Abstract
Precise control of somatic stem cell proliferation is crucial to ensure maintenance of tissue homeostasis in high-turnover tissues. In Drosophila, intestinal stem cells (ISCs) are essential for homeostatic turnover of the intestinal epithelium and ensure epithelial regeneration after tissue damage. To accommodate these functions, ISC proliferation is regulated dynamically by various growth factors and stress signaling pathways. How these signals are integrated is poorly understood. Here, we show that EGF receptor signaling is required to maintain the proliferative capacity of ISCs. The EGF ligand Vein is expressed in the muscle surrounding the intestinal epithelium, providing a permissive signal for ISC proliferation. We find that the AP-1 transcription factor FOS serves as a convergence point for this signal and for the Jun N-terminal kinase (JNK) pathway, which promotes ISC proliferation in response to stress. Our results support the notion that the visceral muscle serves as a functional ‘niche’ for ISCs, and identify FOS as a central integrator of a niche-derived permissive signal with stress-induced instructive signals, adjusting ISC proliferation to environmental conditions.
Keywords: Drosophila, EGF signaling, Intestinal stem cells
INTRODUCTION
In high-turnover tissues, the production of new differentiated cells from stem cells is crucial to maintain homeostasis and prevent attrition. In long-lived organisms, stem cell proliferation has to be precisely regulated to maintain regenerative capacity while preventing overproliferation and cancer (Radtke and Clevers, 2005; Rando, 2006; Rossi et al., 2008; Sharpless and DePinho, 2007). The properties of stem cells are regulated by signals from the environment, the organism and, in many cases, a specialized stem cell niche that provides essential growth factors and thus generates a microenvironment that maintains stem cell function (Barker et al., 2008; Bryder et al., 2006; Crosnier et al., 2006; Gopinath and Rando, 2008; Morrison and Spradling, 2008). All of these inputs need to be integrated within the stem cell population to respond to changing environmental conditions with the production of the appropriate number of differentiated cells. The signaling networks that control stem cell maintenance and proliferation govern the balance between tissue regeneration and tumor prevention in aging animals and are therefore crucial to understand.
In Drosophila melanogaster, the integrity of the midgut epithelium is maintained by multipotent intestinal stem cells (ISCs) (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). The ISC lineage consists of a non-dividing ISC daughter cell [enteroblast (EB)] and two differentiated cell types [enterocytes (EC), the main cell type in the intestine; and enteroendocrine cells (EE)] (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). EB differentiation into ECs and EEs is controlled by Notch and JAK (HOP – FlyBase)/STAT (STAT92E – FlyBase) signaling, the decision between these two cell types being regulated by differential Notch and JAK/STAT signaling activities (Beebe et al., 2010; Jiang et al., 2009; Lin et al., 2009; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Ohlstein and Spradling, 2007). ISC-mediated tissue regeneration is required to maintain tissue homeostasis in the intestinal epithelium after tissue damage due to infection, DNA damage and oxidative stress (Amcheslavsky et al., 2009; Biteau et al., 2008; Buchon et al., 2009a; Jiang et al., 2009; Pitsouli et al., 2009). If control of this regenerative process breaks down, for example in old organisms or under conditions of excessive stress, aberrant stem cell proliferation can occur, leading to dysplasia of the intestinal epithelium. This dysplasia phenotype is characterized by an accumulation of misdifferentiated cells at the basal membrane and by disruption of the apicobasal organization of the epithelium. This effect sensitizes flies to infection (Apidianakis et al., 2009) and shortens lifespan (Biteau et al., 2008; Biteau et al., 2010; Buchon et al., 2009a). Precise regulation of ISC proliferation is thus crucial to limit overproliferation while maintaining regenerative capacity, ensuring long-term functional maintenance of this tissue.
ISC proliferation and self-renewal are regulated by the growth factors insulin and WNT/WG, the JAK/STAT signaling pathway, as well as by the MAP Kinase p38 (Amcheslavsky et al., 2009; Beebe et al., 2010; Buchon et al., 2009a; Jiang et al., 2009; Lee et al., 2009; Lin et al., 2008; Lin et al., 2009; Park et al., 2009). In response to tissue damage, for example after exposure to genotoxic or reactive oxygen species (ROS)-inducing compounds or infection, ISC proliferation is further strongly promoted by multiple stress-responsive signaling systems (JNK, JAK/STAT and PVR/p38) (Amcheslavsky et al., 2009; Apidianakis et al., 2009; Biteau et al., 2008; Buchon et al., 2009a; Buchon et al., 2009b; Chatterjee and Ip, 2009; Choi et al., 2008; Cronin et al., 2009; Jiang et al., 2009). The proper interplay of growth factor and stress signals in ISCs is expected to be important for tissue function and integrity. How these different types of biological information are integrated to regulate proliferation rates of ISCs is unclear.
Here, we show that the EGF Receptor (EGFR) signaling pathway plays a crucial role in the regulation of ISC proliferation. We find that the EGFR ligand Vein is expressed in the circular muscle surrounding the intestinal epithelium, providing a constitutive signal that activates the ERK (Rolled – FlyBase) protein kinase in ISCs and is essential to maintain an optimal ISC proliferative capacity. We show that the transcription factor FOS (KAY – FlyBase) acts as an integrator of this EGFR/ERK signal with stress signals mediated by the Jun N-terminal Kinase (JNK; BSK – FlyBase) pathway in ISCs. FOS is required for EGFR-mediated ISC proliferation, but also mediates the JNK-dependent boost in proliferation rates in response to stress. FOS integrates these two specific signals through distinct phosphorylation sites. Our findings thus identify muscle-derived growth factors as crucial regulators of proliferative competence of ISCs, strengthening the notion that the visceral muscle serves as a functional niche for ISCs. The integration of this permissive signal with stress-response signaling pathways by FOS provides a model for the dynamic regulation of ISC proliferation in homeostatic and stress conditions.
MATERIALS AND METHODS
Drosophila stocks and culture
The following strains were obtained from the Bloomington Drosophila Stock Center: w1118, UAS-RasV12, UAS-RasN17, UAS-DERDN, UAS-DER1act, UAS-RafGOF, rase1B, rase2F, HowGal4 and tub-Gal80ts. UAS-EGFRRNAi (#43267 and #107130), UAS-rasRNAi (#106642), UAS-rolledRNAi (#43123) and UAS-bskRNAi (#34138) were obtained from the Vienna Drosophila RNAi Center. The line esgGal4NP5130 was kindly provided by S. Hayashi (RIKEN Center for Developmental Biology, Kobe, Japan), UAS-BskDN by M. Mlodzik (Mount Sinai Medical Center, New York, NY, USA) and NP1Gal4 by D. Ferrandon (IBMC, Strasbourg, France).
The original UAS-rolledRNAi carries multiple insertions and is named RolledRNAi2x. A single insertion on the second chromosome was isolated and is referred as rolledRNAi.
UAS-fosRNAi and UAS-fos point mutant constructs were previously described (Hyun et al., 2006). UAS-fosRNAi strong and UAS-fosRNAi weak refer respectively to the FI39/15 and FI49 lines (Hyun et al., 2006).
The kay2 allele was described previously (Zeitlinger et al., 1997). The kay3 allele is a transposon insertion in the kayak locus, lethal in trans with the kay1 or kay2 alleles and causing a strong loss-of-function phenotype (not shown).
All flies were raised on standard yeast and molasses-based food, at 25°C and 65% humidity, on a 12-hour light/dark cycle, unless otherwise indicated.
Conditional expression of UAS-linked transgenes
The TARGET system was used to conditionally express UAS-linked transgenes in ISCs and EBs (McGuire et al., 2003). The esg-Gal4, How-Gal4 and NP1-Gal4 drivers were combined with a ubiquitously expressed temperature-sensitive Gal80 inhibitor (tub-Gal80ts). These conditional drivers are termed esgGFPts, HowGal4ts and NP1Gal4ts. Crosses and flies were kept at 18-20°C (permissive temperature), then shifted to 29°C to allow expression of the transgenes.
