might confer beneficial effects of exercise training via regulation of MMPs, we sought to test upstream modulation of TIMP-1. Transforming growth factor-beta (TGF-β1) is an upstream regulator of TIMP-1 and fibrosis (40). Therefore, we hypothesized that exercise training would ameliorate age-related up-regulation of TGF-β1 protein levels in the rat LV. TGF-β1 levels in the LV were indeed 95% higher in OS rats compared with the YS group (Fig. 5C). However, LV TGF-β1 levels were 28% lower in OE rats that had undergone 12 wk of exercise training compared with the OS group. In contrast, no significant exercise effect for TGF-β1 was observed in the YE group. A model of a prospective signaling pathway whereby exercise training protects against age-induced fibrosis is presented in Fig. 6.
Figure 6.
Integrative signaling model based on our results, indicating prospective targets for exercise training in modulating MMPs and fibrosis.
Myofibroblasts are a differentiated cell type of fibroblast produced from progenitor cells in the heart of circulation in response to cytokines, TGF-β1, and inflammatory mediators (41, 42). Myofibroblasts are prevalent with increased ECM remodeling and protein synthesis, often related to wound healing, scar tissue formation, and growth (42). We, therefore, used α-smooth muscle actin (α-SMC) as a marker of myofibroblast expression (43) in the aging heart to determine whether exercise training reduces myofibroblast proliferation. However, we found that no significant exercise or age effects for α-SMC protein expression in the FBNF1 heart, suggesting that suppression of myofibroblast proliferation is not a mechanism of exercise protection.
DISCUSSION
Our results demonstrate that 12 wk of exercise training in FBNF1 rats significantly ameliorates aging-associated increases in extramyocyte space and collagen-positive staining. In addition, exercise training reduced fibrosis when visualized with collagen type I-positive staining and novel NLOM imaging in the hearts of old rats. Exercise training also alleviated age-related down-regulation of active and connective tissue fraction MMPs MMP-1, MMP-2, MMP-3, and MMP-14. Consistent with the inhibitory effect of TIMP-1 on MMP activation, aging elevated TIMP-1, an effect that was virtually abolished by exercise training. We also observed that exercise training attenuated age-induced up-regulation of TGF-β1, an upstream stimulator of TIMP-1. These results are consistent with the hypothesis that habitual exercise training attenuates age-associated collagen type-I accumulation and fibrosis through a signaling pathway that reduces MMP dysregulation through TIMP-1 and TGF-β1. Indeed, this is the first report to identify potential amelioration of fibrosis in the aged heart by alleviating dysregulation of MMP signaling. A discussion of the primary findings follows.
Collagen accumulation, advanced glycation end product (AGE)-related cross-linking, and fibrosis with aging are progressive and associated with reduced cardiac contractility and risk of heart failure (30). Elevation in fibrotic connective tissue might lead to decreased cardiac compliance and impaired diastolic function, therefore increasing the risk of heart failure cited with aging (30, 44). Previously, we demonstrated that exercise training reduces extramyocyte space in the aging Fischer-344 rat strain (37). Here, we observed substantial increases in total collagen staining, collagen type I-positive staining, fibrosis, and decreased linearity of the collagen network with aging in the heart in FBNF1 rats. Together, these findings are consistent with increases in cardiac collagen type I abundance as an integral part of ECM remodeling in response to the aging process (6, 9, 14).
Exercise training protection against fibrosis and altered cardiac function with aging
Consistent with our hypothesis, we found direct evidence that exercise training attenuates or reverses collagen accumulation, using 3 imaging techniques: Masson's trichrome, collagen type I-specific IHC, and NLOM. In addition, attenuation of ECM space and fibrosis by exercise training was directly related to a reduction in collagen, and specifically collagen type I. Reduction in alinearity and amelioration by exercise training of the weblike geometry characteristic of aging was consistent across imaging approaches and dramatic. This is the first study to visualize the protective effects of chronic exercise on geometric remodeling of collagen that occurs in the aging heart. Our invasive, visual evidence of amelioration of collagen and ECM remodeling by exercise training in the aging heart using NLOM, Masson's trichrome, and collagen type I staining is novel and relevant to potential improvement of cardiac function.
