Abstract
Background
Increasing colorectal cancer screening (CRCS) can have a substantial positive impact on morbidity and mortality.
Objectives
The purpose of this report is to describe the development and feasibility testing of a computer-based, theory-guided educational program designed to increase CRCS.
Research Design
This mixed-methods study used focus groups and subsequent randomized controlled trial design.
Subjects
Participants (N = 199) were randomized to an intervention or control group; 75% were African American; mean age was 57.36 (SD = 6.8); 71% were male.
Measures
Previously validated measures on knowledge, beliefs, and screening test adherence were used to establish pre- and post-intervention perceptions. Feasibility was measured by response and completion rates, and participants’ perceptions of the program.
Results
Before feasibility testing, the program was presented to 2 focus groups. Changes were made to the program based on discussion, leading to a visually appealing, easy to understand and navigate, self-paced program. In the RCT pilot test that followed, of the participants in the intervention group, 80% said the education helped them decide to get CRCS; 49% agreed it helped them overcome barriers; 91% agreed it was useful, 68% thought it raised new concerns about cancer, but only 30% said it made them worry about CRC; 95% agreed their doctor's office should continue giving such education, and 99% said they would inform family about the program.
Conclusions
The response rate of 83% demonstrated feasibility of conducting colorectal cancer education in the primary care setting; overall the program was well received; participants averaged 23 minutes to complete it. Participants sought no help from attending data collectors and navigated the revised touch screen program with ease. Computer-based education is feasible in primary care clinics.
Keywords: cancer screening, colorectal cancer, underserved population, intervention development
Despite compelling evidence that screening for colorectal cancer (CRC) can reduce incidence of, and mortality from CRC, and the availability of evidence-based guidelines for, and tests to detect CRC, screening rates remain low and lag behind those for other common cancers.1,2 Hence, it is important to develop and implement effective interventions to promote CRC screening.
Primary care is an ideal setting to facilitate preventive health behaviors. Increasingly, primary care practices are being measured on the rates of delivery of preventive services, including CRC screening.3,4 A challenge for primary care providers is to develop cost-effective strategies to provide integrated, accessible health care services, and to develop sustained partnership with patients;5 one example of such a strategy would be delivering CRC screening to patients in the context of a busy practice. Before the incorporation of such education into clinical practice, it is important to develop the educational program in conjunction with the target population (as described in this report), allowing for the integration of culturally relevant material as well as accounting for user characteristics. The participatory development and feasibility testing we conducted are important to the long-term success of educational programs.
Purpose
The purpose of the parent study was to test the efficacy of a computer-based intervention designed to increase CRC screening test use—that is, fecal occult blood test (FOBT), flexible sigmoidoscopy, or colonoscopy. To reduce respondent burden in data collection and intervention delivery time, we collectively referred to the latter 2 tests as endoscopy. This report focuses on the development and feasibility testing of the computer-based, theory-guided educational program called TIMS© (Tailored Messaging Intervention System) designed to increase CRC screening among patients in primary care clinics. Descriptive data on participants’ knowledge of CRC and screening, perceived risk, perceived self-efficacy regarding FOBT and endoscopy (sigmoidoscopy and colonoscopy) and benefits of, and barriers to each test are also described. Intervention efficacy was tested using an RCT design. This report, however, will focus on the development and feasibility testing only. As such, we focus on the development process and final product, feasibility testing, and baseline characteristics of the sample.
TIMS and Rationale for Using Tailored Messages
Tailored interventions are defined as “any combination of information or change strategies intended to reach one specific person, based on characteristics that are unique to that person, related to the outcome of interest, and derived from an individual assessment.”6 Computerized tailored health education provides respondents with personally adapted feedback about their present health behaviors and factors known to impact such behavior, as well as personally adapted suggestions to motivate individuals to change and maintain healthy behaviors. Reviews of the effectiveness of tailored communications indicate that tailored messages are more likely to be remembered and viewed as relevant.7,8 Health promotion messages may be tailored to beliefs, knowledge, stage of readiness, or any combination of factors.7 Although the efficacy of interactive tailored computerized messaging for CRC screening is still under study, its effectiveness in increasing breast cancer screening, as well as changing diet, exercise, and smoking behavior in both men and women, underscores its potential usefulness.8–10 The process by which TIMS was used to deliver the education is explained in detail later in this article.
