Abstract
An insulin-like signaling pathway mediates the environmental influence on the switch between the C. elegans developmental programs of reproductive growth versus dauer arrest. However, the specific role of endogenous insulin-like peptide (ILP) ligands in mediating the switch between these programs remains unknown. C. elegans has 40 putative insulin-like genes, many of which are expressed in sensory neurons and interneurons, raising the intriguing possibility that ILPs encode different environmental information to regulate the entry into, and exit from, dauer arrest. These two developmental switches can have different regulatory requirements: here we show that the relative importance of three different ILPs varies between dauer entry and exit. Not only do we find that one ILP, ins-1, ensures dauer arrest under harsh environments and that two other ILPs, daf-28 and ins-6, ensure reproductive growth under good conditions, we also show that daf-28 and ins-6 have non-redundant functions in regulating these developmental switches. Notably, daf-28 plays a more primary role in inhibiting dauer entry, whereas ins-6 has a more significant role in promoting dauer exit. Moreover, the switch into dauer arrest surprisingly shifts ins-6 transcriptional expression from a set of dauer-inhibiting sensory neurons to a different set of neurons, where it promotes dauer exit. Together, our data suggest that specific ILPs generate precise responses to dauer-inducing cues, such as pheromones and low food levels, to control development through stimulus-regulated expression in different neurons.
Keywords: Developmental plasticity, Insulin-like peptides, ILP code, Sensory neurons, Caenorhabditis elegans
INTRODUCTION
The environment has long been known to influence physiology. In C. elegans, the nature of its environment determines its developmental program (Golden and Riddle, 1984). Under conditions of abundant food supply, low population density and optimal temperatures, C. elegans develops through four larval stages (L1-L4) to become a reproductive adult (Golden and Riddle, 1982; Golden and Riddle, 1984). However, high population density, food scarcity and/or high temperatures can induce first-stage larvae (L1) to enter a different program, known as dauer arrest (Golden and Riddle, 1982; Golden and Riddle, 1984). Dauers, which are alternative third-stage larvae (L3) and anatomically distinct from L3s grown under optimal conditions, are highly stress-resistant and equipped for long-term survival (Cassada and Russell, 1975; Golden and Riddle, 1982; Golden and Riddle, 1984; Riddle et al., 1981).
The entry into the dauer state depends on a high ratio of a glycosidic pheromone mixture (Butcher et al., 2007; Jeong et al., 2005) to food cues (Golden and Riddle, 1982; Golden and Riddle, 1984). This dauer-inducing pheromone mixture, which is secreted by each animal throughout its life and thus reflects population density, is sensed, together with the food cues, by specific neurons that regulate dauer entry (Bargmann and Horvitz, 1991; Kim et al., 2009; Schackwitz et al., 1996). Conversely, the exit from the dauer state into the last larval stage (L4), prior to becoming fertile adults, is promoted by a subsequent improvement in the environment (Golden and Riddle, 1984), such as an increase in food levels, which is sensed by a different set of neurons (Bargmann and Horvitz, 1991; Ouellet et al., 2008).
An important pathway that mediates the sensory influence on C. elegans development is the insulin DAF-2 pathway (Kimura et al., 1997; Riddle et al., 1981; Vowels and Thomas, 1992). Mutations that downregulate the insulin receptor ortholog DAF-2 (Kimura et al., 1997) lead to dauer arrest, which requires the activity of the FOXO transcription factor DAF-16 (Gottlieb and Ruvkun, 1994; Lin et al., 1997; Ogg et al., 1997; Riddle et al., 1981; Vowels and Thomas, 1992). Moreover, whereas a strong downregulation in DAF-2 signaling induces dauers that arrest constitutively, a weaker reduction of DAF-2 activity leads to transient dauer formation (Gems et al., 1998). This suggests that the DAF-2 pathway determines not only when the animals should enter dauer arrest but also when they should exit from it.
Although there is only one known C. elegans insulin receptor ortholog, DAF-2 (Kimura et al., 1997), there are 40 insulin-like genes that have been predicted to encode insulin-like peptides (ILPs) (Li et al., 2003; Pierce et al., 2001) (see www.wormbase.org). Many of the ILPs are expressed in overlapping subsets of sensory neurons and/or interneurons, including the sensory neurons (Kodama et al., 2006; Li et al., 2003; Pierce et al., 2001) that regulate dauer entry or exit (Bargmann and Horvitz, 1991; Kim et al., 2009; Ouellet et al., 2008; Schackwitz et al., 1996). Interestingly, the mechanism that regulates entry into dauer arrest differs from the mechanism that regulates exit from this state, not only at the neuronal but also at the molecular level (Bargmann and Horvitz, 1991; Kim et al., 2009; Ouellet et al., 2008; Schackwitz et al., 1996; Tissenbaum et al., 2000). Since the endogenous roles of individual ILPs in these developmental switches are unknown, the different ILP gene expression patterns have led us to consider the hypothesis that they encode discrete sets of sensory information to regulate dauer entry versus exit.
Here, we have tested our hypothesis by analyzing the functions of the ILPs daf-28, ins-6 and ins-1 in regulating these two developmental switches. We find that the ILP requirements for dauer entry versus exit are dissimilar. Surprisingly, we observe that the relative importance of daf-28 versus ins-6 in dauer entry is reversed in dauer exit. In addition, we show that environmental information is encoded by these ILPs through cue-driven expression in distinct sensory neurons, which in turn could elicit precise physiological responses by modulating the activities of the affected sensory circuits and/or their target tissues.
MATERIALS AND METHODS
Worm strains and culture
All mutants used in this study were backcrossed six times to the wild-type (N2) strain before any phenotypic analysis was performed. To directly study the relative contributions of the ILPs that have been previously implicated through indirect approaches in regulating the dauer program, we used deletion alleles, with each serving as a molecular null specific for its corresponding ILP (Pierce et al., 2001) (see www.wormbase.org). Worms were continuously fed E. coli OP50 for at least two generations before each assay.
Transgenic worms
Independent lines were generated using standard methods and the ofm-1p::gfp (Miyabayashi et al., 1999) co-injection marker (injected at 25 ng/μl). For controls, we generated wild-type and mutant worms that carry the ofm-1p::gfp co-injection marker alone.
ins-6 rescue lines
To generate the full rescue construct for ins-6 (pQZ11), we used a 4.2 kb fragment of the ins-6 genomic locus that includes the 1.7 kb sequence upstream of its start codon and the 2.1 kb sequence downstream of its stop codon, which was inserted into the pCR-BluntII-TOPO vector (Invitrogen, UK). We injected this construct at two different doses (2 and 25 ng/μl) into wild type or ins-6(tm2416) mutants. We then crossed the resulting extrachromosomal arrays into (1) the ins-6(tm2416); daf-28(tm2308) mutants to assay for rescue of the ins-6-dependent dauer entry phenotype or (2) the ins-6(tm2416); daf-2(e1368) mutants to test for rescue of the ins-6-dependent dauer exit phenotype.
daf-28 rescue lines
To generate the full rescue for daf-28 (pQZ43), we inserted into the pCR-BluntII-TOPO vector a 4.09 kb fragment of the daf-28 genomic locus that spans the 3.3 kb sequence upstream of the start codon to the 70 bp region downstream of the stop codon. This construct was introduced as extrachromosomal arrays at two different doses (2ng/μl and 25 ng/μl) into ins-6(tm2416); daf-28(tm2308) or daf-2(e1368); daf-28(tm2308) mutants.
