Abstract
The study aim was to determine whether low level exposure to organophosphate pesticides (OPs) causes neuropsychological or psychiatric impairment. Methodological weaknesses of earlier studies were addressed by: recruiting participants who had retired on ill health grounds; excluding participants with a history of acute poisoning, medical or psychiatric conditions that might account for ill health; and exploring factors which may render some individuals more vulnerable to the effects of OPs than others. Performance on tests of cognition and mood of 127 exposed sheep farmers (67 working, 60 retired) was compared with 78 unexposed controls (38 working, 40 retired) and published test norms derived from a cross section of several thousand adults in the general population. Over 40% of the exposed cohort reported clinically significant levels of anxiety and depression compared to less than 23% of controls. Exposed subjects performed significantly worse than controls and standardisation samples on tests of memory, response speed, fine motor control, mental flexibility and strategy making, even after controlling for the effects of mood. The pattern was similar for both working and retired groups. The cognitive deficits identified cannot be attributed to mood disorder, malingering, a history of acute exposure or genetic vulnerability in terms of PON1192 polymorphisms. Results suggest a relationship may exist between low level exposure to organophosphates and impaired neurobehavioural functioning and these findings have implications for working practice and for other occupational groups exposed to OPs such as aviation workers and Gulf War veterans.
Keywords: Organophosphates, Neurobehavioural, Neuropsychological, Cognitive dysfunction, Farm workers
1. Introduction
Organophosphates (OPs) are the most widely used group of pesticides and insecticides in the world and are used for a variety of agricultural and domestic purposes. They are also used by industry as solvents, plasticizers, flame retardants and extreme pressure additives (e.g. lubricants) and by the military as pesticides and nerve agents. This means that a very large number of people will be exposed to these chemicals in some form, during their lifetime. OPs are one of the most common causes of poisoning worldwide [40] and questions have been raised about the long term effects these chemicals may have on human health. The immediate effects of high-level exposure to OPs have been well documented and involve inhibition of the enzyme acetylcholinesterase, causing changes in peripheral, autonomic and central nervous system function (cholinergic crisis). However, the effects of long-term low-level exposure to OPs are less clear [8].
A number of occupational groups are exposed to organophosphates on a regular basis (e.g. agricultural workers, horticulturists, pest control operators, chemical plant workers, military personnel and aviation workers) and many individuals complain of chronic ill health following exposure to OPs. For example, a high incidence of physical and psychological symptoms have been reported by commercial airline pilots and cabin crew exposed to OPs in engine oil fumes [19], GulfWar veterans exposed to OP pesticides [26], and sheep farmers who use OP pesticides [1,7,11,12,15,17,20,32,36,37].
Although evidence exists to support the view that high level/acute OP poisoning can cause ill health, the possibility that long-term low-level exposure to OPs in doses below that causing acute toxicity causes ill health is controversial [8]. Previous research has produced inconsistent findings, with some studies finding evidence of ill health and cognitive impairment following low level organophosphate exposure [6,13,14,20,30,34-36] while others have not [2,3,9,17,18]. Methodological differences may account for these inconsistencies, such as examination of different occupational groups with different levels and routes of exposure, use of protective clothing, differences in cultural backgrounds, and different time periods of examination (e.g. following a single episode of exposure, several years of exposure or over a lifetime). Results are easier to interpret and appear more consistent when studies are grouped by design, occupational group, country and time frame of analysis, enabling like to be compared with like.
The purpose of the present study was to determine whether long-term, low level exposure to OPs causes ill health in UK sheep farmers because OPs were used routinely following the introduction of compulsory sheep dipping by the British Government in 1976 until 1992. A number of studies of UK sheep farmers appear in the literature utilising different methodologies including case-series analyses, postal questionnaire surveys and clinical evaluations. Different out-comes have been explored including self-reported symptoms of ill health, neurological abnormalities, genetic polymorphisms which influence OP metabolism, and neuropsychological abnormalities. All but one study [17] suggest a link between exposure to sheep dip and the development of ill health and neurobehavioural problems. For example, Ahmed and Davies [1], Davies, Ahmed and Freer [11], Dunn [12], Tahmaz, Soutar and Cherrie [37] and Solomon et al. [32] looked at the incidence and/or nature of self-reported symptoms among different groups of sheep farmers and found that neuropsychiatric symptoms were common in past users of sheep dip.
Beach et al. [5] and Pilkington et al. [24] looked at abnormalities on neurological examination among sheep farmers and found an association between exposure to sheep dip and neurological symptoms such as neuropathy and reduced sensory discrimination.
Cherry et al. [7], Mackness et al. [21] and Povey et al. [25] investigated whether genetic differences in the ability to metabolise OPs rendered some individuals at greater risk of developing ill health following exposure to sheep dip than others. Human serum paraoxonase 1 (PON1) hydrolyzes and detoxifies a number of OPs, including diazinon, one of the most prevalent OP compounds in sheep dip. All three research groups found PON1 polymorphisms in UK sheep farmers who complain of ill health and conclude that OPs contribute to the self-reported ill health of sheep dippers.
Stephens et al. [36] and Mackenzie Ross et al. [20] found sheep farmers performed more poorly than controls on specific cognitive tests. Stephens et al. compared 146 sheep farmers exposed to OPs in sheep dip with 143 unexposed controls (quarry workers) on neuropsychological tests. The farmers performed significantly worse than controls on tests of sustained attention, syntactic reasoning and speed of information processing; and showed greater vulnerability to psychiatric disorder. Memory functioning appeared intact. However, they provided little information about exposure history and only included participants who were fit enough to be in employment at the time of investigation. They did not allow for the fact that individuals with disabling disease may have retired from work. Mackenzie Ross et al. compared 25 farm workers with a history of apparent low level exposure to sheep dip with 22 non-exposed healthy volunteers on neuropsychological tests. Two thirds of farm workers had retired or reduced their workload on ill health grounds and all were involved in litigation. They performed significantly worse than non-exposed healthy volunteers on tests of mental flexibility, response speed and memory; and over 70% suffered from mood disorder. Although this study included participants who had retired on ill health grounds, the sample size was small and self-selected making it unclear how representative they are of the farming community as a whole. Furthermore, many farm workers appeared to have a history of undiagnosed acute poisoning.