Aging alters the geometry of connective tissue, decreasing linearity of collagenous myocyte sheaths and connective tissue in the heart (9, 16, 17). Hematoxylin and Masson's trichrome stains revealed not only accumulation but also less linear and more weblike geometry of ECM and collagen in the aged heart. We previously found that much of the remodeling of the aging heart occurs toward the endocardial surface (37), important because greater sliding of myocyte sheaths magnifies mechanical stress when impeded (16, 45). Substantial reductions of fibrosis, collagen type I accumulation, and alinearity by exercise training were consistent across imaging techniques. Increased linearity of the collagen network by exercise training may reduce internal work of the aging heart, thus lessening metabolic and blood flow requirements, reducing fibrosis, alleviating arrhythmias, suppressing excess apoptosis, as well as improving function (14, 19, 37).
Previous studies have used biochemical methods to explore the efficacy of exercise training to reduce collagen abundance, HP cross-linking, and alterations in collagen gene expression. Woodiwiss et al. (46) found that 16 wk of habitual voluntary wheel running reduced cardiac stiffness in young rats, without a significant change in collagen or collagen cross-linking. Consistently, we also found no significant changes in collagen-positive imaging with exercise training in young rats. Thomas et al. (6, 29) reported that 10 wk of exercise training reduced collagen cross-linking in the hearts of old Fischer-344 rats, but not in young rats. In a follow-up study, the abundance of collagen per unit dry weight was significantly reduced in the hearts of old Fischer-344 rats (29). Recently, Choi et al. (30) found that exercise training protected against a decline in systolic function, related to an attenuation of decreased solubility of collagen. A mechanism related to lower AGEs was proposed. Long-term exercise training in the aging heart may mimic the cardioprotective effects observed with preconditioning and caloric restriction (47), potentially linked to up-regulation of mitochondrial stress proteins (e.g., MnSOD) and reduced oxidative stress (48).
Studies in human patients suggest a protective role of exercise training on cardiac function, compliance, and, in some cases, diastolic filling. Improvement in diastolic function with exercise training in human studies has been recorded (49, 50). Arbab-Zadeh et al. (44) reported that subjects participating in habitual exercise, with an average age of 70, have significantly less diastolic dysfunction and reduced stiffness during diastole. Prasad et al. (51) found partial protection against age-related abnormalities in filling and relaxation with lifelong exercise. In contrast, Nottin et al. (52) noted that master athletes in their 50s displayed similar changes in LV wall motion during early diastole as the old, sedentary group. Recent findings reviewed by Tanaka and Seals (50) demonstrate that master athletes maintain high cardiovascular function, including compliance and stroke volume, at exceptionally high levels until their 70s. Thus, it is believed that habitual exercise may protect heart function by slowing some of age-induced mechanical remodeling (27, 53).
Exercise alleviation of age-related dysfunction of MMPs
MMPs catalyze degradation of ECM proteins. However, dysregulation of MMPs is now believed to contribute to fibrosis, aging, and heart failure (21, 22, 54). Fedak et al. (21) reported that human heart failure is associated with a large up-regulation of TIMP-1. However, ischemia reperfusion acutely activates MMPs (55). This apparent discrepancy can be explained by differential regulation between aging and hypertension, where MMP activity decreases with aging and increases with hypertension (56). Thus hypertension, ischemia/reperfusion, and heart failure might exhibit acute up-regulation of MMPs, potentially related to scar tissue formation (56). Here, we noted that while the proforms of MMPs either increased (MMP-3) or decreased (MMP-14) with advancing age, the active and ECM forms were consistently reduced by aging (Figs. 3 and 4). These data are consistent with the hypothesis that impaired turnover of ECM proteins might be an important contributory mechanism of accumulation of fibrotic tissue in the heart due to aging.