ILP expression lines
To determine the ins-1 expression pattern, we generated a transcriptional ins-1p::cfp reporter construct (pQZ6) using the Gateway Technology vectors (Invitrogen). In pQZ6, cfp is flanked by the 4.3 kb sequence upstream of the ins-1 start codon and by the 1.1 kb sequence downstream of the ins-1 stop codon. In addition, the 0.8 kb sequence of the largest intron, which might contain regulatory elements required for expression, is fused downstream of the 3′ cis sequences. pQZ6 was injected into wild-type worms at 100 ng/μl. Three independent lines were recovered, which show identical patterns of cfp expression.
To determine the ins-6 expression pattern, we constructed a transcriptional ins-6p::mCherry reporter (pQZ10) using the Gateway system. The mCherry is flanked by the 1.7 kb sequence upstream of the ins-6 start codon and by the 2.0 kb sequence downstream of the ins-6 stop codon. pQZ10 was injected into wild-type worms at 100 ng/μl. Three lines were recovered, which have the same mCherry expression pattern.
Generation of worms genetically ablated for ASJ neurons
To genetically ablate the ASJ neurons, we drove human caspase 1 (ICE, or CASP1) [a gift of V. Maricq (Zheng et al., 1999)] transcription from the 1 kb thioredoxin (trx-1) promoter [gift of P. Swoboda (Miranda-Vizuete et al., 2006)] as trx-1p::ICE in the pPD95.77 vector (pQZ37). trx-1 is specifically expressed in the ASJ neurons (Miranda-Vizuete et al., 2006). We introduced pQZ37 at 100 ng/μl into either ins-6(tm2416); daf-2(e1368) mutants or daf-2(e1368) mutants.
Dauer entry assays
The worms were grown at 25°C and allowed to lay eggs at this temperature for 3-7 hours. The eggs were then allowed to develop either at 25°C or 27°C and scored ~24 hours after egg-laying for L1 arrest phenotypes. Like wild type, we observed no L1 arrest phenotypes for any worms carrying deletions in ins-1, ins-6 and/or daf-28 at these temperatures. Forty-eight hours after the egg-laying midpoint, the fraction of dauers and L4s/adults was counted. Animals carrying transgenes or the daf-2 or daf-16 mutation were grown at 20°C and allowed to lay eggs for 3-7 hours, which were then allowed to develop either at 20°C or shifted to 22.5°C, 25°C or 27°C. At 48 hours after the egg-laying midpoint, the fraction of dauers and L4s/adults was counted. To compare the different genotypes, we used the Wilcoxon Mann-Whitney rank sum test (Hothorn et al., 2008; R Development Core Team, 2009).
Dauer exit assays
The eggs that were laid for 3-7 hours at 20°C were shifted to 25°C. All dauers that were formed 48 hours after the egg-laying midpoint were collected and scored daily for dauer exit. We used JMP 5.1 (SAS) software to determine Kaplan-Meier probability estimates of dauer exit events and for all statistical comparisons. P-values were determined by the log-rank test.
To measure dauer exit in the ASJ-ablated and control animals, eggs that were laid for 4 hours at 20°C were transferred to 25°C on 3.5-cm plates containing 5 ml nematode-growth (NG) agar (Brenner, 1974), 150 μl of a crude dauer pheromone mixture [prepared according to Thomas et al. (Thomas et al., 1993)] and 50 μl E. coli OP50. After 48 hours, all dauers were collected and transferred onto plates lacking the exogenously added pheromone. These dauers were again scored daily at 25°C for exit into the L4 stage.
Analyses of ILP gene expression in response to environmental cues
Dauer pheromone
To assess the effect of dauer pheromone on ILP gene expression, we placed ~100 embryos on 3.5-cm NG agar plates, to which 100-200 μl dauer pheromone and 50 μl E. coli OP50 were added. The concentration of the crude dauer pheromone mixture used in these assays caused ~45-55% of wild-type L1s to form dauers at 25°C. We monitored the ILP gene expression of the different larvae and adults that subsequently developed on these plates.
Starvation
The effect of low food availability on ILP gene expression was tested by several methods. First, we compared the ILP gene expression of well-fed larvae and young adults with those of age-matched larvae (L1 and L4) and young adults from starved plates. Second, we bleached gravid adults to collect a number of eggs that either (1) were placed directly on NG agar plates in the absence of food, which caused the animals to undergo L1 arrest or (2) were permitted to develop to L1 or L2 on plates with food, before being harvested and washed at least twice with M9 buffer (Lewis and Fleming, 1995) to remove the bacteria. These harvested L1 and L2 animals were then placed on plates without food, which caused the animals to arrest as L2 or L3, respectively. The starved animals were all scored for ILP gene expression within a day, and sometimes for several days afterwards.
Temperature
To determine the influence of temperature, the ILP gene expression of animals that developed at 20°C was compared with that of age-matched animals that developed at 27°C from eggs laid at 20°C.
RESULTS
daf-28 has a more prominent role than ins-6 in inhibiting dauer entry
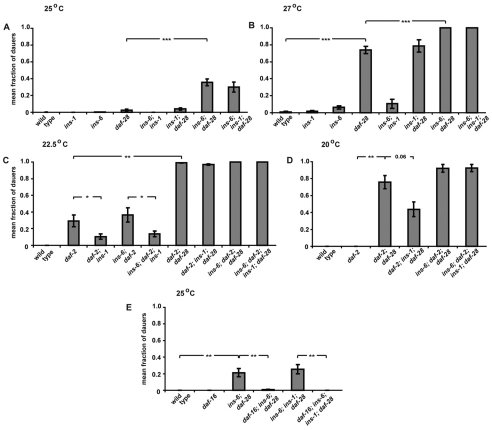
Previous gain-of-function experiments suggested that the ILPs daf-28, ins-6 and ins-1 play a role in dauer entry (Li et al., 2003; Malone et al., 1996; Pierce et al., 2001). However, these earlier studies involved indirect manipulations of ILP function, which do not allow the direct comparison of the endogenous contributions of specific ILPs in regulating not only dauer entry but also dauer exit. Thus, to directly test the role of each ILP in these two processes, we studied deletion mutants in which gene function is completely and specifically eliminated. We first examined the dauer entry phenotypes of the worms carrying the following single or combined deletions: daf-28(tm2308), ins-6(tm2416) and ins-1(nr2091). Like wild type, the ins-6 and ins-1 deletion mutants formed no dauers at 25°C and very few dauers at 27°C, a temperature known to stimulate dauer entry (Fig. 1A,B and see Table S1 in the supplementary material). By contrast, the daf-28 deletion mutants formed few dauers at 25°C and a much larger fraction of dauers at 27°C (Fig. 1A,B and see Table S1 in the supplementary material). In addition, loss of ins-6 enhanced the dauer entry phenotype of daf-28 deletion mutants at both temperatures, whereas removing ins-1 did not (Fig. 1A,B and see Table S1 in the supplementary material). Together, these data indicate that daf-28 acts with ins-6 to inhibit dauer entry, which is consistent with the reported rescue of the dauer formation phenotype of the gain-of-function daf-28(sa191) mutant upon overexpression of wild-type daf-28 or ins-6 (Li et al., 2003). At the same time, by comparing null mutants, we unexpectedly identified a stronger role for daf-28 than ins-6 in inhibiting this process.
