Abstract
Dyslipidemia is associated with a prothrombotic phenotype; however, the mechanisms responsible for enhanced platelet reactivity remain unclear. Proatherosclerotic lipid abnormalities are associated with both enhanced oxidant stress and the generation of biologically active oxidized lipids, including potential ligands for the scavenger receptor CD36, a major platelet glycoprotein. Using multiple mouse in vivo thrombosis models, we now demonstrate that genetic deletion of Cd36 protects mice from hyperlipidemia-associated enhanced platelet reactivity and the accompanying prothrombotic phenotype. Structurally defined oxidized choline glycerophospholipids that serve as high-affinity ligands for CD36 were at markedly increased levels in the plasma of hyperlipidemic mice and in the plasma of humans with low HDL levels, were able to bind platelets via CD36 and, at pathophysiological levels, promoted platelet activation via CD36. Thus, interactions of platelet CD36 with specific endogenous oxidized lipids play a crucial role in the well-known clinical associations between dyslipidemia, oxidant stress and a prothrombotic phenotype.
An important role for increased platelet reactivity in the pathophysiology of occlusive arterial thrombi associated with myocardial infarction and stroke is widely recognized, and subjects with increases in various measures of platelet reactivity are at increased prospective risk for coronary events and death1–5. Increased platelet reactivity and thrombogenic potential are associated with a number of pathophysiological states related to dyslipidemia, including atherosclerosis, diabetes, and metabolic syndrome2,6–10. It has been suggested that the cardiovascular risk associated with dyslipidemia may be due at least as much to effects on thrombogenesis as to long-term effects on atherogenesis2. The mechanisms responsible for enhancing platelet reactivity during dyslipidemia in vivo are still largely unknown, even though control of platelet reactivity is regarded as critical for prevention of coronary artery disease11.
Dyslipidemia is associated with both oxidative stress and the generation of biologically active oxidized lipids. Moreover, enhanced platelet reactivity is associated with the accumulation of oxidized lipids in serum9,12–14. Thus, oxidized lipids may be a link between hyperlipidemia and increased platelet reactivity. Biologically active oxidized phospholipids can initiate and modulate many of the cellular events attributed to the pathogenesis of atherosclerosis15. We have recently isolated and structurally defined a novel family of atherogenic oxidized choline glycerophospholipids (oxPCCD36) that are formed during the oxidization of low-density lipoprotein (LDL) by multiple pathways16 and are present in vivo at sites of enhanced oxidative stress16–18. oxPCCD36 serve as high-affinity ligands for the macrophage scavenger receptor CD36 (ref. 16) and facilitate macrophage foam cell formation through recognition and uptake of oxidized LDL by CD36 (ref. 19). CD36 is implicated in a variety of pathological conditions, including atherosclerosis, diabetes and innate immunity20. Absence of CD36 in a mouse model of atherogenesis results in significant inhibition of atherosclerosis21.
Platelets express scavenger receptors, including CD36 (ref. 22), although their functional role in platelet biology has not been defined. CD36 on macrophages and endothelial cells serves as a signaling molecule20. We therefore hypothesized that interaction of oxPCCD36 with platelet CD36 may alter platelet reactivity, inducing prothrombotic signals associated with dyslipidemia. Here we show that interaction with oxPCCD36 results in enhanced platelet reactivity and a prothrombotic phenotype.
RESULTS
CD36 is involved in thrombosis in vivo during hyperlipidemia
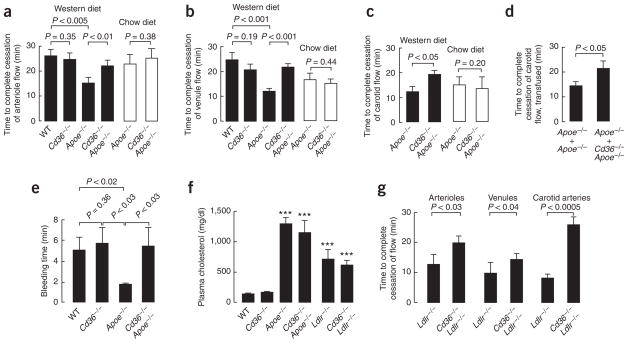
To test the hypothesis that CD36 contributes to a prothrombotic phenotype under conditions of hyperlipidemia, we initially used a mesenteric thrombosis model and intravital microscopy to compare in vivo vessel occlusion times between age-matched groups of male mice fed a Western diet (Fig. 1). The time to thrombotic occlusion of mesenteric arterioles after induction of injury was significantly shorter in hyperlipidemic Apoe−/− mice than in wild-type mice (Fig. 1a), indicating the presence of a pronounced prothrombotic phenotype. Notably, whereas functional deficiency of CD36 had no effect on thrombotic occlusion time in the absence of significant hyperlipidemia (compare wild-type to Cd36−/− mice), the absence of CD36 in the Apoe−/− background significantly protected mice from the hyperlipidemia-related prothrombotic phenotype (Fig. 1a). Parallel studies that quantified thrombotic occlusion times within venules demonstrated a similar distinct hyperlipidemia-related prothrombotic phenotype that was significantly rescued by CD36 deficiency (Fig. 1b). Notably, the absence of CD36 also protected mice from the hyperlipidemia-related prothrombotic phenotype state in carotid arteries—a large, atherosclerosis-prone vessel (Fig. 1c). Finally, hyperlipidemic Apoe−/− mice had accelerated thrombosis in another model examined, tail-cut bleeding time (Fig. 1e). Again, the absence of CD36 markedly protected mice from the hyperlipidemia-associated prothrombotic phenotype (Fig. 1e). Differences in occlusion times observed in these experiments could not be explained by favorable changes in lipid profiles in Apoe−/− Cd36−/− mice as compared to Apoe−/− mice (Fig. 1f) or by altered platelet production, as platelet counts in Apoe−/− Cd36−/− mice (4.01 ± 0.88 × 108/ml) were similar to those of Apoe−/− mice (4.13 ± 0.72 × 108/ml; P > 0.5). Because occlusion times in wild-type and Cd36−/− mice were similar (Fig. 1a,b,e), differences in platelet-collagen interaction can also be ruled out as a potential contributor to the observed impact of CD36 deficiency on the hyperlipidemia-induced prothrombotic phenotype.
Figure 1.
CD36 plays a role in thrombosis in vivo in the setting of hypercholesterolemia. (a–c) Mice of the indicated genotypes were maintained on Western diet (solid bars) and then used for an intravital thrombosis assay. Mesenteric arterioles (a) or venules (b) or carotid arteries (c) were visualized, and in vivo thrombosis times were measured as described in Methods. Mice on chow diet (empty bars) were analyzed in the same way, except that FeCl3 exposure times were increased by 1 min. Data are presented as mean ± s.e.m. for n ≥ 7 for chow diet and n ≥ 8 for Western diet. (d) Apoe−/− mice and Apoe−/− Cd36−/− on a Western diet were depleted of platelets by γ-irradiation, subsequently injected with platelets from either Apoe−/− mice (n = 5) or Apoe−/− Cd36−/− mice (n = 5) and the carotid artery occlusion test was performed as described in Methods. (e) Tail vein bleeding assay was performed as described in Methods. Data are presented as mean ± s.e.m.; numbers of mice used are: wild-type (n = 8), Cd36−/− (n = 5), Apoe−/− (n = 4) and Cd36−/− Apoe−/− (n = 4). (f) Total plasma cholesterol concentration is presented as mean ± s.d. mg/dl for at least 6 animals with the indicated genotype on a Western diet. ***P < 0.001 by t-test as compared to wild-type. (g) Indicated groups of mice were fed a high-cholesterol diet as described in Methods, and in vivo thrombosis times were assessed as in a–c. n ≥ 7 animals in each group. See Supplementary Methods for details.