In our study, exercise training consistently up-regulated active and ECM fraction MMPs, including MMP-1, MMP-2, MMP-3, and MMP-14, mitigating age-related reduction in MMP expression (Figs. 3 and 4). These are highly novel findings that are consistent with a signaling pathway involving MMP regulation eliciting the protective effects of ET against remodeling, collagen accumulation, and fibrosis. MMP-1, MMP-2, MMP-3, and MMP-14 can serve as collagenases and degrade a host of ECM proteins, including aggrecan (MMP-1, MMP-2, and MMP-3), fibronectin (MMP-2, MMP-3, and MMP-14), laminin (MMP-2, MMP-3, and MMP-14), and gelatin (MMP-1, MMP-2, MMP-3, and MMP-14) (10, 18, 57). Elevation of TIMP-1 we observed with aging (Fig. 5A) was indeed consistent with upstream suppression of active and ECM fraction forms of MMP-1, MMP-2, MMP-3, and MMP-14. Furthermore, exercise training substantially reduced TIMP-1 in concert with up-regulation of MMP-1, MMP-2, MMP-3, and MMP-14. Indeed, Thomas et al. (22) reported a significant up-regulation in TIMP-1 and TIMP-2 as a result of heart failure in rats. Fedak et al. (21) postulated that elevation of TIMP-1 and reduction in TIMP-3 contributed to human heart failure. Further, Bonnema et al. (13) found that aging increased TIMP-1, TIMP-4, and MMP-2 profiles, while MMP-9 was lower in the old human heart. Given that TIMP-2, TIMP-3, and TIMP-4 were not responsive to exercise training, these data imply that a TIMP-1/MMP pathway is a viable candidate pathway of exercise protection against age-related fibrosis. In addition, TIMP-1 is revealed as a target candidate for therapeutic development.
Exercise training also mitigated age-associated up-regulation of TGF-β1 in the LV (Fig. 5C). TGF-β1 is a potent stimulator of TIMP-1 and a potential contributor to fibrosis in the aging heart (13, 25, 40). Our data therefore indicate that alleviation of a signaling cascade involving TGF-β1, TIMP-1, and MMPs might be an important mediator of exercise protection against fibrosis as a result of aging. Given increased agreement of the role of TGF-β1 in fibrosis with aging and in the etiology of heart failure (40), the potency of exercise training in attenuating TGF-β1 protein expression has important clinical relevance.
We found that exercise training reduced collagen type I mRNA in the LVs from young rats, but not old (Fig. 2C). This finding was directly related to a downward aging effect in collagen type I gene expression. Indeed, no significant differences were found in Col I mRNA among YE, OS, and OE groups. This is consistent with the notion that aging suppresses collagen I production in the heart, rather than elevating synthesis. In addition, exercise training was not effective at altering collagen I gene expression in the hearts of old rats (Fig. 2C). Previously, Thomas et al. (6) reported that exercise training reduced the decline in LV mRNAs for collagen type I and collagen type III in middle-aged rats. However, they found that exercise was less effective in alleviating reduction of collagen mRNAs in senescent rats. Together, these data are inconsistent with the notion that exercise confers protection against fibrosis and collagen accumulation in the aging heart via a reduction in protein synthesis for type I collagen.
The lack of response of a myofibroblast marker (α-SMC) to exercise and age also indicates that reduction in scar tissue formation was not a likely mechanism of exercise protection in the aged heart. Therefore, it is increasingly likely that exercise protection observed in the current study was related to alterations in pathway signaling (e.g., MMPs–TIMP-1–TGF-β1) that elevate collagen and ECM protein degradation.
Clinical relevance
The clinical importance of habitual exercise in the protection of cardiac function and health is profound. Physical inactivity, similar to aging, results in diminished cardiac function and exacerbates the risk of heart disease (58). Indeed, inactivity appears to accelerate cardiac aging, and is a major determinant in the reduction in cardiac function in older adults (26, 27, 59). Exercise training has been shown to improve maximal aerobic capacity and heart function in older populations, related to improvement in mechanical properties (50). Exercise training also protects against future damage, up-regulation of type I collagen, apoptosis, and impaired mechanical function related to myocardial infarction (60). Notably, exercise training appears to retain its ability to reduce muscle damage, apoptosis, impaired contractile function, and altered mechanical properties as a result of ischemia/reperfusion injury (26, 61, 62). Lack of an intermediate age group is a study limitation that would establish age-related changes as true aging effects, and would identify a potential threshold for fibrosis. Continued investigation into the mechanisms of exercise protection is vital, and will identify therapeutic targets to mitigate fibrosis, cardiovascular disease, and heart failure prevalent with advancing age.