The only study of neuropsychological function in UK sheep farmers exposed to OP pesticides which did not find objective evidence of neurobehavioural impairment, was that reported by Jamal et al. [17]. These authors compared three groups of sheep farmers according to whether they had signs of peripheral neuropathy ( ‘no’, ‘possible’ and ‘probable/definite’ signs) and their performance on neuropsychological tests was related to these groupings. Farmers in the probable/definite group reported more symptoms of emotional distress (anxiety and depression) and showed evidence of reduced processing speed, but no other consistent differences between the groups were found on any of the other neuropsychological measures. The authors conclude that whatever factor was responsible for causing peripheral neuropathy did not cause cognitive impairment. Exposure history was not specified or used as a variable in the analysis and the authors acknowledge that their sample size was too small to allow a meaningful analysis of the relationship between cognitive function and exposure history.
Although the vast majority of earlier studies suggest a link between exposure to sheep dip and the development of neurobehavioural problems, it is unclear whether this is due to a history of acute poisoning or a result of cumulative low level exposure. The present study addresses the methodological weaknesses of earlier studies and sought to determine whether low level exposure to OPs is associated with neuropsychological and psychiatric impairment in UK sheep farmers. Past medical and psychiatric history were taken into account to exclude other possible causes of ill health. This study is the first clinical study to recruit participants who have retired on ill health grounds and to determine in the same cohort of farmers whether variability in PON1 status (plasma level and position 192 functional genotype) renders some individuals more vulnerable to the effects of OPs than others. Participants were expected to show a similar pattern of deficits as that reported by Stephens et al. [36] and Mackenzie Ross et al. [20]. They are hypothesised to show deficits on tests of working and general memory, response speed and mental flexibility with preserved reasoning and general intellectual functioning.
2. Method
2.1. Ethical approval
Ethical approval for this study was granted by the joint University College London (UCL)/UCL Hospital Committee A and by the University of Washington Human Subjects Committee. Written informed consent was provided by all study participants.
2.2. Study design and participants
This study compared the performance of 127 sheep farmers (67 working, 60 retired) to 78 controls (38 working, 40 retired) on measures of cognitive function and mood state. Finding a group of farmers in the UK who do not have a history of exposure to OPs is almost impossible and it was necessary to identify an alternative occupational group that could act as controls. Rural police workers were chosen.
The focus of the project was restricted to the North and South West regions of England which have the highest number of sheep farmers in the UK. Recruitment of the exposed cohort involved writing to farm owners listed on relevant databases (e.g. UK National Business Directory, National Farmers Union membership lists); and telephoning every fifth person on lists held by the Wool Marketing Board. A total of 393 farmers were contacted by telephone and invited to take part and the response rate was 59%. Additionally, some farmers were recruited through advertising or replying to articles in the media.
Controls were recruited by enlisting the help of local constabularies and the National Association of Retired Police Officers (NARPO) who contacted their members by email or newsletter to provide details of our study. Our study was also advertised in Police Press.
Initially 434 farmers came forward (222 retired, 212 working) and 252 police (170 retired, 82 working), however 67% of the farmers and 63% of the controls had to be excluded based on the inclusion/exclusion criteria (Table 1). A further 17 farmers and 4 controls were excluded to in order to establish similar demographic profiles between the groups; 5 farmers’ and 1 policeman’s data were excluded because they showed evidence of poor effort/malingering on a psychometric test which is insensitive to severe brain injury but which is greatly affected by effort [16].
Table 1.
Inclusion and exclusion criteria
| Exposed cohort | Unexposed cohort | |
|---|---|---|
| Inclusion | Aged between 18–70 years old Living in the South West or North of England |
Aged between 18–70 years old Has worked in a rural area in the South West or North of England |
| For the retired cohort, they must have retired on ill health grounds not age or economic reasons. |
Retired on ill health grounds not age or economic reasons |
|
| Exposure to organophosphate pesticides for a a minimum of 5 years prior to 1991 (safety regulations were implemented in 1992). |
No known exposure to organophosphate pesticides. |
|
| NO history of acute intoxication requiring medical intervention. |
||
| Exclusion | History of psychiatric problems prior to exposure, neurological or serious medical problems which might otherwise account for any cognitive or emotional problems identified in this study. |
History of psychiatric or serious medical problems which might otherwise account for any cognitive or emotional problems identified in this study. |
| Substance abuse (including alcohol) |
Substance abuse (including alcohol) |
|
| Those with a history of acute organophosphate intoxication. |
Exposure to organophosphates |
2.2.1. Exclusion criteria
To ensure that any cognitive and emotional problems identified in this study relate to OP exposure, it was important to exclude individuals with a medical or psychiatric history that might otherwise account for their symptoms.
2.3. Procedures and measures
Study participants were visited at home or at their workplace and underwent an extensive neuropsychological assessment and completed a number of questionnaires to establish demographic, mood, health and exposure information.
2.3.1. Exposure history
Farmers underwent an in-depth semi-structured interview about their work and exposure history in order to establish the degree of their exposure to OPs, whether or not they had a history of undiagnosed acute poisoning (i.e. ‘dippers flu’) or diagnosed poisoning (i.e. they sought medical help after dipping.
2.3.2. Measures of psychological function
An extensive psychometric test battery was used comprising measures that are known to be reliable, sensitive and routinely used in clinical practice within the National Health Service. All tests have adequate published reliability, validity and normative data:
The Wechsler Adult Intelligence Scale-III was used to measure current intellectual functioning. It comprises fourteen subtests which measure a variety of verbal and non-verbal functions. Eleven subtests were administered [39].
The Wechsler Memory Scale-III was used to assess working, verbal and visual memory [39].
Trail Making A and B were used to assess motor speed and mental flexibility and the Stroop test was included as a measure of mental flexibility [33].