To assess whether the CD36-dependent effects described above were specific to the setting of hyperlipidemia, we performed occlusion assays in mice fed a chow diet using an increased duration of ferric chloride exposure that induced rates of closure comparable to those observed in mice on Western diet (Fig. 1a–c empty bars). Even though there was greater vessel damage in this series of experiments, the time to occlusion in most cases was increased as compared to Western diet–fed animals, indicating a lower prothrombotic potential in mice on chow diet. Notably, there was no statistically significant difference in the occlusion times in Apoe−/− mice as compared with Apoe−/− Cd36−/− mice in all three types of vessel studied, consistent with our hypothesis that CD36-mediated effects are dependent on hyperlipidemia.
To address any potential issues related to the mixed genetic background of the Cd36−/− and Apoe−/− Cd36−/− mice, we used independently generated, CD36-deficient ‘oblivious’ mice23 that are pure C57BL/6. The occlusion times of Apoe−/− oblivious mice and Apoe−/− Cd36−/− mice on Western diet were very similar (arterioles: 19.1 ± 7.0 min versus 22.3 ± 7.3 min; venules: 22.1 ± 6.4 min versus 21.8 ± 5.0 min; all values are means ± s.d.). The occlusion times of oblivious mice and Cd36−/− mice on Western diet were also similar (arterioles: 21.8 ± 8.8 min versus 24.9 ± 7.9 min; venules: 19.9 ± 8.6 min versus 20.8 ± 7.1 min; all values are means ± s.d.).
To demonstrate that platelet CD36 was responsible for the observed differences, we first depleted Apoe−/− and Apoe−/− Cd36−/− mice of platelets by γ-irradiation, then injected them intravenously with platelets from either Apoe−/− or Apoe−/− Cd36−/− mice and then performed carotid artery occlusion assays. In all cases, both the donor and the recipient mice were maintained on a Western diet. The occlusion time was significantly longer when platelet donors were Apoe−/− Cd36−/− as compared to Apoe−/− (Fig. 1d). This result was independent of the genotype of the recipient mice.
To demonstrate that the effect of CD36 deficiency was not restricted to hyperlipidemia associated with apoE deficiency, we employed another widely used model of murine hyperlipidemia: LDL receptor-deficient mice (Ldlr−/−) fed a high-cholesterol diet. The lipoprotein profile in these mice is different from that of Apoe−/− mice and is similar to that observed in humans with familial hypercholesterolemia. The time to thrombotic occlusion after induction of injury was significantly shorter in Ldlr−/− mice than in wild-type mice in all three types of vessels examined (Fig. 1g). The absence of CD36 in the hyperlipidemic Ldlr−/− background again significantly protected mice from the prothrombotic state (Fig. 1g). Taken together, these results demonstrate that platelet CD36 may have a disease-modifying role by reducing the hyperlipidemia-associated prothrombotic state, and that CD36 can contribute to thrombosis in various blood vessels, including large, atherosclerosis-prone arteries.
CD36 deficiency in hyperlipidemia modulates platelet reactivity
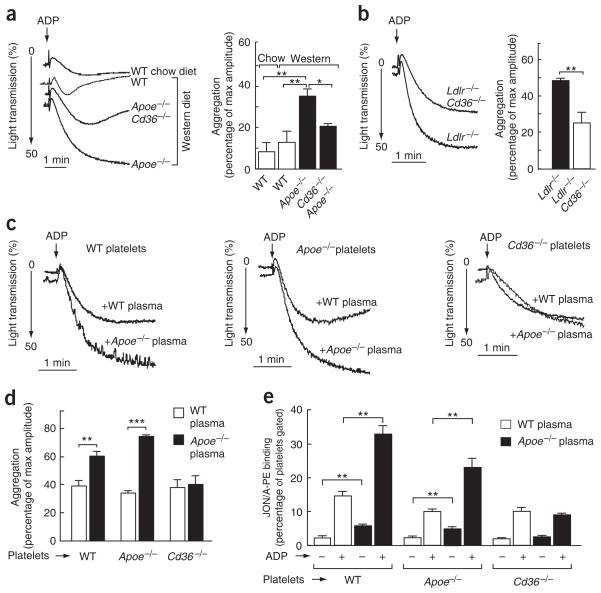
It has previously been reported that platelets from hyperlipidemic animals and humans have an increased in vitro aggregation response. To determine whether this augmented response was CD36 dependent, the aggregation of platelets in response to a suboptimal dose of adenosine diphosphate (ADP) was examined in different groups of mice. We observed a significant increase in the extent and rate of aggregation of platelets from hyperlipidemic Apoe−/− mice compared with wild-type mice (Fig. 2a). This response was significantly blunted in platelets from Apoe−/− Cd36−/− mice (Fig. 2a). No significant differences were observed between platelets from wild-type and Cd36−/− mice on normal chow (data not shown), again indicating that CD36 plays an important modulatory role in platelet function only in the setting of hyperlipidemia. Similar results were obtained when platelets from hyperlipidemic Ldlr−/− mice were compared to platelets from Ldlr−/− Cd36−/− mice (Fig. 2b).
Figure 2.
CD36 deficiency blunts platelet responses to agonists in hypercholesterolemic plasma. (a,b) Platelet aggregation in platelet-rich plasma from mice of the indicated genotypes on the indicated diets was induced by ADP and optically monitored. Representative aggregation curves are shown. Bar graph shows aggregation results expressed as maximal amplitude of aggregation (mean ± s.e.m.; n = 3 for WT, n = 4 for Apoe−/−, n = 6 for Apoe−/− Cd36−/−, n = 3 for Ldlr−/− and n = 4 for Ldlr−/− Cd36−/−). (c) Platelets were isolated by gel filtration of pooled blood from wild-type and Cd36−/− mice on a chow diet and Apoe−/− mice on a Western diet as described in Methods. Citrated, pooled, platelet-poor plasma from either wild-type or Apoe−/− mice on a Western diet was isolated and combined with platelets of the indicated genotype at a 1:1 ratio. Aggregation was induced by ADP and optically monitored. Representative aggregation curves are shown. (d) Bar graph shows aggregation results from c expressed as maximal amplitude of aggregation (mean ± s.e.m.; n = 3 for WT, n = 3 for Apoe−/− and n = 4 for Cd36−/−). (e) Flow cytometric analysis of integrin αIIbβ3 activation on mouse platelets. Platelets and citrated, platelet-poor plasma were isolated and combined at a 1:2 ratio (vol/vol), stimulated with ADP and integrin αIIbβ3 activation was assessed using JON/A, a monoclonal, phycoerythrin-conjugated antibody for mouse αIIbβ3 in the activated conformation (mean ± s.e.m.; n = 3 for WT, n = 3 for Apoe−/−, n = 4 for Cd36−/−). *P < 0.05, **P < 0.01 and ***P < 0.001. See Supplementary Methods for details.
