Abstract
Previous studies demonstrated that a subset of synMuv B mutants ectopically misexpress germline-specific P-granule proteins in their somatic cells, suggesting a failure to properly orchestrate a soma/germline fate decision. Surprisingly, this fate confusion does not affect viability at low to ambient temperatures. Here, we show that, when grown at high temperature, a majority of synMuv B mutants irreversibly arrest at the L1 stage. High temperature arrest (HTA) is accompanied by upregulation of many genes characteristic of germ line, including genes encoding components of the synaptonemal complex and other meiosis proteins. HTA is suppressed by loss of global regulators of germline chromatin, including MES-4, MRG-1, ISW-1 and the MES-2/3/6 complex, revealing that arrest is caused by somatic cells possessing a germline-like chromatin state. Germline genes are preferentially misregulated in the intestine, and necessity and sufficiency tests demonstrate that the intestine is the tissue responsible for HTA. We propose that synMuv B mutants fail to erase or antagonize an inherited germline chromatin state in somatic cells during embryonic and early larval development. As a consequence, somatic cells gain a germline program of gene expression in addition to their somatic program, leading to a mixed fate. Somatic expression of germline genes is enhanced at elevated temperature, leading to developmentally compromised somatic cells and arrest of newly hatched larvae.
Keywords: synMuv B, Gem line, Chromatin, Larval arrest, Temperature, Caenorhabditis elegans
INTRODUCTION
The development of an organism relies on a series of cell fate decisions. One of the most pivotal of these decisions is that between soma and germ line. It is important to specify a small number of germ cells within a mostly somatic organism in order to ensure both the survival of the individual and its fertility. The single-cell embryo has a number of attributes of the germ line, including an inherited chromatin state compatible with gene expression patterns in germ cells. In mammals, erasure of germline character is one of the first steps in embryogenesis; primordial germ cells are newly induced later in development (for a review, see Saitou and Yamaji, 2010). In Caenorhabditis elegans and Drosophila, germ cells are segregated away from somatic cells very early in development and the erasure of germline characteristics in the soma appears to be more gradual (for a review, see Nakamura and Seydoux, 2008). In all cases, the somatic cells of embryos face the challenge of reprogramming their chromatin away from a germline state and towards a somatic state.
Synthetic multivulva class B (synMuv B) proteins are a conserved class of transcriptional repressors that have been implicated in the soma/germline fate decision. They were originally classified based on the synthetic multivulva phenotype displayed when a class B mutation is combined with a class A or class C mutation (for a review, see Fay and Yochem, 2007). synMuv B proteins include the single worm homolog of retinoblastoma, LIN-35; E2F components, EFL-1 and DPL-1; a worm homolog of HP1, HPL-2; and members of the worm NuRD complex, MEP-1 and LET-418. synMuv B mutants also show a number of distinct phenotypes as single mutants, several of which suggest that somatic cells have acquired germline-like characteristics, including enhanced RNAi, transgene silencing and expression of germ-granule proteins (Lehner et al., 2006; Unhavaithaya et al., 2002; Wang et al., 2005). Surprisingly, these germline characteristics do not appear to have deleterious affects on somatic cell development, as synMuv B single mutants grow to adulthood when cultured at low to ambient temperatures (Andersen et al., 2008).
In the present study, we show that elevated temperature causes synMuv B mutants to arrest as young larvae. This high temperature arrest (HTA) phenotype is only seen in those synMuv B mutants that ectopically express numerous germline-specific proteins, including P-granule components and meiosis proteins, in their soma. The number of ectopically expressed genes and the level of ectopic expression are both enhanced by elevated temperature. Ectopic expression of germline genes and HTA are suppressed by loss of MES-4, the MES-2/3/6 complex and other germline chromatin modifiers. Our findings suggest that synMuv B(+) function antagonizes a germline chromatin state in somatic cells, and that this chromatin-level regulation is inherently sensitive to temperature and is necessary for worm viability. We identified the intestine as the somatic tissue responsible for synMuv B HTA. We hypothesize that the intestine is more susceptible to a germline fate transformation than other somatic tissues and that a partial intestine-to-germline transformation impairs the crucial role of the intestine in nutrient uptake.
MATERIALS AND METHODS
Strains and culture
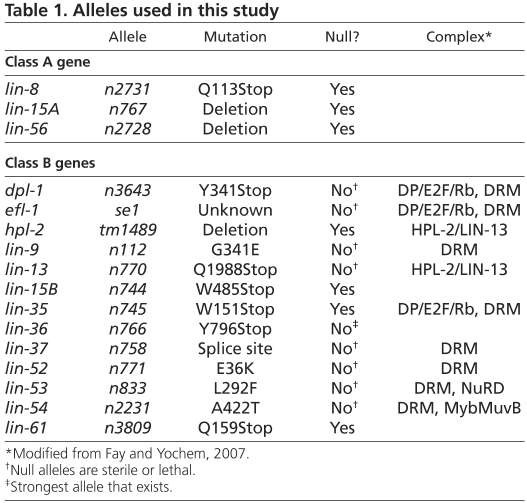
C. elegans were cultured (Brenner, 1975) at 20°C unless otherwise noted. N2 (Bristol) was used as wild type (WT). SS0991 containing the transgene bnEx56[elt-2p::lin-35:GFP +rol-6] and SS1009 lin-35(n745); bnEx56[elt-2p::lin-35:GFP +rol-6] were created for this study. The OLB11 strain rde-1(ne219);dusIs[elt-2p::rde-1 +rol-6] was a gift from Olaf Bossinger (Pilipiuk et al., 2009). Mutant alleles used are listed below and in Table 1:
LGI: lin-35(n745), lin-53(n833), lin-61(n3809)
LGII: lin-8(n2731), dpl-1(n3643), lin-56(n2728)
LGIII: lin-13(n770), lin-37(n758), lin-9(n112), lin-52(n771), hpl-2(tm1489), lin-36(n766), mrg-1(qa6200)
LGIV: lin-54(n2231)
LGV: efl-1(se1), rde-1(ne219), mes-4(bn85)
LGX: lin-15A(n767), lin-15B(n744).
Table 1.
Alleles used in this study
Larval arrest assays
L4 larvae were placed at either 24°C or 26°C for ~18 hours and then moved to new plates and allowed to lay embryos for 5 hours. Progeny were scored.
Immunocytochemistry
Worms were fixed using methanol/acetone (Strome and Wood, 1983). L1 larvae were obtained by hatching embryos in the absence of food in M9 buffer and fixed when starved or after feeding for ~5 hours. Primary antibody dilutions were: 1:30,000 for rabbit anti-PGL-1 (Kawasaki et al., 1998), 1:10,000 for rat anti-PGL-3 (Kawasaki et al., 2004), 1:5000 for rabbit anti-GLH-1 (Gruidl et al., 1996), 1:1000 for guinea pig anti-HTP-3 (MacQueen et al., 2005) and 1:1000 for mouse anti-ELT-2 (gift of Jim McGhee, University of Calgary, Calgary, Alberta, Canada). Secondary antibodies conjugated to Alexa Fluor 488 or 594 (Molecular Probes) were used at 1:300 for 2 hours at room temperature. Images were acquired with a Volocity spinning disk confocal system (Perkin-Elmer/Improvision, Norwalk, CT, USA) fitted on a Nikon Eclipse TE2000-E inverted microscope.
Microarray analysis
Total RNA was isolated from starved synchronized L1 worms born at 26°C. Isolation of RNA and linear amplification were performed as described (Chi and Reinke, 2006). Fluorescently labeled cDNA samples were prepared and hybridized to long oligomer microarrays for the C. elegans genome from the Washington University Genome Sequencing Center as previously described (Reinke et al., 2000). Microarray normalization and analysis were performed with analysis tools in Bioconductor (www.bioconductor.org) using R statistical programming language. For each gene in each microarray hybridization experiment, the ratio of RNA levels from the two samples was transformed into a log2 value and the mean log2 ratio was calculated. The log2 ratios were normalized by print-tip Loess normalization (Dudoit and Yang, 2002). All genes with a false discovery rate of ≤5% (q≤0.05) (Storey and Tibshirani, 2003) and a mean fold-change ratio of ≥1.5 were selected for further analysis. Microarray data have been deposited to Gene Expression Omnibus with accession numbers GSE26823, GSE26824 and GSE26825.