The Graded Naming Test [22] was used to assess naming and a verbal fluency test [33] was used to assess expressive language.
The Grooved Pegboard test was used to assess fine motor control [38].
The California Computerised Assessment Package was used to assess simple and choice reaction time [23].
The Medical Symptom Validity Test was used as a measure of cognitive effort/symptom validity [16].
Ten key areas of cognitive function were identified and tests were grouped in a way that would reflect participants’ abilities in these areas (Table 2).
Table 2.
Key cognitive domains and associated neuropsychological tests.
| Cognitive domain | Tests | Cognitive domain | Tests |
|---|---|---|---|
| Working memory | WAIS-III digit span39 | Mental flexibility | Trails B33 |
| WAIS-III digit span | Stroop33 | ||
| Backwards | |||
| WAIS-III arithmetic | CALCAP choice23 | ||
| WMS-III LNS39 | |||
| Visual memory | WMS-III visual | Strategy making | Verbal fluency33 |
| Immediate memory | |||
| WMS-III visual | Verbal ability | WAIS-III Vocabulary | |
| Delayed memory | WAIS-III comprehension | ||
| Graded naming22 | |||
| Auditory memory | WMS-III auditory | Visuo-spatial skills | WAIS-III block design |
| Immediate memory | WMS-III spatial span | ||
| WMS-III auditory | |||
| Delayed memory | |||
| WMS-III auditory | |||
| Recognition delayed | |||
| Response speed | WAIS-III digit symbol | Verbal and visual | WAIS-III picture arrangement |
| Trails A33 | Reasoning | WAIS-III comprehension | |
| CALCAP simple23 | WAIS-III similarities | ||
| Fine motor control | Grooved Pegboard38 |
2.3.3. PON1 status
Study participants were asked to provide a sample of venous blood for analysis of PON1 polymorphisms and activity level. PON1 status was determined under conditions that allow for determination of in vivo rates of diazoxon detoxification [27,28].
2.4. Statistical analyses
Two factor ANOVAs were used to compare Exposure Groups (exposed vs. control) and Working Status (farmers vs. controls). Age was added as a covariate (ANCOVA) when variables were not aged-scaled. Variables that were not normally distributed were transformed to meet parametric test assumptions. To minimize potential Type I errors, multivariate tests were used where appropriate (MANOVA/MANCOVA). Pillai’s trace was reported in these cases, as it is the most reliable multivariate statistic for unequal sample sizes.
3. Results
3.1. Sources of recruitment and reasons for retirement
All working and retired controls (rural police workers) were recruited by advertising. The primary reason for retirement given by police workers was musculo-skeletal injury (78%). A small number (7%) retired due to stress which had fully resolved by the time they took part in this study; and 15% retired on other ill health grounds such as breathing difficulties, gout, benign tumour, chronic fatigue and arthritis.
Working and retired farmers were recruited by a combination of purposive sampling (written correspondence and phone calls) and advertising. The majority of working farmers (81%) were recruited by purposive sampling and 19% responded to advertisements. The majority of retired farmers (68%) were recruited by advertisement as many were no longer listed on union/business membership lists. Having said that, over a third (32%) of retired farmers were recruited by purposive sampling. Around half of the retired cohort had retired from farming completely (51%) whilst the remainder had semi-retired. None used sheep dip after retiring, the average number of years having elapsed since the last time they dipped sheep being 9 years for working farmers and 11 years for retired farmers. The primary reasons for retirement given by farmers (77%) was a constellation of non-specific symptoms including chronic fatigue, headaches, cognitive impairment, muscular and joint pain, numbness and chemical sensitivity. A quarter of these farmers attributed their symptoms to pesticide (sheep dip) poisoning. The remainder of the retired sample (23% ) had retired on other ill health grounds such as musculo-skeletal injury, breathing difficulties and prostate problems.
3.2. Demographic information
Demographic characteristics of farmers and controls appear in Table 3. Premorbid intelligence was estimated using a measure that is unlikely to have been affected by cognitive damage (matrix reasoning) as previous research suggests organophosphate exposed individuals may have impaired reading ability [20]. Farmers and controls were successfully matched for gender, education and premorbid IQ, but not age. As the majority of psychometric test data used in the analysis were age-corrected, it is unnecessary to match the groups on age.
Table 3.
Demographic information for the control and exposed participants in both the working and retired groups.
| Exposed group |
Control group |
|
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Agea | 54.73 (9.42) | 51.73 (7.36) |
| Years in education | 11.57 (2.08) | 11.95 (1.59) |
| Matrix reasoning | 12.52 (2.53) | 12.61 (2.19) |
| Gender | 102M, 25F | 68M, 10F |
Significant difference between groups using an independent t-test.
Tables 4a-4c summarize the effect of Exposure Group on broad cognitive domains and summarize the results of univariate analyses examining the effect of Exposure Group on the individual psychological tests that comprise these domains. There were no effects of Working Status (and no significant interactions), so descriptive statistics were collapsed across this variable.
Table 4a.
IMPAIRED domains – main effects of group on performance on memory tests and descriptive statistics and univariate effects of exposure group on cognitive performance.
| Cognitive area | Exposed |
Control |
Tests | F value | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |||
| Working memory | M | 12.03*** | ||||||
| Digit span | 125 | 9.36 | 2.36 | 76 | 11.21 | 2.71 | A | 25.30*** |
| Digit span backward | 124 | 4.6 | 1.16 | 76 | 5.32 | 1.33 | A | 15.70*** |
| Letter–number (LNS) |
127 | 9.48 | 2.62 | 78 | 11.69 | 2.44 | A | 34.53*** |
| Arithmetic | 122 | 11.43 | 2.78 | 75 | 11.48 | 2.89 | A | 0.03 |
| Visual memory | M | 7.54*** | ||||||
| Visual immediate | 127 | 90.91 | 15.95 | 78 | 98.71 | 16.65 | A | 11.06*** |
| Visual delayed | 125 | 93.04 | 14.78 | 77 | 101.34 | 14.45 | A | 15.00*** |
| Auditory memory | M | 5.11** | ||||||
| Auditory immediate | 127 | 98.52 | 16.07 | 78 | 107.32 | 13.69 | A | 15.35*** |
| Auditory delayed | 125 | 99.87 | 14.86 | 78 | 107.23 | 13.98 | A | 13.09*** |
| Auditory recognition | 125 | 101.08 | 15.69 | 78 | 106.03 | 13.3 | A | 5.74** |
p<.001;
p<.01;
p<.05.