Next, platelets from wild-type and Cd36−/− mice on chow diet and from hyperlipidemic Apoe−/− mice fed Western diet were separated from plasma by gel filtration, combined with citrated platelet-poor plasma from either wild-type or hyperlipidemic Apoe−/− mice, and tested in an aggregation assay. The aggregation response of platelets from Apoe−/− mice in plasma from wild-type mice was similar to that observed with platelets and plasma from wild-type mice (Fig. 2c,d). Platelet hyperreactivity was induced by adding hyperlipidemic plasma from Apoe−/− mice to platelets isolated from either Apoe−/− or wild-type mice (Fig. 2c,d). Thus, a factor present in the hyperlipidemic plasma was sufficient to confer a hypercoagulable (enhanced aggregation response) state. This effect required platelet CD36, because no enhanced aggregation was noted in Cd36−/− platelets in the presence of hyperlipidemic (Apoe−/−) plasma (Fig. 2c,d). In a parallel set of experiments, we found that activation of platelet fibrinogen receptor integrin αIIbβ3 in response to ADP was significantly increased in the presence of hyperlipidemic plasma, but only in CD36-expressing platelets (Fig. 2e). Notably, even without agonist added, a significant increase in the level of activated αIIbβ3 was observed when the platelets were incubated with hyperlipidemic plasma (Fig. 2e). These results demonstrate that the observed platelet hyperreactivity has a CD36 requirement and is associated with the presence of a procoagulant agent in hyperlipidemic plasma.
oxPCCD36 are markedly increased in hyperlipidemic plasma
Recent studies have reported the isolation and structural characterization of a novel family of oxidized choline glycerophospholipids (oxPCCD36) that are enriched within atheromas and serve as specific high-affinity ligands for macrophage CD36 (refs. 16,17; the structures and names of oxPCCD36 molecular species are shown at the top of Table 1). Because hyperlipidemia is associated with enhanced indices of oxidant stress, we hypothesized that oxPCCD36 species may accumulate in plasma during hyperlipidemic conditions, where they may serve as ligands for platelet CD36 and thereby modulate platelet reactivity.
Table 1.
oxPCCD36 are markedly increased in the plasma of mice fed a diet enriched in cholesterol
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| HOdiA | KOdiA | HOOA | KOOA | HDdiA | KDdiA | HODA | KODA | |
| Chow, WT (n = 5) | 0.06 ± 0.02 | 0.28 ± 0.05 | 0.36 ± 0.07 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.10 ± 0.02 | 0.17 ± 0.08 |
| Chow, Apoe −/− (n = 5) | 0.16 ± 0.14 | 0.67 ± 0.54 | 0.44 ± 0.39 | 0.17 ± 0.19 | 0.25 ± 0.14 | 0.30 ± 0.23 | 0.32 ± 0.22 | 0.50 ± 0.42 |
| Western, Apoe −/− (n = 5) | 0.64 ± 0.13 | 3.00 ± 0.62 | 0.66 ± 0.10 | 1.82 ± 0.20 | 1.15 ± 0.50 | 1.38 ± 0.68 | 1.11 ± 0.21 | 2.26 ± 0.10 |
| Fold increase over WT | 10.7 | 10.7 | 1.8 | 30.3 | 28.8 | 34.5 | 11.1 | 13.3 |
| P value of increase | P < 0.0001 | P < 0.0001 | P < 0.0006 | P < 0.0001 | P < 0.001 | P < 0.002 | P < 0.0001 | P < 0.0001 |
| Chow, Ldlr −/− (n = 8) | 0.10 ± 0.08 | 0.40 ± 0.20 | 0.39 ± 0.22 | 0.13 ± 0.08 | 0.11 ± 0.06 | 0.16 ± 0.11 | 0.30 ± 0.16 | 0.24 ± 0.16 |
| Western, Ldlr −/− (n = 5) | 0.60 ± 0.24 | 2.21 ± 0.56 | 1.70 ± 0.87 | 0.56 ± 0.17 | 0.50 ± 0.26 | 0.79 ± 0.30 | 1.63 ± 0.35 | 1.44 ± 0.66 |
| Fold increase over Ldlr −/−, chow | 6.0 | 5.5 | 4.4 | 4.3 | 4.5 | 4.9 | 5.4 | 6.0 |
| P value of increase | P < 0.0002 | P < 0.0001 | P < 0.001 | P < 0.0001 | P < 0.001 | P < 0.0002 | P < 0.0001 | P < 0.0004 |
The plasma concentrations of the indicated oxidized PC species (in μmol/l) in overnight-fasted, age-matched males fed chow or Western diet for 12 weeks were determined by LC/ESI/MS/MS analysis as described in Methods. Data are expressed as mean ± s.d. for 5–8 animals in each group. Abbreviations: HDdiA-PC and HOdiA-PC, the 9-hydroxy-10-dodecenedioic acid and 5-hydroxy-8-oxo-6-octenedioic acid esters of 2-lysoPC; HODA-PC and HOOA-PC, the 9-hydroxy-12-oxo-10-dodecenoic acid and 5-hydroxy-8-oxo-6-octenoic acid esters of 2-lysoPC; KODA-PC and KOOA-PC, the 9-keto-12-oxo-10-dodecenoic acid and 5-keto-8-oxo-6-octenoic acid esters of 2-lysoPC; KDdiA-PC and KOdiA-PC, the 9-keto-10-dodecendioic acid and 5-keto-6-octendioic acid esters of 2-lysoPC; WT, wild type.