METHODS
All participants were recruited from the Chicago metropolitan area and were patients at the Internal Medicine Clinic at the University of Illinois-Chicago Medical Center or the primary care clinics at the Jesse Brown Veterans Administration Medical Center (Chicago). We estimate that the university clinic serves about 16,000 female and 14,000 male patients aged 50 or older each year. Additionally, the ethnicity breakdown is estimated to be 58% African American, and 16% white. At the VA in the year before the study was implemented, the clinics together served over 8000 patients aged 50 and older with 6% of those being female, and approximately 75% being African American. The developmental phase and pilot testing of TIMS were approved by the Institutional Review Boards at both clinical sites.
How TIMS Works
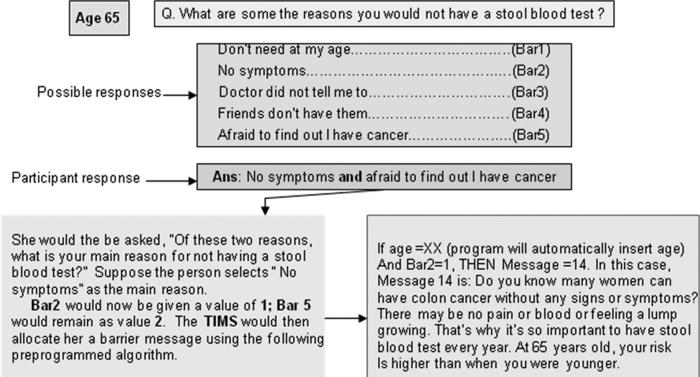
Tailoring by computer relies on preprogrammed algorithms to match a specific response with a message. For example, Figure 1 (Sample Tailored Algorithm for FOBT Barriers) depicts a sample algorithm for a respondent regarding stool blood test barriers.
FIGURE 1.
Sample tailored algorithm for FOBT barriers.
In TIMS, participants answered a series of questions on the computer, using a touch-screen response format. Computer-based education both tailored and nontailored have shown successful changes in a variety of preventive and illness-related health behaviors.11–17 Touch-screen and interactive computer programs are endorsed as a practical, private, and user-friendly method of collecting health data and delivering education.18–22 Items on knowledge of CRC and screening, perceived risk of CRC, self-efficacy regarding FOBT and endoscopy (sigmoidoscopy and colonoscopy), and benefits of and barriers to each screening test were assessed via computer. Responses to these items were collated in real time by TIMS and based on preprogrammed algorithms, relevant messages—ie, tailored to a participant's responses were presented on screen to those in the intervention groups (Fig. 1). Constructs selected for tailoring were based on the Health Belief Model.23
Based on their responses, participants received tailored messages on knowledge of CRC and screening, perceived barriers to each CRC screening test (FOBT, sigmoidoscopy, and colonoscopy), benefits of each CRC screening test, perceived risk of CRC and self-efficacy regarding each CRC screening test. Questions on stages of readiness to change behavior were based on the Transtheoretical Model of Change.24 Respondents also received messages related specifically to their stages of change (defined in subsequent sections in this article). Questions also captured the participant's age, gender, and race/ethnicity, which were then embedded in the tailored messages. The messages are drawn from an existing message library, previously developed with National Cancer Institute funding.25 Figure 1 contains an example of a tailored message. The tailored message library can be obtained by contacting the lead author.