A=ANOVA, AC=ANCOVA, M=MANOVA, MC=MANCOVA.
Table 4c.
INTACT domains – main effects of group on performance on tests of verbal and visuospatial ability, reasoning and general intellectual function and descriptive statistics and univariate effects of exposure group on cognitive performance.
| Cognitive area | Exposed |
Control |
Tests | F value | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |||
| Verbal ability | M | 1.81 | ||||||
| Vocabulary | 125 | 10.33 | 2.41 | 76 | 10.96 | 1.87 | A | 3.45 |
| Comprehension | 119 | 10.84 | 2.64 | 75 | 11.35 | 2.12 | A | 2.75 |
| Graded naming | 127 | 12.36 | 1.28 | 76 | 12.78 | 1.1 | A | 4.41* |
| Visuo-spatial ability | M | 1.65 | ||||||
| Block design | 125 | 11.98 | 2.84 | 76 | 12.17 | 3.19 | A | 0.22 |
| Spatial span | 126 | 10.07 | 2.64 | 78 | 10.87 | 3.19 | A | 3.21 |
| Verbal/visual reasoning | M | 2.45 | ||||||
| Picture arrangement | 122 | 9.9 | 2.67 | 69 | 10.96 | 2.78 | A | 6.58* |
| Similarities | 124 | 10.47 | 2.6 | 76 | 10.92 | 1.87 | A | 2.75 |
| Comprehension | 119 | 10.84 | 2.64 | 75 | 11.35 | 2.12 | A | 1.66 |
| General intelligence | ||||||||
| WAIS-III full scale IQ | 122 | 104.79 | 11.76 | 74 | 110.03 | 10.83 | A | 10.21** |
p<.001;
p<.01;
p<.05.
A=ANOVA, AC=ANCOVA, M=MANOVA, MC=MANCOVA.
3.3. Impaired versus intact cognitive domains
Farmers (both working and retired groups) were significantly impaired on measures of memory, response speed, fine motor control, mental flexibility and strategy making; and this pattern was similar for both the working and retired groups. They were not significantly impaired on measures of verbal abilities, visuo-spatial abilities and visual and verbal reasoning, suggesting these areas were largely intact. Although a significant main effect of Exposure Group on overall Full Scale WAIS scores (F = 10.21, p <.01) was found, suggesting farmers have significantly lower intellectual ability than controls, this was a result of patchy under functioning on certain WAIS subtests (see above). Their overall general intellectual ability is relatively well preserved.
Three psychometric tests could not be analysed using parametric tests: the CALCAP simple test of response speed and the Stroop and CALCAP choice tests of mental flexibility. Instead the percentage of participants who obtained normal/abnormal scores was examined and analysed using chi square. A significantly higher proportion of retired farmers performed abnormally on CALCAP compared to controls (simple χ2=5.15, p<.05; choice χ2=10.59, p=.001). However working farmers and controls performed similarly on this measure (simple χ2=1.91, ns; choice χ2=1.96, ns). A significantly higher proportion of farmers performed abnormally on the Stroop test compared to controls in both the working (χ2=7.64, p<.01) and retired (χ2=10.71, p=.001) groups.
3.4. Farmers versus normative comparison standards: re-analysis of the data using an alternative comparison group
To examine whether the patterns of deficit observed in the initial analysis could have been driven by selection of an inappropriate control group (i.e. police workers), the above analyses were repeated using normative comparison standards [22,23,33,38,39]. The overall findings of this study are the same whether we compare exposed farmers with rural police workers or with published test norms derived from a cross section of thousands of adults in the general population.
3.5. Mood disorder
Hospital Anxiety and Depression Scale [31]: 46.9% of farmers had scores above clinical cut-offs for depression compared to only 6.5% controls; and 41.5% of farmers scored in the clinical range for anxiety compared to only 22.1% of controls. Chi square revealed that a significantly higher proportion of farmers scored in the clinical range for anxiety (χ2 = 7.25, p<.01) and depression (χ2 = 33.97, p<.001).
As previous research has shown that depression and anxiety may be related to poor performance on certain psychometric tests [4] the above analyses on cognitive performance were re-run with the effects of mood partialled out. Results showed that the pattern of impairment remained the same in all cases.
3.6. Exposure history
Participants in this study were exposed to OPs over a number of years as a result of sheep dipping and were asked to report retrospectively on their exposure history (see Table 5).
Table 5.
Quantifying exposure.
| Exposure indices | Mean | Standard deviation | Range |
|---|---|---|---|
| No. of years spent working with OPs | |||
| Working group | 23.05 | 10.72 | 8–49 |
| Retired group | 26.11 | 15.17 | 5–66 |
| No. of days spent working with OPs | |||
| Working group | 3.77 | 6.90 | 0.5–30 |
| Retired group | 2.84 | 3.54 | 1–21 |
| Years since last dip | |||
| Working group | 9.48 | 8.78 | 0–37 |
| Retired group | 11.26 | 7.65 | 0–42 |
3.7. ‘Dippers flu’
A number of participants (33.8% of the working cohort and 50.9% of the retired group) reported that throughout their working life they suffered repeated episodes of flu-like symptoms (e.g. fatigue, muscle pain, headaches and general malaise) following exposure to OPs. The cause and nature of ‘dippers flu’ has not been established scientifically, but the symptoms have much in common with those associated with mild exposure to organophosphate compounds. The chi-square test revealed no significant difference between working and retired farmers in terms of how many reported dippers flu.