Assigning HTA categories
For each HTA candidate gene, we evaluated expression patterns based on microarray identification of genes with germline-enriched expression (Reinke et al., 2004), SAGE (serial analysis of gene expression) of germline, muscle, neuronal and intestinal tissue (Meissner et al., 2009; Wang et al., 2009), and in situ hybridization data from the NEXTDB database (http://nematode.lab.nig.ac.jp/db2/index.php). Germline-enriched genes included those present in the Reinke et al. data set and those with 2× more SAGE tags in germline than in other tissues. Intestine-enriched genes included those with 2× more SAGE tags in intestine than in other tissues. Tissue-specific genes included genes with SAGE tags only in that tissue.
Quantitative RT-PCR
Total RNA was isolated using Trizol (Invitrogen) from three biological replicates of synchronized L1s born at either 20°C or 26°C. cDNA synthesis and quantitative PCR (qPCR) were carried out as described (Updike and Strome, 2009). All experiments were normalized to act-2.
RNAi
RNAi by feeding was carried out using clones from the Ahringer library (Fraser et al., 2000) except for mes-2, which was made in the Strome laboratory. Feeding experiments were carried out on NGM plates containing 0.2% lactose and ampicillin as described above for larval arrest assays. dsRNA for injection was made as described by Cui et al. (Cui et al., 2006). Young adults were injected with 850 ng/μl of dsRNA and placed at 26°C. Worms were allowed to recover for 16-18 hours before single worms were placed on individual plates for 24 hours. Progeny were scored after 4 days.
RESULTS
A subset of synMuv B mutants arrest as larvae at high temperature
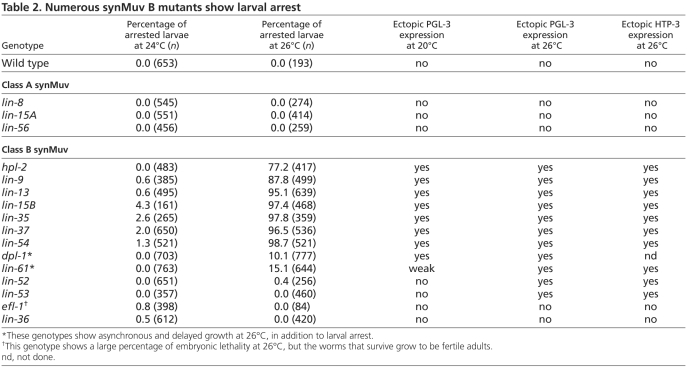
Several synMuv B mutants display the fascinating phenotype of ectopically expressing germline-specific P-granule proteins in their somatic tissues (Unhavaithaya et al., 2002; Wang et al., 2005). Two synMuv B mutants, mep-1 and let-418, arrest development as young larvae but, surprisingly, the remaining synMuv B mutants can grow to adulthood and thus do not appear to be negatively impacted by the misexpression of germline traits in somatic cells (Andersen et al., 2008; Unhavaithaya et al., 2002). To investigate this further, we assessed the viability of synMuv B mutants at different temperatures. We and others have observed that numerous mutants in germline processes display their mutant phenotypes exclusively or most strongly at elevated temperature (e.g. Conine et al., 2010; Kawasaki et al., 1998; Spike et al., 2008). Indeed, we found that when grown at elevated temperature (26°C) numerous synMuv B mutants arrest development as young larvae. Of the 13 synMuv B mutants tested (see alleles in Table 1), seven arrested with high penetrance at 26°C, two arrested with low penetrance at 26°C, and four did not arrest at 26°C (Table 2). The three synMuv A mutants tested did not arrest at 26°C (Table 2). All genotypes tested showed no or very low levels of arrest when grown just two degrees cooler (24°C). Thus, the viability of a subset of synMuv B mutants is severely compromised at high temperature (26°C) but not at lower temperatures (24°C and below). We refer to this as high temperature arrest (HTA) and focus in this paper on elucidating the gene expression and cellular defects that lead to HTA.
Table 2.
Numerous synMuv B mutants show larval arrest
Analysis of lin-35 and lin-37 mutants showed that HTA was observed only when larvae lacked both a maternal load (M) and zygotic expression (Z) of synMuv B(+) product (i.e. see M–Z− in Table S1 in the supplementary material). Neither M+Z− nor M–Z+ mutant larvae arrested at 26°C (see Table S1 in the supplementary material). Thus, either maternally or zygotically expressed synMuv B(+) gene function can promote larval viability.
The temperature-sensitive period for HTA is during late embryogenesis and early larval development and HTA is not reversible
To determine the temperature-sensitive period for HTA, we performed temperature-shift experiments on lin-13, lin-15B and lin-35 mutants (see Fig. S1 in the supplementary material). Downshifting gravid adults or mixed stage embryos from 26°C to 20°C rescued the growth of all L1s; downshifting hatched L1s did not rescue their growth. These results suggest that the temperature-sensitive period for HTA is after embryogenesis and that once arrest has occurred it is not reversible. Upshifting gravid adults from 20°C to 26°C led to arrest of all progeny, upshifting mixed stage embryos and L1s led to arrest of most progeny, and upshifting after L1 did not lead to arrest. These results suggest that the temperature-sensitive period is during embryogenesis and L1. Thus, both downshift and upshift experiments indicate a temperature-critical period in the hours of development leading up to the HTA arrest stage.
Ectopic expression of P-granule proteins in synMuv B mutants is enhanced at high temperature
To investigate whether ectopic P-granule protein expression in synMuv B mutants contributes to the HTA phenotype, we determined whether P-granule protein expression in the somatic cells of synMuv B mutants, like larval arrest, is sensitive to temperature. We examined all mutants for ectopic P-granule formation at 20°C and 26°C using three different P-granule components: PGL-1, PGL-3 and GLH-1 (Fig. 1; data not shown). Previous work suggested that only a subset of the mutants that show HTA in our experiments display ectopic expression of PGL-1 at 20°C (Wang et al., 2005). Examining PGL-3 at 20°C, we observed ectopic expression in all synMuv B genotypes that show high or low penetrance HTA, although ectopic PGL-3 expression in lin-61 was fairly weak (Table 2). We did not observe ectopic PGL-3 expression at 20°C in synMuv B mutants that did not show HTA or in synMuv A mutants (Table 2).
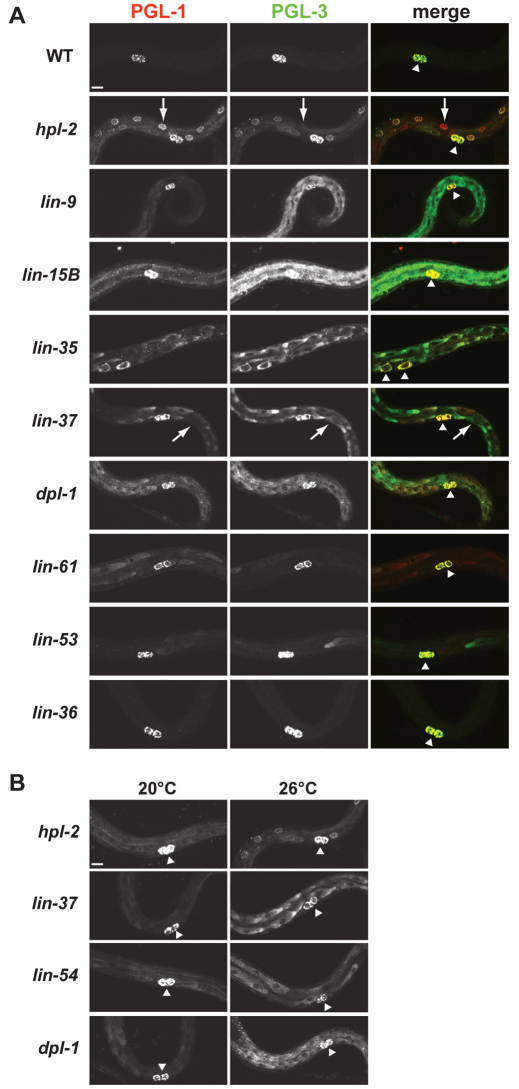
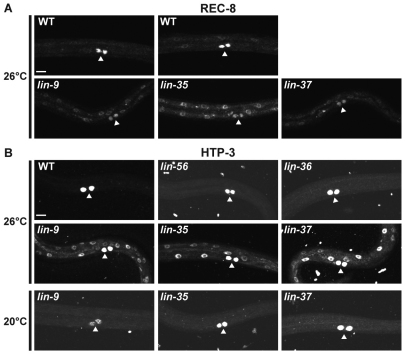
Fig. 1.