Adaptation of TIMS Based on Focus Group Discussion
Focus groups can be a powerful means to test or evaluate a new product. In these groups the intent was to refine TIMS to increase its acceptability by the target population. Because TIMS was initially developed for implementation in a US state with few minority residents, this resulted in an initial target population that was predominantly white. Before implementation in Chicago we engaged members of the new target population in participatory research. TIMS was presented to 2 focus groups in Chicago; participants were from the target primary care clinics (described under Methods) where the program would be tested. Recruitment occurred through flyers placed in the clinics. A semi-structured discussion guide was used to facilitate discussion during the groups; questions focused on explaining the TIMS program and eliciting feedback from participants. TIMS was presented to the participants using an LCD projector. Separate groups of men (n = 3) and women (n = 4) met in a room at the UIC-College of Nursing. We did not attempt to achieve data saturation as we might in a true qualitative design. Rather the goal was to understand how to adapt the program before launching it in Chicago clinics. Time and funding limited us from presenting the revised program to more focus groups. Acceptability of the program, as will be discussed later in this article, was high. Discussion was led by the principal investigator of the study (UM) who is an experienced group facilitator. Refreshments were provided and participants received $25.00 in compensation.
Discussants identified a variety of problems with the educational program, fitting within the themes of: navigation, colors, font, and graphics. Researchers and programmers discussed changes to the program. Two team members then independently reviewed the revised TIMS. TIMS was pilot tested after several iterations. The specific problems identified and the corresponding revisions made to TIMS are available in the online Appendix (located on the Medical Care website, www.lww-medicalcare.com). It is important to not that, overall, the formative discussion phase of this study and the subsequent revision of TIMS led to a computer-based educational program that was visually appealing, easy to understand and navigate, self-paced, and culturally sensitive to the target population.
Feasibility Testing
Participants were recruited from both clinical sites. Eligibility criteria included: age 50 or older, not adherent with CRC screening, no personal history of CRC, not been advised by their provider to abstain from CRC screening, and English-speaking. Persons with a family history of a known hereditary cancer syndrome, irritable bowel disease, or who met high risk criteria (ie, first-degree relative diagnosed with CRC before 50 years, or 2 or more first degree relatives with CRC) were excluded because the focus of this study was screening among average-risk individuals. High-risk patients may have dissimilar beliefs that impact their behavior differently. Because all participants were recruited in primary care clinics where they were already receiving health care, lack of access to health care was not an issue for this sample.
Trained research assistants distributed flyers to patients in clinic waiting rooms. Their base was a table with a poster about the study and a basket of crackers and candy. If a patient expressed interest, they were given an eligibility criteria sheet to complete. The data collector reviewed the consent form with eligible patients, then assigned each one a unique identification number which was already randomized to study group. For this report, we focus on the descriptive data on knowledge and beliefs and feasibility (recruitment, completion, and perceptions of TIMS). Data collectors entered the identification and group numbers into the computer and handed the participant one of 2 Toshiba® NoteBook laptops with swivel screens and touch screen pens. The basic instructions given by assistants were to read the information on the screen and use the touch screen pen to respond. When the participant completed the session, the data were automatically saved. On the return of the laptop, they were given a choice of a $15 gift card to a local department or grocery store.
TIMS as described previously, was self-paced, with a touch-screen format that enabled a respondent to move between screens when ready. Qualitative information gathered by the assistant was based on questions asked by participants or observations of participants. These data were recorded in paper and pencil format for analysis. The educational session took approximately 23 minutes to complete, and minimal assistance was requested by respondents.
For the intervention group, computer algorithms (programmed in SQL) read individual preintervention data and brought up screens with relevant text messages. Specific constructs tailored on were derived from past work, and included knowledge, perceived risk, benefits, barriers, self-efficacy, and stage of readiness.13 The attention control group only completed the self-administered computerized survey and received no education. Standard care at the clinic was available to all participants.