Fig. 1.
daf-28 acts with ins-6 to inhibit dauer entry, whereas ins-1 promotes it. (A,B) The mean fractions of wild type and insulin-deletion C. elegans mutants that form dauers at 25°C (A) or 27°C (B). Each mean ± s.e.m. includes at least three independent trials of ~100 worms per trial. The detailed statistical comparisons between the dauer entry phenotypes of different genotypes under different conditions in this and subsequent figures can be found in Table S1 in the supplementary material. (C,D) The effect of different insulin deletions on the dauer entry of daf-2(e1368) mutants at 22.5°C (C) and 20°C (D). (E) The dauer entry of ins-6; daf-28 deletion mutants is suppressed by daf-16(mu86) at 25°C. *, P≤0.05; **, P≤0.01; ***, P≤0.001.
Because daf-28 and ins-6 might encode ligands for the DAF-2 receptor, we tested the effect of these two ILPs on the temperature-sensitive dauer entry phenotype of the reduction-of-function daf-2(e1368) mutants. At temperatures that induce few or no daf-2(e1368) dauers, loss of daf-28 strongly enhanced dauer entry in these daf-2 mutants, whereas the ins-6 deletion had little or no effect (Fig. 1C,D and see Table S1 in the supplementary material). Since daf-2 requires the activity of daf-16 to regulate dauer formation (Riddle et al., 1981), we next tested whether the same is true for ins-6; daf-28 double mutants. We found that the dauer entry of ins-6; daf-28 mutants was suppressed by loss of daf-16 (Fig. 1E and see Table S1 in the supplementary material), which suggests that DAF-28 and INS-6 activate the DAF-2 receptor to inhibit dauer entry via inhibition of DAF-16.
ins-1 promotes dauer entry
Next, we analyzed how ins-1 interacts with daf-28 and ins-6 in the presence of wild-type or downregulated daf-2 activity. Unlike daf-28 and ins-6, deletion of ins-1 suppressed dauer entry in daf-2(e1368) at 22.5°C (Fig. 1C and see Table S1 in the supplementary material). Likewise, loss of ins-1 decreased the number of dauers formed by ins-6; daf-2(e1368) mutants (Fig. 1C and see Table S1 in the supplementary material). By contrast, an ins-1 deletion only suppressed the dauer entry of the daf-2; daf-28 double mutants at a lower temperature, 20°C, which is a weaker dauer-inducing condition, and not at 22.5°C, a stronger dauer-inducing condition (Fig. 1C,D and see Table S1 in the supplementary material). Consistent with these observations, loss of ins-1 also had no effect on dauer entry in ins-6; daf-2(e1368); daf-28 triple mutants, which all formed dauers at both temperatures (Fig. 1C,D and see Table S1 in the supplementary material). Thus, these findings suggest that ins-1 functions to promote dauer entry, which is in agreement with the increased dauer formation previously observed in weak reduction-of-function daf-2 mutants that overexpress wild-type ins-1 (Pierce et al., 2001). Yet, these present studies also suggest that ins-1 only weakly antagonizes the activity of the DAF-2 pathway in regulating this switch between the developmental programs.
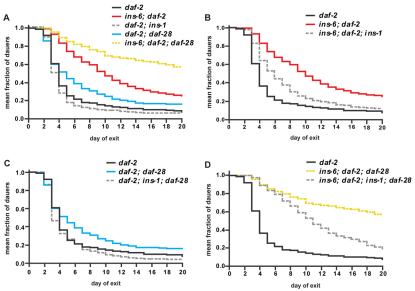
ins-6 has a more prominent role than daf-28 in promoting dauer exit
DAF-2 signaling also regulates exit from dauer arrest (Gems et al., 1998; Kao et al., 2007). For example, through a loss-of-function mutation, daf-28 has been shown to affect exit from the dauer state (Kao et al., 2007). To determine whether ins-6 and ins-1 also regulate dauer exit, we analyzed the effects of loss of these two ILPs, either singly or in combination and with or without daf-28, on the dauer exit phenotype of daf-2(e1368) mutants, which are known to form dauers at 25°C that exit after a few days (Gems et al., 1998). We found that deletion of ins-6 in daf-2(e1368) mutants strongly inhibited dauer exit, but removal of daf-28 only slightly delayed it (Fig. 2A and see Table S2 in the supplementary material). Moreover, removal of both ins-6 and daf-28 in daf-2 mutants caused the greatest delay in dauer exit (Fig. 2A and see Table S2 in the supplementary material). Thus, unpredicted from previous studies on dauer entry (daf-28 and ins-6) and dauer exit (daf-28) (Kao et al., 2007; Li et al., 2003; Malone et al., 1996), these data indicate that although both ILPs act together to promote dauer exit, ins-6 has a more significant role than daf-28 in this process.
Fig. 2.
ins-6 acts with daf-28 to promote dauer exit, whereas ins-1 inhibits it. (A-D) The rates of dauer exit at 25°C of animals carrying different combinations of insulin deletions in a daf-2(e1368) mutant background. Each curve represents cumulative data from six independent trials. All curves are significantly different from that of the daf-2(e1368) control (P≤0.001, log-rank test). The complete statistical comparisons between the dauer exit phenotypes of the different groups of animals in this and subsequent figures are shown in Table S2 in the supplementary material.
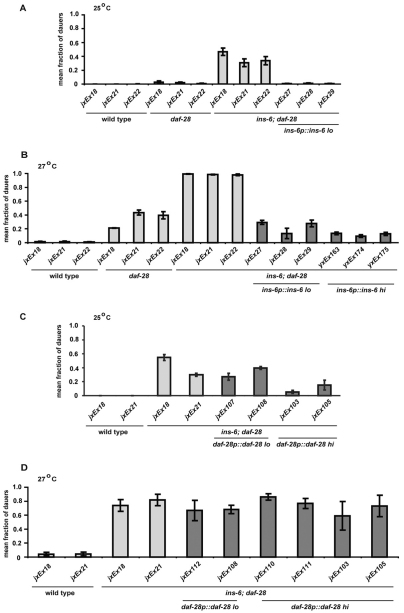
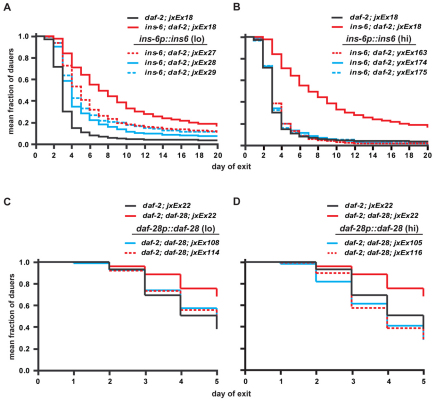
This difference in the relative importance of ins-6 in dauer entry versus exit was also reflected by the ins-6 levels required to rescue dauer entry versus those needed to rescue dauer exit. A low level of ins-6 was sufficient to rescue the dauer entry of ins-6; daf-28 double mutants back to that of daf-28 single mutants (Fig. 3A,B and see Table S1 in the supplementary material). Conversely, the dauer exit phenotype of ins-6; daf-2 double mutants could only be fully rescued to the exit phenotype of daf-2 single mutants with high levels of ins-6 (Fig. 4A,B and see Table S2 in the supplementary material). It should be noted that high ins-6 levels did not completely rescue the dauer entry of ins-6; daf-28 double mutants back to wild type (Fig. 3B and see Table S1 in the supplementary material), which again suggests that these ILPs do not act completely redundantly with each other.
Fig. 3.