synMuv B mutants show temperature-sensitive expression of P-granule proteins in somatic cells. (A) PGL-1 (red) and PGL-3 (green) staining in wild-type (WT) and synMuv B mutant L1s at 26°C. Wild-type L1s display staining only in the two primordial germ cells (PGCs) Z2 and Z3 (arrowheads), whereas mutants show a range of ectopic expression. In some cells the levels of PGL-1 and PGL-3 differ (arrows). (B) PGL-3 staining in synMuv B mutants at 20°C and 26°C. PGCs (arrowheads). Scale bar: 10 μm.
Ectopic expression of P-granule proteins in somatic cells of synMuv B mutants is elevated at 26°C compared with 20°C (Fig. 1B; Table 2; see Table S2 in the supplementary material). Different genotypes manifest high temperature enhancement in different ways. hpl-2 and lin-13 showed an increased level and more punctate staining of P-granule proteins; the punctae were often perinuclear, as observed in germ cells (Fig. 1; see Table S2 in the supplementary material; data not shown). Other genotypes, such as lin-37, lin-54 and dpl-1, showed increased levels of ectopic P-granule staining that remained diffuse even at high temperature (Fig. 1; see Table S2 in the supplementary material). Some genotypes, such as lin-9, lin-15B and lin-35, displayed such high levels of ectopic P-granule protein expression at 20°C that increased expression at 26°C was not obvious. Surprisingly, two genotypes that did not display HTA or show ectopic P-granule protein staining at 20°C, lin-52 and lin-53, showed ectopic PGL-3 expression at 26°C (Table 2; Fig. 1A; data not shown). Thus, all genotypes that demonstrate HTA also have ectopic P-granule expression at 20°C and most show enhanced expression at 26°C. However, ectopic expression is not sufficient to cause HTA, as lin-52 and lin-53 display ectopic P-granule expression at 26°C but do not show HTA.
HTA can be suppressed by loss of MES and MRG-1 function
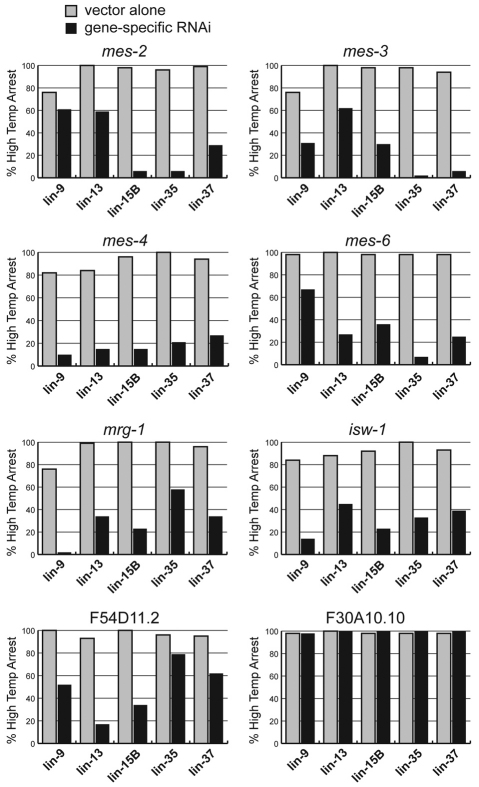
Loss of the germline chromatin modifiers MES-4, MES-2, MES-3, and MES-6 can suppress somatic expression of germline traits in synMuv B mutants (Cui et al., 2006; Unhavaithaya et al., 2002; Wang et al., 2005). If somatic expression of germline traits in synMuv B mutants underlies the HTA phenotype, then loss of MES(+) function should also suppress the HTA phenotype. To test this, we used RNAi to deplete MES function in five synMuv B backgrounds that show HTA (Fig. 2). We also used RNAi to deplete two other germline chromatin modifiers, MRG-1 and ISW-1, which have been shown previously to suppress the synthetic multivulva phenotype of synMuv mutants (Cui et al., 2006). Depletion of any of these six germline chromatin proteins suppressed the HTA phenotype of the five synMuv B mutants tested (Fig. 2). The ability of specific depletions to suppress the HTA phenotype indicates that HTA is not due to general sickliness at elevated temperature, and reveals that the wild-type functions of MES proteins, MRG-1, and ISW-1 promote HTA. Extending the RNAi findings to genetic tests using lin-15B mutants demonstrated that maternally supplied MES-4(+) and zygotically produced MRG-1(+) promote HTA (see Table S3 in the supplementary material).
Fig. 2.
Loss of germline chromatin modifiers suppresses high temperature arrest (HTA). L4 synMuv B mutants of the five genotypes shown were transferred to either empty vector or gene-specific RNAi bacteria at 26°C, and progeny were scored for HTA. n=41-146.
RNAi of MES proteins, MRG-1, and ISW-1 in an otherwise wild-type background results in sterility, raising the possibility that loss of or impairment of the germ line can suppress the HTA phenotype. We tested RNAi of two additional genes, F30A10.10 and F54D11.2, that are known to suppress the synMuv phenotype but have different effects on germline development (Cui et al., 2006). RNAi of F30A10.10 caused a high percentage of sterility in wild-type worms tested (100% sterility, n=321) but did not suppress HTA, whereas F54D11.2 RNAi caused a low percentage of sterility in wild-type worms (11.9% sterility, n=292) but was a strong suppressor of HTA (Fig. 2; data not shown). Thus, RNAi of genes that result in sterility is not sufficient to suppress the HTA phenotype. Only certain germline-required products are involved in arrest.
Numerous germline genes are upregulated in lin-35 mutant larvae and downregulated in lin-35; mes-4(RNAi) larvae
To investigate the possibility that HTA-prone synMuv B mutant larvae globally misexpress genes whose expression is characteristic of the germ line, we performed microarray analysis. We compared RNA accumulation profiles from lin-35 versus wild-type and lin-15B versus wild-type starved L1s at 26°C. Compared with wild type, 1327 genes were significantly upregulated in lin-15B mutant L1s (≥1.5-fold change, q≤0.05, see Materials and methods). In lin-35 mutant L1s, 667 genes were significantly upregulated, 344 (52%) of which were also upregulated in lin-15B (see Table S4 in the supplementary material). Fewer genes are downregulated in the two mutants compared with wild type: 753 and 137 genes in lin-15B and lin-35, respectively (see Table S5 in the supplementary material). Only 31 downregulated genes are shared between them. The high percentage of shared upregulated genes in lin-15B and lin-35 larvae suggests a common HTA pathway in synMuv B mutants.
We took advantage of the fact that mes-4 RNAi can suppress the HTA phenotype to investigate whether a subset of misregulated genes underlies the HTA phenotype. Given our working model that loss of MES-4 can suppress the HTA phenotype by re-establishing a more wild-type gene expression pattern in somatic cells, we looked for genes that are misregulated in lin-35 and expressed at closer to wild-type levels in lin-35; mes-4(RNAi). We identified 128 genes that were upregulated in lin-35 compared with wild type and downregulated in lin-35; mes-4(RNAi) compared with lin-35, and two genes that were downregulated in lin-35 and upregulated in lin-35; mes-4(RNAi) (see Table S6 in the supplementary material). These data suggest that larval arrest in lin-35 mutants is mainly caused by increased expression of certain genes and that depletion of MES-4 suppresses the HTA phenotype by downregulating at least some of those genes. We call this set of genes HTA candidate genes.