MARCM and flip-out clones
Positively marked clones were generated by somatic recombination using the following MARCM stocks: hsFlp;FRT40A tub-Gal80;tub-Gal4,UAS-GFP (gift from B. Ohlstein, Columbia University, New York, NY, USA); hsFlp;FRT42D tub-Gal80;tub-Gal4,UAS-GFP; hsFlp;tub-Gal4,UAS-GFP;FRT82B tubGal80 (gift from N. Perrimon, Harvard University, Boston, MA, USA). Virgins from the appropriate MARCM stock were crossed to the following lines: FRT40A bsk170b; UAS-DERDN;FRT82B; FRT82B rase1B; FRT82B rase2F; UAS-rlRNAi;FRT82B; FRT82B kay2; FRT82B kay3; FRT40A;UAS-fosAA; FRT40A;UAS-fos7A; FRT42D EgfrtopCO and FRT42D Egfrtsla (gifts from N. Baker, Albert Einstein College of Medicine, Bronx, NY, USA).
Flip-out stock is hsFlp;act>CD2>Gal4,UAS-GFP.
2- to 4-day-old mated female flies were heat-shocked for 45 minutes at 37°C to induce somatic recombination. Clones were observed 7 days after induction.
Immunostaining and microscopy
Intact guts were fixed at room temperature for 45 minutes in 100 mM glutamic acid, 25 mM KCl, 20 mM MgSO4, 4 mM sodium phosphate, 1 mM MgCl2, 4% formaldehyde. All subsequent incubations were done in PBS, 0.5% BSA, 0.1% TritonX-100 at 4°C.
The following primary antibodies were used: mouse anti-dpErk (Sigma; 1:100); mouse anti-BrdU (Becton Dickson; 1:200); mouse anti-Prospero, anti-Armadillo and anti-β-galactosidase (Developmental Studies Hybridoma Bank; 1:250, 1:100 and 1:500, respectively); rabbit anti-pH3 (Upstate; 1:1000). Fluorescent secondary antibodies were obtained from Jackson ImmunoResearch. Hoechst was used to stain DNA.
Confocal images were collected using a Leica SP5 confocal system and processed using the Leica software and Adobe Photoshop.
Western blot
Intact guts were dissected and proteins extracted in Laemmli buffer, separated on 10% acrylamide gel and transferred according to standard procedures. Antibodies directed against dpERK (M8159; 1:1000 dilution) and ERK (M5670; 1:5000) are from Sigma. Total proteins from four guts and one gut were used for dpERK and total ERK, respectively.
Analysis of gene expression in the gut
Young mated females were transferred for 7 days at 29°C to allow the expression of RNAi constructs. Total RNA from six dissected guts was extracted using Trizol (Invitrogen), according to manufacturer instructions. cDNA was synthesized using an oligo-dT primer. Real-time PCR was performed on a Bio-Rad iQ5 detection system using the following primers (5′ to 3′): EGFR forward TGGCGATCGTTAAGTCATCCCTGT; EGFR reverse TGCACTGATCCGAGCAAATGGTTC; vein forward TTCCGAGCTAATAGTGCGCTCCTT; vein reverse ATAGACCTCGTTGATGTCCGGGAT; actin5c forward CTCGCCACTTGCGTTTACAGT; actin5c reverse TCCATATCGTCCCAGTTGGTC. Relative expression of Egfr and vein were normalized to Actin5C levels.
Paraquat and bleomycin treatments
For all stress experiments, young mated females were cultured on standard food at 29°C for 2 days, in order to induce transgene expression. Flies were then starved for 6 hours in empty vials at room temperature and re-fed with a sucrose solution (5%; mock) with or without 5 mM paraquat or 10 μg/ml bleomycin sulfate. Flies were maintained at 29°C and dissected 24-48 hours later.
Statistical analysis
For all experiments, the data is represented as mean ± s.e.m. All P-values were calculated using unpaired two-tailed Student's t-test.
RESULTS
EGFR activity is essential for ISC proliferation
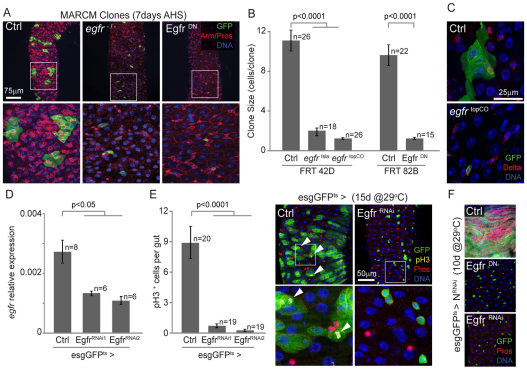
EGF signaling is crucial for the development of the adult midgut in Drosophila (Jiang and Edgar, 2009). The expression of multiple EGF-like ligands in a temporally and spatially defined manner ensures the proper proliferation of progenitors during larval and pupal development. Recent studies indicated that expression of these ligands is maintained in the adult intestine, suggesting that epithelial regeneration might be influenced by EGFR signaling (Buchon et al., 2009b; Jiang and Edgar, 2009). To test this hypothesis, we generated Egfr homozygous mutant ISC clones using the temperature-sensitive allele Egfrtsla (Kumar et al., 1998), or a null allele [EgfrtopCO (Clifford and Schupbach, 1989)], as well as clones overexpressing a dominant negative form of the EGF receptor (EGFRDN), in the posterior midgut using somatic recombination through the mosaic analysis with a repressible cell marker (MARCM) method (Lee and Luo, 1999). In wild-type flies, GFP-marked ISC cell clones grew to ~10-12 cells within 7 days (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006) (Fig. 1A,B). However, Egfr mutant clones and clones expressing DERDN showed very limited growth, mostly consisting of single cells that maintain the expression of Delta, a specific marker for ISCs in the posterior midgut, indicating that ISC proliferation was significantly reduced, whereas ISC survival was not affected (Fig. 1A-C). Accordingly, these single cell clones were maintained in the intestinal epithelium for at least 15 days (see Fig. S1A,B in the supplementary material). EGFR is thus essential for ISC proliferation, but is not required for ISC survival.
Fig. 1.
EGF receptor activity is essential for ISC proliferation. (A-C) MARCM clones overexpressing a dominant-negative form of EGFR (DERDN) and homozygous mutant clones for Egfr (egfr−/−) fail to grow, demonstrating that components of the EGFR activity is required for ISC proliferation. Boxed areas are magnified in the lower panels. Quantification of the clone size, measured by the number of cells per clone, 7 days after clone induction [7d after heat shock (AHS)] is shown in B. Error bars represent s.e.m. FRT 42D, MARCM using Flip recombination target at 42D; FRT 82B, MARCM using Flip recombination target at 82B. Staining for the ISC-specific marker Delta (red), indicates that Egfr-null single cell clones (egfrtopCO) are non-dividing ISCs (C). (D) Egfr expression in the intestine, 5 days after induction of expression of dsRNA constructs against Egfr, using the esgGal4 driver. Expression of the two distinct transgenes significantly alters Egfr expression compared with controls. (E) Knockdown of Egfr in ISCs and EBs using the temperature-sensitive driver esgGFPts (esgGal4;tubGal80ts) is sufficient to prevent age-related induction of proliferation and intestinal dysplasia. Proliferation was quantified by counting the number of pH3+ cells per gut after immunostaining, 15 days after transgenes induction at 29°C. Representative confocal images are shown to illustrate the reduced number of pH3+ cells (indicated by arrowheads) and limited intestinal dysplasia observed in esgGFPts>EgfrRNAi flies. Boxed areas are magnified in the lower panels. (F) Reduction of Egfr activity prevents NotchRNAi-induced tumor formation. EgfrDN or EgfrRNAi transgenes were expressed together with NRNAi using esgGFPts. Ten days after induction, tumors composed of esg+ and PROS+ cells accumulate in the posterior midgut of control flies (NRNAi alone), whereas the intestinal epithelium architecture is preserved when EGFR activity is inhibited. In A, C, E and F, GFP expression is shown in green, cell boundaries are stained using β-Catenin/Armadillo (red, membrane), EEs are marked by the expression of Prospero (red nuclei) and DNA is labeled using Hoechst (blue). EB, enteroblast; EE, enteroendocrine cells; ISC, intestinal stem cell; MARCM, mosaic analysis with a repressible cell marker.