Measures
The perceived risk (perception of vulnerability for developing CRC) and self-efficacy for FOBT, endoscopy (confidence in one's ability to perform all the steps to complete each screening test) and knowledge (screening guidelines, treatment, early detection, and general risk factors) scales were previously tested for reliability and validity.25–27 Psychometrics was further confirmed in our predominantly African American sample. The items on benefits, barriers, and knowledge constituted indices which were internally consistent. The final outcome of the RCT was completion of an FOBT, sigmoidoscopy, or colonoscopy, which will be reported elsewhere (manuscript in progress). Standardized definitions and measures of the screening tests were used.28 Perceived risk and self-efficacy for FOBT and endoscopy were operationalized as interval level measures and used as such in parametric testing. For this study we defined feasibility as recruitment, completion of TIMS, and participants’ perceptions of TIMS.
Stage of Readiness for CRC Screening Adoption
Queries about past behavior and intent to screen, based on separate algorithms, assessed stage of readiness. The 3 stages for FOBT use are defined below as an example. The only difference in the stage definitions for the other 2 screening tests was time intervals measured—5 years for sigmoidos-copy and 10 years for colonoscopy, as recommended by the American Cancer Society.29
Precontemplation
Never had FOBT or last FOBT over a year ago, and not thinking about having FOBT in the next 4 weeks.
Contemplation
Never had FOBT or last FOBT over a year ago, and thinking about having FOBT in the next 4 weeks. Action. FOBT within the past year.
RESULTS
Data Analysis
Responses entered into TIMS were saved in an EXCEL file, retrieved, and converted to SPSS (Version 15) files for analysis. There was minimal missing data as all questions had to be answered before a respondent could advance through the program.
Sample
There were no significant differences in demographic characteristics by study group at preintervention, indicating successful randomization (Table 1). Mean age was 56 (SD = 5.9) and 57 (SD = 7.6) in the control and intervention groups, respectively. The majority was African American, reported not having a partner (widowed, divorced, or single), were unemployed, had some college or technical degree, and had health insurance (Table 1).
TABLE 1.
Sociodemographic Characteristics of the Sample Stratified by Study Group (n = 199)
| Demographic Variables | Intervention Group (n = 101) n (%) | Control Group* (n = 98) n (%) |
|---|---|---|
| Gender | ||
| Female | 32 (56.1) | 25 (43.9) |
| Male | 69 (48.6) | 73 (51.4) |
| Race | ||
| African American | 78 (52.3) | 71 (47.7) |
| All other race/ethnicity | 23 (46.0) | 27 (54.0) |
| Marital status | ||
| With partner | 33 (51.6) | 31 (48.4) |
| Without partner | 68 (51.1) | 65 (48.9) |
| Education | ||
| Less than HS diploma | 22 (59.5) | 15 (40.5) |
| HS diploma or GED | 23 (51.5) | 22 (48.9) |
| Some college or technical school degree | 42 (50.6) | 41 (49.4) |
| Bachelors degree | 6 (40.0) | 9 (60) |
| Post college | 8 (44.0) | 10 (55.6) |
| Employment | ||
| Working full or part time | 85 (50.0) | 85 (50.0) |
| Not working | 16 (55.1) | 13 (44.8) |
| Insurance | ||
| Have insurance | 57 (52.8) | 51 (47.2) |
| Did not have insurance | 44 (48.4) | 47 (51.6) |
| Income | ||
| ≤$15,000 | 55 (54.5) | 46 (45.5) |
| $15,001–$30,000 | 12 (44.4) | 15 (55.6) |
| $30,001–$50,000 | 8 (57.1) | 6 (42.9) |
| $50,001–$75,000 | 4 (44.4) | 5 (55.6) |
| >$75,000 | 7 (36.8) | 12 (63.2) |
| Site | ||
| VA clinics | 58 (52.3) | 53 (47.7) |
| UIC clinic | 43 (48.9) | 45 (51.1) |
No significant differences between groups on any variable.