To assign HTA candidate genes to different tissue classes, we used a combination of data derived from microarray analysis of worms containing and lacking a germ line; SAGE analysis of transcripts in dissected germ lines and GFP-sorted intestine, muscle and neuronal cells; and expression data from the NEXTDB in situ hybridization database (Meissner et al., 2009; Reinke et al., 2004; Wang et al., 2009) (http://nematode.lab.nig.as.jp/db2/index.php) (Fig. 3B; see Table S6 in the supplementary material). The largest category of HTA candidate genes (38%) is genes that have germline character. The germline category includes not only the P-granule gene pgl-3 but also a number of well known meiotic genes such as syp-3, him-3 and rec-8. The second largest category (27%) is genes expressed in the intestine. Genes with germline- and intestine-specific expression are highly represented in the list of genes that are misregulated in lin-35 mutants (Grishok et al., 2008; Kirienko and Fay, 2007). We found that these categories are even more highly represented in the HTA candidate set (see Fig. S2 in the supplementary material). These findings suggest that upregulation of germline-specific and intestine-specific genes might play a key role in the HTA pathway and that reduced expression of those genes (e.g. after depletion of MES-4) rescues synMuv B L1s from arrest.
Fig. 3.
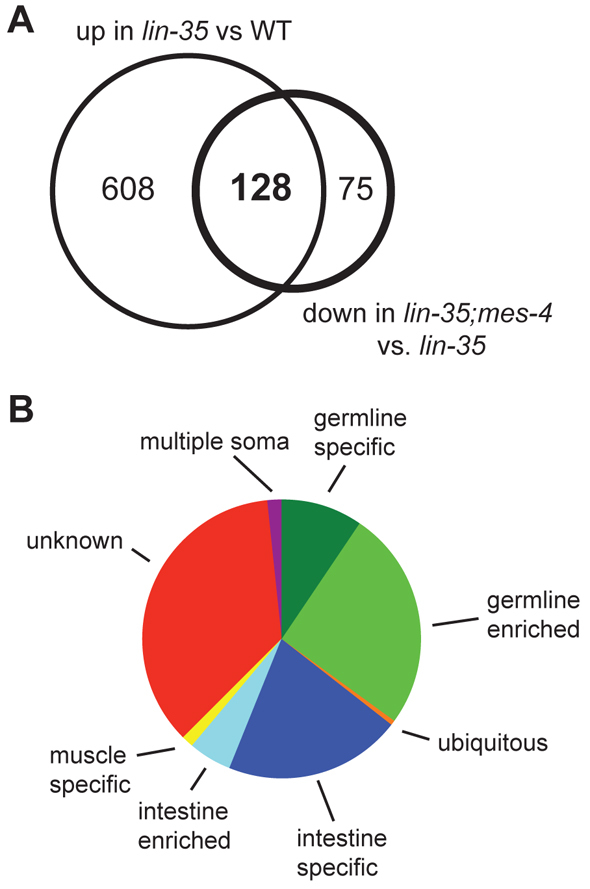
Germline and intestine genes are the predominant category of high temperature arrest (HTA) candidate genes. (A) The overlap of genes significantly upregulated in lin-35 versus wild type (WT) and those significantly downregulated in lin-35;mes-4(RNAi) versus lin-35 defines the HTA candidate genes. (B) Categories of HTA candidate genes based on published expression analysis (Reinke et al., 2004; Meissner et al., 2009; Wang et al., 2009) (see Materials and methods).
Meiosis proteins are expressed in somatic cells of synMuv B mutants at high temperature
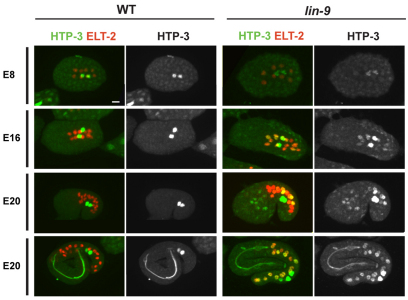
To test whether upregulation of HTA candidate genes in the germline class reflects inappropriate expression in somatic tissues of synMuv B mutants, we immunostained L1s for the protein products of germline genes. We focused on proteins involved in meiosis, a process normally restricted to germ cells and a category containing seven HTA candidate genes. We observed ectopic or increased somatic staining of three meiotic proteins, REC-8, HTP-1 and HTP-3, in the same synMuv B mutants that display ectopic expression of P-granule proteins (Table 2; Fig. 4). REC-8, a meiotic cohesin, was present at low levels in intestinal cells in wild-type L1s (Fig. 4A). However, we observed significantly more intestinal cells expressing REC-8 and generally higher levels of REC-8 in synMuv B mutants compared with wild type (see Fig. S3 in the supplementary material). HTP-1 and HTP-3, related components of the axial element of the synaptonemal complex, showed dramatic turn-on in the soma of all synMuv B mutants that show ectopic expression of PGL-3 (Table 2; Fig. 4B; data not shown). The somatic expression of HTP-1 and HTP-3 was dependent on growth at high temperature. Not all meiotic proteins tested were detectably expressed in the soma of synMuv B mutants (e.g. SYP-1, HIM-8 and ZIM-1); notably, these proteins were also not detectably expressed in the primordial germ cells (PGCs) of L1s. By contrast, most germline genes that showed ectopic somatic expression were also expressed in the PGCs: PGL-3, PGL-1, GLH-1 and HTP-3. The only exception to this was HTP-1. Ectopic expression of meiosis and P-granule proteins became visible during mid-to-late embryogenesis (Fig. 5; data not shown). Using ELT-2 expression to count E-derived intestinal cells, we found that maternally loaded HTP-3 protein disappeared from somatic cells in wild-type and synMuv B mutant embryos between the E8 and E16 cell stage. During this transition HTP-3 levels began to increase in PGCs in wild-type embryos, whereas in synMuv B mutant embryos HTP-3 levels increased in PGCs and also in patches of somatic cells (Fig. 5). In summary, arrest-prone synMuv B mutant L1s display high levels of somatic expression of several proteins whose expression is normally restricted to or enriched in the PGCs, and this misexpression requires growth at high temperature and begins at around the time of zygotic expression of these proteins in the PGCs. These findings are consistent with elevated levels of germline proteins in somatic cells causing larval arrest.
Fig. 4.
Meiosis genes are expressed in the soma of synMuv B mutants at 26°C. (A) REC-8 staining is bright in the primordial germ cells (PGCs; arrowheads) and undetectable or dim in the soma of wild type (WT), whereas synMuv B mutants show enhanced somatic staining. (B) HTP-3 staining is only detected in the PGCs (arrowheads) of wild type, whereas synMuv B mutants show somatic staining also, but only at 26°C. Scale bar: 10 μm.
Fig. 5.
Ectopic expression of HTP-3 begins in embryogenesis. HTP-3 (green) and ELT-2 (red) staining of wild-type (WT) and lin-9 mutant embryos at progressively later stages. HTP-3 staining is limited to the primordial germ cells (PGCs) in wild type after the E8 stage, but appears in additional somatic cells in lin-9 embryos. Scale bar: 10 μm.