To examine whether the patterns of deficit observed in the initial analysis could have been driven by participants with undiagnosed acute exposure, the data were re-analysed after removing all farmers who had experienced dippers flu. The same areas of deficit remained after their removal, with only one minor change on a measure of response speed. In the initial analysis a significantly higher proportion of farmers were observed to have abnormal CALCAP (simple) scores compared to the controls in the retired group, but not in the working group. When those with dippers flu were excluded from the analysis this pattern was reversed.
3.8. Relationship between cognitive tests and exposure indices
To investigate whether the observed cognitive deficits were related to exposure history, correlational analyses were carried out. To avoid Type 1 error the number of variables entered into the correlation matrix were limited. Instead of entering scores from all of the individual subtests included in the test battery, composite z-scores were calculated so that a participant’s performance in the 10 cognitive domains described in the group analyses (please see Table 2), could be examined in relation to exposure history. To avoid bias and determine the false discovery rate, all 10 cognitive domains were entered into the analyses and not just the domains shown to be impaired in the group analyses. Instead of entering data from multiple exposure indices, duration of exposure was considered the most relevant variable (i.e. the number of years spent working with sheep dip). As we hypothesise that increased contact to OPs will result in worse cognitive performance, 1-tailed tests are reported.
Spearman’s correlations revealed significant, negative correlations between duration of exposure and auditory memory (rs=−.15, p<.05), visual memory (rs=−.25, p<.01), verbal ability (rs=−.21, p<.01) and strategy making (rs=−.18, p<.05) indicating an association between duration of exposure and impairments in these areas. A significant positive correlation was also found with fine motor control (rs=.20, p<.05). The latter correlation was positive as high scores on the test of fine motor control are indicative of poor performance. No other significant correlations were found. In summary, 5 out of 10 significant correlations were found, all in the expected direction and all consistent with the findings from the group analyses and with our hypotheses. These findings are unlikely to have occurred by chance.
3.9. Potential susceptibility to OPs: genetic data
Participants’ PON1 phenotype (Q/Q; Q/R; R/R) and arylesterase activity levels appear in Table 6.
Table 6.
PON1 status in study participants according to work status.
| Frequency of phenotypes |
Arylesterase activity |
|||||
|---|---|---|---|---|---|---|
| QR | RR | Mean | SD | Range | ||
| Farmers | ||||||
| Working | 26 | 21 | 5 | 150.38 | 33.67 | 71–239 |
| Retired | 19 | 26 | 5 | 153.84 | 37.71 | 88–243 |
| Controls | ||||||
| Working | 16 | 15 | 1 | 143.33 | 27.10 | 92–207 |
| Retired | 11 | 11 | 3 | 141.09 | 29.76 | 91–207 |
There are similar numbers of participants in each phenotype group, with similar levels of arylesterase. No one included in this study had arylesterase levels less than 81.5 units/ml suggesting there were no poor metabolizers in this study (see circle, Fig. 1), possibly because we excluded participants with a history of acute symptoms following exposure to OPs that required medical intervention (Fig. 1). Indeed, mean arylesterase levels were higher than that reported in other populations [10,27-29].
Fig. 1.
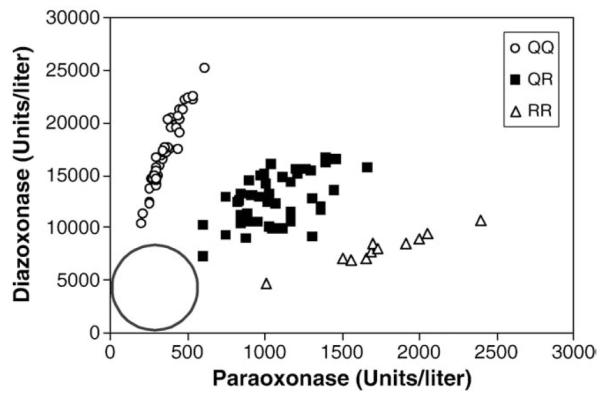
PON1 status of UK sheep farmers with a history of low level exposure to organophosphates in sheep dip. PON1 status was determined by plotting the initial rates of diazoxon hydrolysis vs. paraoxon hydrolysis for each plasma sample as originally described by Richter and Furlong in 1999 [27] and further validated by Richter et al. in 2009 [28]. This activity assay is superior to DNA analyses which provide only the DNA sequence at a specific polymorphic site (codon 192) and not plasma enzyme levels. This PON1 status analysis provides both the functional position 192 polymorphism and the level of PON1, which in the case of diazinon/diazoxon exposure is more important than the position 192 genotype.
4. Discussion
The present study compared the neuropsychological performance of 127 agricultural workers with a history of low level exposure to organophosphate pesticides with 78 non-exposed controls. Information was also obtained about physical and mental health. As far as we are aware, this is the first clinical study of farmers to take account of the ‘healthy worker’ effect by including a cohort of agricultural workers who have retired or changed occupation on ill health grounds. The overall aim of the study was to establish whether low level exposure to OPs is associated with disabling neuropsychological and emotional functioning. A further aim was to establish whether individuals who have retired on ill health grounds constitute a particular subgroup of individuals who are more susceptible to the effects of OPs than others.
A range of emotional, physical and cognitive problems were identified in agricultural workers. In terms of emotional and physical health, over 40% of the exposed cohort complained of clinically significant levels of anxiety and depression compared to less than 23% of controls, the highest rates of distress being found in retired farmers. Farmers also report a range of physical symptoms which they describe as being moderate to severe, the most prominent being fatigue, memory problems, joint stiffness, sleep disturbance, irritability and feeling mentally slowed down.
In terms of cognitive function, general intellectual ability, reasoning, visuo-spatial and verbal ability were relatively well preserved, but agricultural workers obtained lower scores on tests of response speed, working, verbal and visual memory, mental flexibility and fine motor control, than controls. These differences remained after controlling for Type 1 errors, depression and anxiety. Hence, these findings are unlikely to have occurred by chance or to be due to the confounding effects of mood disorder.
Is there a subgroup of individuals who are more susceptible to the effects of OPs than others who have retired from the profession?