Inhibition of dauer entry requires low levels of ins-6 and high levels of daf-28. (A,B) The mean fractions of dauers at 25°C (A) or 27°C (B) in ins-6; daf-28 mutants rescued with low (2 ng/μl, lo) or high (25 ng/μl, hi) levels of ins-6 are compared with those of wild-type C. elegans or with insulin-deletion mutants that carry the ofm-1p::gfp co-injection marker alone (jxEx18, jxEx21 or jxEx22). (C,D) The mean fractions of dauers at 25°C (C) or 27°C (D) in ins-6; daf-28 mutants rescued with low (2 ng/μl) or high (25 ng/μl) levels of daf-28 are compared with those of wild type carrying the co-injection marker alone. The daf-28 levels needed to fully rescue the dauer entry phenotype of the ins-6; daf-28 mutants are higher than 25 ng/μl. Error bars represent s.e.m.
Fig. 4.
Unlike dauer entry, higher ins-6 levels and lower daf-28 levels are required to promote dauer exit. (A,B) The rates of dauer exit of ins-6; daf-2(e1368) mutants that were rescued with low (A) or high (B) ins-6 levels are compared with those of daf-2(e1368) or ins-6; daf-2(e1368) mutants that carry the ofm-1p::gfp co-injection marker alone (jxEx18). Each curve represents the cumulative data from at least seven trials. The low-expressing ins-6 rescue lines are significantly different from the daf-2 control (P<0.0001), whereas the high-expressing ins-6 rescue lines behave the same as the daf-2 control. (C,D) The rates of dauer exit of daf-2(e1368); daf-28 mutants that were rescued with low (C) or high (D) daf-28 levels are compared with those of daf-2(e1368) or daf-2(e1368); daf-28 mutants that carry the jxEx22 co-injection marker. Each curve represents the cumulative data from three trials. See Table S2 in the supplementary material for a comparison of the rescue lines with other, additional control lines.
Similarly, we showed that the daf-28 levels that were necessary to rescue the two phenotypes also reflected the greater requirement for daf-28 in dauer entry versus exit. A higher level of daf-28 was needed to rescue the dauer entry of ins-6; daf-28 mutants (Fig. 3C,D and see Table S1 in the supplementary material), whereas a lower level of daf-28 was sufficient to rescue the dauer exit phenotype of daf-2; daf-28 double mutants back to that of daf-2 single mutants (Fig. 4C,D and see Table S2 in the supplementary material). Together, our data indicate that the ILP regulation of dauer entry is different from that of dauer exit: higher daf-28 levels are required to inhibit entry than to promote exit, whereas the reverse is true for ins-6 (see Fig. 6B).
Fig. 6.
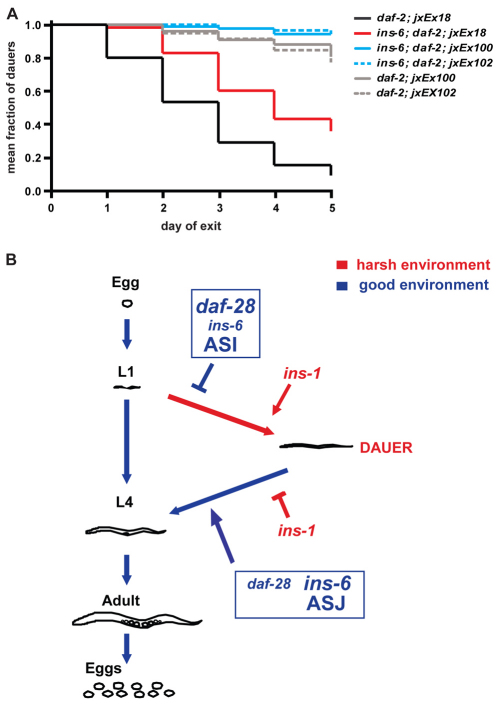
ins-6 acts primarily from ASJ to promote dauer exit. (A) The rates of dauer exit of daf-2(e1368) single and ins-6; daf-2(e1368) double mutants, in which ASJ is genetically ablated (jxEx100 and jxEx102). Control lines are daf-2(e1368) and ins-6; daf-2(e1368) mutants that carry the ofm-1p::gfp co-injection marker alone (jxEx18). The slight difference observed between the dauer exit phenotypes of daf-2 and ins-6; daf-2 mutants upon ASJ ablation might be due to the incomplete loss of ASJ in all animals, as this neuron can still sometimes be seen in some of the post-dauers (data not shown). (B) A model for daf-28, ins-6 and ins-1 function in dauer regulation. daf-28 and ins-6 inhibit dauer entry and promote dauer exit, whereas ins-1 promotes dauer entry and inhibits dauer exit. Whereas daf-28 plays a more prominent role in inhibiting dauer entry (depicted with daf-28 in larger font), ins-6 has a more primary role in promoting dauer exit (depicted with ins-6 in larger font). The sensory neurons in which daf-28 and/or ins-6 might function to regulate the different developmental switches are also shown.
ins-1 inhibits dauer exit
Overexpression of ins-1 has been shown to increase dauer formation (Pierce et al., 2001). However, it is unclear from that study whether ins-1 regulates dauer entry or exit or both. Since we already showed that ins-1 promotes dauer entry (Fig. 1C,D and see Table S1 in the supplementary material), we next analyzed ins-1 for a role in dauer exit. We observed that loss of ins-1, which had little effect on dauer exit in daf-2 single mutants (Fig. 2A and see Table S2 in the supplementary material), enhanced dauer exit in all other daf-2 mutants that lack ins-6 and/or daf-28 (Fig. 2B-D and see Table S2 in the supplementary material). This suggests that ins-1 not only plays a role in dauer entry but also in dauer exit and that the wild-type ins-1 function is to ensure dauer arrest under harsh environments.
ins-6 expression switches between two sensory neurons to control dauer entry versus exit
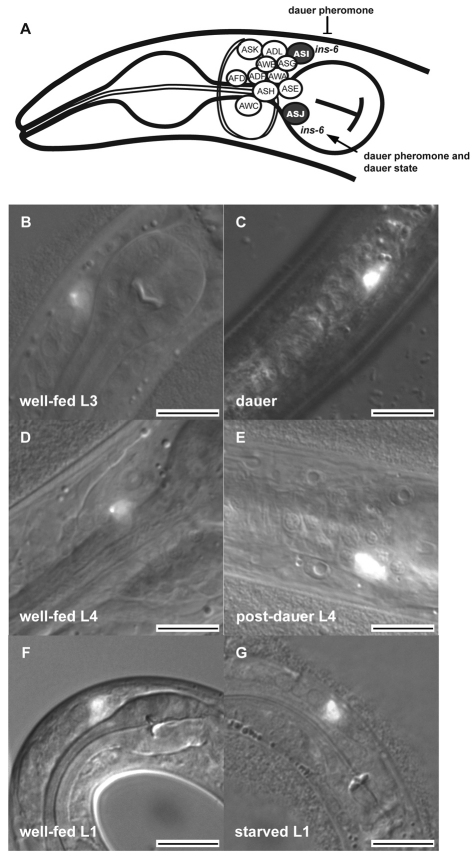
The switch between reproductive growth and dauer arrest is regulated by specific sensory neurons (Bargmann and Horvitz, 1991; Schackwitz et al., 1996) that express some ILP genes (Li et al., 2003; Pierce et al., 2001). Dauer entry is inhibited by the sensory neurons ADF, ASI and ASG (Bargmann and Horvitz, 1991) and is promoted by the sensory neurons ASJ and ASK (Kim et al., 2009; Schackwitz et al., 1996). However, dauer exit is inhibited by the sensory neurons IL2 and promoted by the sensory neurons ASJ and AWC (Bargmann and Horvitz, 1991; Ouellet et al., 2008).