HTA-prone synMuv B mutants show elevated levels of germline gene expression at 20°C and further enhancement at 26°C
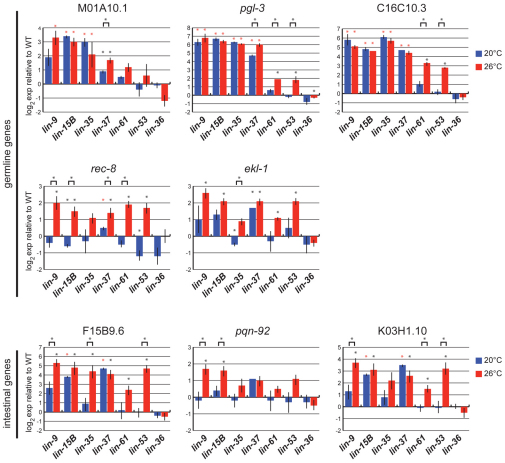
If upregulation of HTA candidate genes underlies the HTA phenotype, then we would expect to see significant upregulation of these genes in synMuv B genotypes that arrest, and no or low upregulation in synMuv B genotypes that do not arrest. We used quantitative RT-PCR to test these predictions. We examined seven synMuv B mutants from the four phenotype classes defined in Table 2. qPCR was carried out for five HTA candidate genes in the germline class, comparing wild type and synMuv B mutants born at either 20°C or 26°C. We observed two distinct expression patterns for these germline genes. The first pattern is demonstrated by M01A10.1, pgl-3 and C16C10.3, which showed a significantly higher level of upregulation in the HTA-prone synMuv B mutants than in the other synMuv B mutants (Fig. 6). The higher level of expression of these genes in HTA-prone genotypes was generally not dependent on temperature. The second pattern is demonstrated by rec-8 and ekl-1 (Fig. 6). These two genes were significantly upregulated at 26°C compared with 20°C in the four HTA-prone synMuv B mutants and also in the low and no HTA mutants lin-61 and lin-53. Thus, mutants that have a high percent of arrest have elevated expression of some germline genes at 20°C, and elevated expression of additional germline genes at 26°C. In synMuv B mutants that do not arrest or arrest at low frequency, fewer germline genes are upregulated. To test whether elevated expression of any single gene causes HTA, we performed RNAi knock-down in a lin-13 background of the 93 HTA candidate genes that are represented in the Ahringer RNAi library. We did not observe rescue of HTA (data not shown). Therefore, we hypothesize that it is the combined overexpression of numerous germline genes in somatic cells that leads to HTA.
Fig. 6.
Genes are more highly misexpressed in high temperature arrest (HTA)-prone genotypes. Quantitative RT-PCR of mRNA levels of eight HTA candidate genes in seven synMuv B mutant L1s relative to wild-type L1s at 20°C and 26°C. lin-9, lin-15B, lin 35 and lin-37 are HTA-prone mutants; lin-61 shows HTA at low frequency; lin-53 and lin-36 do not show HTA. Expression of the act-2 gene was used as an internal control. Black asterisks indicate a significant difference between the given genotype and wild type. Red asterisks indicate a significant difference between the given genotype and both wild type and lin-53. Black asterisks over a bracket indicate a significant difference between expression at 20°C and 26°C (P<0.05). Histograms are based on the mean of three biological replicates; error bars indicate s.e.m.
Given that the second largest group of HTA candidate genes is genes expressed in the intestine, we also analyzed the mRNA levels of three HTA candidate genes in the intestine category: F15B9.6, pqn-92 and K03H1.10 (Fig. 6). These genes showed a pattern of expression similar to rec-8 and ekl-1. Although there was some overexpression at 20°C, for most genotypes overexpression was more pronounced at 26°C. Furthermore, the HTA-prone synMuv B mutants and the low and no HTA mutants showed similar overexpression levels at 26°C. Although the overexpression of these genes might contribute to the HTA phenotype, their lack of differential expression between genotypes suggests that their misexpression alone is not responsible for HTA.
The intestine is the main tissue responsible for HTA
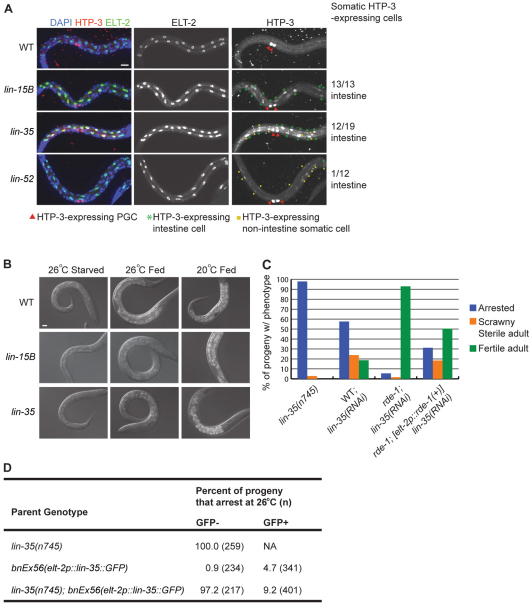
Wild-type C. elegans L1s that are born in the absence of food undergo starvation-induced arrest, raising the possibility that synMuv B mutant L1s born at high temperature also arrest owing to starvation. In fact, when staining for expression of germline proteins in synMuv B mutants, we noted that the predominant site of somatic expression was the intestine (Fig. 7A). lin-52 is an interesting exception: it showed ectopic expression of germline genes predominantly in non-intestinal somatic cells and did not show HTA (Fig. 7A, Table 2). As discussed above, the second largest defined category of HTA candidate genes is intestine genes. These results led us to speculate that compromised intestine function in synMuv B mutants might lead to starvation and HTA.
Fig. 7.
The intestine is responsible for high temperature arrest (HTA). (A) ELT-2 staining (green) highlights intestinal nuclei. In lin-15B and lin-35 mutant L1s the majority of somatic nuclei with HTP-3 staining (red) are intestinal nuclei. By contrast, in lin-52 mutant L1s the majority of somatic nuclei with HTP-3 signal are not intestinal. Red arrowheads indicate primordial germ cells (PGCs). Scale bar: 10 μm. (B) Gut granules, seen as increased birefringent material by differential interference contrast imaging, accumulate in the intestines of wild-type worms after 12 hours of feeding at either 26°C or 20°C. lin-15B and lin-35 mutant L1s are able to accumulate gut granules after feeding at 20°C but not after feeding at 26°C. Scale bar: 10 μm. See Fig. S4 in the supplementary material for autofluorescence images of gut granules. (C) Phenotypes of progeny of lin-35 mutant mothers, or after RNAi of lin-35 in wild type (WT) or RNAi-resistant rde-1 mutants or rde-1 mutants expressing RDE-1(+) in their intestine at 26°C. (D) A transgene array carrying lin-35(+) driven by the elt-2 promoter rescues HTA. From lin-35(n745); bnEx51[elt-2p::lin-35::GFP] mothers, the GFP− progeny that did not inherit the transgene arrested at high frequency at 26°C, whereas the GFP+ progeny that inherited the transgene arrested at low frequency.
We assessed the health of the intestine by examining gut granules, lysosomal organelles that are the site of fat storage in the C. elegans intestine (Schroeder et al., 2007). In wild-type L1s there is a marked increase in gut granule material after feeding (Schroeder et al., 2007) (Fig. 7B and see Fig. S4 in the supplementary material). lin-35 and lin-15B mutants born and fed at 20°C displayed this increase in gut granule material, but mutants born and fed at 26°C did not (Fig. 7B and see Fig. S4 in the supplementary material). Their intestines contained only small gut granules, similar to starved wild-type L1s. These findings suggest that synMuv B mutants experience nutritional deprivation at 26°C.
If defective intestinal function causes the arrest of synMuv B larvae, then loss of synMuv B expression solely in the intestine should lead to HTA. To test this, we took advantage of the OLB11 strain, which expresses RDE-1(+) in the intestine and enables RNAi solely in the intestine of RNAi-resistant rde-1 mutant animals (Pilipiuk et al., 2009). RNAi by injection of lin-35 dsRNA into wild type was able to cause larval arrest, although with lower penetrance (58%) than observed in mutants (i.e. 98% in lin-35(n745) homozygotes; Fig. 7C). Some escapers developed into small scrawny sterile adults, whereas others developed into fertile adults (Fig. 7C). Injection of lin-35 dsRNA into RNAi-resistant rde-1 mutants resulted in a majority of fertile adults, and only 5% larval arrest. Injection of lin-35 dsRNA into RNAi-resistant rde-1 mutants expressing RDE-1(+) in the intestine led to an intermediate phenotype: 31% larval arrest and a significant number of scrawny sterile adults. These results demonstrate that loss of LIN-35 function in the intestine alone is sufficient to cause HTA.