Multiple structurally distinct and specific oxidized phospholipids in plasma samples of mice fed a chow diet or a diet enriched in cholesterol were quantified by liquid chromatography–electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS) analyses. Significant increases in concentration (typically over an order of magnitude) of each of the CD36 ligands derived from the oxidation of 1-hexadecanoyl-2-eicosatetra-5′,8′,11′,14′-enoyl-sn-glycero-3-phosphocholine (PAPC) and 1-hexadecanoyl-2-octadecadi-9′,12′-enoyl-sn-glycero-3-phosphocholine (PLPC) were noted in hyperlipidemic Apoe−/− and Ldlr−/− mice, with the collective plasma concentration of oxPCCD36 molecular species exceeding 10 μM (Table 1). Plasma levels of oxPCCD36 were not affected by the absence of CD36 in either the wild-type or the Apoe−/− background (P > 0.5, data not shown).
oxLDL and oxPCCD36 bind to platelets through CD36
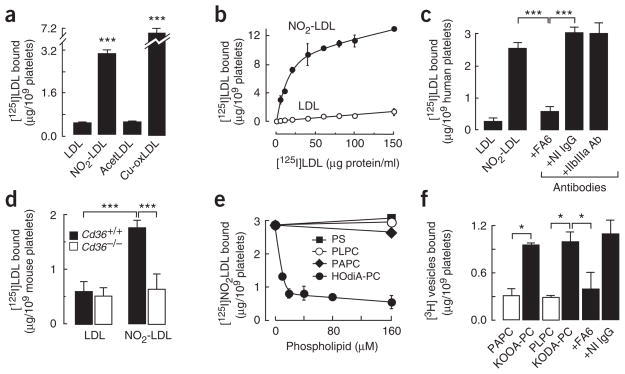
LDL oxidized by the myeloperoxidase-hydrogen peroxide-nitrite ( ) system (NO2-LDL) is a carrier of oxPCCD36 and represents an in vivo–relevant model of oxidized LDL that selectively binds to CD36 (refs. 16,17,19,24). Platelets isolated from human blood bound significant amounts of [125I]NO2-LDL, whereas platelet binding of native [125I]LDL or acetylated [125I]LDL (a ligand for the scavenger receptor class A) was low (Fig. 3a). LDL oxidized by alternative methods (for example, Cu2+-mediated oxidation) also bound to platelets. Competition experiments demonstrated that [125I]NO2-LDL binding was highly specific and was mediated by oxidized lipids (data not shown). Substantial binding of [125I]NO2-LDL to platelets was observed in platelet-rich plasma (data not shown), indicating that NO2-LDL can bind to platelets in conditions resembling those in vivo. [125I]NO2-LDL binding by isolated human platelets was saturable (Fig. 3b), with half-maximal binding observed at 6 μg/ml (12 nM), indicative of a high-affinity interaction. The maximum amount of bound [125I]NO2-LDL corresponded to ≈26,000 binding sites per platelet, which is close to the amount of CD36 on the surface of human platelets25. An inhibitory monoclonal antibody to CD36, FA6, significantly blocked binding of [125I]NO2-LDL to platelets, whereas an isotype-matched non-immune antibody or antibody to an alternative major platelet glycoprotein, αIIbβ3, had no effect (Fig. 3c). [125I]NO2-LDL binding to wild-type mouse platelets was significantly higher than that to Cd36−/− mouse platelets (Fig. 3d). In contrast, binding of native [125I]LDL to platelets from wild-type or Cd36−/− mice was low in both cases. Collectively, these results are consistent with platelet recognition of NO2-LDL through a specific and saturable process mediated by CD36.
Figure 3.
Platelet CD36 specifically binds oxPCCD36 and LDL oxidized by system. (a) [125I]LDL modified by the indicated methods was incubated with platelets isolated from humans in Tyrode’s buffer in the presence of 2 mM Ca2+ at room temperature. Unbound [125I]LDL was separated from platelets and the bound radioactivity was quantified. NO2-LDL, LDL oxidized by the MPO-H2O2-NO2− system; AcetLDL, acetylated LDL; Cu-oxLDL, Cu2+-oxidized LDL. (b) Concentration dependence of [125I]NO2-LDL binding by platelets. Binding of [125I]LDL was used as a control. (c) Human platelets were fixed with paraformaldehyde45 and incubated with [125I]NO2-LDL in the presence or absence of the indicated antibodies (20 μg/ml), and the bound radioactivity was assessed. (d) Binding of native [125I]LDL and [125I]NO2-LDL by isolated platelets from Cd36−/− and wild-type mice was assessed as in a. (e) Binding of [125I]NO2-LDL to human platelets in the presence of various concentrations of the indicated phospholipids was determined as in a. (f) Direct binding of vesicles containing 40 mol % of PAPC (for KOOA-PC) or 40 mol % of PLPC (for KODA-PC), 40 mol % of the indicated specific native phospholipid or oxPCCD36, 20 mol % of cholesterol, and trace amounts of [3H]DPPC (1,2-dihexadecanoyl-sn-glycero-3-phosphocholine) were generated and then incubated with platelets alone or, in the case of KODA-PC–containing vesicles, in the presence of either CD36-specific monoclonal antibody (FA6) or nonimmune, isotype-matched antibody (NI IgG). Unbound vesicles were separated and platelet-bound radioactivity was then quantified. Results represent the mean ± s.d. of three independent experiments. *P < 0.05, **P < 0.01 and ***P < 0.001. See Supplementary Methods for details.
We next used a competition assay to examine whether the PAPC and PLPC preparations oxidized by the system (NO2-PAPC and NO2-PLPC), as well as the individual structurally defined oxPCCD36 shown to be enriched within plasma of hyperlipidemic mice (Table 1), serve as ligands for platelet CD36. oxPCCD36 (data for HOdiA-PC are shown), NO2-PAPC and NO2-PLPC were all shown to completely inhibit [125I]NO2-LDL binding to platelets, whereas native PAPC or PLPC vesicles had no effect (Fig. 3e and data not shown). To directly demonstrate that oxPCCD36 bind to platelets via CD36, small unilamellar vesicles containing a carrier phospholipid and low mole percent synthetic oxPCCD36 species were made. Vesicles containing oxPCCD36 readily bound to platelets in a CD36-dependent manner; in contrast, vesicles containing only native PAPC or PLPC bound weakly (Fig. 3f). Thus, CD36 on both human and mouse platelets is important in the recognition of oxidized lipoproteins through its binding of specific oxidized phospholipids formed by physiologically relevant oxidation pathways.
oxPCCD36 induce platelet activation in a CD36-dependent manner
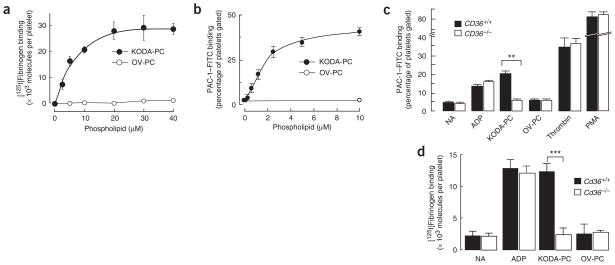
Having shown that oxidized LDL and oxPCCD36 bind platelets via CD36, we next tested whether this interaction could induce platelet activation. Platelet activation is characterized by a conformational change in the integrin αIIbβ3, enabling fibrinogen binding and platelet aggregation. We found that NO2-LDL, NO2-PAPC and NO2-PLPC all induced platelet binding of fibrinogen comparable to that induced by the physiological platelet agonist ADP, whereas native LDL or unoxidized phospholipids had no effect (data not shown). We showed that pure synthetic individual oxPCCD36 induced αIIbβ3 activation of fibrinogen binding in a concentration-dependent manner by two independent methods (by assessing the platelet binding of [125I]fibrinogen and the binding of FITC-labeled monoclonal antibody PAC-1 that is specific for activated integrin αIIbβ3) (Fig. 4a,b). The (patho)-physiological relevance of oxPCCD36-dependent platelet activation was underscored by the observation that activation occurred at oxPCCD36 concentrations within the range seen during hyperlipidemia in vivo (Fig. 4a,b; data for KODA-PC, one of the oxPCCD36 species, are shown; compare with data in Table 1). As a control, parallel studies used oxovaleroyl phosphatidylcholine (OV-PC), a structural analog of oxPCCD36 and biologically active phospholipid formed during PAPC oxidation26 for which no CD36 binding activity has been observed when it is presented in free, non–protein-bound form16 (notably, OV-PC as a protein adduct acquires the ability to bind to CD36; ref. 27). Platelets exposed to OV-PC were not activated, even at high concentrations of OV-PC (Fig. 4a,b).