Feasibility Analysis
For this study we defined feasibility as recruitment, completion of TIMS, and participants’ perceptions of TIMS.
Recruitment
Patients in the UIC clinics were recruited inside the waiting room area. At the VA, due to space optimization (to reach all 4 clinics), we set up a table in the hall outside the clinics. This led to recruitment of a few people (about 10%) who were not there for a primary care visit but received health care from the VA. Between sites, 199 of the 240 persons contacted, agreed to participate for a response rate of 83%; 111 were from the VA clinics and 88 from UIC. There were no significant differences in sociodemographics between sites.
Completion of TIMS
Participants navigated the program with ease with minimal requests for assistance (less than 1% asked for any assistance). Patients in the study were not interrupted at any step (consent process, completing TIMS) by being called in to their clinic appointments allowing for completion of TIMS at the time of study enrollment. Staff at the clinics were not distracted or asked to do anything other than routine patient care.
Participants’ Perceptions of TIMS
Questions about the TIMS intervention were asked of 75 postintervention respondents from the intervention group by phone, 6 weeks later (see Table 2). Overall, the intervention was well received, with 94.5% indicating that the doctor's office should continue giving CRC education to people. TIMS did not raise undue concerns about CRC and 77% said they remembered most of the education. Most people said they would share this information with others (family, friends, coworkers), and almost 95% of the sample agreed that their doctors’ offices should continue giving out such education.
TABLE 2.
Intervention Group Participants’ Perceptions of TIMS (n = 75)
| Yes |
No |
|||
|---|---|---|---|---|
| Items | N | % | N | % |
| Education helped decide to get CRC screening | 60 | 80.0 | 15 | 20.0 |
| Education helped overcome reasons to not get CRC screening | 37 | 49.3 | 36 | 48.0 |
| Education was useful | 68 | 90.7 | 6 | 8.0 |
| Education raised new concerns | 51 | 68.0 | 24 | 32.0 |
| Education made you feel worried about CRC screening | 23 | 30.7 | 51 | 68.0 |
| Did anything about the education stand out | 35 | 46.7 | 40 | 53.3 |
| Would you change anything about the education | 9 | 12.5 | 63 | 87.5 |
| Would you tell others to use educational program if available | 72 | 98.6 | 1 | 1.4 |
Stage of Readiness, Knowledge and Perceived Risk, and Self-Efficacy Preintervention
The majority of the sample was in precontemplation stage (as expected) for both sigmoidoscopy and colonoscopy; for FOBT however, the majority was in contemplation (Table 3). The mean levels for knowledge, perceived risk and self-efficacy, presented in Table 4, indicates there were no significant differences between groups.
TABLE 3.
Preintervention Stage of Readiness
| Preintervention |
||
|---|---|---|
| Stage of Readiness | N | % |
| FOBT | ||
| Contemplation | 111 | 55.8 |
| Precontemplation | 87 | 43.7 |
| Action | — | |
| Sigmoidoscopy | ||
| Contemplation | 53 | 26.6 |
| Precontemplation | 144 | 72.4 |
| Action | — | — |
| Colonoscopy | ||
| Contemplation | 115 | 57.8 |
| Precontemplation | 83 | 41.7 |
| Action | — | — |
— indicates no one was in action preintervention; FOBT, fecal occult blood test.
TABLE 4.
Knowledge, Perceived Risk, and Perceived Self-Efficacy at Preintervention
| Variable (Scale Range) | Intervention Group M (SD) | Control Group M (SD) |
|---|---|---|
| Knowledge of CRC & screening (0–4) | 0.64 (0.84) | 0.91 (0.82) |
| Perceived risk of CRC (0–15) | 3.6 (4.0) | 3.9 (3.9) |
| FOBT self-efficacy (0–35) | 21.6 (8.5) | 25.8 (6.8) |
| END self-efficacy (0–65) | 41.3 (14.1) | 47.7 (11.6) |
FOBT indicates fecal occult blood test; END, endoscopy.