Analysis of EGFR-deficient single cell-clones further suggests that EGFR signaling is not required for EE or EC differentiation: as somatic recombination occurs in the G2 stage of the cell cycle of asymmetrically dividing ISCs, only 50% of the marked cells generated by the MARCM method became clone-generating ISCs, whereas the other 50% became EBs, which will undergo differentiation into a single GFP-positive EE or EC. When Egfr-null or EGFRDN-expressing clones were generated, single GFP-positive EEs and ECs were observed in frequencies comparable with wild-type conditions (see Fig. S1C in the supplementary material).
To confirm further that EGFR is required for ISC proliferation, we used an inducible system to express two independent dsRNA constructs directed against the Egfr mRNA (EgfrRNAi) in ISCs and EBs [using esgGal4 together with ubiquitously expressed temperature-sensitive Gal80, tubGal80ts; combined with UAS-GFP, this system is termed esgGFPts here (Micchelli and Perrimon, 2006)]. Expression of either one of the EgfrRNAi constructs efficiently repressed Egfr expression in the intestine (Fig. 1D), confirming that Egfr is expressed in ISCs and/or EBs. We used the mitotic marker phosphorylated histone H3 (pH3) to assess the frequency of ISC divisions in the intestine (Choi et al., 2008; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006) (Fig. 1E). Because in young flies only a few pH3+ cells can be detected (suggesting that unchallenged ISCs divide rarely or slowly), we assessed the requirement of EGFR for ISC proliferation in backgrounds with well-established increases in ISC proliferation rates: in aging flies; after oxidative challenge (exposure to the ROS-inducing compound paraquat); or when Notch signaling was disrupted in ISCs and EBs. In old and ROS-challenged flies, ISC proliferation was strongly increased owing to activation of stress signaling pathways. Loss of Notch prevents EB differentiation into ECs, and causes unchecked expansion of ISCs and EEs into tumor-like structures (Biteau et al., 2008; Choi et al., 2008; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). When EGFR expression was knocked down, the prevalence of pH3+ cells and the accumulation of esgGFP+ cells at 15 days of age was significantly reduced, whereas paraquat-induced proliferation of ISCs was inhibited by expression of EGFRDN (Fig. 1E; see Fig. S1D in the supplementary material). Similarly, the formation of Notch mutant ISC and EE tumors was significantly impaired, indicating that loss of EGFR is sufficient to prevent ISC proliferation independently of Notch signaling (Fig. 1F). Altogether, our results demonstrate that the activity of the EGF receptor is thus essential for ISC proliferation under normal conditions, as well as in response to stress or mitogenic signals, without affecting ISC survival or differentiation in the ISC lineage.
The MAPK signaling pathway is required for ISC proliferation
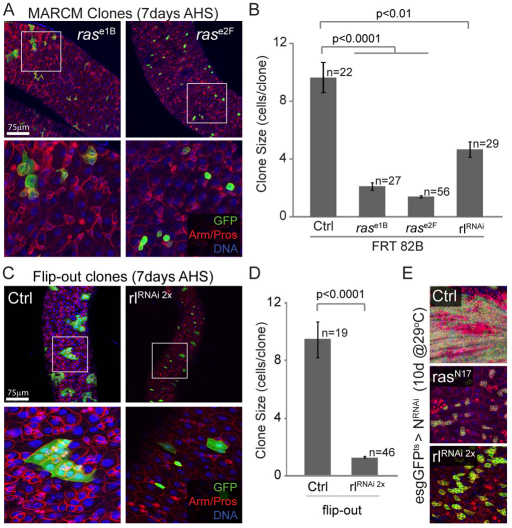
EGFR signaling is transduced by the MAPK signaling pathway in Drosophila, including the small GTPase RAS (encoded by Drosophila Ras85D) and the MAP kinase ERK [encoded by Drosophila gene rolled (rl)] (Shilo, 2005). We assessed whether these downstream components are also required for stem cell proliferation using the MARCM and flip-out lineage tracing techniques (Lee and Luo, 1999; Pignoni and Zipursky, 1997). Similar to Egfr loss-of-function conditions, ISCs homozygous for Ras loss-of-function alleles or expressing a dominant negative form of RAS or rolledRNAi failed to generate multi-cell clones (Fig. 2A-D). Inhibition of RAS and ERK is also sufficient to inhibit NRNAi- and paraquat-induced proliferation (Fig. 2E; see Fig. S1D in the supplementary material), confirming that the MAPK/ERK pathway is required to maintain proliferative competence of ISCs.
Fig. 2.
Components of the MAPK signaling pathway are required for ISC proliferation. (A) MARCM clones homozygous for Ras loss-of-function alleles fail to grow compared with control clones (see Fig. 1A). Boxed areas are magnified in the lower panels. (B) Quantification of clone size 7 days after induction, including MARCM clones expressing one copy of the rolledRNAi construct. Error bars represent s.e.m. (C,D) Flip-out clones overexpressing two copies of the rolledRNAi construct mostly remain as single stem cells, confirming the essential role of ERK in ISC proliferation. The clone size, measured by the number of cells per clone, 7 days after clone induction, is quantified in D. (E) Inhibition of Ras and ERK prevents NotchRNAi-induced tumor formation. Confocal images of posterior midguts co-expressing a dominant form of Ras (rasN17) or two copies of the rolledRNAi construct with NRNAi in ISCs and EBs, 10 days after induction at 29°C. In A, C and E, GFP expression is shown in green, cell boundaries are stained using β-Catenin/Armadillo (red, membrane), EEs are marked by the expression of Prospero (red nuclei) and DNA is labeled using Hoechst (blue). EB, enteroblast; EE, enteroendocrine cells; ISC, intestinal stem cell; MARCM, mosaic analysis with a repressible cell marker.