Figure 4.
oxPCCD36 activates platelet fibrinogen receptor integrin αIIbβ3 in a CD36-dependent manner. (a) Human platelets isolated by gel filtration were incubated with increasing concentrations of oxPCCD36 or OV-PC and αIIbβ3 activation was assessed on the basis of the binding of 125I-labeled fibrinogen. (b) Human platelets isolated by gel filtration were incubated with increasing concentrations of oxPCCD36 or OV-PC. After addition of agonist, FITC-labeled PAC-1 mouse monoclonal antibody (specific for activated integrin αIIbβ3) was added at a dilution of 1:100 and αIIbβ3 activation was assessed by FACS analysis. (c) Human platelets were isolated from CD36+/+ or CD36−/− donors and analyzed for αIIbβ3 activation as in b. The final concentrations of stimuli used were 10 μM ADP, 20 μM lipid oxidized phospholipids, 0.5 U/ml thrombin and 10 nM PMA. NA, no additions. (d) Washed platelets from wild-type or Cd36−/− mice were incubated with 10 μM ADP or 20 μM synthetic oxidized phospholipids, and platelet activation was assessed by 125I-labeled fibrinogen binding. NA, no additions. Results represent the mean ± s.d. of three independent experiments. Statistical significance was determined by t-test: *P < 0.05, **P < 0.01, ***P < 0.001. See Supplementary Methods for details.
Although human CD36−/− platelet responses to multiple agonists, including ADP, thrombin and phorbol 12-myristate 13-acetate (PMA), were indistinguishable from those of CD36-expressing platelets, no activation of αIIbβ3 was observed in platelets from CD36-deficient donors in response to 0.5–20 μM of oxPCCD36 (data for 20 μM is shown) (Fig. 4c). This specific lack of response to oxPCCD36 was also reproduced with platelets isolated from Cd36−/− mice as compared to those from wild-type mice (Fig. 4d).
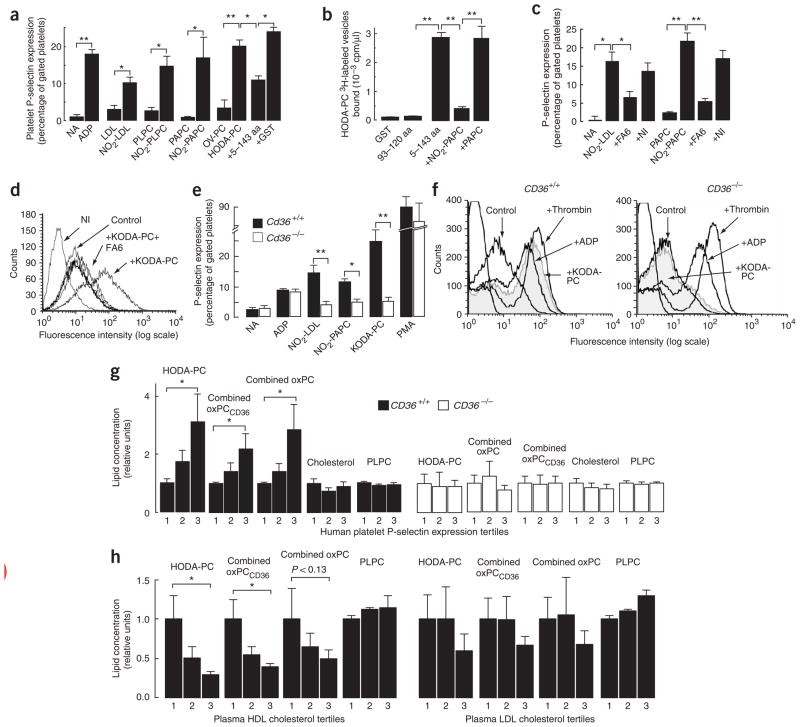
Another critical component of platelet activation is the secretion of granular contents, a process that exposes P-selectin on the platelet surface. Platelet P-selectin mediates the formation of platelet-monocyte aggregates, which have been implicated in several vascular disease processes28–30. NO2-LDL, NO2-PAPC and NO2-PLPC vesicles were all potent inducers of P-selectin expression (Fig. 5a). oxPCCD36 (data for HODA-PC, another type of oxPCCD36, are shown), but not OV-PC, induced platelet P-selectin expression as potently as did ADP (Fig. 5a). Native LDL, PAPC and PLPC vesicles had no effect on P-selectin expression. Similar results were obtained using either washed platelets or platelet-rich plasma (data not shown). The CD36-dependence of these effects was confirmed using a recombinant human CD36–glutathione S-transferase fusion peptide corresponding to amino acids 5–143 of the extracellular domain of CD36 that was shown previously to bind oxLDL31. This peptide, but not a control peptide (93–120 amino acids), demonstrated high-affinity recognition of specific oxPCCD36 (Fig. 5b) and [125I]NO2-LDL, and it significantly inhibited the activation of platelets by oxPCCD36 (Fig. 5a).
Figure 5.
oxPCCD36 induce platelet P-selectin expression in a CD36-dependent manner. (a) Isolated human platelets were incubated with the indicated stimuli and P-selectin expression was assessed as described in Methods. NA, no additions. Results represent the mean ± s.d. of three independent experiments. 5–144 aa, CD36-GST fusion peptide with amino acids 5–144 of CD36; GST, glutathione S-transferase. (b) Binding of 3H-labeled small unilamellar vesicles containing oxPCCD36 (HODA-PC) to recombinant CD36-GST fusion peptides immobilized on glutathione-Sepharose was assessed in the presence or absence of the indicated competitors. 93–120 aa, CD36-GST fusion peptide with amino acids 93–120 of CD36. (c,d) Effect of the Fab fragment of the CD36-blocking monoclonal antibody on platelet activation induced by (c) NO2-LDL and NO2-PAPC and (d) oxPCCD36. NI, nonimmune isotype-matched antibody. (e) Platelet P-selectin expression was determined using FITC-conjugated antibody to mouse P-selectin. (f) Human platelets isolated from CD36+/+ or CD36−/− donors were treated with agonists and P-selectin expression was assessed. (b,c,e) Data are presented as mean ± s.d. of three independent experiments; (d,f) data are presented as a typical result of at least three independent experiments. (g) Human platelets from CD36+/+ or CD36−/− donors (n = 3) were combined with human citrated plasma from various donors, stimulated with ADP, and P-selectin expression was assessed. Samples were divided into tertiles according to P-selectin expression, the mean plasma sample lipid levels were assigned a relative value of 1 for the lowest P-selectin tertile, and the means ± s.e.m. are shown for all P-selectin tertiles. (h) Human plasma samples (n = 24) were analyzed for levels of multiple oxidized phospholipids, HDL cholesterol and LDL cholesterol. Samples were divided into tertiles according to HDL cholesterol or LDL cholesterol concentrations. The mean oxidized phospholipid levels were assigned relative values of 1 for the lowest lipoprotein tertile, and corresponding mean ± s.e.m. are shown for all lipoprotein tertiles. *P < 0.05, **P < 0.01, ***P < 0.001. See Supplementary Methods for details.
