Abstract
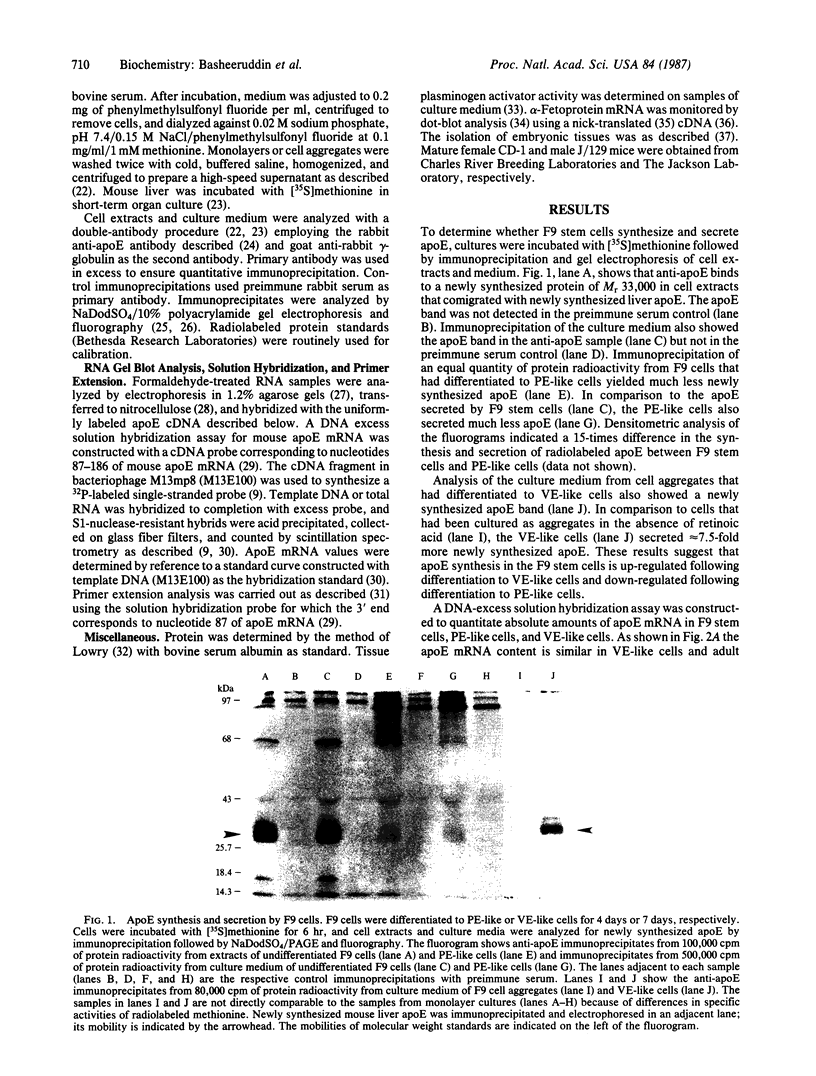
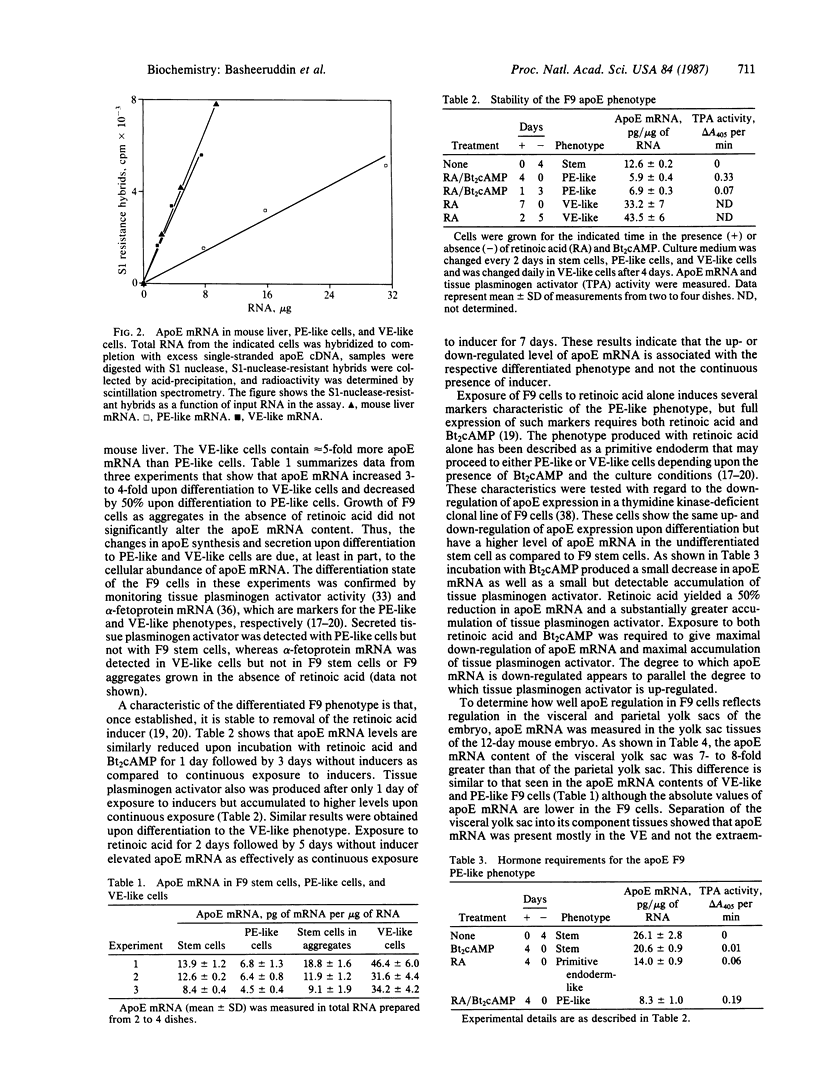
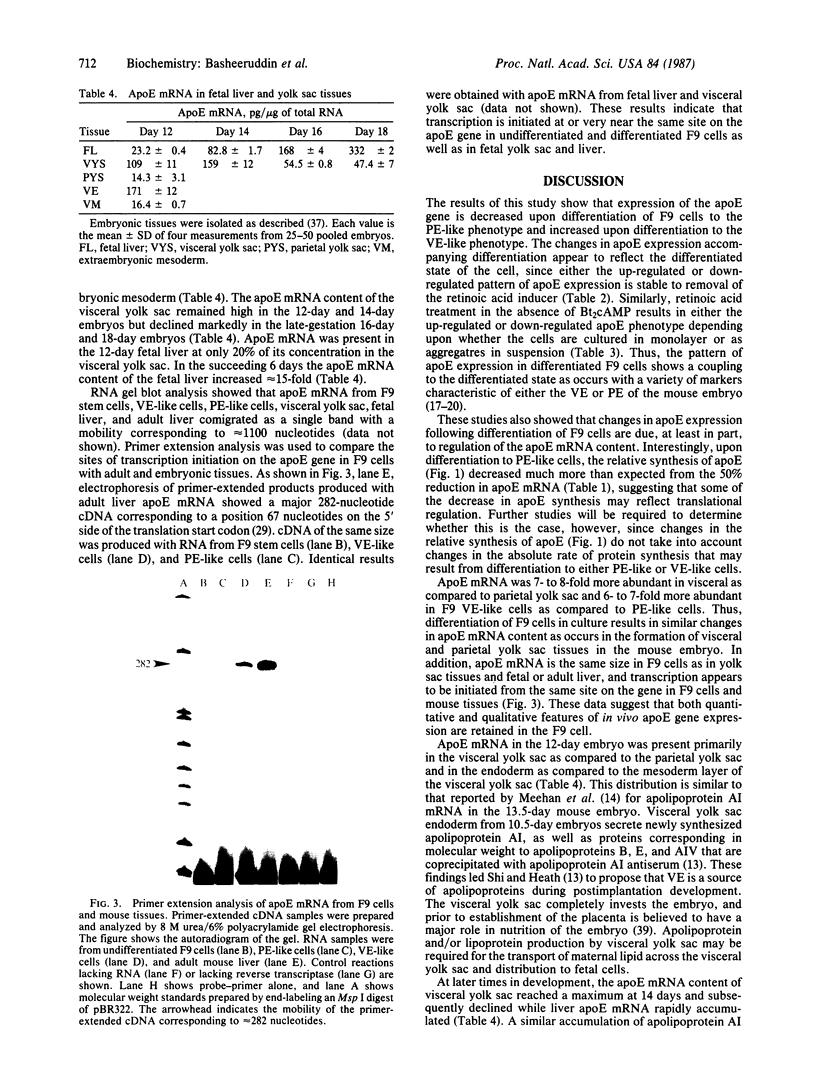
Apolipoprotein E (apoE) expression was studied in F9 embryonal carcinoma cells that differentiate in response to retinoic acid into cells resembling either parietal endoderm or visceral endoderm of the parietal or visceral yolk sac. F9 cells secreted newly synthesized apoE when incubated with radiolabeled amino acid. Upon differentiation to parietal endoderm-like cells, apoE synthesis and secretion were markedly down-regulated. In contrast, apoE secretion was up-regulated upon differentiation to visceral endoderm-like cells. These changes in apoE expression were due, at least in part, to regulation of apoE mRNA abundance since visceral endoderm-like cells contained 5- to 7-fold more apoE mRNA than parietal endoderm-like cells. The apoE phenotype reflected the differentiated state of the F9 cell since either the up-regulated or down-regulated pattern was stable to the removal of the retinoic acid inducer. To determine how well apoE regulation in F9 cells reflects regulation in visceral and parietal yolk sacs, apoE mRNA was measured in yolk sac tissues of the 12-day mouse embryo. Visceral yolk sac contained 109 +/- 11 pg of apoE mRNA per micrograms of RNA while parietal yolk sac contained 14.3 +/- 3.1 pg/micrograms of RNA. This 7- to 8-fold difference is similar to the difference in the apoE mRNA contents of visceral endoderm-like and parietal endoderm-like F9 cells. RNA gel blot analysis showed that apoE mRNA is the same size in F9 cells as in yolk sac tissues and fetal or adult liver. In addition, primer-extension analysis showed that transcription is initiated at or near the same site on the apoE gene in F9 cells and mouse tissues. These data suggest that both quantitative and qualitative features of apoE gene expression in development are retained in the F9 cell. The F9 cell should provide a useful system to study the developmental activation of endogenous apolipoprotein genes as well as exogenous apolipoprotein genes introduced by transfection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade-Gordon P., Strickland S. Interaction of heparin with plasminogen activators and plasminogen: effects on the activation of plasminogen. Biochemistry. 