Expression of the EGFR ligand Vein in the muscle is partially required for ISC proliferation
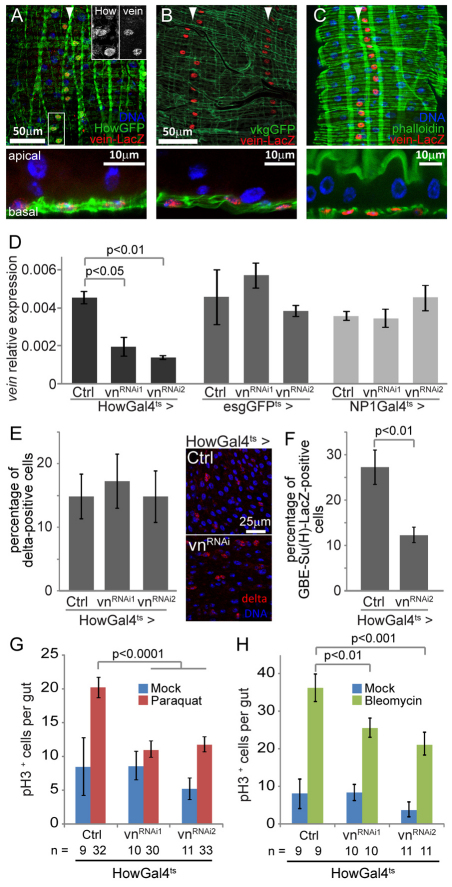
The requirement of EGFR/ERK signaling activity for ISC proliferation suggests that an EGF-like ligand is secreted from a neighboring source, generating a niche-like microenvironment for ISCs that maintains proliferative competence. During the development of the adult intestine, the muscle surrounding the intestinal epithelium expresses the neuregulin homolog vein, one of the Drosophila EGFR ligands, and it has been suggested that this expression is preserved in adult intestines (Jiang and Edgar, 2009). Using a reporter construct expressing nuclear β-galactosidase, we confirmed that vein promoter activity is maintained in the adult visceral muscle, as indicated by the colocalization of β-galactosidase positive nuclei with GFP expressed under the control of the muscle-specific HowGal4 driver and with phalloidin staining, as well as with a basement membrane component, vkgGFP (also known as collagen IV) (Fig. 3A-C). Importantly, expression of two independent veinRNAi constructs in the muscle using the HowGal4 driver was sufficient to strongly reduce the expression of vein in the whole intestine, as determined by qRT-PCR, whereas expressing these dsRNA constructs in ISCs/EBs or ECs had no effect (Fig. 3D). The visceral muscle is thus the primary source of Vein in the adult midgut and is a candidate for providing the signal required for ISC proliferation. We first tested this idea by assessing whether vein expression in the muscle is required for ISC proliferation under normal conditions. Indeed, inhibition of vein expression in the muscle for 5 days did not affect the number of ISCs (identified by the expression of Delta; Fig. 3E), but significantly reduced the number of EBs in the intestinal epithelium (identified by the expression of the GBE-Su(H)-lacZ reporter; Fig. 3F), suggesting that ISC division is reduced in these conditions. We assessed further the requirement for vein expression in stress-induced ISC proliferation. Compared with wild-type controls, the number of pH3+ cells detected after exposure to paraquat or to the genotoxic compound bleomycin (Amcheslavsky et al., 2009) was significantly lower in animals expressing veinRNAi in the muscle (Fig. 3G,H). vein expression in the muscle is thus required for optimal ISC proliferation.
Fig. 3.
The EGFR ligand vein is expressed in the intestinal muscle and is required for ISC proliferation. (A-C) The Vein-lacZ transcriptional reporter is expressed in muscle. Nuclear β-galactosidase expression is detected by immunostaining (red), in longitudinal rows of cells located basally, along the entire posterior midgut (indicated by arrowheads). Cells positive for the Vein-lacZ reporter also express GFP when driven with the muscle-specific HowGal4 driver (A, inserts show single channel images of the boxed area), demonstrating that Vein-lacZ is expressed in muscle. Co-localization with vkgGFP (a GFP fusion with the basement membrane component collagen IV) and phalloidin (staining F-actin) further demonstrates the basal position of β-galactosidase+ cells in the epithelium (B,C). (D) vein mRNA can be detected in the intestine and is expressed in muscle. Expression of dsRNA constructs against Vein in the muscle (HowGal4ts driver) is sufficient to significantly reduce mRNA level in the intestine, whereas expression of vnRNAi constructs in ISCs or EBs (esgGFPts driver) or in enterocytes (NP1Gal4ts driver) has no effect. The expression of vein is measured by real-time RT-PCR, relative to the expression of Actin5c. (E) vein knockdown in the muscle does not affect ISC maintenance. The proportion of Delta-positive cells is similar in the epithelium of control and HowGal4ts>vnRNAi flies 5 days after transgenes induction. Representative images are shown to illustrate the maintenance of small Delta-positive cells in the epithelium of HowGal4ts>vnRNAi flies (shown in red). (F) vein knockdown reduces the number of EBs. The proportion of cells positive for the EB marker GBE-Su(H)-lacZ is significantly lower in the intestine of HowGal4ts>vnRNAi flies compared with wild-type controls. (G,H) Knocking down vein expression in the muscle limits stress-induced proliferation. The number of pH3-positive cells, in mock-treated flies or after exposure to paraquat or bleomycin (for 24 and 48 hours, respectively) was measured in the epithelium of control and HowGal4ts>vnRNAi flies. EB, enteroblast; ISC, intestinal stem cell.
ERK activation is a permissive signal required to promote cell proliferation
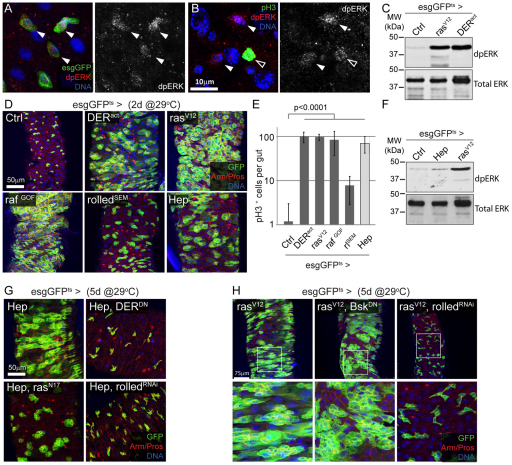
To monitor the activity of the EGFR signaling pathway in the intestinal epithelium, we detected the active, double-phosphorylated (dp) form of ERK using immunohistochemistry (Gabay et al., 1997). In the posterior midgut of young flies, dpERK could be detected in ISCs (Fig. 4A) and enteroendocrine cells (not shown), but not in ECs. Interestingly, the vast majority of these cells were dpERK positive (>80% of esg-positive cells), even though under unstressed conditions most ISCs were not or only slowly dividing (Biteau et al., 2008; Choi et al., 2008; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006) (Fig. 4E; in young flies, only 10% of esg+ cells incorporate BrdU within 48 hours, not shown). This suggests that the level of ERK activity in ISCs under such basal, non-stressed conditions is not sufficient to promote high rates of cell division. The lack of mitotic activity in the majority of cells does not seem to be due to fluctuations of ERK activity, as the intensity of dpERK staining in pH3+ and pH3 ISCs was indistinguishable (Fig. 4B). Although we cannot formally exclude the possibility that ERK is activated very transiently during early phases of the cell cycle, we deduce that ERK activation in these cells provides a permissive, but not inductive, signal for proliferation. Interestingly, however, expression of an oncogenic form of RAS is sufficient to promote widespread ISC division (Apidianakis et al., 2009), indicating that strong EGFR gain-of-function conditions can overcome a threshold and provide a sufficient signal for ISC division. To confirm this interpretation, we expressed activated forms of EGFR [DER-ellipse (Baker and Rubin, 1989)], RAS [rasV12 (Karim and Rubin, 1998)], RAF [rafGOF (Brand and Perrimon, 1994)] and ERK [rolledSEM (Brunner et al., 1994)] using the esgGFPts driver. Expression of any of these constructs for 2 days resulted in strongly increased levels of activated ERK in the intestine (Fig. 4C) and correspondingly very high numbers of pH3+ cells in the intestinal epithelium accompanied by a dramatic expansion of esg+ cells (Fig. 4D,E). Strong activation of ERK is thus sufficient to promote ISC proliferation. Importantly, however, expression of the JNK Kinase Hemipterous [HEP (Biteau et al., 2008)] strongly increased ISC proliferation without significantly increasing ERK phosphorylation in the intestine (Fig. 4E,F), suggesting that, as in other biological contexts, RAS and HEP signal through independent signaling pathways to induce ISC proliferation (Ciapponi et al., 2001; Kockel et al., 2001; Luo et al., 2007; Suzanne et al., 2001; Weston and Davis, 2002). These findings further support the notion that elevated ERK phosphorylation is not required to induce ISC proliferation under stress conditions, and that the basal activity of ERK that is maintained by muscle-derived Vein constitutes a permissive signal for ISC division. In this model, EGFR signaling is required to maintain proliferative competence of ISCs, whereas activation of JNK signaling or other stress-responsive signaling pathways is required to stimulate proliferation of ISCs in response to oxidative stress, tissue damage or infection (Amcheslavsky et al., 2009; Apidianakis et al., 2009; Biteau et al., 2008; Buchon et al., 2009a; Buchon et al., 2009b; Chatterjee and Ip, 2009; Choi et al., 2008; Cronin et al., 2009; Jiang et al., 2009). Accordingly, JNK [encoded by the Drosophila gene basket (bsk)] is required for oxidative stress and DNA damage-induced proliferation of ISCs, but not for proliferation under homeostatic conditions: inhibition of JNK, by expressing either dominant-negative bsk [BskDN (Weber et al., 2000) or a dsRNA against bsk [BskRNAi (Hull-Thompson et al., 2009)] in ISCs and EBs significantly decreased paraquat- and bleomycin-induced proliferation (see Fig. S2B,C in the supplementary material), whereas ISCs homozygous for the bsk loss-of-function alleles bsk2 and bsk170B generated normally sized clones in unchallenged flies (see Fig. S2A in the supplementary material). Confirming the permissive versus inductive nature of EGFR/ERK and JNK signaling in ISC proliferation, JNK activity was not required for RAS-induced overproliferation of ISCs (Fig. 4H). Conversely, EGFR signaling is required for JNK-induced proliferation (Fig. 4G; see Fig. S3 in the supplementary material).