Perceived Benefits and Barriers Preintervention
The questions related to benefits and barriers for FOBT and endoscopy were open-ended in that a list was provided. If a participant selected more than one reason, those reasons appeared together on the next screen with a request to select the main reason. For the postintervention phone survey, if participants indicated more than one benefit or barrier, the data collector asked them to choose the main reason—the other reasons were marked as secondary choices. To find cancer was chosen by 40% as the primary benefit item for FOBT; peace of mind was the most common secondary benefit choice (29%). With regard to barriers, no recommendation from the doctor was chosen by 40% of the sample as the primary barrier; for a secondary choice, worry about finding cancer and fear of finding cancer were chosen by 10% and 9.5% respectively. For endoscopy, the primary benefit of endoscopy was to find cancer early (42%). To find polyps early (33%), peace of mind (31%), take control of health (25%), and to save life (29%) were all commonly checked secondary benefits.
DISCUSSION
Development of TIMS
The substantive changes made to TIMS in the development phase underscore the need to pilot test interventions with target populations. Additionally, the data also speak to the need to develop computer-based education based on the needs of the end-user rather than the programmers or data analysts.
Feasibility
It is feasible to conduct computer-based CRC education in busy primary care clinics. Both our response rate (83%) and the fact that no one was interrupted during the intervention to see the provider, support feasibility. The high response rate decreases the element of nonresponse bias in our results. Tailored messages were accurately delivered to respond to knowledge, benefits, barriers, self-efficacy and perceived risk responses. Participants’ perceptions of TIMS were favorable as 90% of the sample indicated they found the information useful. Overall, the high response rate, participants being able to complete the program on their own, and the lack of disruption of patient flow in the clinics are all supportive of feasibility, indicating it is feasible to conduct CRC education in these primary care clinics, and that the reach of the program was high.
Descriptive data showed that in this predominantly African American sample, there were no differences between participants in the intervention or control groups in knowledge or beliefs. Stages of readiness were as expected—the majority being in precontemplation.
Lessons Learned
One important finding was related to the kind of patients that can be recruited to such studies. Some participants were not at the VA (when recruited to the study) for a primary care visit but rather had appointments at specialty clinics or were visiting someone. These patients may not have had an opportunity to discuss cancer screening that day when the education was most fresh for them. We recommend that future research screen out such patients or tailor further to the challenges associated with comorbidities faced by them and consider other implementation factors that would maximize the effectiveness of such an intervention. Additionally, we recommend giving all study participants an FOBT kit at the conclusion of the intervention thereby eliminating the need to return to a primary care provider for this test.
The substantive changes made after the focus groups reinforced, once again, the importance of pilot testing interventions with each target sample, regardless of how much testing may have been done with other target populations (online Appendix). The TIMS was initially developed for a middle-class white audience. Based on feedback from focus group discussants (members of the new target population), TIMS needed to be revised.
Pilot testing is an important step in intervention development and one that could potentially save a researcher money and time. We recommend that all interventions be developed with a degree of latitude that allows for changes to be made based on pilot-test data. Additionally, computer-based programs must be developed with a keen eye to user characteristics rather than the needs of the research team or the ease of programming. For example, features such as the keyboard and the program completion indicator were either difficult to use or too distracting (see online Appendix).
Cultural sensitivity or relevance has been interpreted in many different ways.8 One important principle guiding any research program aiming for culturally sensitivity is that the answer to this cultural relevance lies with the target population. Pretesting (as in the focus groups of this study) or pilot testing can both be informative as to the degree and type of cultural relevance required by the target population. Developing culturally relevant interventions is an important aspect of successful interventions in cancer control. The disparate cancer related outcomes for African Americans further underscores the imperative of formative work such as the current pilot study. From 1992 to 2002, CRC incidence rates in African Americans as a group were 9% higher than for whites, and mortality rates were also higher (28.6%) compared with whites (21.1%).30 In addition to being disproportionately affected by CRC,31,32 African Americans also are less likely to be screened within the appropriate time intervals33,34 and more likely to present with later stage disease (proximal cancers and well-differentiated tumors),34 which may correspond to decreased survival rates for African Americans.31 This exploratory pilot study provides an excellent foundation from which to test the efficacy of TIMS with a larger, more ethnically diverse sample of individuals who are nonadherent with CRC screening. Further refinement is recommended if working with groups other than this study sample.