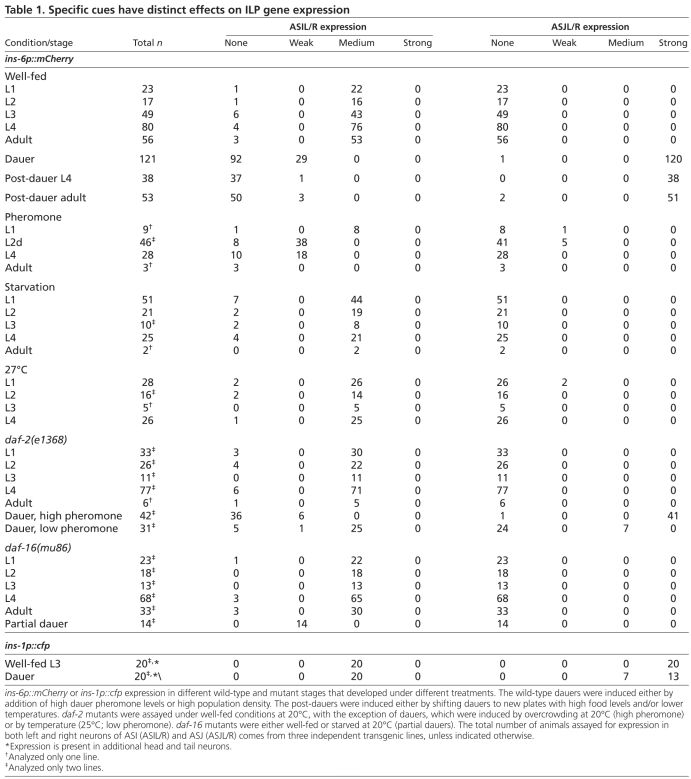
Although daf-28 is expressed in ASI and ASJ of well-fed animals and is downregulated in both neurons by low food availability, a dauer pheromone mixture or entry into dauer arrest (Li et al., 2003), the cells from which ins-1 or ins-6 might act to regulate the developmental switches are unknown. ins-1 is expressed in many neurons, including those that regulate entry into and exit from the dauer program (Kodama et al., 2006; Tomioka et al., 2006). Unlike daf-28, the switch in developmental programs had little or no effect on ins-1 expression in ASI and ASJ (Table 1), as determined from a cfp transcriptional reporter fused to the ins-1 5′ and 3′ cis regulatory sequences.
Table 1.
Specific cues have distinct effects on ILP gene expression
By contrast, we observed that ins-6 expression, which was based on an mCherry transcriptional reporter fused to the upstream and downstream regulatory regions of ins-6 (ins-6p::mCherry), is rare in non-neuronal tissues and restricted to the ASI neurons of well-fed larvae and adults (Fig. 5). This is different from the previously described expression of ins-6 in many neurons (Pierce et al., 2001), including ASI (A.C. and J.A., data not shown), which was determined with a gfp or an mCherry transcriptional reporter fused only to the ins-6 upstream sequences. This suggests that sequences downstream of ins-6 contain element(s) that repress its expression in other cells.
Fig. 5.
ins-6 transcription switches between ASI and ASJ in response to dauer pheromone and dauer arrest. (A) The twelve sensory neurons in the C. elegans amphid sensory organ (White et al., 1986). The ASI and ASJ neurons are indicated in black. Dauer pheromone inhibits ins-6 transcription in ASI, whereas both the pheromone and the dauer state activate ins-6 in ASJ. (B,D,F) ins-6p::mCherry is expressed in the ASI of well-fed L3 (B), L4 (D) and L1 (F) larvae. (C,E) ins-6p::mCherry becomes expressed in the ASJ of a dauer larva (C) and remains on in the ASJ of a post-dauer L4 larva (E). (G) ins-6p::mCherry is unaffected in ASI and is not activated in the ASJ of a starved L1 larva. All animals are oriented with their anterior to the lower left and their dorsal side up. Scale bars: 10 μm.
Interestingly, the switch into dauer arrest shifted the transcription of ins-6p::mCherry from ASI to ASJ (Fig. 5B,C, Table 1). In addition, as the animals started to exit from dauer, ASJ expression of ins-6p::mCherry appeared to become stronger (A.C. and J.A., data not shown) in response to the improved environment. We also saw this activation of ins-6 in the ASJ of worms carrying a transcriptional reporter fused only to the ins-6 upstream sequences (A.C. and J.A., data not shown). Together, our data suggest that ins-6 functions in ASI to inhibit dauer entry, and that it also functions in ASJ to promote dauer exit.
Since overexpression of ins-6 from either ASI or ASJ was able to rescue both the dauer entry phenotype of ins-6; daf-28 mutants and the dauer exit phenotype of ins-6; daf-2 mutants (see Figs S1 and S2 and Tables S1 and S2 in the supplementary material), we asked whether endogenous ins-6 acts primarily in ASJ to promote dauer exit. We analyzed worms in which the ASJ neurons were genetically ablated through the ASJ-specific expression of human caspase 1 (Zheng et al., 1999). As expected, ASJ ablation delayed the exit of daf-2 single-mutant dauers (Fig. 6A and see Table S2 in the supplementary material). Nonetheless, killing ASJ also largely abolished the difference between the dauer exit phenotypes of daf-2 single and ins-6; daf-2 double mutants (Fig. 6A and see Table S2 in the supplementary material). These findings support the hypothesis that ins-6 expression shifts to ASJ to induce the switch from dauer arrest to reproductive growth.
The dauer pheromone and the dauer state have distinct effects on ins-6 expression
We next asked which cues would downregulate ins-6p::mCherry in ASI and which cues would induce it in ASJ. We observed that high concentrations of the dauer-inducing pheromone downregulated ins-6p::mCherry in the ASI of pre-dauer (L2d) or L4 larvae or adults (Table 1). However, the dauer pheromone mixture by itself induced little or no ins-6p::mCherry expression in the ASJ of different stages of well-fed larvae and adults (Table 1). Surprisingly, induction of dauer arrest by shifting daf-2(e1368) mutants to 25°C, but under low pheromone levels, was also insufficient to fully activate ins-6p::mCherry in ASJ (Table 1). Indeed, the switch in ins-6p::mCherry from ASI to ASJ was only completely executed in dauers that were induced by high levels of dauer pheromone, either by the direct addition of the pheromone mixture or by high population density (Table 1). Since we also never observed ins-6p::mCherry expression in the ASJ of partial dauers, our data suggest that the combined activities of the dauer state and the dauer pheromone, which presumably induces a stronger arrest, are required for this expression shift (Table 1).
In addition, we found that starvation alone or high temperature (27°C) had little or no effect on ins-6p::mCherry expression, as compared with well-fed worms at 20°C (Fig. 5F,G, Table 1). Moreover, we detected no significant effect on ins-6p::mCherry expression in continuously well-fed animals lacking daf-16 or having reduced daf-2 activity (Table 1). Together, our findings suggest that the downregulation of ins-6p::mCherry in ASI is a specific response to the dauer pheromone cue, whereas the switch in neuronal expression is a distinct response that is specific to the coordinated action of the pheromone and the dauer state.
ins-6 and daf-28 play only a minor role in regulating lifespan
Since our observations suggested that ins-6, daf-28 and ins-1 have discrete, non-redundant functions in regulating two developmental programs, we asked whether they also affect lifespan, which is known to be regulated by DAF-2 (Kenyon et al., 1993; Kimura et al., 1997). Unlike daf-2 reduction-of-function mutants, we found that ins-6, daf-28 or ins-1 alone had little or no effect on adult lifespan (see Fig. S3 in the supplementary material). Similarly, loss of both ins-6 and daf-28 had little effect on the lifespan of animals that did not undergo dauer formation (see Fig. S3B,D in the supplementary material). However, ins-6; daf-28 double mutants that formed transient dauers did live longer than double mutants that never became dauers (see Fig. S3B,D in the supplementary material). This suggests that post-dauer adults are not physiologically the same as well-fed adults that have not undergone dauer arrest. Consistent with this idea, we found that ins-6p::mCherry surprisingly persisted in ASJ and was absent in the ASI of post-dauer L4s, young adults and 5-day-old adults (Fig. 5E, Table 1), which is different from the situation for continuously well-fed animals that expressed ins-6p::mCherry in ASI and never in ASJ (Fig. 5D, Table 1). Our findings also suggest that, to regulate lifespan, other ILPs with a more primary role than ins-6, daf-28 and ins-1 in this process are required to modulate DAF-2 signaling.