We reasoned that, if loss of synMuv B expression in the intestine alone is sufficient to cause HTA, then intestine-specific expression of synMuv B(+) might be sufficient to rescue synMuv B mutant larvae from HTA. We generated worms with intestine-specific expression of LIN-35(+) tagged with GFP. The extrachromosomal array carrying the LIN-35::GFP transgene was inherited by ~60% of progeny, enabling us to compare lin-35 mutant siblings that expressed LIN-35(+) in their intestine (GPF+) with siblings that did not (GFP−). Only 9.2% of GFP+ larvae arrested, compared with 97.2% of GFP− larvae (Fig. 7D). In animals with mosaic expression of LIN-35::GFP in their intestine, we noted that individual GFP+ intestinal cells no longer expressed detectable levels of germline proteins (see Fig. S5 in the supplementary material). Thus, expression of LIN-35(+) in the intestine is sufficient to reduce germline gene expression in intestinal cells and protect larvae from HTA.
DISCUSSION
We have shown that C. elegans mutants lacking synMuv B chromatin regulator function arrest development as larvae when grown at elevated temperature. This high temperature arrest (HTA) is accompanied, and probably caused by, aberrant temperature-sensitive expression of numerous germline-specific genes in somatic cells. Based on the restored larval growth after loss of key germline chromatin regulators, we hypothesize that an essential role of the synMuv B proteins is to antagonize or erase a germline chromatin state in somatic cells and in that way enable somatic cells to undergo normal somatic development. The intestine is the somatic tissue most prone to misexpression of germline genes and is the tissue responsible for HTA.
The subset of synMuv B mutants that show HTA defines a new category of synMuv B genes
synMuv B genes were originally named and grouped based upon the synthetic multivulva (synMuv) phenotype displayed by synMuv A; synMuv B double mutants (for a review, see Fay and Yochem, 2007). synMuv B mutations in the absence of synMuv A mutations also influence fertility, cell cycle, RNAi and larval development (Beitel et al., 2000; Bender, A. M. et al., 2004; Chi and Reinke, 2006; Lehner et al., 2006; Mani and Fay, 2009; Ouellet and Roy, 2007; Wang et al., 2005). Like HTA, these phenotypes are limited to subsets of synMuv B genes. The subset of synMuv B genes that show a penetrant HTA phenotype does not coincide with any previously defined subset of synMuv B genes. For example, numerous HTA-prone synMuv B mutants also display increased levels of RNAi. However, dpl-1 mutants display enhanced RNAi but only minimal HTA, and lin-37 mutants do not display enhanced RNAi but show highly penetrant HTA (Wang et al., 2005). lin-35 has also been shown to affect intestinal function in a synthetic fashion when combined with a mutation in slr-2 (Kirienko et al., 2008). However, other HTA-prone synMuv B mutants, such as lin-9, lin-15B and lin-37, do not display this same synthetic phenotype with slr-2. Therefore, the HTA phenotype appears to define a new subset of synMuv B genes that are necessary on their own for larval survival at high temperature.
Biochemical and genetic analyses have identified several complexes containing synMuv B family members, including the DRM, DP/E2F/Rb and HPL-2/LIN-13 complexes (Coustham et al., 2006; Harrison et al., 2006; Poznic, 2009). Members of all three complexes are associated with temperature-sensitive larval arrest. Of the seven synMuv B proteins whose loss causes a strong HTA phenotype, four (LIN-9, LIN-35, LIN-37 and LIN-54) are members of the DRM complex (Harrison et al., 2006). Other core components of the DRM complex, such as LIN-52 and LIN-53, do not show HTA. It is possible that loss of some members of the DRM complex render it temperature-sensitive, whereas loss of other members does not. Arguing against this scenario is the lin-9(n112) mutant, which destabilizes the DRM complex even at low temperature (Harrison et al., 2006) but does not arrest at low temperature. Thus, it seems likely that loss of the DRM complex in lin-9, lin-35, lin-37 and lin-54 mutants is not the underlying cause of HTA. Because efl-1 mutants do not show HTA and dpl-1 mutants show only weak HTA, it seems unlikely that disruption of the canonical DP/E2F/Rb complex underlies HTA. Loss of either HPL-2 or LIN-13 results in HTA, implicating the HPL-2/LIN-13 complex in regulation of gene expression in somatic cells. Finally, LIN-15B has not been found in a complex with other synMuv B proteins but results in strong HTA and so is also an important contributor to gene regulation in somatic cells.
The close developmental paths and physical association of the germ line and intestine might render the intestine more susceptible to expression of germline traits
The intestine appears more prone than other tissues to expression of germline genes in synMuv B mutants, and is the tissue responsible for HTA, at least in a lin-35 background. What features of the intestine make it susceptible to showing germline traits? The germ line and intestine arise from embryonic cells (P4 and E) that are special in several ways: they are descended from sister cells (P2 and EMS) in the four-cell embryo that directly signal each other, the P and E lineage stay in close association with each other throughout embryogenesis, and P4 and E clonally generate single tissues (Altun and Hall, 2009; McGhee, 2007). Expression of germline genes in the intestine of synMuv B mutants begins in mid-to-late embryogenesis [see Unhavaithaya et al. (Unhavaithaya et al., 2002) and this paper] when the PGCs themselves begin to express at least some germline genes (Kawasaki et al., 1998; Spencer et al., 2010) and when the PGCs are reported to have lobes that extend into intestinal cells (Sulston et al., 1983). Perhaps as a result of the similar lineage and tight physical connection between the intestine and germ line, the intestine might be poised to initiate a germline program of gene expression during embryogenesis. In wild-type embryos, synMuv B(+) proteins participate in repressing that program.
Creating a germline-like chromatin environment contributes to HTA
synMuv B mutants show numerous germline traits in somatic cells, including expression of germline genes, increased RNAi efficiency and silencing of some transgenes (Lehner et al., 2006; Unhavaithaya et al., 2002; Wang et al., 2005). This implies that their somatic cells, especially intestinal cells, have acquired at least some germline identity. This does not represent a switch in cell fate, as synMuv B mutants still express elt-2 specifically in the intestine [see Unhavaithaya et al. (Unhavaithaya et al., 2002) and this paper]. Instead, synMuv B mutant somatic cells appear to be hybrid in nature, with features of both germ line and soma. This hybrid nature renders intestinal physiology sensitive to temperature. Our tests of whether depletion of any single misexpressed germline gene could restore intestinal health gave negative results. What did restore intestinal health was depletion of individual germline chromatin regulators, including the MES proteins, MRG-1, and ISW-1. Thus, creating a chromatin environment characteristic of germ cells contributes to HTA. It is unclear whether it is the chromatin state itself or the resulting misexpression of numerous genes that causes HTA.
Our current view of MES-4 function offers an attractive model for the antagonistic relationship between MES-4 and synMuv B regulation of chromatin in somatic cells. MES-4 is a histone methyltransferase (HMT) that methylates histone H3 on lysine 36 (H3K36me) and whose loss from the maternal germ line causes PGCs to die in progeny (Bender et al., 2006; Capowski et al., 1991). Unlike most other studied H3K36 HMTs, which are recruited to genes through association with elongating RNA polymerase II (RNA Pol II), MES-4 can associate with genes independently of RNA Pol II (Bender et al., 2006; Rechtsteiner et al., 2010). In early embryos, MES-4 resides on genes that were expressed in the maternal germ line, even genes that are no longer expressed in embryos (Rechtsteiner et al., 2010). These and other results (Furuhashi et al., 2010) suggest that MES-4 is a maintenance HMT that propagates the memory of gene expression from the maternal germ line to PGCs. The persistence of maternally loaded MES-4 in somatic cells until mid-to-late embryogenesis raises the dilemma of how somatic cells avoid following a germline path. We hypothesize that the synMuv B proteins antagonize or cause the erasure of MES-4 marks on germline genes in somatic cells. In the absence of synMuv B(+) function, intestinal cells, and to a lesser and more variable extent other somatic cells, initiate a germline pattern of gene expression in mid-to-late embryogenesis at the time when germline genes are turned on in the PGCs.