1986 Jul 15;25(14):4033–4040. doi: 10.1021/bi00362a007. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Havel R. J., Goldstein J. L. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7545–7549. doi: 10.1073/pnas.78.12.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Blue M. L., Ostapchuk P., Gordon J. S., Williams D. L. Synthesis of apolipoprotein AI by peripheral tissues of the rooster. A possible mechanism of cellular cholesterol efflux. J Biol Chem. 1982 Sep 25;257(18):11151–11159. [PubMed] [Google Scholar]
- Blue M. L., Williams D. L., Zucker S., Khan S. A., Blum C. B. Apolipoprotein E synthesis in human kidney, adrenal gland, and liver. Proc Natl Acad Sci U S A. 1983 Jan;80(1):283–287. doi: 10.1073/pnas.80.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dawson P. A., Schechter N., Williams D. L. Induction of rat E and chicken A-I apolipoproteins and mRNAs during optic nerve degeneration. J Biol Chem. 1986 May 5;261(13):5681–5684. [PubMed] [Google Scholar]
- Driscoll D. M., Getz G. S. Extrahepatic synthesis of apolipoprotein E. J Lipid Res. 1984 Dec 1;25(12):1368–1379. [PubMed] [Google Scholar]
- Elshourbagy N. A., Boguski M. S., Liao W. S., Jefferson L. S., Gordon J. I., Taylor J. M. Expression of rat apolipoprotein A-IV and A-I genes: mRNA induction during development and in response to glucocorticoids and insulin. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8242–8246. doi: 10.1073/pnas.82.23.8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy N. A., Liao W. S., Mahley R. W., Taylor J. M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci U S A. 1985 Jan;82(1):203–207. doi: 10.1073/pnas.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. L. Origin and differentiation of extraembryonic tissues in the mouse. Int Rev Exp Pathol. 1983;24:63–133. [PubMed] [Google Scholar]