One of the aims of this study was to determine whether individuals who have retired on ill health grounds constitute a particular subgroup of individuals who are more susceptible to the effects of OPs than others. Following a major review of the literature concerning the health effects of exposure to OPs, a UK Government advisory committee, the Committee on Toxicity (COT) [8] suggested that farmers who have retired on ill health grounds may constitute a subgroup of individuals who are more susceptible to the effects of OPs than others and that previous studies may have underestimated the risk associated with exposure to OPs by failing to include retired workers.
Although differences were found in this study between working and retired farmers on subjective, self report measures of mental and physical health, no significant differences were found on objective measures of cognitive function or in terms of PON1 status. This was surprising given the views of COT and given the fact that a number of different recruitment methods were used in this study that could have resulted in selection bias and differences between working and retired cohorts. For example, most of the retired farmers volunteered to take part in the study after reading about it in an advertisement and those with neurobehavioural symptoms would be more likely/motivated to take part in the study than working farmers recruited via purposive sampling methods.
However, our study findings indicate that individuals who have retired on ill health grounds are not at increased risk of suffering cognitive impairment following exposure to OPs, although they may report more physical and emotional problems.
Do the findings relate to low level exposure or acute poisoning?
Another issue in this study concerning the effects of low level exposure to OPs, is the possibility that ‘dippers flu’ may reflect undiagnosed acute toxicity. Further analyses were undertaken to determine whether differences between farmers and controls could have been driven by inclusion of individuals with a history of undiagnosed acute toxicity. However, the same areas of deficit remained even after removal of study participants with a history of ‘dippers flu’.
Have the findings arisen because an inappropriate control group was used? Do they relate to exposure history?
A potential weakness of this study design which could limit the conclusions that can be drawn was the choice of control population (i.e. rural police workers). Finding a group of farmers in the UK who do not have a history of exposure to OPs is almost impossible and it was necessary to identify an alternative occupational group that could act as controls. Several were considered and rural police workers were chosen. Police workers differ from farmers in terms of the exact nature of the work they undertake, lifestyle, economic status and life experiences. Differences between farmers and police workers in terms of physical health and emotional well-being could be due to an unidentified confounder that was not controlled for in this study and not exposure history; but differences between farmers and controls in terms of cognitive function are unlikely to be due to confounding variables as the cohorts were matched as far as possible in terms of characteristics which may affect cognitive function (age, gender, educational level, premorbid IQ). Nevertheless, in order to check the validity of our findings the analyses were repeated using normative comparison standards derived from a cross section of several thousand adults in the general population. The overall findings are the same whether exposed farmers are compared to rural police workers or with published test norms routinely used in clinical practice.
Furthermore, statistical analyses were carried out to look at the relationship between exposure history and cognitive function. Unfortunately, there is no biomarker of long term exposure to organophosphate pesticides. Exposure had to be estimated via self-report which is problematic as it may be distorted by inaccuracies of memory and response bias. Hence, the accuracy of the exposure information provided by farmers is open to question, which reduces the chance of finding significant associations. Nevertheless, a number of significant correlations were observed between duration of exposure and verbal and visual memory, verbal ability, strategy making and fine motor control. Although the correlations were weak, they were in the expected direction, consistent with findings from the group analyses and consistent with study hypotheses.
4.1. Comparisons with previous research
The pattern of results observed in the current study is consistent with findings from two other studies of neuropsychological function in UK sheep farmers. Both Stephens et al. [36] who examined working sheep farmers and Mackenzie Ross et al. [20] who examined a self selected sample of 25 UK sheep farmers who had retired on ill health grounds found an association between exposure to organophosphate pesticides and neurobehavioural impairment.
The only study of neuropsychological function in UK sheep farmers exposed to OP pesticides which did not find objective evidence of significant neurobehavioural impairment, was that reported by Jamal et al. [17]. However, the study design was unusual as it assumed that there should be a relationship between peripheral nerve damage (i.e. neuropathy) and central nervous system damage (i.e. cognitive impairment) but the mechanisms underlying these conditions may differ. Furthermore, exposure history was not specified or used as a variable in the analysis and the authors acknowledge that their sample size was too small to allow a meaningful analysis of the relationship between cognitive function and exposure history.
In summary, both correlation and group analyses suggest that a relationship may exist between low level exposure to organophosphates and impaired neurobehavioural functioning. Few differences were found between working and retired groups. The cognitive deficits identified in the exposed cohort cannot be attributed to mood disorder, malingering, a history of acute exposure, or genetic vulnerability in terms of PON1 polymorphisms. The pattern of deficits is consistent with reports from two previous neuropsychological studies of UK sheep farmers.
The present findings suggest OP pesticides are more harmful than previously thought, even at low levels of exposure. This has implications for working practice and policies and guidelines about the use of organophosphate chemicals and for other occupational groups who are exposed to oganophosphate chemicals on a regular basis, such as military personnel and commercial airline pilots and cabin crew. Cabin air on commercial aircraft is supplied from engines and is sometimes contaminated with hydraulic fluids and jet engine oils which contain a large number of chemicals including organophosphates, which are added as lubricants. A recent review of Gulf War Syndrome concluded that ill health was due to exposure to OP pesticides [26].
All of these occupational groups contain individuals who report chronic ill health which they attribute to exposure to OPs. Follow-up studies are needed to determine whether symptoms persist, improve or worsen. At present, there are no treatment protocols for individuals who report chronic ill health following exposure to OPs and there is a need for prospective treatment trials.
Supplementary Material
Table 4b.