Fig. 4.
The MAPK signaling pathway is active in ISCs and its activation is sufficient to promote ISC proliferation. (A) The active, double-phosphorylated (dp) form of ERK can be detected by immunostaining in the ISCs under normal conditions (red, left-hand panel; monochrome, right-hand panel). (B) Similar dpERK staining is observed in dividing (pH3-positive; open arrowhead) and non-dividing ISCs (solid arrowhead). (C) Expression of activated forms of the EGFR (DERact) or RAS (rasV12), using the esgGFPts driver, dramatically increases the level of activated ERK in the intestine. dpERK is detected by Western blot from dissected midguts 2 days after transgene induction at 29°C. Levels of total ERK protein serve as loading control. (D,E) Activation of the EGFR/MAPK and JNK signaling pathways is sufficient to induce ISC proliferation, resulting in intestinal dysplasia. Activated forms of DER (DERact), RAS (rasV12), RAF (rafGOF) and ERK (rolledSEM; rlSEM) or HEP (Hep) were expressed for 2 days in ISCs using the temperature-sensitive driver (esg-Gal4,UAS-GFP;tub-Gal80ts). Proliferation rates in the midgut were quantified (E) by counting the number of pH3+ cells per gut in the same genetic conditions. (F) Activation of JNK in ISCs and EBs does not significantly elevate dpERK levels. dpERK is detected by Western blot from dissected midguts 2 days after transgene induction at 29°C. Levels of total ERK protein serve as loading control. (G) Overexpression of HEP in ISCs and EBs results in dyplastic phenotype in the intestinal epithelium (esgGFPts>Hep). Co-expression of dominant forms of EGFR (DERDN) and ras (rasN17), or a dsRNA construct directed against ERK (rolledRNAi), is sufficient to prevent HEP-induced dysplasia. (H) RAS-induced dysplasia specifically requires ERK activity. Inhibiting ERK (rolledRNAi) prevents the expansion of esg+ cells observed when RasV12 is expressed under the control of the esgGFPts driver. Co-expression of a JNK dominant form (BskDN) does not affect this phenotype. Boxed areas are enlarged in lower panels. EB, enteroblast; ISC, intestinal stem cell.
The transcription factor FOS is required for JNK- and ERK-induced ISC proliferation
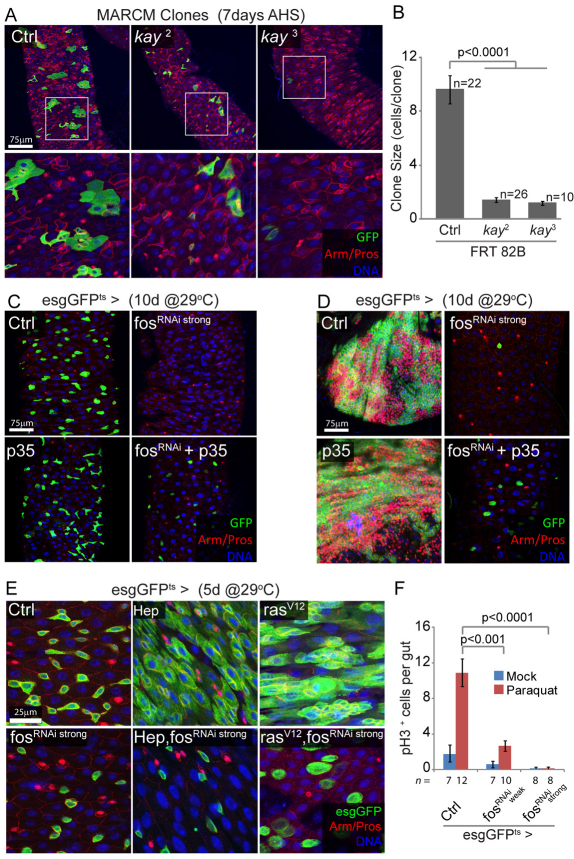
The proliferative response of ISCs both to activation of JNK and of EGFR/ERK signaling pathways raises the possibility that a common downstream effector might mediate these responses and thus integrate permissive and inductive signals. The AP-1 transcription factor FOS is a well-described target of JNK signaling in Drosophila and has been shown to also respond to EGFR signaling in various biological contexts, such as developing imaginal discs (Ciapponi et al., 2001). FOS might thus serve as a convergence point for JNK and ERK responses in ISCs. To test this idea, we first assessed the requirement for FOS in proliferating ISCs. We generated homozygous mutant clones for the fos loss-of-function alleles kay2 and kay3. The resulting GFP-positive clones failed to grow, and often remained restricted to a single stem cell (Fig. 5A,B). This result suggests that FOS is required for ISC proliferation. kay2 [a hypomorphic mutant allele that does not result in cell lethality (Zeitlinger et al., 1997)] clones were induced at frequencies comparable to wild-type clones, but clones homozygous for the strong loss-of-function allele kay3 (a P-element insertion into the kay locus) were recovered at much lower frequency, suggesting that strongly reducing FOS function in ISCs affects stem cell survival. To confirm these effects of fos loss-of-function on ISC proliferation and survival, we used two distinct transgenic constructs allowing mild or strong expression of dsRNA directed against FOS [fosRNAi (Hyun et al., 2006)]. Flip-out clones expressing fosRNAi weak show much reduced growth compared with wild-type clones (see Fig. S4A,B in the supplementary material). In addition, expression of fosRNAi strong for 10 days in ISCs and EBs using esgGal4 results in the complete loss of esg+ cells in the intestinal epithelium. This cell loss could be rescued by overexpressing the anti-apoptotic protein p35, demonstrating that strong knockdown of FOS in ISCs results in their death by apoptosis (Fig. 5C). Similarly, strong inhibition of FOS in a Notch loss-of-function background (esgGFPts>NRNAi) prevented ISC overproliferation and resulted in the apoptotic death of esg-positive cells. However, preventing apoptosis of these Notch mutant ISCs did not restore proliferation (Fig. 5D), supporting the conclusion that FOS activity is essential for both ISC proliferation and survival, and demonstrating that these functions of FOS are separable.
Fig. 5.