The control group in this study received the baseline questionnaire making this an attention control group which probably raised awareness about CRC screening. Future research must account for the minimal intervention that may be delivered with an attention control group that receives a questionnaire preintervention. By assessing the characteristics of individuals who respond to a minimal intervention we may be able to further tailor interventions to reduce the “bulkiness” or size of our education.14 TIMS will be further tested in similar and diverse populations to further refine its content and delivery.
Acknowledgments
Supported by a grant from The National Cancer Institute (CA10056).
REFERENCES
- 1.American Cancer Society . Cancer facts and figures. American Cancer Society; Atlanta, GA: 2007. [Google Scholar]
- 2.Meissner HI, Breen N, Klabunde CN, et al. Patterns of colorectal cancer screening uptake among men and women in the united states. Cancer Epidemiol Biomarkers Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine . Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- 4.Klabunde CN, Lanier D, Breslau ES, et al. Improving colorectal cancer screening in primary care practice: innovative strategies and future directions. J Gen Intern Med. 2007;22:1195–1205. doi: 10.1007/s11606-007-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safran DG, Kosinski M, Tarlov AR, et al. The primary care assessment survey: tests of data quality and measurement performance. Med Care. 1998;36:728–739. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Kreuter MW, Farrell D, Olevitch L, et al. Tailoring Health Messages: Customizing Communication with Computer Technology. Lawrence Erlbaum; Mahwah, NJ: 2000. [Google Scholar]
- 7.Skinner CS, Campbell MK, Rimer BK, et al. How effective is tailored print communication? Ann Behav Med. 1999;21:290–298. doi: 10.1007/BF02895960. [DOI] [PubMed] [Google Scholar]
- 8.Kreuter MW, Wray RJ. Tailored and targeted health communication: strategies for enhancing information relevance. Am J Health Behav. 2003;27(Suppl 3):S227–S232. doi: 10.5993/ajhb.27.1.s3.6. [DOI] [PubMed] [Google Scholar]
- 9.Smeets T, Brug J, de Vries H. Effects of tailoring health messages on physical activity. Health Educ Res. 2008;23:402–413. doi: 10.1093/her/cyl101. [DOI] [PubMed] [Google Scholar]
- 10.Champion VL, Springston JK, Zollinger TW, et al. Comparison of three interventions to increase mammography screening in low income african american women. Cancer Detect Prev. 2006;30:535–544. doi: 10.1016/j.cdp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Green MJ, Biesecker BB, McInerney AM, et al. An interactive computer program can effectively educate patients about genetic testing for breast cancer susceptibility. Am J Med Genet. 2001;103:16–23. doi: 10.1002/ajmg.1500. [DOI] [PubMed] [Google Scholar]
- 12.Green MJ, McInerney AM, Biesecker BB, et al. Education about genetic testing for breast cancer susceptibility: patient preferences for a computer program or genetic counselor. Am J Med Genet. 2001;103:24–31. doi: 10.1002/ajmg.1501. [DOI] [PubMed] [Google Scholar]
- 13.Gustafson DH, Hawkins R, Pingree S, et al. Effect of computer support on younger women with breast cancer. J Gen Intern Med. 2001;16:435–445. doi: 10.1046/j.1525-1497.2001.016007435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones JM, Nyhof-Young J, Friedman A, et al. More than just a pamphlet: development of an innovative computer-based education program for cancer patients. Patient Educ Couns. 2001;44:271–281. doi: 10.1016/s0738-3991(00)00204-4. [DOI] [PubMed] [Google Scholar]