DISCUSSION
The large number of ILP genes in C. elegans and the spatiotemporal diversity of their expression patterns, which includes partly overlapping subsets of sensory neurons (Li et al., 2003; Pierce et al., 2001), raise the likelihood that different ILPs regulate different processes in response to diverse stimuli. In this study, we show that the ILP regulation of dauer entry versus exit is more complex than previously thought. We observe not only that daf-28 and ins-6 have growth-promoting activities that oppose those of ins-1, but also that the relative requirement for daf-28 and ins-6 switches between dauer entry and exit. Accordingly, our data suggest that ILPs function as a combinatorial code to regulate C. elegans development in response to complex sensory cues.
Individual ILPs encode specific cues to regulate distinct switches in developmental programs
In C. elegans, the ratio between food cues and dauer pheromone, which reflect food availability and population density, determines whether L1 larvae will undergo dauer arrest or whether dauers will exit from arrest (Golden and Riddle, 1982; Golden and Riddle, 1984). The perception of the food and pheromone cues by a specific subset of sensory neurons that either inhibit (ASI, ADF and ASG) or promote (ASJ and ASK) dauer entry or promote (ASJ and AWC) or inhibit (IL2) dauer exit (Bargmann and Horvitz, 1991; Kim et al., 2009; Macosko et al., 2009; Ouellet et al., 2008; Schackwitz et al., 1996) in turn regulates the secretion of growth-modulatory signals. For example, low food levels and high dauer pheromone concentrations repress the expression in ASI of the TGFβ daf-7, which is required for reproductive development under growth-inducing conditions (Ren et al., 1996; Schackwitz et al., 1996). However, re-introduction of food to a dauer population induces dauer exit and resumption of daf-7 expression and reproductive development (Ren et al., 1996).
Similarly, food cues activate transcription of the ILP daf-28 in ASI and ASJ, whereas the dauer pheromone suppresses it in both neurons (Li et al., 2003). However, food levels and dauer pheromone have different effects on other ILPs (Fig. 5, Table 1). For example, both cues have little or no effect on ins-1 mRNA levels in ASI and ASJ (Table 1). By contrast, high levels of dauer pheromone specifically repress the transcription of ins-6 in ASI, whereas food levels do not affect ins-6 expression in this neuron (Fig. 5, Table 1). Interestingly, other cues derived from dauer arrest-induced physiological changes, together with high pheromone levels, trigger ins-6 transcription in ASJ (Fig. 5, Table 1), a change in expression that alone is insufficient to promote dauer exit. This suggests that (1) the animal initiates ins-6 expression in this dauer exit-promoting neuron to facilitate its activation as soon as environmental conditions improve and (2) ins-6 activity is also regulated post-transcriptionally by other cues, perhaps such as increases in food levels. Thus, it is possible that different sensory cues regulate ILP function not only at the level of transcription but also at the level of translation and/or secretion. Indeed, this might be the case for ins-1, for which we observe no transcriptional changes in response to food or pheromone signals (Table 1). The specificity in the effects of different cues on the spatiotemporal expression of daf-28, ins-6 and ins-1 suggests a mechanism through which these ILPs encode environmental information and consequently regulate dauer entry versus exit.
Our findings also suggest that ins-6 and daf-28 encode ligands that have differing requirements in activating the DAF-2 pathway during the two developmental switches, whereas ins-1 antagonizes the activity of this pathway (Fig. 6B). These ligands might modulate DAF-2 signaling in a context-dependent manner. For example, besides having been shown to act as an antagonist of the pathway in regulating the dauer program (Pierce et al., 2001) (this paper) and food-associated thermotactic behavior (Kodama et al., 2006), ins-1 can also act like a DAF-2 agonist in salt-chemotactic learning behavior (Tomioka et al., 2006). Thus, to control a particular process, ins-1 and other ILPs may act from specific neurons to regulate DAF-2 signaling in a specific subset of cells, in either an autocrine or paracrine manner. Previous mosaic analyses of daf-2 function have already raised the possibility that different cells have different DAF-2 activities (Apfeld and Kenyon, 1998; Wolkow et al., 2000). Yet, none of our experiments has ruled out the possibility that some of these ILPs, which are predicted to have diverse structures [e.g. DAF-28 or INS-6 compared with INS-1 (Pierce et al., 2001)], will bind receptors other than DAF-2.
It should also be noted that, unlike daf-2 reduction-of-function mutants (Kenyon et al., 1993; Larsen et al., 1995), ins-6, daf-28 and ins-1, which function combinatorially to regulate development (Fig. 6B), have little or no effect on lifespan (see Fig. S3 in the supplementary material). Since lifespan is influenced by many types of cues and sensory neurons (Alcedo and Kenyon, 2004; Apfeld and Kenyon, 1999; Lee and Kenyon, 2009; Libert et al., 2007; Poon et al., 2010), it is not surprising that many cue-responsive ILPs would be involved in regulating DAF-2 activity to affect longevity.
ins-6 activity depends on spatial context
The switch in ins-6 expression from ASI to ASJ upon dauer arrest (Fig. 5, Table 1) suggests that ins-6 activity, and perhaps that of other ILPs, depends on the spatial context of its expression. Indeed, the expression of ins-6 in the dauer-inhibiting ASI neurons of well-fed larvae is consistent with ins-6 function in suppressing entry into dauer arrest (Fig. 6B). Similarly, ins-6 expression in ASJ upon dauer arrest is in keeping with its role in promoting dauer exit (Fig. 6B), i.e. INS-6 is secreted from ASJ to induce exit.
However, exogenous ins-6 expression in either ASI or ASJ not only inhibits entry into, but also promotes exit from, dauer (see Figs S1 and S2 and Tables S1 and S2 in the supplementary material). Although this might be due to much higher than endogenous expression of ins-6, daf-2(e1368) dauers formed under low pheromone still exit from arrest when ins-6 remains primarily expressed in ASI and does not completely switch to ASJ (Table 1). Nonetheless, high pheromone-induced ASJ-ablated daf-2 single-mutant and ins-6; daf-2 double-mutant dauers have very similar exit phenotypes (Fig. 6A and see Table S2 in the supplementary material). Since high pheromone-induced dauers with intact ASJ do exhibit the neuronal switch in ins-6 expression (Table 1), the ASJ ablation experiment suggests that ins-6 acts from ASJ under this condition, and not from other neurons, to promote exit.