FOS is required for stem cell survival and proliferation, downstream of JNK and RAS. (A,B) FOS is required in ISCs for clone formation. Posterior midguts showing MARCM clones homozygous for fos (kay) loss-of-function alleles, 7 days after induction. Mutant clones remain smaller than controls, often limited to single stem cells, and are recovered less frequently for the kay3 allele. Boxed areas are enlarged in lower panels. Clone size (number of cells per clone) is quantified in B. FRT 82B, MARCM using Flip recombination target at 82B. (C) Prolonged inhibition of FOS results in ISC death by apoptosis. Expression of the fosRNAi construct that causes strong knockdown of FOS expression, using the esgGFPts driver, leads to the disappearance of esg+ cells. This phenotype is rescued when the anti-apoptotic protein p35 is co-expressed, whereas expression of p35 alone has no effect. (D) A similar experiment performed in N loss-of-function background demonstrates that p35 expression rescues cell death but is unable to restore NRNAi-induced proliferation and tumor formation, suggesting that FOS affects both survival and proliferative capacity of ISCs. (E) FOS is required for HEP- and RAS-induced ISC proliferation. Intestines 5 days after induction of HEP or rasV12 together with fosRNAi, using the temperature-sensitive esgGal4 driver. The HEP- and rasV12-induced expansion of esg+ cells is entirely blocked by fosRNAi, demonstrating that FOS is required downstream of JNK and RAS. Note that knocking down FOS does not block RAS-induced cell growth. (F) Paraquat-induced ISC proliferation requires FOS. Expression of two different fosRNAi constructs, using esgGFPts (for 2 days at 29°C prior to treatment), significantly reduces Paraquat-induced proliferation in the intestinal epithelium, as shown by the limited number of pH3+ cells per gut 48 hours after paraquat exposure. In A, C and D GFP is shown in green, armadillo (Arm) outlines cell boundaries (red), prospero (Pros) identifies EEs (nuclear red), DNA is shown in blue. AHS, after heat shock; EE, enteroendocrine cells; ISC, intestinal stem cell; MARCM, mosaic analysis with a repressible cell marker.
Next, we wanted to investigate whether FOS relays EGFR/ERK and JNK signaling in the regulation of stem cell proliferation. To test this idea directly, we assessed the effect of impairing FOS function on JNK- or RAS-induced proliferation. When FosRNAi was expressed together with either HEP or RasV12 using the esgGal4 driver, no expansion of esg+ cells or increase in pH3+ cells was observed (Fig. 5E; see Fig. S4C in the supplementary material). Note that, when RasV12 and FosRNAi were co-expressed, the size of the esg+ cells increased, probably owing to fos-independent effects of RAS activation on cell growth. Similarly, we found that FOS is required for the paraquat-induced increase in the frequency of pH3+ cells and BrdU incorporation in the intestinal epithelium (Fig. 5F; see Fig. S4D in the supplementary material).
Distinct phosphorylation sites in FOS mediate JNK- and RAS/ERK-induced ISC proliferation
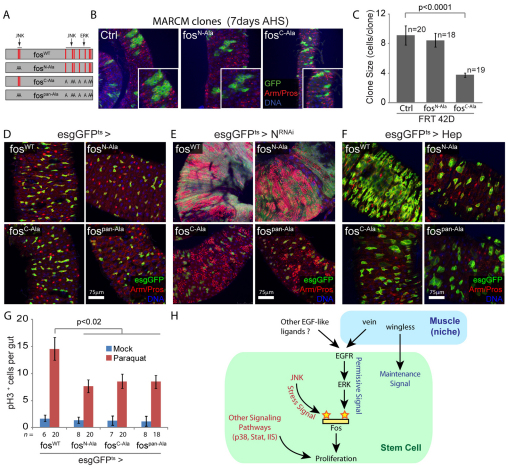
These results strongly suggest that FOS is essential for both JNK and EGFR-mediated proliferation in ISCs. It remains possible, however, that FOS acts primarily as an ERK target and is thus generally required for ISC proliferation, but does not directly respond to the JNK-mediated inductive signal. Interestingly, a function of FOS directly downstream of JNK and ERK in Drosophila has been suggested, as the two kinases can phosphorylate FOS on overlapping and distinct sites (Ciapponi et al., 2001). This suggests a potential mechanism by which FOS might integrate permissive and instructive signals to regulate ISC proliferation. In vitro, JNK phosphorylates two residues located in the N-terminal part of the FOS protein, whereas both ERK and JNK can phosphorylate residues in the C-terminal domain (Ciapponi et al., 2001) (Fig. 6A). The importance of these distinct phosphorylation sites for FOS function in vivo has been tested in developmental contexts. Expression of a mutant form of FOS carrying alanine substitutions of the N-terminal phosphorylation sites (FOSN-Ala) dominantly interferes with JNK-dependent thorax closure, but has no effect on ERK-dependent wing vein formation. Conversely, expression of a mutant form in which the C-terminal phosphorylation sites are replaced (FOSC-Ala), recapitulates ERK mutant phenotypes in the developing wing, without affecting JNK function during thorax closure (Ciapponi et al., 2001). These mutants thus provide unique tools to selectively perturb JNK- or ERK-specific signaling to FOS.
Fig. 6.
FOS integrates JNK and ERK signaling pathways through distinct phosphorylation sites. (A) Schematic representation of the FOS protein and the different mutants carrying substitution of JNK and/or ERK phosphorylation sites. Red lines indicate phosphorylation sites. (B,C) Posterior midguts showing MARCM clones overexpressing fosC-Ala and fosN-Ala, 7 days after induction. Expression of FOS carrying substitution ERK phosphorylation sites (fosC-Ala) prevents the formation of large clones. Insets show enlarged cells. Clone size (number of cells per clone) is quantified in C. (D) Expression of FOS mutant forms in ISCs and EBs does not affect the architecture of the posterior midgut. (E) ERK phosphorylation sites are required for N loss-of-function tumor formation. Intestines 10 days after induction of NRNAi and fos mutants carrying substitution of JNK and/or ERK phosphorylation sites. Expression of the mutant forms lacking ERK phosphorylation prevents NRNAi-induced ISC overproliferation. (F) ERK and JNK phosphorylation sites are required for HEP-induced proliferation. Representative confocal images of intestines 5 days after induction of JNK and HEP together with the wild-type or mutant forms of FOS in ISCs/EBs. The HEP-induced expansion of esg+ cells is blocked by all the FOS mutant forms. (G) Overexpression of FOS mutant forms partially prevents paraquat-induced stem cell proliferation, as shown by reduced number of pH3+ cells, 48 hours after paraquat exposure. In B, D and E, GFP is shown in green, armadillo (Arm) outlines cell boundaries (red), prospero (Pros) identifies EEs (nuclear red), DNA is shown in blue. (H) Model representing the role of the EGFR signaling pathway and circular muscle acting as a niche for ICS and the integration of EGFR and JNK signaling by FOS in ISCs to regulate proliferation. EB, enteroblast; EE, enteroendocrine cells; ISC, intestinal stem cell; MARCM, mosaic analysis with a repressible cell marker.
To assess the effect of ERK or JNK-specific FOS phosphorylation on ISC proliferation, we generated MARCM clones overexpressing these different phosphorylation point mutants of FOS and tested their influence on ISC proliferation under normal conditions. Expression of FOS mutant for ERK C-terminal phosphorylation sites (FOSC-Ala) prevented the formation of large clones in the posterior midgut, whereas overexpression of FOSN-Ala had no effect on clone size (Fig. 6B,C). Similarly, expressing FOS mutants in which the ERK phosphorylation sites were replaced, strongly reduced NRNAi-induced proliferation and tumor formation, whereas mutating JNK phosphorylation sites had no effect (Fig. 6D-F). This strongly suggests that ERK-dependent phosphorylation of FOS is a permissive signal required for stem cell proliferation, and confirms that JNK-dependent regulation of FOS is not required under normal conditions.
To further confirm this model, we assessed the consequences of expressing these FOS variants in conditions in which ISC proliferation is induced by JNK. The expression of any of the three mutant forms of FOS (JNK-specific, FOSN-Ala; ERK-specific, FOSC-Ala; and combined, FOSpan-Ala) was sufficient to prevent HEP-induced expansion of esg+ cells (Fig. 6F), and significantly reduced paraquat-induced proliferation (Fig. 6G), further supporting the notion that JNK-mediated phosphorylation of FOS is required in addition to ERK phosphorylation to mediate the instructive signal promoting ISC proliferation in response to stress.