Acknowledgments
Funding for this study was provided by support from the American Heart Association (0555064Y, 0855158F), the U.S. National Institutes of Health (AR054084), and the Sydney and J. L. Huffines Institute for Sports Medicine.
REFERENCES
- 1. Centurione, Di L., Giulio C., Cacchio M., Rapino M., Bosco D., Grifone G., Sabatini N., Bianchi G., Antonucci A., Cataldi A. (2005) Correlations between protein kinase c (zeta) signaling and morphological modifications during rat heart development and aging. Mech. Ageing Dev. 124, 957–966 [DOI] [PubMed] [Google Scholar]
- 2. Capasso J. M., Palackal T., Olivetti G., Anversa P. (1990) Severe myocardial dysfunction induced by ventricular remodeling in aging rat hearts. Am. J. Physiol. 259, H1086–H1096 [DOI] [PubMed] [Google Scholar]
- 3. Gielen S., Adams V., Niebauer J., Schuler G., Hambrecht R. (2005) Aging and heart failure–similar syndromes of exercise intolerance? Implications for exercise-based interventions. Heart Fail. Monit. 4, 130–136 [PubMed] [Google Scholar]
- 4. Pugh K. G., Wei J. Y. (2001) Clinical implications of physiological changes in the aging heart. Drugs Aging 18, 263–276 [DOI] [PubMed] [Google Scholar]
- 5. Stewart S., MacIntyre K., Capewell S., McMurray D. (2003) Heart failure and the aging population: an increasing burden in the 21st century? Heart 89, 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas D. P., Zimmerman S. D., Hansen T. R., Martin D. T., McCormick R. J. (2000) Collagen gene expression in rat left ventricle: interactive effect exercise training. J. Appl. Physiol. 89, 1462–1468 [DOI] [PubMed] [Google Scholar]
- 7. Berlew B. S. Diastolic dysfunction in the elderly—the interstitial issue. (2004) Am. J. Geriatr. Cardiol. 13, 29–38 [DOI] [PubMed] [Google Scholar]
- 8. Bing O. H., Conrad C. H., Boluvt M. O., Robinson R. G., Brooks W. W. (2002) Studies of prevention, treatment, and mechanisms of heart failure in the aging spontaneously hypertensive rat. Heart Failure Rev. 7, 71–88 [DOI] [PubMed] [Google Scholar]
- 9. Masson S., Latinim R., Saliom M., Fiordaliso F. (2005) Cardiac fibrosis and aging. In Fibrogenesis: Cellular and Molecular Basis (Razzaque M.S., ed.) pp 97–103, Kluwer Academic Publishers, New York [Google Scholar]
- 10. Goldsmith E. C., Borg T. K. (2002) The dynamic interaction of the extracellular matrix in cardiac remodeling. J. Card. Fail. 8, S314–S318 [DOI] [PubMed] [Google Scholar]
- 11. Aumailley M., Gayraud B. (1998) Structure and biological activity of the extracellular matrix. J. Mol. Med. 76, 253–265 [DOI] [PubMed] [Google Scholar]
- 12. Kassiri Z., Khokha R. (2005) Myocardial extra-cellular matrix and its regulation by metalloproteinases and their inhibitors. Thromb. Haemost. 93, 212–219 [DOI] [PubMed] [Google Scholar]
- 13. Bonnema D. D., Webb C. S., Pennington W. R., Stroud R. E., Leonardi A. E., Clark L. L., McClure C. D., Finklea L., Spinale F. G., Zile M. R. (2007) Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J. Card. Fail. 13, 530–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eghbali M., Eghbali M., Robinson T. F., Seifter S., Blumenfeld O. O. (1989) Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc. Res. 23, 723–729 [DOI] [PubMed] [Google Scholar]
- 15. Camelliti P., Borg T. K., Kohl P. (2005) Structural and functional characterization of cardiac fibroblasts. Cardiovasc. Res. 65, 40–51 [DOI] [PubMed] [Google Scholar]
- 16. DeSouza R. R. (2002) Aging of myocardial collagen. Biogerontology 3, 325–335 [DOI] [PubMed] [Google Scholar]