MES-2, MES-3 and MES-6 might influence expression of germline genes in synMuv B mutants via their effect on MES-4. MES-2/3/6 constitutes the worm version of Polycomb Repressive Complex 2, which methylates H3K27 (Bender, L. B. et al., 2004). In wild type, MES-2/3/6(+) action helps keep MES-4 restricted to certain chromosomal regions (Bender, L. B. et al., 2004). Perhaps MES-2/3/6-mediated H3K27 methylation helps focus MES-4 binding on germline-expressed genes. Loss of this focusing effect in mes-2, mes-3 or mes-6 mutants might cause MES-4 to spread onto other genes and less effectively promote expression of germline genes in the soma of synMuv B mutant embryos. Consistent with this view, the suppression of HTA by RNAi depletion of MES-2, MES-3 or MES-6 is substantially weaker than by RNAi depletion of MES-4.
Overexpression of intestinal genes in the intestine of synMuv B mutants
The two main categories of HTA candidate genes are germline and intestine genes. We suggested above that expression of germline genes in the soma is a result of failure to block the action of MES-4 in the soma and that this misexpression of germline genes is crucial to the HTA phenotype. Why should intestinal genes also be highly represented? We propose that the misregulation of intestinal genes might in fact reflect the sensitivity specifically of the intestine to loss of gene expression regulation in synMuv B mutants. In support of this, intestine-specific genes are highly represented not only in those genes whose expression is upregulated in lin-35 and lin-15B mutants but also those that are downregulated (see Fig. S2, Table S4 and Table S5 in the supplementary material). This is in contrast to germline genes, which are abundant only in the upregulated category. Additionally, although some muscle- and neuron-specific genes are upregulated in lin-35 and lin-15B mutants, none of the five of those genes we tested was ectopically expressed in the intestine of mutant L1s (L.N.P., unpublished). Thus, the intestine in synMuv B mutants aberrantly turns on germline genes and might suffer from compromised regulation of genes that are normally expressed in intestine, but does not appear to be generally permissive to expression of genes normally restricted to other somatic tissues.
synMuv B proteins buffer gene expression across different temperatures
Many genes are misexpressed at low temperature in synMuv B mutants (Grishok et al., 2008; Kirienko and Fay, 2007). Our data show that elevated temperature enhances the misexpression of some of those genes. Although this temperature sensitivity might reflect an underlying temperature sensitivity of crippled synMuv B complexes, it is difficult to envisage how this could be suppressed by loss of MES-4 or other germline chromatin regulators. Instead, we propose that synMuv B proteins contribute to the maintenance of gene expression at constant levels across different temperatures by the creation of a generally closed chromatin state. Links between temperature, chromatin state and gene expression are apparent in both plants and Drosophila. Recent work in Arabidopsis has shown that incorporation of the histone variant H2A.Z at low temperatures and eviction at high temperatures is crucial for the changes in gene expression that underlie high temperature growth phenotypes (Kumar and Wigge, 2010). Work in Drosophila has shown that growth at high temperature suppresses position effect variegation (Hartmann-Goldstein, 1967). These data suggest that at high temperature there is a decreased ability of heterochromatin to form or spread. Thus, it appears that closed or repressive chromatin states might be inherently sensitive to temperature. We hypothesize that loss of synMuv B repressive function at low temperature causes some opening of chromatin and upregulation of some genes, and at high temperature, at which repressive chromatin states might be inherently less stable, causes chromatin to take on an even more open state, leading to increased levels of gene misexpression.
Supplementary Material
Acknowledgements
We thank John Tamkun, Needhi Bhalla and members of the Strome lab for helpful discussions; Olaf Bossinger for the OLB11 strain and the pOLB10 plasmid containing the elt-2 promoter; Jim McGhee for the ELT-2 antibody; Abby Dernburg for the HTP-1 and HTP-3 antibodies; Needhi Bhalla for the SYP-1, HIM-8 and ZIM-1 antibodies; and Erik Andersen and the Horvitz lab for numerous synMuv strains. Some C. elegans strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. This work was funded by Ruth Kirschstein National Research Service Award postdoctoral fellowships GM083548 to L.N.P. and GM69084 to C.A.S. and NIH grant GM34059 to S.S.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial Share Alike License (http://creativecommons.org/licenses/by-nc-sa/3.0), which permits unrestricted non-commercial use, distribution and reproduction in any medium provided that the original work is properly cited and all further distributions of the work or adaptation are subject to the same Creative Commons License terms.
Note added in proof
Janic et al. recently reported that Drosophila L(3)MBT [Lethal (3) Malignant Brain Tumor], a homolog of the C. elegans synMuv B protein LIN-61, antagonizes expression of germline genes in somatic cells, specifically in brain tumor cells (Janic et al., 2010).
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.059501/-/DC1
References
- Altun Z. F., Hall D. H. (2009). Alimentary system, intestine. In WormAtlas, doi:10.3908/wormatlas.1.4 [Google Scholar]
- Andersen E. C., Saffer A. M., Horvitz H. R. (2008). Multiple levels of redundant processes inhibit Caenorhabditis elegans vulval cell fates. Genetics 179, 2001-2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitel G. J., Lambie E. J., Horvitz H. R. (2000). The C. elegans gene lin-9,which acts in an Rb-related pathway, is required for gonadal sheath cell development and encodes a novel protein. Gene 254, 253-263 [DOI] [PubMed] [Google Scholar]
- Bender A. M., Wells O., Fay D. S. (2004). lin-35/Rb and xnp-1/ATR-X function redundantly to control somatic gonad development in C. elegans. Dev. Biol. 273, 335-349 [DOI] [PubMed] [Google Scholar]
- Bender L. B., Cao R., Zhang Y., Strome S. (2004). The MES-2/MES-3/MES-6 complex and regulation of histone H3 methylation in C. elegans. Curr. Biol. 14, 1639-1643 [DOI] [PubMed] [Google Scholar]
- Bender L. B., Suh J., Carroll C. R., Fong Y., Fingerman I. M., Briggs S. D., Cao R., Zhang Y., Reinke V., Strome S. (2006). MES-4: an autosome-associated histone methyltransferase that participates in silencing the X chromosomes in the C. elegans germ line. Development 133, 3907-3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. (1975). The genetics of Caenorhabditis elegans. Genetics 77, 71-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capowski E. E., Martin P., Garvin C., Strome S. (1991). Identification of grandchildless loci whose products are required for normal germ-line development in the nematode Caenorhabditis elegans. Genetics 129, 1061-1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W., Reinke V. (2006). Promotion of oogenesis and embryogenesis in the C. elegans gonad by EFL-1/DPL-1 (E2F) does not require LIN-35 (pRB). Development 133, 3147-3157 [DOI] [PubMed] [Google Scholar]
- Conine C. C., Batista P. J., Gu W., Claycomb J. M., Chaves D. A., Shirayama M., Mello C. C. (2010). Argonautes ALG-3 and ALG-4 are required for spermatogenesis-specific 26G-RNAs and thermotolerant sperm in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 107, 3588-3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustham V., Bedet C., Monier K., Schott S., Karali M., Palladino F. (2006). The C. elegans HP1 homologue HPL-2 and the LIN-13 zinc finger protein form a complex implicated in vulval development. Dev. Biol. 297, 308-322 [DOI] [PubMed] [Google Scholar]