IMPAIRED domains – main effects of group on performance on tests of response speed and mental flexibility and descriptive statistics and univariate effects of exposure group on cognitive performance.
| Cognitive area | Exposed |
Control |
Tests | F value | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |||
| Response speed | ||||||||
| Digit symbol | 126 | 8.84 | 2.55 | 75 | 10.81 | 2.15 | A | 37.81*** |
| Trails A | 127 | 41.03 | 15.3 | 74 | 32.27 | 13.05 | AC | 19.88*** |
| CALCAP simple | Χ 2 | see text | ||||||
| Fine motor control | MC | 22.83*** | ||||||
| Grooved Pegboard dominant hand (RT) |
124 | 92.65 | 24.51 | 74 | 75.08 | 11.89 | AC | 43.90*** |
| Grooved Pegboard non-dominant hand (RT) |
123 | 96.49 | 22.47 | 73 | 81.12 | 13.92 | AC | 30.68*** |
| Mental Flexibility | ||||||||
| Trails B | 124 | 96.26 | 44.74 | 77 | 71.03 | 30.79 | AC | 23.10*** |
| Stroop | Χ 2 | See text | ||||||
| CALCAP choice | Χ 2 | See text | ||||||
| Strategy making | ||||||||
| Verbal fluency | 127 | 32.88 | 11.51 | 77 | 44.21 | 10.47 | AC | 46.94*** |
pb.001;
pb.01;
pb.05.
A=ANOVA, AC=ANCOVA, M=MANOVA, MC=MANCOVA.
Acknowledgements
We would like to thank the Department of Environment, Food and Rural Affairs (DEFRA) and the National Institute of Environmental Health Sciences (ES009883, ES04696 and ES07033; Dr Furlong) for supporting this work. DEFRA was involved in the study design, but not in data collection, analysis, interpretation or report writing. We would also like to thank Miss Julia Britton and Miss Tessa Hughes for assistance with recruitment and data collection; Miss Rebecca Richter for technical support; Miss Alison Dunevein for assistance with data entry and management; Professor John Henry, Consultant Toxicologist for provision of information about the medical aspects of organophosphate poisoning; and Dr Andy Field for statistical advice.
Footnotes
5. Conflict of interest statement None of the authors have any conflicts of interest to declare.
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ntt.2010.03.004.
References
- [1].Ahmed GM, Davies DR. Chronic organophosphate exposure: towards the definition of a neuropsychiatric syndrome. Journal of Nutritional and Environmental Medicine. 1999;7:169–176. [Google Scholar]
- [2].Albers JW, Berent S, Garabrant DH, Giordani B, Schweitzer SJ, Garrison RP, Richardson RJ. The effects of occupational exposure to chlorpyrifos on the neurologic examination of central nervous system function: a prospective cohort study. Journal of Occupational & Environmental Medicine. 2004;48:367–379. doi: 10.1097/01.jom.0000121127.29733.5c. [DOI] [PubMed] [Google Scholar]
- [3].Ames RG, Steenland K, Jenkins B, Chrislip D, Russo J. Chronic neurologic sequelae to cholinesterase inhibition among agricultural pesticide applicators. Archives of Environmental Health. 1995;50(No6):440–444. doi: 10.1080/00039896.1995.9935980. [DOI] [PubMed] [Google Scholar]
- [4].Baddeley AD, Wilson BA, Watts FN, editors. Handbook of Memory Disorders. John Wiley & Sons; England: 1995. [Google Scholar]
- [5].Beach JR, Spurgeon A, Stephens R, Heathfield T, Calvert IA, Levy LS, Harrington JM. Abnormalities on neurological examination among sheep farmers exposed to organophosphorous pesticides. Occupational and Environmental Medicine. 1996;53:520–525. doi: 10.1136/oem.53.8.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bosma H, van Boxtel MPJ, Ponds RWHM, Houx PJ, Jolles J. Pesticide exposure and risk of mild cognitive dysfunction. The Lancet. 2000;356:912–913. doi: 10.1016/s0140-6736(00)02685-4. [DOI] [PubMed] [Google Scholar]
- [7].Cherry N, Mackness M, Durrington P, Povey A, Dippnall M, Smith T, Mackness B. Paraoxonase (PON1) polymorphisms in farmers attributing ill health to sheep dip. The Lancet. 2002;359:763–764. doi: 10.1016/s0140-6736(02)07847-9. [DOI] [PubMed] [Google Scholar]
- [8].COT report: Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (Department of Health) Organophosphates. 1999. Crown Copyright.
- [9].Daniell W, Barnhart S, Demers P, Costa LG, Eaton DL, Miller M, Rosenstock L. Neuropsychological performance among agricultural pesticide applicators. Environmental Research. 1992;59:217–228. doi: 10.1016/s0013-9351(05)80241-5. [DOI] [PubMed] [Google Scholar]
- [10].Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE. The effect of the human serum paraxonase polymorphism is reversed with diazoxon, soman and sarin. Nature Genetics. 1996;14:334–336. doi: 10.1038/ng1196-334. [DOI] [PubMed] [Google Scholar]
- [11].Davies DR, Ahmed GM, Freer T. Chronic organophosphate induced neuropsychiatric disorder (COPIND): results of two postal questionnaire surveys. Journal of Nutritional and Environmental Medicine. 1999;9:123–134. [Google Scholar]
- [12].Dunn G. Report on an Analytical Study of OP Sheep Dips. UK Veterinary Medicines Directorate; 2002. [Google Scholar]
- [13].Farahat TM, Abdelrasoul GM, Amr MM, Shebl MM, Farahat FM. Neurobehavioural effects among workers occupationally exposed to organophosphorus pesticides. Occupational and Environmental Medicine. 2003;60:279–286. doi: 10.1136/oem.60.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fiedler N, Kipen H, Kelly-McNeil K. Long term use of organophosphates and neuropsychological performance. American Journal of Industrial Medicine. 1997;32:487–496. doi: 10.1002/(sici)1097-0274(199711)32:5<487::aid-ajim8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [15].Fletcher T, MacLehose R, Hurley F, Cherrie J, Cowie H, Jamal G, Julu P. SHAPE Report: Survey of Health Complaints Among Sheep-Dippers. Department for Environment, Food and Rural Affairs; 2005. [Google Scholar]
- [16].Green P. Manual for the Medical Symptom Validity Test. Green’s publishing Inc; Edmonton, Canada: 2004. [Google Scholar]
