Abstract
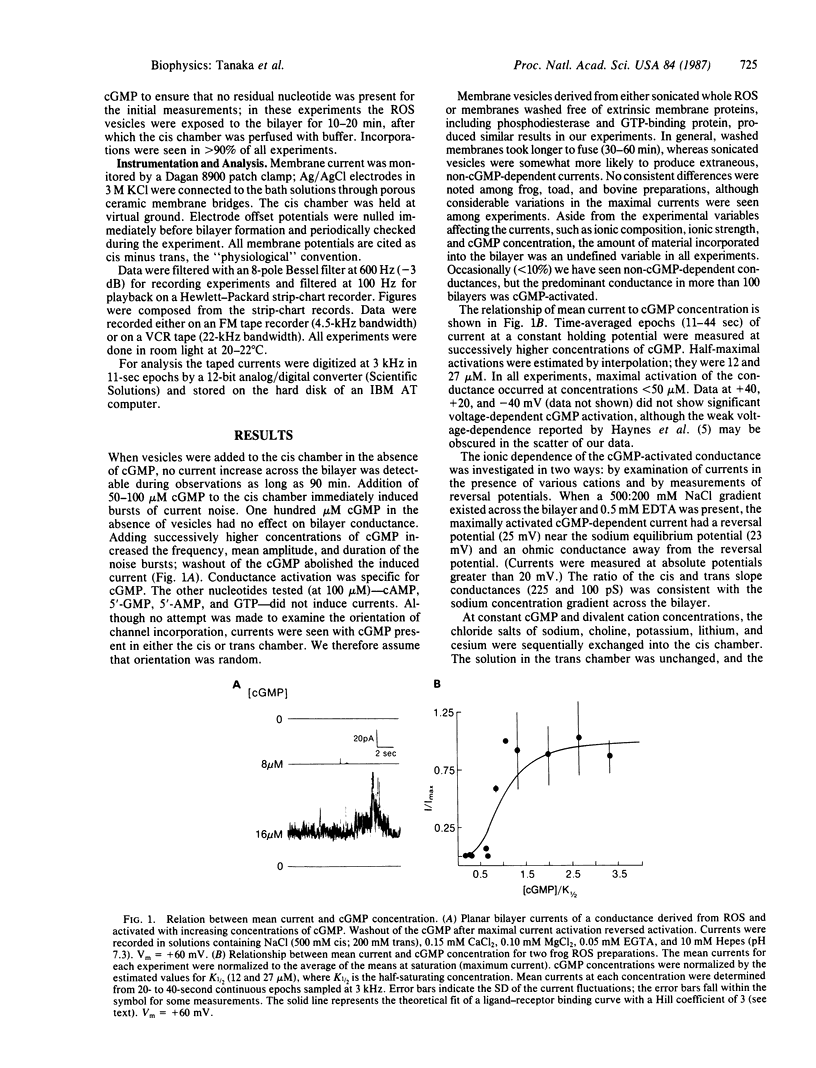
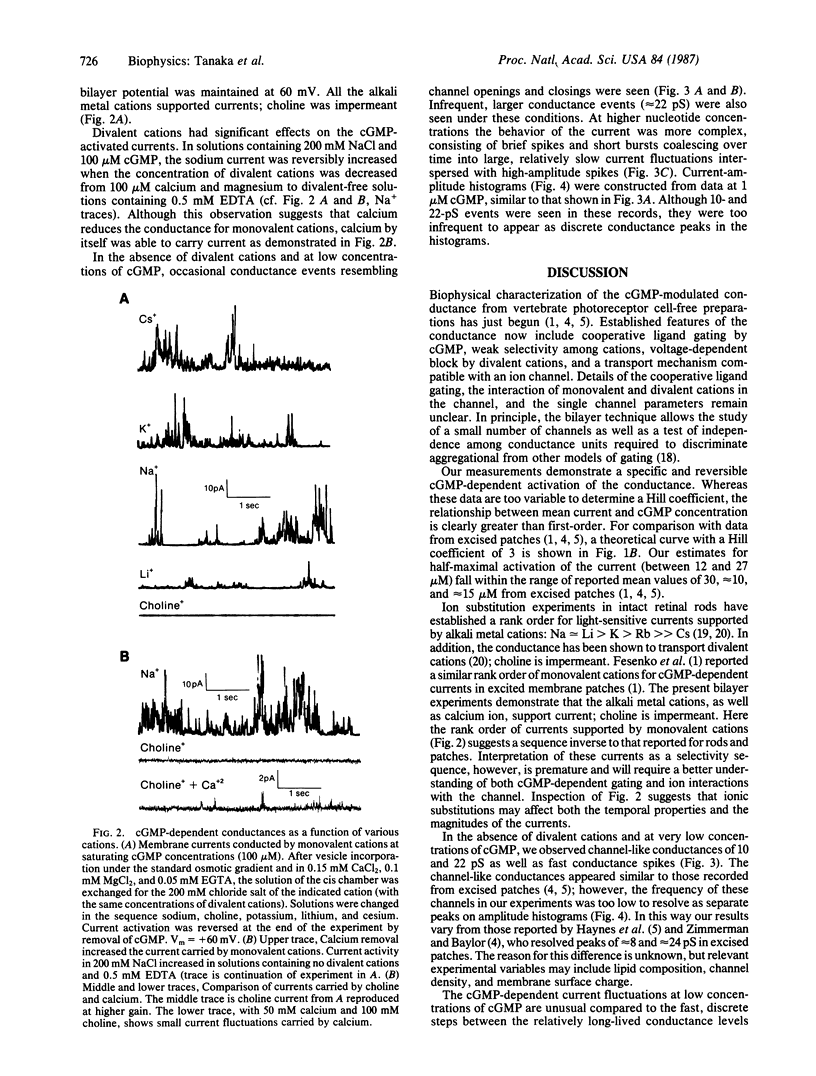
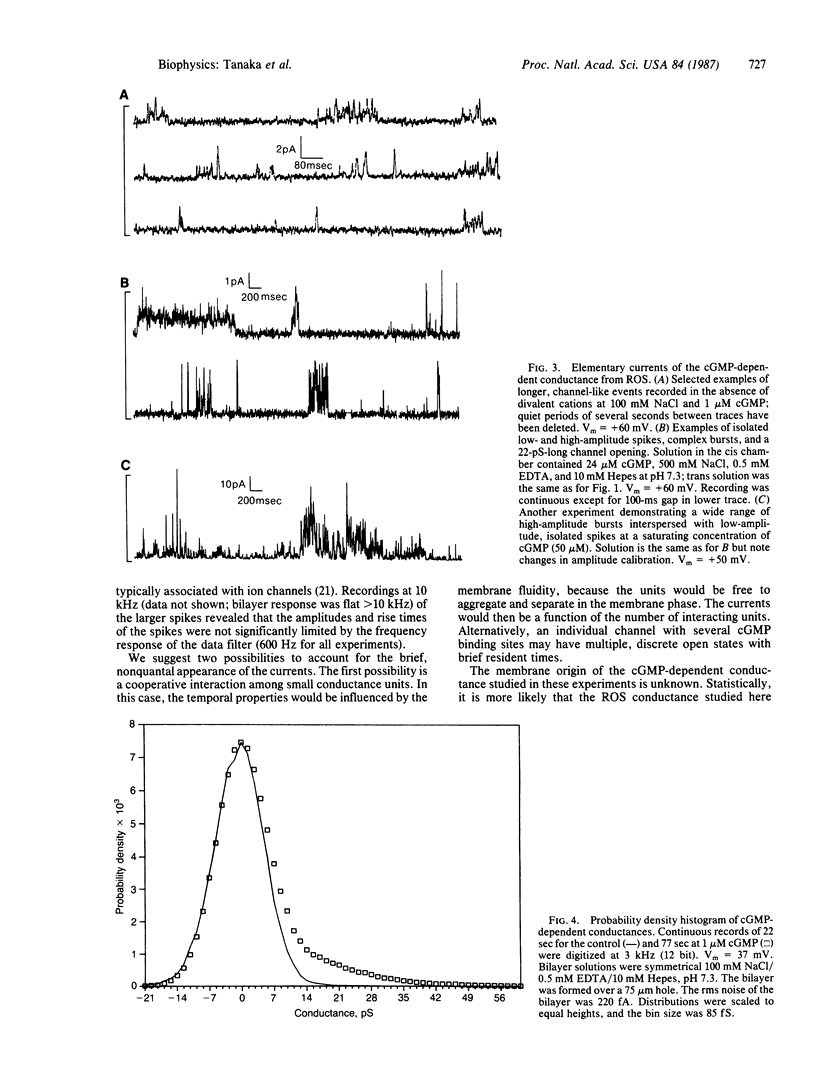
The light-modulated current of vertebrate retinal rods flows through a 3',5'-cyclic GMP-dependent conductance located in the outer segment plasma membrane. We report the incorporation into planar bilayers of a conductance derived from vertebrate rod outer segment membranes specifically activated by cGMP but not by cAMP, 5'-GMP, GTP, or 5'-AMP. When the mean currents were measured as a function of increasing cGMP concentration, maximal activation occurred at concentrations less than 50 microM. Washout of cGMP rapidly reversed the effect. The apparent half-saturating concentrations were between 12 and 27 microM. Sodium, lithium, cesium, and potassium supported current in the presence of low concentrations of Ca2+, Mg2+, and 100 microM cGMP; choline did not. Removal of the divalent cations reversibly increased the currents. When calcium was the only current-carrying cation, attenuated currents were seen. These experiments support the hypothesis that calcium is a permeant blocker of the conductance. At low concentrations of cGMP in solutions also containing 0.5 mM EDTA, brief current spikes occurred with amplitudes from 0.5 to 4 pA at 50 mV. These spikes differed from the well-defined, unitary conductance steps usually associated with the opening and closing of ion channels. Occasionally we saw longer-lasting channel-like events; however, amplitude