CD36 was necessary for the induction of P-selectin expression by oxPCCD36, as NO2-PAPC and pure synthetic oxPCCD36 did not induce expression when CD36 was blocked by the Fab fragment of FA6 (Fig. 5c,d). Moreover, mouse and human CD36−/− platelets showed significantly reduced P-selectin exposure compared to CD36-expressing platelets in response to NO2-LDL, NO2-PAPC and pathophysiologically relevant levels of the prototypic oxPCCD36 KODA-PC (Fig. 5e,f).
oxPCCD36 in human plasma modulate platelet reactivity
We then sought to determine whether oxPCCD36 were present in human plasma and whether these amounts were sufficient to promote platelet activation. Under conditions developed to minimize oxidation of lipids during their isolation from plasma17, we quantified the amounts of multiple distinct oxidized phospholipids in normotriglyceridemic plasma samples from donors (n = 24) with a wide range of plasma LDL cholesterol (range of 36–243 mg/dl) recruited from a Preventive Cardiology Clinic. We detected substantial amounts of oxPCCD36 and its oxidation products (such as OV-PC and G-PC, the glutaric acid ester of 2-lyso-PC) in all samples. There was considerable interindividual variability in the amounts of the multiple oxPCCD36 species monitored, with the concentration of an individual oxPCCD36 species ranging from undetectable to 1.8 μM, and the combined oxPCCD36 concentration ranging from approximately 0.7 to 4.6 μM. The combined concentration of all oxidized phospholipids measured, including those that are products of oxPCCD36 degradation, ranged from 5.4 to 51 μM. Thus, the concentrations of oxPCCD36 in human plasma are precisely within the concentration range demonstrated to promote prothrombotic activity in both mice and humans. We then tested whether agonist-induced platelet P-selectin expression correlated with plasma levels of oxPCCD36, as well as whether any observed correlation was CD36 dependent. The effect of plasma from multiple donors on agonist-induced P-selectin expression was assessed on platelets isolated from CD36-positive or CD36-deficient human donors. Considerable, reproducible variations in the plasma effect on platelet response to agonists were observed. Plasma samples that reproducibly supported the highest P-selectin expression in CD36-positive platelets had significantly higher concentrations of specific oxidized phospholipids (Fig. 5g, left graph), compared to plasma samples that reproducibly supported the lowest P-selectin expression. HODA-PC is shown as a characteristic example of an individual oxPCCD36; similar patterns were observed with virtually all oxPCCD36 (Fig. 5g, left graph). Notably, the responses of CD36-deficient platelets failed to correlate with plasma levels of oxPCCD36 (Fig. 5g, right graph). There was no correlation between the platelet response to agonists with the concentrations of two major unoxidized phospholipids in plasma, PAPC and PLPC (Fig. 5g and data not shown). In addition, total cholesterol in the plasma did not correlate with the platelet responses. These results are consistent with a role for circulating oxPCCD36-platelet interactions as modulators of platelet responsiveness to physiological agonists.
We next tested whether oxPCCD36 levels in human plasma samples correlate with high-density lipoprotein (HDL) and LDL cholesterol levels. Although no significant correlations were noted between LDL cholesterol and oxPCCD36, a highly significant correlation was noted between oxPCCD36 (both individually and in combination) and HDL cholesterol (Fig. 5h). Data for the relationship between HODA-PC and HDL is shown as a typical example; similar patterns were observed with virtually all oxPCCD36. The Spearman correlation coefficient for HODA-PC was 0.622 (P < 0.0006), and that for combined oxPCCD36 was 0.522 (P < 0.005). HODA-PC concentrations were more than three times higher in plasma from subjects in the lowest HDL tertile, and combined oxPCCD36 concentrations were, on average, about 2.5 times higher. In contrast to the oxidized phospholipid data, the concentrations of unoxidized phospholipids (data for PLPC are shown) showed modest increases with increasing HDL concentration. These findings demonstrate that in relatively normolipidemic human plasma it is HDL, not LDL, that controls oxPCCD36 concentrations.
DISCUSSION
The present studies support a previously unknown role for platelet CD36 as a sensor of oxidative stress and modulator of platelet reactivity, activation and thrombosis under hyperlipidemic conditions in vivo. Whereas macrophage CD36 involvement in fatty-streak formation and atherogenesis is well described19,21,32, the involvement of this receptor in platelet function has not to our knowledge been unequivocally shown. Multiple studies have shown that dyslipidemia, a major risk factor for atherosclerosis, is associated with enhanced platelet reactivity and increased thrombogenic potential2,7,8,10. The studies reported herein demonstrate that the engagement of platelet CD36 by structurally defined endogenous ligands plays a causal role in facilitating the development of a dyslipidemia-associated prothrombotic state.
Using two different mouse models of hyperlipidemia and several assays for in vivo thrombosis, we confirmed that hyperlipidemia induces a pronounced prothrombotic state; these findings are consistent with prior observations in Apoe−/− mice33,34. The present study markedly extends the analysis of this phenomenon by identifying a specific platelet receptor (CD36) and specific endogenous lipid ligands associated with oxidant stress (oxPCCD36) as participants in this important pathophysiologic process. The observation that CD36-positive platelets in hyperlipidemic plasma showed increased aggregation responses at suboptimal agonist concentration in vitro provides a direct link between accelerated thrombosis and platelet thrombogenic status. There was no significant reduction in response to the agonist in platelets from Cd36−/− mice as compared to wild-type mice on a normal chow diet, in line with our hypothesis that the role of CD36 in thrombosis is restricted to the dyslipidemic milieu, where there is enhanced oxidative stress that can generate specific ligands for CD36 (ref. 17). Furthermore, rescue of the hyperlipidemia-induced prothrombotic phenotype by Cd36 deletion was observed in mesenteric arterioles and venules as well as in carotid arteries, indicating that this pathway contributes to thrombosis at both low and high shear stress rates, and in both large and small vessels.
We previously identified a family of oxidized choline glycerophospholipids with an sn-2 acyl group that incorporates a structural motif for supporting high-affinity CD36 recognition, a terminal γ-hydroxy (or oxo)-α,β-unsaturated carbonyl (oxPCCD36). oxPCCD36 are formed during LDL oxidation by multiple pathways, are present in vivo at sites of enhanced oxidative stress16–18 and promote foam cell formation through CD36 (refs. 16,17). We now extend these observations to demonstrate that oxPCCD36 accumulate in the plasma of hyperlipidemic mice at concentrations up to 40-fold higher than those found in normolipidemic mice, and that they are found in substantial amounts in human plasma.