- 17. Gazoti Debessa C. R., Mesiano Malfrino L. B., Rodrigues de Souza R. (2001) Age related changes of the collagen network of the human heart. Mech. Ageing Dev. 122, 1049–1058 [DOI] [PubMed] [Google Scholar]
- 18. Jugdutt B. I. (2003) Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Curr. Drug Targets Cardiovasc. Haematol. Disord. 3, 1–30 [DOI] [PubMed] [Google Scholar]
- 19. Tsuruda T., Costello-Boerrigter L. C., Burnett J. C. (2004) Matrix metalloproteinases: pathways of induction by bioactive molecules. Heart Fail. Rev. 9, 53–61 [DOI] [PubMed] [Google Scholar]
- 20. Van Linthout S., Seeland U., Riad A., Eckhardt O., Hohl M., Dhayat N., Richter U., Fischer J. W., Böhm M., Pauschinger M., Schultheiss H. P., Tschöpe C. (2008) Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol. 103, 319–327 [DOI] [PubMed] [Google Scholar]
- 21. Fedak P. W., Altamentova S. M., Weisel R. D., Nili N., Ohno N., Verma S., Lee T. Y., Kiani C., Mickle D. A., Strauss B. H., Li R. K. (2003) Matrix remodeling in experimental and human heart failure: a possible regulatory role for TIMP-3. Am. J. Physiol. 284, H626–H634 [DOI] [PubMed] [Google Scholar]
- 22. Thomas C. V., Coker M. L., Zellner J. L., Handy J. R., Crumbley A. J., Spinale F. G. (1998) Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation 97, 1708–1715 [DOI] [PubMed] [Google Scholar]
- 23. Lovelock J. D., Baker A. H., Dong J. F., Bergeron A. L., McPheat W., Sivasubrananian N., Mann D. L. (2005) Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am. J. Physiol. 288, H461–H468 [DOI] [PubMed] [Google Scholar]
- 24. Tikellis C., Cooper M. E., Twigg S. M., Bursn W. C., Tolcos M. (2004) Connective tissue growth factor is up-regulated in the diabetic retina: amelioration by angiotensin-converting enzyme inhibition. Endocrinology 145, 860–866 [DOI] [PubMed] [Google Scholar]
- 25. Tsutsui H., Matsushima. S., Kinugawa S., Ide T., Inoue N., Ohta Y., Yokota T., Hamaguchi S., Sunagawa K. (2007) Angiotensin II type 1 receptor blocker attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Hypertens. Res. 30, 439–449 [DOI] [PubMed] [Google Scholar]
- 26. Taylor R. P., Starnes J. W. (2003) Age, cell signaling and cardioprotection. Acta Physiol. Scand. 178, 107–116 [DOI] [PubMed] [Google Scholar]
- 27. Goldspink D. F. (2005) Ageing and activity: their effects on the functional reserve capacities of the heart and vascular smooth and skeletal muscles. Ergonomics 48, 1334–1351 [DOI] [PubMed] [Google Scholar]
- 28. Deley G., Kervio G., Van Hoecke J., Verges B., Grassi B., Casillas J. M. (2007) Effects of a one-year exercise training program in adults over 70 years old: a study with a control group. Aging Clin. Ex. Res. 19, 310–315 [DOI] [PubMed] [Google Scholar]
- 29. Thomas D. P., Cotter T. A., Li X., McCormick J., Gosselin L. E. (2001) Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. Eur. J. Appl. Physiol. 85, 164–169 [DOI] [PubMed] [Google Scholar]
- 30. Choi S. Y., Chang H. J., Choi S. I., Kim K. I., Cho Y. S., Youn T. J., Chung W. Y., Chae I. H., Choi D. J., Kim H. S., Kim C. H., Oh B. H., Kim M. H. (2009) Long-term exercise training attenuates age-related diastolic dysfunction: association of myocardial collagen cross-linking. J. Korean Med. Sci. 24, 32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olfert I. M., Balouch J., Mathieu-Costello O. (2004) Oxygen consumption during maximal exercise in Fischer 344 x Brown Norway F1 hybrid rats. J. Gerontol. Biol. Sci. Med. Sci. 59, 801–808 [DOI] [PubMed] [Google Scholar]