These observations also suggest that temperature-induced, low pheromone-exposed daf-2(e1368) dauers might represent a weaker arrest, thus explaining the absence of the ins-6 shift from ASI to ASJ (Table 1). By contrast, the high pheromone-induced dauers might reflect a stronger arrest, which induces the full dauer transcriptional program, including the altered ins-6 expression (Table 1). ins-6 may become specifically upregulated in ASJ after a strong arrest because ASJ has the receptors that sense such an arrest and the inputs that induce exit from it. Our data further raise the possibility that the circuit that induces exit after a weak arrest, which might or might not be similar to a dauer entry-regulating circuit, is different from the circuit that induces exit after a stronger arrest. Accordingly, INS-6, as well as other ILPs, might act locally as part of different developmental circuits that are remodeled in response to cues, such as the combination of dauer pheromone with the dauer state.
Post-dauer adults are distinct from continuously well-fed adults
Our observation that ins-6 is expressed in a different sensory neuron in post-dauer adults suggests that these animals might exhibit a different physiology from continuously fed adults. Consistent with this hypothesis, the duration of the dauer state has been shown to correlate positively with the number of reproductive defects in post-dauer adults (Kim and Paik, 2008). Moreover, ins-6; daf-28 post-dauer adults live 11% longer than the continuously fed double mutants (see Fig. S3 in the supplementary material). This is similar to a recent observation that wild-type post-dauer adults live longer than their continuously fed counterparts (Hall et al., 2010). This suggests that (1) the dauer state causes a physiological change that is sufficient to induce a small but significant extension in adult lifespan and (2) this lifespan extension is not necessarily dependent on ins-6 and daf-28.
The relevance of multiple ILPs in other animals
The concept that ILPs encode environmental information to regulate physiology might be true not only for C. elegans but also for other animals, such as Drosophila or mammals. Drosophila has seven known ILPs, dilp1-7 (Ilp1-7 – FlyBase), which are expressed in neuronal and/or non-neuronal cells (Brogiolo et al., 2001; Ikeya et al., 2002; Rulifson et al., 2002; Slaidina et al., 2009; Yang et al., 2008). Intriguingly, the neurons that express some of these ILPs send or receive projections from subesophageal ganglion interneurons, which in turn receive information (Melcher and Pankratz, 2005; Rulifson et al., 2002; Yang et al., 2008) from gustatory neurons that innervate chemosensory structures within the fly mouthparts (Scott et al., 2001). In addition, some of these neuronally expressed ILPs (dilp2, dilp3 and dilp5) have been proposed to regulate growth and metabolism in a nutrient level-dependent manner, whereas others do not (Broughton et al., 2008; Grönke et al., 2010; Ikeya et al., 2002; Min et al., 2008; Zhang et al., 2009). Some are also required in other processes, such as in selecting an optimal environment for egg laying, which is dilp7 dependent (Yang et al., 2008).
By comparison, mammals have seven to ten known members of the insulin/relaxin superfamily that are expressed in non-overlapping cells, including neurons with known sensory-associated functions (Ayer-le Lievre et al., 1991; Bathgate et al., 2002; Liu and Lovenberg, 2008; Meyts et al., 2009; Sherwood, 2004). The roles of insulin, IGF1 and IGF2 in mammalian metabolism, growth, differentiation and lifespan have been studied in great detail (Kenyon, 2005; Nakae et al., 2001; Sherwood, 2004), and some relaxins have been found to regulate reproductive, as well as non-reproductive, processes (Sherwood, 2004). However, the functions of other members of this family are less clear. Since the effects of ILP signaling on physiology (e.g. growth and lifespan) are conserved from worms to mammals (Blüher et al., 2003; Holzenberger et al., 2003; Kenyon et al., 1993; Taguchi et al., 2007), our study raises the possibility that specific subsets of mammalian ILPs also act together to regulate specific processes in response to different sets of environmental cues.
Supplementary Material
Acknowledgements
We thank M. Thomas for technical assistance; I. Katic and M. Regenass for help in generating some of the transgenic lines; W. Maier for the crude dauer pheromone mixture; C. Kenyon, S. Mitani, the Caenorhabditis Genetics Center and the C. elegans Gene Knockout Consortium for strains; C. Bargmann, A. Fire, V. Maricq and P. Swoboda for molecular reagents; M. Pietrzak for DNA sequencing; and Q. Ch'ng, I. Katic, M. Noll, M. Pankratz, the J.A. lab and H. Cornils for discussions and/or critical comments on the manuscript. This work was supported by grants from the Esther A. and Joseph Klingenstein Fund, the March of Dimes Foundation, the Alfred P. Sloan Foundation, the John Merck Fund and NIH (R01 DC009852) to Y.Z. and the Novartis Research Foundation to J.A. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.060905/-/DC1
References
- Alcedo J., Kenyon C. (2004). Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41, 45-55 [DOI] [PubMed] [Google Scholar]
- Apfeld J., Kenyon C. (1998). Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. Cell 95, 199-210 [DOI] [PubMed] [Google Scholar]
- Apfeld J., Kenyon C. (1999). Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804-809 [DOI] [PubMed] [Google Scholar]
- Ayer-le Lievre C., Stahlbom P. A., Sara V. R. (1991). Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus. Development 111, 105-115 [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Horvitz H. R. (1991). Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science 251, 1243-1246 [DOI] [PubMed] [Google Scholar]
- Bathgate R. A. D., Samuel C. S., Burazin T. C. D., Layfield S., Claasz A. A., Reytomas I. G. T., Dawson N. F., Zhao C., Bond C., Summers R. J., et al. (2002). Human relaxin gene 3 (H3) and the equivalent mouse relaxin (M3) gene. J. Biol. Chem. 277, 1148-1157 [DOI] [PubMed] [Google Scholar]
- Blüher M., Kahn B. B., Kahn C. R. (2003). Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299, 572-574 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E. (2001). An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11, 213-221 [DOI] [PubMed] [Google Scholar]
- Broughton S., Alic N., Slack C., Bass T., Ikeya T., Vinti G., Tommasi A. M., Driege Y., Hafen E., Partridge L. (2008). Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE 3, e3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher R. A., Fujita M., Schroeder F. C., Clardy J. (2007). Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem. Biol. 3, 420-422 [DOI] [PubMed] [Google Scholar]
- Cassada R. C., Russell R. L. (1975). The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46, 326-342 [DOI] [PubMed] [Google Scholar]
- Gems D., Sutton A. J., Sundermeyer M. L., Albert P. S., King K. V., Edgley M. L., Larsen P. L., Riddle D. L. (1998). Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150, 129-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L. (1982). A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 218, 578-580 [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L. (1984). The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 102, 368-378 [DOI] [PubMed] [Google Scholar]
- Gottlieb S., Ruvkun G. (1994). daf-2, daf-16 and daf-23: genetically interacting genes controlling dauer formation in Caenorhabditis elegans. Genetics 137, 107-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönke S., Clarke D.-F., Broughton S., Andrews T. D., Partridge L. (2010). Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 6, e1000857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. E., Beverly M., Russ C., Nusbaum C., Sengupta P. (2010). A cellular memory of developmental history generates phenotypic diversity in C. elegans. Curr. Biol. 20, 149-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzenberger M., Dupont J., Ducos B., Leneuve P., Geloen A., Even P. C., Cervera P., Le Bouc Y. (2003). IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421, 182-187 [DOI] [PubMed] [Google Scholar]
- Hothorn T., Hornik K., van de Wiel M. A., Zeileis A. (2008). Implementing a class of permutation tests: the coin package. J. Stat. Softw. 28, 1-23 [Google Scholar]