DISCUSSION
Our findings establish a crucial role for EGF signaling in the regulation of ISC proliferation, and thus support the notion that the visceral muscle surrounding the intestinal epithelium has the characteristics of a functional niche. vein expression in the muscle maintains the competence of ISCs to enter rapid proliferation in responses to stress and JNK signaling, and is thus expected to regulate epithelial homeostasis. Interestingly, we find that both the EGFR-mediated permissive signal and the JNK-derived inductive signal are relayed by FOS, establishing an integrated molecular mechanism for the control of ISC proliferation (Fig. 6H).
The visceral muscle: a niche for ISCs?
Many stem cell populations are regulated by their microenvironments, and larval ISC progenitors are regulated by a transient niche (Mathur et al., 2010). However, ISCs in adult flies apparently lack such a closely associated cell population within the intestinal epithelium. By contrast, control of ISC maintenance by muscle-derived Wingless suggested this tissue as a potential functional niche for adult ISCs (Lin and Xi, 2008; Lin et al., 2008). Our results support and extend this idea by identifying a second growth factor derived from the visceral muscle that controls ISC proliferation. In its regulation of stem cell function through Wingless and Vein, and in the close association of ISCs and muscle cells, the muscle thus shares characteristics of stem cell niches in other systems, yet it also differs from these in important ways. In mammals, as well as in the Drosophila and C. elegans gonads, the niche of most stem cell populations maintains stem cell quiescence and prevents differentiation (Jones and Wagers, 2008; Morrison and Spradling, 2008; Voog and Jones, 2010). The EGF signal originating from the muscle, however, maintains the capacity of ISCs to divide, allowing these cells to respond to stimulating signals while not affecting ISC differentiation. Interestingly, EGFR signaling has not been described so far as crucial for interactions between the niche and stem cell populations in other systems, and our findings raise the possibility that this signaling pathway might also regulate the function of other stem cell populations in both invertebrates and vertebrates.
Potential function for additional EGF-like ligands
Whereas knocking down the expression of vein in the muscle partially affects the ability of ISCs to proliferate under normal conditions and in response to stress, the inhibition of EGFR completely abolishes stem cell division. This might reflect the inefficiency of the veinRNAi constructs used in our study, but might also suggest a contribution of other EGFR ligands to the regulation of ISC function. Accordingly, a genome-wide analysis of the transcriptional response of the adult intestine to bacterial infection suggests that expression of vein, as well as of two other genes encoding EGFR ligands, Keren and spitz, is increased after immune challenge (Buchon et al., 2009b). However, the potential role for these additional EGF-like ligands in regulating ISC function remains to be investigated and the cells expressing spitz and Keren in the adult intestine have yet to be identified.
Integration of mitogenic and stress signals by FOS
ISC function is regulated by systemic [insulin-like peptides expressed by neurosecretory cells in the brain (Amcheslavsky et al., 2009)], muscle-derived [vein and wingless (this study) (Lin et al., 2008)], local [unpaired cytokines expressed by ECs (Buchon et al., 2009a; Buchon et al., 2009b; Chatterjee and Ip, 2009; Cronin et al., 2009; Jiang et al., 2009; Lin et al., 2009)] and cell-intrinsic [JNK and PVR/p38 signaling (Biteau et al., 2008; Buchon et al., 2009a; Choi et al., 2008; Park et al., 2009)] signals. These multiple signals are integrated in ISCs to adapt their proliferation rate and differentiation program to environmental and physiological challenges. To fully understand stem cell regulation in this high-turnover tissue, the molecular structure of this signaling network has to be unraveled. Our findings introduce the transcription factor FOS as a crucial regulator of ISC proliferation that integrates mitogenic and stress signals, and indicate that JNK and ERK regulate FOS activity directly by phosphorylation on distinct residues, controlling ISC proliferation in a combinatorial fashion. This signal-specific mode of FOS regulation by ERK and JNK in Drosophila had previously been described in the context of morphogenetic movements (in which FOS is regulated by JNK) and of eye and wing growth during development (in which it is regulated by ERK and JNK) (Ciapponi et al., 2001).
How FOS promotes ISC proliferation remains unclear. In developing imaginal discs, inhibition of FOS causes an accumulation of cells in the G2/M phase of the cell cycle, probably owing to a loss of Cyclin B expression, an essential regulator of the G2/M transition (Hyun et al., 2006). Interestingly, in ISCs, expression of FosRNAi not only inhibits stress-induced accumulation of pH3+ cells, but also represses BrdU incorporation (see Fig. S4D in the supplementary material), indicating that FOS regulates entry into S phase. In these cells, FOS might thus regulate the transcription of essential S phase components. Further studies will be required to identify such ISC-specific FOS target genes.
AP-1 and intestinal homeostasis
The maintenance of stem cells in a primed state, ready to respond to inductive mitogenic stress signals, is likely to be crucial for high-turnover tissues like the intestinal epithelium, which require rapid activation of stem cell division for an efficient regenerative response to tissue damage. At the same time, this enhanced mitogenic potential of ISCs might contribute to the loss of tissue homeostasis in the aging gut (Biteau et al., 2008), and contribute to cancer formation in mammalian intestinal epithelia (Barker et al., 2009; van der Flier and Clevers, 2009). Interestingly, a conserved role of AP-1 transcription factors and JNK signaling in the regulation of intestinal stem cell proliferation and intestinal cancer is emerging in mice. JNK activation is sufficient to induce cell proliferation in the intestinal crypt and increases tumor incidence and tumor growth in an inflammation-induced colon cancer model (Sancho et al., 2009). These effects of JNK signaling are mediated by the FOS binding partner JUN, as shown by the requirement for JNK-mediated phosphorylation of JUN for APCmin/+-induced tumorigenesis (Nateri et al., 2005). Strikingly, ISC-specific activation of WNT signaling, by mutating APC or expressing an active form of β-catenin or wingless itself, is sufficient to induce the formation of tumor-like stem cell clusters in the fly intestine (Lee et al., 2009; Lin et al., 2008). A potential interaction of WNT signaling with JNK and JUN or FOS in ISCs remains to be tested in Drosophila. Interestingly, increased FOS activity has also recently been shown to be sufficient to promote hematopoietic stem cell self-renewal in mice, further illustrating the conserved function of FOS in the regulation of stem cell function (Deneault et al., 2009). AP-1 transcription factors are thus emerging as conserved essential regulators of stem cell function and our findings provide an important starting point for further studies characterizing stem cell-specific signaling networks that integrate mitogenic, survival and stress signals to control stem cell maintenance, quiescence and proliferation, and thus influence the balance between regeneration and tumor suppression in high turnover tissues.