To support our hypothesis that oxPCCD36 link dyslipidemia and enhanced platelet reactivity, we demonstrated that various forms of oxLDL and multiple structurally specific oxPCCD36 (but not structural oxPC analogs lacking CD36 binding activity) bound to human and mouse platelets, and that binding was dependent on CD36. Ligand binding to platelet CD36 resulted in platelet activation, as assessed by activation of integrin αIIbβ3 as well as by an increase in P-selectin surface expression. The physiological relevance of these results is further supported by (i) the finding that oxPCCD36 levels in human and mouse plasma precisely span the concentration range in which biological effects on platelet responses are observed and (ii) the demonstration that a statistically significant correlation exists between the CD36-dependent platelet response to agonists and plasma levels of oxPCCD36. Because anti-inflammatory and antioxidant effects of HDL are well documented35, our finding of a link between elevated oxPCCD36 and low HDL suggests that it is an interaction between CD36 and oxidative stress, not merely dyslipidemia (or elevated LDL), that is responsible for enhanced platelet reactivity in vivo. It is thus tempting to speculate that individuals with high circulating plasma oxPCCD36 are at an increased risk for thrombosis.
CD36 is involved in a diverse array of physiological and pathological processes in vivo, including lipid sensing and metabolism, innate immune responses, angiogenesis, uptake of apoptotic cells, atherogenesis and diabetes20,23,36,37. These various functional capacities arise as a result of the multiple distinct ligands with which CD36 interacts, the numerous downstream effectors upon which CD36 acts and the variety of cell types that express CD36. Surprisingly, even though CD36 was recognized as a major platelet glycoprotein more than three decades ago, its role in platelet physiology has remained obscure. Human CD36-negative platelets exhibit a mild defect in the initial stages of adhesion to fibrillar collagen I (ref. 38). Notably, VLDL increases platelet response to collagen in a CD36-dependent manner; however, the mechanism of this effect remains unclear39. Platelet CD36 is associated with non-receptor tyrosine kinases of the Src family40, which have previously been implicated in platelet activation by oxidized LDL41. The present findings suggest that platelet CD36 might serve as a sensor of specific oxidized phospholipids generated during oxidative stress, providing a mechanism for CD36 involvement in the prothrombotic phenotype associated with dyslipidemia, oxidant stress and atherosclerosis. CD36 engagement may induce an activating signal that is additive (or synergistic) with signals from other receptors and may result in platelet activation by subthreshold concentrations of physiological agonists. Thus, CD36 joins a growing number of platelet receptors and ligands that may potentiate members of the platelet activation cascade, including Gas6 and its receptors (ref. 42), CD40 ligand and αIIbβ3 (ref. 43) and Eph kinases and ephrins44. Unlike other ‘co-receptor’ ligands, which are primarily localized to the platelet surface during the initial phase of platelet aggregation, CD36 ligands are likely to be presented to the platelet surface before ‘classical’ platelet agonists. Thus, CD36 may serve to ‘prime’ or sensitize the platelet for subsequent activation, and this may contribute to platelet hyperreactivity.
METHODS
Sources of materials
Na[125I] was from ICN Pharmaceutical, Inc. Native unoxidized phospholipids were from Avanti Polar Lipids. Anti-CD36 monoclonal antibody FA6-152 was from Immunotech. The Fab fragment of FA6 was generated by papain digestion. Conjugated anti–murine P-selectin and anti–murine activated αIIbβ3 (clone JON/A) monoclonal antibodies were from Emfret Analytics. Specific anti–human P-selectin murine monoclonal antibody was either generated using purified human platelets as antigen and conjugated to FITC or purchased from BD Biosciences/Pharmingen. FITC-conjugated PAC-1 was from Becton Dickinson. All other reagents were obtained from Sigma Aldrich unless otherwise specified.
Experimental animals
We generated Cd36−/− Apoe−/− mice by crossing Cd36−/− and Apoe−/− mice (Taconic) as described32 and established the lines from littermates after crossing heterozygotes, so that all Cd36−/− lines were back-crossed six or more times to the C57BL/6 genetic background. The resulting genetic background was 99.22% C57BL/6 and 0.78% 129Sv. Mice were fed normal chow for 4 weeks, followed by a Western diet (cholate-free, 21% (wt/wt) adjusted calories from anhydrous milk fat and 0.2% (wt/wt) cholesterol, Harlan Teklad, TD 88137) for 12 weeks. Ldlr−/− Cd36−/− mice were generated by crossing Cd36−/− and Ldlr−/− mice (Jackson Laboratory). At the age of 8–10 weeks, mice were fed a high-fat (18% wt/wt), high-cholesterol (1.125% wt/wt) diet (Research Diets D12108). All strains are background matched. All procedures were approved by the Institutional Animal Case and Use Committee of Cleveland Clinic. Total serum cholesterol was determined as described21.
Lipoproteins and phospholipid vesicle preparation and modification
LDL was isolated, labeled with Na[125I] and additionally modified as described24. Phospholipid vesicle preparation was performed as described16. Briefly, we prepared stock solutions (2 mg/ml) of small unilamellar vesicles comprising PLPC or PAPC with varying mol % of synthetic oxidized phospholipids in argon-sparged sodium phosphate buffer (67 mM, pH 7.4) by extruding the mixtures through a 0.1-μm polycarbonate filter using an Avanti Mini-Extruder Set (Avanti Polar Lipids, Inc.) at 37 °C. For direct binding experiments, we added 3H-labeled dipalmitoylphosphatidylcholine (25 μCi/mg) to phospholipids. We generated NO2-PAPC and NO2-PLPC by oxidation of PAPC and PLPC vesicles by the system24.
Synthesis of phospholipids
We performed total syntheses of the oxidized phospholipids and then purified them by flash silica column chromatography or high-performance liquid chromatography (HPLC), as described elsewhere16,26. We confirmed the structures by multinuclear magnetic resonance and high-resolution mass spectrometry16,26. We routinely analyzed synthetic lipids by HPLC with on-line electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS). If lipids were found to be less than 95% pure, they were re-isolated before use.
Mass spectrometric analysis of phospholipids
We used LC/ESI/MS/MS to separate and to quantify plasma oxPC species16,18. Briefly, we added 4 ng of internal standard, ditetradecyl phosphatidylcholine (DTPC) to 200 μl plasma and extracted phospholipids three times following the method of Bligh and Dyer as described16. We rapidly dried combined extracts under nitrogen flow, resuspended them in 120 μl 85% (vol/vol) methanol and stored them under argon at −80 °C until analysis was performed no more than 24 h later. We introduced 40 μl of the extract onto a 2690 HPLC system (Waters) and separated phospholipids through a C18 column (2 × 150 mm, 5 μm octadecyl silane, Phenomenex) under gradient conditions at flow rate of 0.2 ml/min as described16,18. We introduced the HPLC column effluent onto a Micromass Quattro Ultima triple quadrupole mass spectrometer (Micromass) and analyzed it by positive electrospray ionization in the multiple reaction monitoring (MRM) mode. Following collision-induced fragmentation with argon gas, phosphatidylcholine molecules produced a diagnostic product ion with mass-to-charge ratio (m/z) 184, corresponding to the protonated phosphocholine headgroup. The ion pairs for the MRM transitions monitored were each molecular ion [MH]+ of the phosphotidylcholines and the diagnostic product ion (m/z 184). We constructed calibration curves with a fixed amount of DTPC and varying amounts of each synthetic phosphatidylcholine.