- 32. Kwak H. B., Kim J. H., Lawler J. M. (2008). Responses of caspase-8 and caspase-12 pathways to 12 weeks of exercise training in aging rat skeletal muscle. FASEB J. 22, 753.7 [Google Scholar]
- 33. Chung E., Dorton B. J., Diffee G. M. (2006) Regional myosin heavy chain isoform expression in response to exercise training in old rat myocardium. FASEB J. 20, A1447 [Google Scholar]
- 34. Zheng D., MacLean P. S., Pohnert S. C., Knight J. B., Olson A. L., Winder W. W., Dohm G. L. (2001) Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J. Appl. Physiol. 91, 1073–1083 [DOI] [PubMed] [Google Scholar]
- 35. Kim J. H., Kwak H. B., Leeuwenburgh C., Lawler J. M. (2008) Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp. Gerontol. 43, 317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee P. F., Yeh A. T., Bayless K.J. (2009) Nonlinear optical microscopy reveals invading endothelial cells anisotropically alter three-dimensional collagen matrices. Exp. Cell. Res. 315, 396–410 [DOI] [PubMed] [Google Scholar]
- 37. Kwak H.-B., Song W., Lawler J. M. (2006) Exercise-training ameliorates age- induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the aging rat heart. FASEB J. 20, 791–793 [DOI] [PubMed] [Google Scholar]
- 38. Martinez D. A., Vailas A. C., Vanderby R., Jr., Grindeland R. E. (2007) Temporal extracellular matrix adaptations in ligament during wound healing and hindlimb unloading. Am. J. Physiol. 293, R1552–R1560 [DOI] [PubMed] [Google Scholar]
- 39. Hwang H. S., Cirrincione G., Thomas D. P., McCormick R. J., Boluyt M. O. (2007) Aldosterone antagonism fails to attenuate age-associated left ventricular fibrosis. J. Gerontol. A Biol. Sci. Med. Sci. 62, 382–388 [DOI] [PubMed] [Google Scholar]
- 40. Chen M. M., Lam A., Judith A., Abraham J. A., Schreiner G. F., Joly A. H. (2000) CTGF expression is induced by TGF-β in cardiac fibroblasts and cardiac yocytes: a potential role in heart fibrosis. J. Mol. Cell. Cardiol. 32, 1805–1819 [DOI] [PubMed] [Google Scholar]
- 41. Gabbiani G. (2003) The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500–503 [DOI] [PubMed] [Google Scholar]
- 42. Tomasek J. J., Gabbiani G., Hinz B., Chaponnier C., Brown R. A. (2002) Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat. Rev. Mol. Cell. Biol. 3, 349–363 [DOI] [PubMed] [Google Scholar]
- 43. Serini G., Gabbiani G. (1999) Mechanisms of myofibroblast activity and phenotypic modulation. Exp. Cell Res. 250, 273–283 [DOI] [PubMed] [Google Scholar]
- 44. Arbab-Zadeh A., Dijk E., Prasad A., Fu Q., Torres P., Zhang. R., Thomas J.D., Palmer D., Levine BD. (2004) Effect of aging and physical activity on left ventricular compliance. Circulation 110, 1799–1805 [DOI] [PubMed] [Google Scholar]
- 45. Lumens J., Delhaas T., Arts T., Cowan B. R., Young A. A. (2006) Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using MRI. Am. J. Physiol. 291, H1573–1579 [DOI] [PubMed] [Google Scholar]
- 46. Woodiwiss A. J., Oosthuyse T., Norton G. R. (1998) Reduced cardiac stiffness following exercise is associated with preserved myocardial collagen characteristics in the r at. Eur. J. Appl. Physiol. 78, 148–154 [DOI] [PubMed] [Google Scholar]
- 47. Rohrbach S., Niemann B., Abushouk A. M. A., Holtz J. (2006) Caloric restriction and mitochondrial function in the aging myocardium. Exp. Gerontol. 41, 525–531 [DOI] [PubMed] [Google Scholar]