- Ikeya T., Galic M., Belawat P., Nairz K., Hafen E. (2002). Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 12, 1293-1300 [DOI] [PubMed] [Google Scholar]
- Jeong P. Y., Jung M., Yim Y. H., Kim H., Park M., Hong E., Lee W., Kim Y. H., Kim K., Paik Y. K. (2005). Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 433, 541-545 [DOI] [PubMed] [Google Scholar]
- Kao G., Nordenson C., Still M., Rönnlund A., Tuck S., Naredi P. (2007). ASNA-1 positively regulates insulin secretion in C. elegans and mammalian cells. Cell 128, 577-587 [DOI] [PubMed] [Google Scholar]
- Kenyon C. (2005). The plasticity of aging: insights from long-lived mutants. Cell 120, 449-460 [DOI] [PubMed] [Google Scholar]
- Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. (1993). A C. elegans mutant that lives twice as long as wild type. Nature 366, 461-464 [DOI] [PubMed] [Google Scholar]
- Kim K., Sato K., Shibuya M., Zeiger D. M., Butcher R. A., Ragains J. R., Clardy J., Touhara K., Sengupta P. (2009). Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science 326, 994-998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Paik Y.-K. (2008). Developmental and reproductive consequences of prolonged non-aging dauer in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 368, 588-592 [DOI] [PubMed] [Google Scholar]
- Kimura K. D., Tissenbaum H. A., Liu Y., Ruvkun G. (1997). daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277, 942-946 [DOI] [PubMed] [Google Scholar]
- Kodama E., Kuhara A., Mohri-Shiomi A., Kimura K. D., Okumura M., Tomioka M., Iino Y., Mori I. (2006). Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev. 20, 2955-2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P., Albert P. S., Riddle D. L. (1995). Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139, 1567-1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Kenyon C. (2009). Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr. Biol. 19, 715-722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Fleming J. T. (1995). Basic culture methods. In Caenorhabditis elegans: Modern Biological Analysis of an Organism, vol. 48 (ed. Epstein H. F., Shakes D. C.), pp. 3-29 San Diego, CA: Academic Press; [Google Scholar]
- Li W., Kennedy S. G., Ruvkun G. (2003). daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 17, 844-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S., Zwiener J., Chu X., VanVoorhies W., Roman G., Pletcher S. D. (2007). Regulation of Drosophila life span by olfaction and food-derived odors. Science 315, 1133-1137 [DOI] [PubMed] [Google Scholar]
- Lin K., Dorman J. B., Rodan A., Kenyon C. (1997). daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278, 1319-1322 [DOI] [PubMed] [Google Scholar]
- Liu C., Lovenberg T. W. (2008). Relaxin-3, INSL5, and their receptors. Results Probl. Cell Differ. 46, 213-237 [DOI] [PubMed] [Google Scholar]
- Macosko E. Z., Pokala N., Feinberg E. H., Chalasani S. H., Butcher R. A., Clardy J., Bargmann C. I. (2009). A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458, 1171-1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone E. A., Inoue T., Thomas J. H. (1996). Genetic analysis of the roles of daf-28 and age-1 in regulating Caenorhabditis elegans dauer formation. Genetics 143, 1193-1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher C., Pankratz M. J. (2005). Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 3, e305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyts P. D., Gauguin L., Svendsen A. M., Sarhan M., Knudsen L., Nøhr J., Kiselyov V. V. (2009). Structural basis of allosteric ligand-receptor interactions in the insulin/relaxin peptide family. Ann. N. Y. Acad. Sci. 1160, 45-53 [DOI] [PubMed] [Google Scholar]
- Min K. J., Yamamoto R., Buch S., Pankratz M., Tatar M. (2008). Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell 7, 199-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Vizuete A., González J. C. F., Gahmon G., Burghoorn J., Navas P., Swoboda P. (2006). Lifespan decrease in a Caenorhabditis elegans mutant lacking TRX-1, a thioredoxin expressed in ASJ sensory neurons. FEBS Lett. 580, 484-490 [DOI] [PubMed] [Google Scholar]
- Miyabayashi T., Palfreyman M. T., Sluder A. E., Slack F., Sengupta P. (1999). Expression and function of members of a divergent nuclear receptor family in Caenorhabditis elegans. Dev. Biol. 215, 314-331 [DOI] [PubMed] [Google Scholar]
- Nakae J., Kido Y., Accili D. (2001). Distinct and overlapping functions of insulin and IGF-I receptors. Endocr. Rev. 22, 818-835 [DOI] [PubMed] [Google Scholar]
- Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L., Tissenbaum H. A., Ruvkun G. (1997). The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994-999 [DOI] [PubMed] [Google Scholar]
- Ouellet J., Li S., Roy R. (2008). Notch signalling is required for both dauer maintenance and recovery in C. elegans. Development 135, 2583-2592 [DOI] [PubMed] [Google Scholar]
- Pierce S. B., Costa M., Wisotzkey R., Devadhar S., Homburger S. A., Buchman A. R., Ferguson K. C., Heller J., Platt D. M., Pasquinelli A. A., et al. (2001). Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 15, 672-686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon P. C., Kuo T.-H., Linford N. J., Roman G., Pletcher S. D. (2010). Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol. 8, e1000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2009). R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; [Google Scholar]
- Ren P., Lim C.-S., Johnsen R., Albert P. S., Pilgrim D., Riddle D. L., (1996). Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science 274, 1389-1391 [DOI] [PubMed] [Google Scholar]
- Riddle D. L., Swanson M. M., Albert P. S. (1981). Interacting genes in nematode dauer larva formation. Nature 290, 668-671 [DOI] [PubMed] [Google Scholar]
- Rulifson E. J., Kim S. K., Nusse R. (2002). Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296, 1118-1120 [DOI] [PubMed] [Google Scholar]
- Schackwitz W. S., Inoue T., Thomas J. H. (1996). Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17, 719-728 [DOI] [PubMed] [Google Scholar]
- Scott K., Brady J. R., Cravchik A., Morozov P., Rzhetsky A., Zuker C., Axel R. (2001). A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104, 661-673 [DOI] [PubMed] [Google Scholar]
- Sherwood O. D. (2004). Relaxin's physiological roles and other diverse actions. Endocr. Rev. 25, 205-234 [DOI] [PubMed] [Google Scholar]
- Slaidina M., Delanoue R., Gronke S., Partridge L., Léopold P. (2009). A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev. Cell 17, 874-884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A., Wartschow L. M., White M. F. (2007). Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317, 369-372 [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Birnby D. A., Vowels J. J. (1993). Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics 134, 1105-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum H. A., Hawdon J., Perregaux M., Hotez P., Guarente L., Ruvkun G. (2000). A common muscarinic pathway for diapause recovery in the distantly related nematode species Caenorhabditis elegans and Ancylostoma caninum. Proc. Natl. Acad. Sci. USA 97, 460-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka M., Adachi T., Suzuki H., Kunitomo H., Schafer W. R., Iino Y. (2006). The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron 51, 613-625 [DOI] [PubMed] [Google Scholar]
- Vowels J. J., Thomas J. H. (1992). Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics 130, 105-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S. (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 314, 1-340 [DOI] [PubMed] [Google Scholar]
- Wolkow C. A., Kimura K. D., Lee M. S., Ruvkun G. (2000). Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science 290, 147-150 [DOI] [PubMed] [Google Scholar]
- Yang C.-H., Belawat P., Hafen E., Jan L. Y., Jan Y.-N. (2008). Drosophila egg-laying site selection as a system to study simple decision-making processes. Science 319, 1679-1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Liu J., Li C. R., Momen B., Kohanski R. A., Pick L. (2009). Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc. Natl. Acad. Sci. USA 106, 19617-19622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Brockie P. J., Mellem J. E., Madsen D. M., Maricq A. V. (1999). Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor Neuron 24, 347-361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.