- Gmür R., Solter D., Knowles B. B. Independent regulation of H-2K and H-2D gene expression in murine teratocarcinoma somatic cell hybrids. J Exp Med. 1980 Jun 1;151(6):1349–1359. doi: 10.1084/jem.151.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS M. L., HARKNESS R. D. Changes in the physical properties and in the collagen and hexosamine contents of the foetal membranes during pregnancy in the rat. J Physiol. 1956 Jun 28;132(3):482–491. doi: 10.1113/jphysiol.1956.sp005541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L., Taylor A., Adamson E. Cell interactions modulate embryonal carcinoma cell differentiation into parietal or visceral endoderm. Nature. 1981 May 21;291(5812):235–237. doi: 10.1038/291235a0. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Hammer R. E., Tilghman S. M., Brinster R. L. Developmental regulation of alpha-fetoprotein genes in transgenic mice. Mol Cell Biol. 1985 Jul;5(7):1639–1648. doi: 10.1128/mcb.5.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Meehan R. R., Barlow D. P., Hill R. E., Hogan B. L., Hastie N. D. Pattern of serum protein gene expression in mouse visceral yolk sac and foetal liver. EMBO J. 1984 Aug;3(8):1881–1885. doi: 10.1002/j.1460-2075.1984.tb02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman T. C., Dawson P. A., Rudel L. L., Williams D. L. Quantitation of apolipoprotein E mRNA in the liver and peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2452–2457. [PubMed] [Google Scholar]
- Rajavashisth T. B., Kaptein J. S., Reue K. L., Lusis A. J. Evolution of apolipoprotein E: mouse sequence and evidence for an 11-nucleotide ancestral unit. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8085–8089. doi: 10.1073/pnas.82.23.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reue K. L., Quon D. H., O'Donnell K. A., Dizikes G. J., Fareed G. C., Lusis A. J. Cloning and regulation of messenger RNA for mouse apolipoprotein E. J Biol Chem. 1984 Feb 25;259(4):2100–2107. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shelburne F., Hanks J., Meyers W., Quarfordt S. Effect of apoproteins on hepatic uptake of triglyceride emulsions in the rat. J Clin Invest. 1980 Mar;65(3):652–658. doi: 10.1172/JCI109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelness G. S., Williams D. L. Apolipoprotein II messenger RNA. Transcriptional and splicing heterogeneity yields six 5'-untranslated leader sequences. J Biol Chem. 1984 Aug 10;259(15):9929–9935. [PubMed] [Google Scholar]