histograms did not resolve discrete conductance levels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bodoia R. D., Detwiler P. B. Patch-clamp recordings of the light-sensitive dark noise in retinal rods from the lizard and frog. J Physiol. 1985 Oct;367:183–216. doi: 10.1113/jphysiol.1985.sp015820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A., Sorbi R. T. Cyclic GMP and the permeability of the disks of the frog photoreceptors. J Physiol. 1979 Oct;295:171–178. doi: 10.1113/jphysiol.1979.sp012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A. Effect of cGMP and cations on the permeability of cattle retinal disks. Eur J Biochem. 1985 May 2;148(3):599–606. doi: 10.1111/j.1432-1033.1985.tb08882.x. [DOI] [PubMed] [Google Scholar]
- Cavaggioni A., Sorbi R. T. Cyclic GMP releases calcium from disc membranes of vertebrate photoreceptors. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3964–3968. doi: 10.1073/pnas.78.6.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbs W. H., Pugh E. N., Jr Cyclic GMP can increase rod outer-segment light-sensitive current 10-fold without delay of excitation. Nature. 1985 Feb 14;313(6003):585–587. doi: 10.1038/313585a0. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Conner J. D., Bodoia R. D. Gigaseal patch clamp recordings from outer segments of intact retinal rods. Nature. 1982 Nov 4;300(5887):59–61. doi: 10.1038/300059a0. [DOI] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Haynes L. W., Kay A. R., Yau K. W. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986 May 1;321(6065):66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- Haynes L., Yau K. W. Cyclic GMP-sensitive conductance in outer segment membrane of catfish cones. Nature. 1985 Sep 5;317(6032):61–64. doi: 10.1038/317061a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. J Physiol. 1985 Jan;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch K. W., Kaupp U. B. Cyclic GMP directly regulates a cation conductance in membranes of bovine rods by a cooperative mechanism. J Biol Chem. 1985 Jun 10;260(11):6788–6800. [PubMed] [Google Scholar]
- Matthews H. R., Torre V., Lamb T. D. Effects on the photoresponse of calcium buffers and cyclic GMP incorporated into the cytoplasm of retinal rods. Nature. 1985 Feb 14;313(6003):582–585. doi: 10.1038/313582a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Harkness J., Parkes J. H., Gonzalez-Oliva C., Liebman P. A. Kinetic studies suggest that light-activated cyclic GMP phosphodiesterase is a complex with G-protein subunits. Biochemistry. 1986 Feb 11;25(3):651–656. doi: 10.1021/bi00351a021. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Cation selectivity of light-sensitive conductance in retinal rods. Nature. 1984 May 24;309(5966):352–354. doi: 10.1038/309352a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman A. L., Baylor D. A. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986 May 1;321(6065):70–72. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]