- [17].Jamal GA, Hansen S, Pilkington A, Buchanan D, Gillham RA, Abdel-Azis M, Julu POO, Al-Rawas SF, Hurley F, Ballantyne JP. A clinical neurological, neurophysiological and neuropsychological study of sheep farmers and dippers exposed to organophosphate pesticides. Occupational and Environmental Medicine. 2002;59:434–441. doi: 10.1136/oem.59.7.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].London L, Myers JE, Nell V, Taylor T, Thompson ML. An investigation into neurologic and neurobeavioural effects of long-term agricultural use among deciduous fruit farm workers in the Western Cape, South Africa. Environmental Research. 1997;73:132–145. doi: 10.1006/enrs.1997.3715. [DOI] [PubMed] [Google Scholar]
- [19].Ross S.J. Mackenzie. Cognitive function following exposure to contaminated air on commercial aircraft: a case series of 27 pilots seen for clinical purposes. Journal of Nutritional & Environmental Medicine Occupational Health & Safety: Australia & New Zealand. 2008;17(2):111–126. [Google Scholar]
- [20].Ross S.J. Mackenzie, Clark JS, Harrison V, Abraham KM. Cognitive impairment following exposure to organophosphate pesticides: a pilot study. Journal of Occupational Health & Safety: Australia & New Zealand. 2007;23(2):133–142. [Google Scholar]
- [21].Mackness B, Durrington P, Povey AC, et al. Paraoxonase and susceptibility to organophosphorous poisoning in farmers dipping sheep. Pharmacogenetics. 2003;13:81–88. doi: 10.1097/00008571-200302000-00004. [DOI] [PubMed] [Google Scholar]
- [22].McKenna P, Warrington EK. The Graded Naming Test: Manual. NFER-Nelson; Windsor: 1983. [Google Scholar]
- [23].Miller EN. California Computerised Assessment Package Manual (CALCAP) Second edition Eric N Miller and Norland Software; USA: 2002. [Google Scholar]
- [24].Pilkington A, Buchanan D, Jamal GA, Gillham R, Hansen S, Kidd M, Hurley JF, Soutar CA. An epidemiological study of the relations between exposure to organophosphate pesticides and indices of chronic peripheral neuropathy and neuropsychological abnormalities in sheep farmers and dippers. Occupational and Environmental Medicine. 2001;58:702–710. doi: 10.1136/oem.58.11.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Povey AC, Mackness MI, Durrington PN, Dippnall M, Smith AE, Mackness B, Cherry NM. Paraoxonase polymorphisms and self reported chronic ill health in farmers dipping sheep. Occupational Medicine. 2005;55:282–286. doi: 10.1093/occmed/kqi128. [DOI] [PubMed] [Google Scholar]
- [26].RAC Report: Research Advisory Committee on Gulf War Veterans’ Illnesses. US Government Printing Office; Washington D.C.: Gulf War Illness and the Health of Gulf War Veterans. Scientific Findings and Recommendations. 2008
- [27].Richter RJ, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics. 1999;9:745–753. [PubMed] [Google Scholar]
- [28].Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicology and Applied Pharmacology. 2009;235:1–9. doi: 10.1016/j.taap.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Roest M, van Himbergen TM, Barendrecht AB, Peeteres PHM, van der Schouw YT, Voorbij HAM. Genetic and environmental determinants of the PON1 phenotype. European Journal of Clinical Investigation. 2007;37:187–196. doi: 10.1111/j.1365-2362.2007.01769.x. [DOI] [PubMed] [Google Scholar]
- [30].Roldan-Tapia L, Parron T, Santed F. Sanchez. Neuropsychological effects of long-term exposure to organophosphate pesticides. Neurotoxicology & Teratology. 2005;27(2):259–266. doi: 10.1016/j.ntt.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [31].Snaith RP, Zigmund AS. The Hospital Anxiety and Depression Scale: Manual. NFER-Nelson; 1983. [Google Scholar]
- [32].Solomon C, Poole J, Palmer KT, Peveler R, Coggon D. Neuropsychiatric symptoms in past users of sheep dip and other pesticides. Occupational and Environmental Medicine. 2007;64:259–266. doi: 10.1136/oem.2005.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Spreen O, Strauss E. A Compendium of Neuropsychological Tests. Oxford University Press; New York: 1991. [Google Scholar]
- [34].Srivastava AK, Gupta BN, Bihar V, Mathur N, Srivastava LP, Pangety BS, Bharti RS, Kumar P. Clinical, biochemical and neurobehavioral studies in workers engaged in the manufacture of quinalphos. Food and Chemical Toxicology. 2000;38:65–69. doi: 10.1016/s0278-6915(99)00123-4. [DOI] [PubMed] [Google Scholar]
- [35].Stephens R, Sreenivasan B. Neuropsychological effects in long-term low level organophosphate exposure in orchard sprayers in England. Archives of Environmental Health. 2004;59(11):566–574. doi: 10.1080/00039890409603435. [DOI] [PubMed] [Google Scholar]
- [36].Stephens R, Spurgeon A, Calvert IA, Beach J, Levy LS, Berry H, Harrington JM. Neuropsychological effects of long-term exposure to organophosphate in sheep dip. The Lancet. 1995;345(6):1135–1139. doi: 10.1016/s0140-6736(95)90976-1. [DOI] [PubMed] [Google Scholar]
- [37].Tahmaz N, Soutar A, Cherrie JW. Chronic fatigue and organophosphate pesticides in sheep farming: a retrospective study amongst people reporting to a UK pharmacovigilance scheme. Annals of Occupational Hygiene. 2003;47:261–267. doi: 10.1093/annhyg/meg042. [DOI] [PubMed] [Google Scholar]
- [38].Trites R. Grooved Pegboard Test. Royal Ottawa Hospital; Ottawa, Ontario, Canada: 1977. [Google Scholar]
- [39].Wechsler D. Manual for the Wechsler Adult Intelligence Scale-III (UK) and Manual for the Wechsler Memory Scale-III (UK) Psychological Corporation; USA: 1998. [Google Scholar]
- [40].World Health Organisation/UNEP Working Group; Geneva: WHO Report: Public Health Impact of Pesticides Used in Agriculture. 1990
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