Supplementary Material
Acknowledgements
We thank Dirk Bohmann, Jason Karpac and Christine Hochmuth for comments on the manuscript. This work was supported by the National Institute on Aging (NIH RO1 AG028127), NYSTEM (grant # N08G-048) and the Ellison Medical Foundation (AG-SS-2224-08) to H.J., as well as an AFAR/Ellison Medical Foundation postdoctoral fellowship to B.B. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.056671/-/DC1
References
- Amcheslavsky A., Jiang J., Ip Y. T. (2009). Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4, 49-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y., Pitsouli C., Perrimon N., Rahme L. (2009). Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc. Natl. Acad. Sci. USA 106, 20883-20888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. E., Rubin G. M. (1989). Effect on eye development of dominant mutations in Drosophila homologue of the EGF receptor. Nature 340, 150-153 [DOI] [PubMed] [Google Scholar]
- Barker N., van de Wetering M., Clevers H. (2008). The intestinal stem cell. Genes Dev. 22, 1856-1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., Ridgway R. A., van Es J. H., van de Wetering M., Begthel H., van den Born M., Danenberg E., Clarke A. R., Sansom O. J., Clevers H. (2009). Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608-611 [DOI] [PubMed] [Google Scholar]
- Beebe K., Lee W. C., Micchelli C. A. (2010). JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev. Biol. 338, 28-37 [DOI] [PubMed] [Google Scholar]
- Biteau B., Hochmuth C. E., Jasper H. (2008). JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3, 442-455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B., Karpac J., Supoyo S., Degennaro M., Lehmann R., Jasper H. (2010). Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 6, e1001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. (1994). Raf acts downstream of the EGF receptor to determine dorsoventral polarity during Drosophila oogenesis. Genes Dev. 8, 629-639 [DOI] [PubMed] [Google Scholar]
- Brunner D., Oellers N., Szabad J., Biggs W. H., 3rd, Zipursky S. L., Hafen E. (1994). A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 76, 875-888 [DOI] [PubMed] [Google Scholar]
- Bryder D., Rossi D. J., Weissman I. L. (2006). Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am. J. Pathol. 169, 338-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Broderick N. A., Chakrabarti S., Lemaitre B. (2009a). Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 23, 2333-2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Broderick N. A., Poidevin M., Pradervand S., Lemaitre B. (2009b). Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200-211 [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Ip Y. T. (2009). Pathogenic stimulation of intestinal stem cell response in Drosophila. J. Cell. Physiol. 220, 664-671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N. H., Kim J. G., Yang D. J., Kim Y. S., Yoo M. A. (2008). Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell 7, 318-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciapponi L., Jackson D. B., Mlodzik M., Bohmann D. (2001). Drosophila Fos mediates ERK and JNK signals via distinct phosphorylation sites. Genes Dev. 15, 1540-1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford R. J., Schupbach T. (1989). Coordinately and differentially mutable activities of torpedo, the Drosophila melanogaster homolog of the vertebrate EGF receptor gene. Genetics 123, 771-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin S. J., Nehme N. T., Limmer S., Liegeois S., Pospisilik J. A., Schramek D., Leibbrandt A., Simoes Rde M., Gruber S., Puc U., et al. (2009). Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325, 340-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C., Stamataki D., Lewis J. (2006). Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet. 7, 349-359 [DOI] [PubMed] [Google Scholar]
- Deneault E., Cellot S., Faubert A., Laverdure J. P., Frechette M., Chagraoui J., Mayotte N., Sauvageau M., Ting S. B., Sauvageau G. (2009). A functional screen to identify novel effectors of hematopoietic stem cell activity. Cell 137, 369-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay L., Seger R., Shilo B. Z. (1997). In situ activation pattern of Drosophila EGF receptor pathway during development. Science 277, 1103-1106 [DOI] [PubMed] [Google Scholar]
- Gopinath S. D., Rando T. A. (2008). Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell 7, 590-598 [DOI] [PubMed] [Google Scholar]
- Hull-Thompson J., Muffat J., Sanchez D., Walker D. W., Benzer S., Ganfornina M. D., Jasper H. (2009). Control of metabolic homeostasis by stress signaling is mediated by the lipocalin NLaz. PLoS Genet. 5, e1000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J., Becam I., Yanicostas C., Bohmann D. (2006). Control of G2/M transition by Drosophila Fos. Mol. Cell. Biol. 26, 8293-8302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Edgar B. A. (2009). EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136, 483-493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., McEwen D. G., Edgar B. A. (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. L., Wagers A. J. (2008). No place like home: anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 9, 11-21 [DOI] [PubMed] [Google Scholar]
- Karim F. D., Rubin G. M. (1998). Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development 125, 1-9 [DOI] [PubMed] [Google Scholar]
- Kockel L., Homsy J. G., Bohmann D. (2001). Drosophila AP-1: lessons from an invertebrate. Oncogene 20, 2347-2364 [DOI] [PubMed] [Google Scholar]
- Kumar J. P., Tio M., Hsiung F., Akopyan S., Gabay L., Seger R., Shilo B. Z., Moses K. (1998). Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development 125, 3875-3885 [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L. (1999). Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451-461 [DOI] [PubMed] [Google Scholar]
- Lee W. C., Beebe K., Sudmeier L., Micchelli C. A. (2009). Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development 136, 2255-2264 [DOI] [PubMed] [Google Scholar]
- Lin G., Xi R. (2008). Intestinal stem cell, muscular niche and Wingless signaling. Fly 2, 310-312 [DOI] [PubMed] [Google Scholar]
- Lin G., Xu N., Xi R. (2008). Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455, 1119-1123 [DOI] [PubMed] [Google Scholar]
- Lin G., Xu N., Xi R. (2009). Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of Drosophila intestinal stem cells. J. Mol. Cell Biol. 2, 37-49 [DOI] [PubMed] [Google Scholar]
- Luo X., Puig O., Hyun J., Bohmann D., Jasper H. (2007). Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. EMBO J. 26, 380-390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur D., Bost A., Driver I., Ohlstein B. (2010). A transient niche regulates the specification of Drosophila intestinal stem cells. Science 327, 210-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. E., Le P. T., Osborn A. J., Matsumoto K., Davis R. L. (2003). Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302, 1765-1768 [DOI] [PubMed] [Google Scholar]
- Micchelli C. A., Perrimon N. (2006). Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475-479 [DOI] [PubMed] [Google Scholar]
- Morrison S. J., Spradling A. C. (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598-611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nateri A. S., Spencer-Dene B., Behrens A. (2005). Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437, 281-285 [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. (2006). The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470-474 [DOI] [PubMed] [Google Scholar]
- Ohlstein B., Spradling A. (2007). Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315, 988-992 [DOI] [PubMed] [Google Scholar]
- Park J.-S., Kim Y.-S., Yoo M.-A. (2009). The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila. Aging 1, 637-651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignoni F., Zipursky S. L. (1997). Induction of Drosophila eye development by decapentaplegic. Development 124, 271-278 [DOI] [PubMed] [Google Scholar]
- Pitsouli C., Apidianakis Y., Perrimon N. (2009). Homeostasis in infected epithelia: stem cells take the lead. Cell Host Microbe 6, 301-307 [DOI] [PubMed] [Google Scholar]
- Radtke F., Clevers H. (2005). Self-renewal and cancer of the gut: two sides of a coin. Science 307, 1904-1909 [DOI] [PubMed] [Google Scholar]
- Rando T. A. (2006). Stem cells, ageing and the quest for immortality. Nature 441, 1080-1086 [DOI] [PubMed] [Google Scholar]
- Rossi D. J., Jamieson C. H., Weissman I. L. (2008). Stems cells and the pathways to aging and cancer. Cell 132, 681-696 [DOI] [PubMed] [Google Scholar]
- Sancho R., Nateri A. S., de Vinuesa A. G., Aguilera C., Nye E., Spencer-Dene B., Behrens A. (2009). JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 28, 1843-1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N. E., DePinho R. A. (2007). How stem cells age and why this makes us grow old. Nat. Rev. Mol. Cell Biol. 8, 703-713 [DOI] [PubMed] [Google Scholar]
- Shilo B. Z. (2005). Regulating the dynamics of EGF receptor signaling in space and time. Development 132, 4017-4027 [DOI] [PubMed] [Google Scholar]
- Suzanne M., Perrimon N., Noselli S. (2001). The Drosophila JNK pathway controls the morphogenesis of the egg dorsal appendages and micropyle. Dev. Biol. 237, 282-294 [DOI] [PubMed] [Google Scholar]
- van der Flier L. G., Clevers H. (2009). Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241-260 [DOI] [PubMed] [Google Scholar]
- Voog J., Jones D. L. (2010). Stem cells and the niche: a dynamic duo. Cell Stem Cell 6, 103-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber U., Paricio N., Mlodzik M. (2000). Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development 127, 3619-3629 [DOI] [PubMed] [Google Scholar]
- Weston C. R., Davis R. J. (2002). The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 12, 14-21 [DOI] [PubMed] [Google Scholar]
- Zeitlinger J., Kockel L., Peverali F. A., Jackson D. B., Mlodzik M., Bohmann D. (1997). Defective dorsal closure and loss of epidermal decapentaplegic expression in Drosophila fos mutants. EMBO J. 16, 7393-7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.