- Sherrill B. C., Innerarity T. L., Mahley R. W. Rapid hepatic clearance of the canine lipoproteins containing only the E apoprotein by a high affinity receptor. Identity with the chylomicron remnant transport process. J Biol Chem. 1980 Mar 10;255(5):1804–1807. [PubMed] [Google Scholar]
- Shi W. K., Heath J. K. Apolipoprotein expression by murine visceral yolk sac endoderm. J Embryol Exp Morphol. 1984 Jun;81:143–152. [PubMed] [Google Scholar]
- Shi W. K., Hopkins B., Thompson S., Heath J. K., Luke B. M., Graham C. F. Synthesis of apolipoproteins, alphafoetoprotein, albumin, and transferrin by the human foetal yolk sack and other foetal organs. J Embryol Exp Morphol. 1985 Feb;85:191–206. [PubMed] [Google Scholar]
- Stein P., Barra Y., Jay G., Strickland S. Expression of a secreted transplantation antigen gene during murine embryogenesis. Mol Cell Biol. 1986 Oct;6(10):3397–3400. doi: 10.1128/mcb.6.10.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland S. Mouse teratocarcinoma cells: prospects for the study of embryogenesis and neoplasia. Cell. 1981 May;24(2):277–278. doi: 10.1016/0092-8674(81)90313-5. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Williams D. L., Dawson P. A. Immunochemical measurement of apolipoprotein synthesis in cell and organ culture. Methods Enzymol. 1986;129:254–271. doi: 10.1016/0076-6879(86)29074-6. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Dawson P. A., Newman T. C., Rudel L. L. Apolipoprotein E synthesis in peripheral tissues of nonhuman primates. J Biol Chem. 1985 Feb 25;260(4):2444–2451. [PubMed] [Google Scholar]
- Williams D. L., Newman T. C., Shelness G. S., Gordon D. A. Measurement of apolipoprotein mRNA by DNA-excess solution hybridization with single-stranded probes. Methods Enzymol. 1986;128:671–689. doi: 10.1016/0076-6879(86)28099-4. [DOI] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Determinants of hepatic uptake of triglyceride-rich lipoproteins and their remnants in the rat. J Biol Chem. 1980 Jun 10;255(11):5475–5480. [PubMed] [Google Scholar]
- Yeoh G. C., Morgan E. H. Albumin and transferrin synthesis during development in the rat. Biochem J. 1974 Nov;144(2):215–224. doi: 10.1042/bj1440215. [DOI] [PMC free article] [PubMed] [Google Scholar]