- Cui M., Kim E. B., Han M. (2006). Diverse chromatin remodeling genes antagonize the Rb-involved SynMuv pathways in C. elegans. PLoS Genet. 2, e74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudoit S., Yang Y. H. (2003). Bioconductor R packags for exploratory analysis and normalization of cDNA microarray data. In The Analysis of Gene Expression Data: Methods and Software (ed. Parmigiani G., Garrett E. S., Irizarry R. A., Zeger S. L.), pp. 73-101 New York, NY: Springer; [Google Scholar]
- Fay D. S., Yochem J. (2007). The SynMuv genes of Caenorhabditis elegans in vulval development and beyond. Dev. Biol. 306, 1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A. G., Kamath R. S., Zipperlen P., Martinez-Campos M., Sohrmann M., Ahringer J. (2000). Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408, 325-330 [DOI] [PubMed] [Google Scholar]
- Furuhashi H., Takasaki T., Rechtsteiner A., Li T., Kimura H., Checchi P. M., Strome S., Kelly W. G. (2010). Trans-generational epigenetic regulation of C. elegans primordial germ cells. Epigenetics Chromatin 3, 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grishok A., Hoersch S., Sharp P. A. (2008). RNA interference and retinoblastoma-related genes are required for repression of endogenous siRNA targets in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105, 20386-20391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruidl M. E., Smith P. A., Kuznicki K. A., McCrone J. S., Kirchner J., Roussell D. L., Strome S., Bennett K. L. (1996). Multiple potential germ-line helicases are components of the germ-line-specific P granules of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93, 13837-13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. M., Ceol C. J., Lu X., Horvitz H. R. (2006). Some C. elegans class B synthetic multivulva proteins encode a conserved LIN-35 Rb-containing complex distinct from a NuRD-like complex. Proc. Natl. Acad. Sci. USA 103, 16782-16787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Goldstein I. J. (1967). On the relationship between heterchromatization and variegation in Drosophila, with special reference to temperature-sensitive periods. Genet. Res. 10, 143-159 [DOI] [PubMed] [Google Scholar]
- Janic A., Mendizabal L., Llamazares S., Rossell D., Gonzalez C. (2010). Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science 330, 1824-1827 [DOI] [PubMed] [Google Scholar]
- Kawasaki I., Shim Y. H., Kirchner J., Kaminker J., Wood W. B., Strome S. (1998). PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell 94, 635-645 [DOI] [PubMed] [Google Scholar]
- Kawasaki I., Amiri A., Fan Y., Meyer N., Dunkelbarger S., Motohashi T., Karashima T., Bossinger O., Strome S. (2004). The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics 167, 645-661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirienko N. V., Fay D. S. (2007). Transcriptome profiling of the C. elegans Rb ortholog reveals diverse developmental roles. Dev. Biol. 305, 674-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirienko N. V., McEnerney J. D., Fay D. S. (2008). Coordinated regulation of intestinal functions in C. elegans by LIN-35/Rb and SLR-2. PLoS Genet. 4, e1000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. V., Wigge P. A. (2010). H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140, 136-147 [DOI] [PubMed] [Google Scholar]
- Lehner B., Tischler J., Fraser A. G. (2006). RNAi screens in Caenorhabditis elegans in a 96-well liquid format and their application to the systematic identification of genetic interactions. Nat. Protoc. 1, 1617-1620 [DOI] [PubMed] [Google Scholar]
- MacQueen A. J., Phillips C. M., Bhalla N., Weiser P., Villeneuve A. M., Dernburg A. F. (2005). Chromosome sites play dual roles to establish homologous synapsis during meiosis in C. elegans. Cell 123, 1037-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani K., Fay D. S. (2009). A mechanistic basis for the coordinated regulation of pharyngeal morphogenesis in Caenorhabditis elegans by LIN-35/Rb and UBC-18-ARI-1. PLoS Genet. 5, e1000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D. (2007). The C. elegans intestine. In WormBook (ed. The C. elegans Research Community), pp. 1-36 doi/10.1895/wormbook.1.133.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed]
- Meissner B., Warner A., Wong K., Dube N., Lorch A., McKay S. J., Khattra J., Rogalski T., Somasiri A., Chaudhry I., et al. (2009). An integrated strategy to study muscle development and myofilament structure in Caenorhabditis elegans. PLoS Genet. 5, e1000537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A., Seydoux G. (2008). Less is more: specification of the germline by transcriptional repression. Development 135, 3817-3827 [DOI] [PubMed] [Google Scholar]
- Ouellet J., Roy R. (2007). The lin-35/Rb and RNAi pathways cooperate to regulate a key cell cycle transition in C. elegans. BMC Dev. Biol. 7, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipiuk J., Lefebvre C., Wiesenfahrt T., Legouis R., Bossinger O. (2009). Increased IP3/Ca2+ signaling compensates depletion of LET-413/DLG-1 in C. elegans epithelial junction assembly. Dev. Biol. 327, 34-47 [DOI] [PubMed] [Google Scholar]
- Poznic M. (2009). Retinoblastoma protein: a central processing unit. J. Biosci. 34, 305-312 [DOI] [PubMed] [Google Scholar]
- Rechtsteiner A., Ercan S., Takasaki T., Phippen T. M., Egelhofer T. A., Wang W., Kimura H., Lieb J. D., Strome S. (2010). The histone H3K36 methyltransferase MES-4 acts epigenetically to transmit the memory of germline gene expression to progeny. PLoS Genet. 6, e1001091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke V., Smith H. E., Nance J., Wang J., Van Doren C., Begley R., Jones S. J., Davis E. B., Scherer S., Ward S., et al. (2000). A global profile of germline gene expression in C. elegans. Mol. Cell 6, 605-616 [DOI] [PubMed] [Google Scholar]
- Reinke V., Gil I. S., Ward S., Kazmer K. (2004). Genome-wide germline-enriched and sex-biased expression profiles in Caenorhabditis elegans. Development 131, 311-323 [DOI] [PubMed] [Google Scholar]
- Saitou M., Yamaji M. (2010). Germ cell specification in mice: signaling, transcription regulation, and epigenetic consequences. Reproduction 139, 931-942 [DOI] [PubMed] [Google Scholar]
- Schroeder L. K., Kremer S., Kramer M. J., Currie E., Kwan E., Watts J. L., Lawrenson A. L., Hermann G. J. (2007). Function of the Caenorhabditis elegans ABC transporter PGP-2 in the biogenesis of a lysosome-related fat storage organelle. Mol. Biol. Cell 18, 995-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer W. C., Zeller G., Watson J. D., Henz S. R., Watkins K. L., McWhirter R. D., Petersen S., Sreedharan V. T., Widmer C., Jo J., et al. (2011). A spatial and temporal map of C. elegans gene expression. Genome Res. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike C. A., Bader J., Reinke V., Strome S. (2008). DEPS-1 promotes P-granule assembly and RNA interference in C. elegans germ cells. Development 135, 983-993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey J. D., Tibshirani R. (2003). Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol. Biol. 224, 149-157 [DOI] [PubMed] [Google Scholar]
- Strome S., Wood W. B. (1983). Generation of asymmetry and segregation of germ-line granules in early C. elegans embryos. Cell 35, 15-25 [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119 [DOI] [PubMed] [Google Scholar]
- Unhavaithaya Y., Shin T. H., Miliaras N., Lee J., Oyama T., Mello C. C. (2002). MEP-1 and a homolog of the NURD complex component Mi-2 act together to maintain germline-soma distinctions in C. elegans. Cell 111, 991-1002 [DOI] [PubMed] [Google Scholar]
- Updike D. L., Strome S. (2009). A genomewide RNAi screen for genes that affect the stability, distribution and function of P granules in Caenorhabditis elegans. Genetics 183, 1397-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Kennedy S., Conte D., Jr, Kim J. K., Gabel H. W., Kamath R. S., Mello C. C., Ruvkun G. (2005). Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature 436, 593-597 [DOI] [PubMed] [Google Scholar]
- Wang X., Zhao Y., Wong K., Ehlers P., Kohara Y., Jones S. J., Marra M. A., Holt R. A., Moerman D. G., Hansen D. (2009). Identification of genes expressed in the hermaphrodite germ line of C. elegans using SAGE. BMC Genomics 10, 213 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.