- 48. Lawler J. M., Kwak H.-B., Kim J.-H., Suk M.-H. (2009) Exercise training upregulates MnSOD while reducing pro-oxidant signaling in the aging rat left ventricle. Am. J. Physiol. 296, R1496–R1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takemoto K. A., Bernstein L., Lopez J. F., Marshak D., Rahimtoola S. H., Chandraratna P. A. (1992) Abnormalities of diastolic filling of the left ventricle associated with aging are less pronounced in exercise-trained individuals. Am. Heart. J. 124, 143–148 [DOI] [PubMed] [Google Scholar]
- 50. Tanaka H., Seals D.R. (2008) Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J. Physiol. 586, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prasad A., Popovic Z. B., Arbab-Zadeh. A., Fu Q., Palmer D., Dijk E., Greenberg N. L., Garcia M. J., Thomas J. D., Levine B. D. (2007) The effects of aging and physical activity on Doppler measures of diastolic function. Am. J. Cardiol. 99, 1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nottin S., Nguyen L. D., Terbah M., Obert P. (2004) Long-term endurance training does not prevent the age-related decrease in left ventricular relaxation properties. Acta Physiol. Scand. 181, 209–215 [DOI] [PubMed] [Google Scholar]
- 53. Torella D., Rota M., Nurzynska D., Musso E., Monsen A., Shiraishi I., Zias E., Walsh K., Rosenzweig A., Sussman M. A., Urbanek K., Nadal-Ginard B., Kanjstura J., Anversa P., Leri A. (2004) Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circul. Res. 94, 514–524 [DOI] [PubMed] [Google Scholar]
- 54. Sivasubramanian N., Coker M. L., Kurrelmeyer K. M., MacLellan W. R., DeMayo F. J., Spinale F. G., Mann D. L. (2001) Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 104, 826–831 [DOI] [PubMed] [Google Scholar]
- 55. Lalu M. M., Pasini E., Schulze C. J., Ferrari-Vivaldi M., Ferrari-Vivaldi G., Bachetti T., Schulz R. (2005) Ischaemia-reperfusion injury activates matrix metalloproteinases in the human heart. Eur. Heart. J. 26, 27–35 [DOI] [PubMed] [Google Scholar]
- 56. Robert V., Besse S., Sabr,i A., Silvestre J. S., Assayag P., Nguyen V. T., Swynghedauw B, Delcayre C. (1997) Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab. Invest. 76, 729–738 [PubMed] [Google Scholar]
- 57. Vincenti M. P. (2001) The matrix metalloproteinase (MMP) and tissue inhibitor metalloproteinase (TIMP) genes. Matrix Metalloproteinase Protoc. Springer Protoc. 151, 5. [DOI] [PubMed] [Google Scholar]
- 58. Booth F. W., Gordon S., Carlson C. J., Hamilton M. T. (2000) Waging war on modern chronic diseases: primary prevention through exercise biology. J. Appl. Physiol. 88, 774–787 [DOI] [PubMed] [Google Scholar]
- 59. Lunardi M., Galetta F., Volterrani C., Giaconi A., Azzarelli A., Bernardi D., Giusti C. (1993) The effect of physical exercise on the response to exertion in the elderly. G. Ital. Cardiol. 23, 673–677 [PubMed] [Google Scholar]
- 60. Jin H., Yang R., Li W., Ryan A. M., Ogasawara A. K., Van Pebrorgh J., Paoni N. F. (2002) Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am. J. Physiol. 279, H2994–H3002 [DOI] [PubMed] [Google Scholar]
- 61. Quindry J., French J., Hamilton K., Lee Y., Mehta J. L., Powers S. K. (2005) Exercise training provides cardioprotection against ischemia-reperfusion induced apoptosis in young and old animals. Exp. Gerontol. 40, 416–425 [DOI] [PubMed] [Google Scholar]
- 62. Starnes J. W., Taylor R. P., Park Y. (2003) Exercise improves postischemic function in aging hearts. Am. J. Physiol. 285, H347–H351 [DOI] [PubMed] [Google Scholar]