- 15.Kim SP, Knight SJ, Tomori C, et al. Health literacy and shared decision making for prostate cancer patients with low socioeconomic status. Cancer Invest. 2001;19:684–691. doi: 10.1081/cnv-100106143. [DOI] [PubMed] [Google Scholar]
- 16.Nicholas D, Huntington P, Williams P, et al. Health information: an evaluation of the use of touch screen kiosks in two hospitals. Health Info Libr J. 2001;18:213–219. doi: 10.1046/j.1471-1842.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 17.Sutherland LA, Campbell M, Ornstein K, et al. Development of an adaptive multimedia program to collect patient health data. Am J Prev Med. 2001;21:320–324. doi: 10.1016/s0749-3797(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 18.Baratiny GY, Campbell EM, Sanson-Fisher RW, et al. Collecting cancer risk factor data from hospital outpatients: use of touch-screen computers. Cancer Detect Prev. 2000;24:501–507. [PubMed] [Google Scholar]
- 19.DePalma A. Prostate cancer shared decision: a CD-ROM educational and decision-assisting tool for men with prostate cancer. Semin Urol Oncol. 2000;18:178–181. [PubMed] [Google Scholar]
- 20.Pearson J, Jones R, Cawsey A, et al. The accessibility of information systems for patients: use of touchscreen information systems by 345 patients with cancer in scotland. Proc AMIA Symp. 1999:594–598. [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkie DJ, Huang HY, Berry DL, et al. Cancer symptom control: feasibility of a tailored, interactive computerized program for patients. Fam Community Health. 2001;24:48–62. [PubMed] [Google Scholar]
- 22.Wofford JL, Currin D, Michielutte R, et al. The multimedia computer for low-literacy patient education: a pilot project of cancer risk perceptions. Med Gen Med. 2001;3:23. [PubMed] [Google Scholar]
- 23.Janz NK, Champion VL, Strecher VJ. The health belief model. In: Glanz K, Lewis FM, Rimer BK, editors. Health Behavior and Health Education: Theory, Research and Practice. 3rd ed. Jossey-Bass; San Francisco, CA: 1997. [Google Scholar]
- 24.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 25.Menon U, Belue R, Sugg Skinner C, et al. Perceptions of colon cancer screening by stage of screening test adoption. Cancer Nurs. 2007;30:178–185. doi: 10.1097/01.NCC.0000270706.80037.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon U, Champion V, Monahan PO, et al. Health belief model variables as predictors of progression in stage of mammography adoption. Am J Health Promot. 2007;21:255–261. doi: 10.4278/0890-1171-21.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rawl SR, Champion VL, Menon U, et al. Validation of scales to measure benefits and barriers to colorectal cancer screening: scale development. J Psychosocial Oncol. 2001;19:47. [Google Scholar]
- 28.Vernon SW, Meissner H, Klabunde C, et al. Measures for ascertaining use of colorectal cancer screening in behavioral, health services, and epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2004;13:898–905. [PubMed] [Google Scholar]
- 29.American Cancer Society . Cancer Facts and Figures 2008. American Cancer Society; Atlanta, GA: 2008. [Google Scholar]
- 30.Ries LAG, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2004, based on November 2006 data submission. Available at: http://seer.cancer.gov/csr/1975_20042007.
- 31.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 32.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 33.Cooper GS, Koroukian SM. Racial disparities in the use of and indications for colorectal procedures in medicare beneficiaries. Cancer. 2004;100:418–424. doi: 10.1002/cncr.20014. [DOI] [PubMed] [Google Scholar]
- 34.Mostafa G, Matthews BD, Norton HJ, et al. Influence of demographics on colorectal cancer. Am Surg. 2004;70:259–264. [PubMed] [Google Scholar]