Intravital thrombosis
We isolated platelets from platelet-rich mouse plasma by gel filtration on a Sepharose 2B column (Sigma-Aldrich), then fluorescently labeled platelets with calcein (Molecular Probes) and injected them into syngeneic male mice (4–5×106 platelets per g) via the lateral tail vein. We exposed the vessel of choice in mice anesthetized with ketamine and xylazine, visualized mesenteric arterioles (50–80 μm in diameter) or venules (60–80 μm diameter) or the carotid artery on a Leica DM LFS microscope with water immersion objectives and recorded images with a high-speed, color, cooled digital camera (QImaging Retiga EXi Fast 1394) with Streampix high-speed acquisition software. We recorded the resting blood vessel for 3 min, then created a vessel-wall injury by application of a 1.5 × 1.5 mm square of Whatman filter paper soaked in FeCl3 solution to the surface of the vessel (we used 12.5% FeCl3 for 3 min on mesenteric vessels and 10% FeCl3 for 2 min on carotid arteries in hyperlipidemic animals; we increased FeCl3 exposure times by 1 min in chow diet–fed animals). Then we removed the paper, covered the vessel with saline at 37 °C and recorded platelet vessel wall interactions for 30 min, or until full occlusion occurred and lasted for more than 30 s. Experiments with platelet depleted mice were performed as follows. Apoe−/− mice and Apoe−/− Cd36−/− mice on a Western diet were depleted of platelets by γ-irradiation (11 Gy) and when platelet counts dropped below 5% of normal the mice were injected with 12.5 × 106/g body weight platelets from either Apoe−/− mice (n = 5) or Apoe−/− Cd36−/− mice (n = 5). Carotid artery occlusion test was performed by application of 15% FeCl3 for 3 min.
Clinical samples
All human blood products were obtained using protocols approved by the Institutional Review Boards of the Cleveland Clinic and The Blood Center of Wisconsin. Informed consent was obtained from all donors.
Statistical analysis
In most assays, we used the two-sample t-test to evaluate statistical significance. In intravital thrombosis experiments, we used the nonparametric log-rank test. Spearman’s correlation coefficient was calculated with GraphPad Prism 4.0c.
Supplementary Material
Acknowledgments
We thank E. Plow for thoughtful comments and criticisms and W. Feng, W. Li and V. Verbovetskaya for technical assistance. This work was supported in part by National Institutes of Health grants HL077213 and HL053315 (E.A.P.) and by a Scientist Development Grant from the American Heart Association (E.A.P.), HL70621 and HL076491 (S.L.H.), HL 70083 (M.F.), HL072942 and HL46403 (R.L.S. and M.F.), HL071625, HL073311 and HL077107 (T.V.B.), HL53315 (R.G.S.), Cleveland Clinic Specialized Centers for Clinically Oriented Research (P01 HL077107, S.L.H.; and P50 HL81011, R.L.S. and M.F.) and the Cleveland Clinic Foundation General Clinical Research Center (M01 RR018390).
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Trip MD, Cats VM, van Capelle FJ, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990;322:1549–1554. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 2.Lacoste L, et al. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–3177. doi: 10.1161/01.cir.92.11.3172. [DOI] [PubMed] [Google Scholar]
- 3.Kabbani SS, et al. Platelet reactivity characterized prospectively: a determinant of outcome 90 days after percutaneous coronary intervention. Circulation. 2001;104:181–186. doi: 10.1161/01.cir.104.2.181. [DOI] [PubMed] [Google Scholar]
- 4.Vanschoonbeek K, et al. Thrombin-induced hyperactivity of platelets of young stroke patients: involvement of thrombin receptors in the subject-dependent variability in Ca2+ signal generation. Thromb Haemost. 2002;88:931–937. [PubMed] [Google Scholar]
- 5.Kabbani SS, et al. Usefulness of platelet reactivity before percutaneous coronary intervention in determining cardiac risk one year later. Am J Cardiol. 2003;91:876–878. doi: 10.1016/s0002-9149(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho AC, Colman RW, Lees RS. Platelet function in hyperlipoproteinemia. N Engl J Med. 1974;290:434–438. doi: 10.1056/NEJM197402212900805. [DOI] [PubMed] [Google Scholar]
- 7.Stuart MJ, Gerrard JM, White JG. Effect of cholesterol on production of thromboxane b2 by platelets in vitro. N Engl J Med. 1980;302:6–10. doi: 10.1056/NEJM198001033020102. [DOI] [PubMed] [Google Scholar]
- 8.Davi G, et al. Increased thromboxane biosynthesis in type IIa hypercholesterolemia. Circulation. 1992;85:1792–1798. doi: 10.1161/01.cir.85.5.1792. [DOI] [PubMed] [Google Scholar]
- 9.Davi G, et al. Increased levels of soluble P-selectin in hypercholesterolemic patients. Circulation. 1998;97:953–957. doi: 10.1161/01.cir.97.10.953. [DOI] [PubMed] [Google Scholar]
- 10.Cipollone F, et al. Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia: effects of statin therapy. Circulation. 2002;106:399–402. doi: 10.1161/01.cir.0000025419.95769.f0. [DOI] [PubMed] [Google Scholar]
- 11.Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance: an emerging clinical entity. Eur Heart J. 2005 doi: 10.1093/eurheartj/ehi684. [DOI] [PubMed] [Google Scholar]
- 12.Salonen JT, et al. Effects of antioxidant supplementation on platelet function: a randomized pair-matched, placebo-controlled, double-blind trial in men with low antioxidant status. Am J Clin Nutr. 1991;53:1222–1229. doi: 10.1093/ajcn/53.5.1222. [DOI] [PubMed] [Google Scholar]
- 13.Vericel E, Januel C, Carreras M, Moulin P, Lagarde M. Diabetic patients without vascular complications display enhanced basal platelet activation and decreased antioxidant status. Diabetes. 2004;53:1046–1051. doi: 10.2337/diabetes.53.4.1046. [DOI] [PubMed] [Google Scholar]
- 14.Morita H, Ikeda H, Haramaki N, Eguchi H, Imaizumi T. Only two-week smoking cessation improves platelet aggregability and intraplatelet redox imbalance of long-term smokers. J Am Coll Cardiol. 2005;45:589–594. doi: 10.1016/j.jacc.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 15.Berliner JA, Watson AD. A role for oxidized phospholipids in atherosclerosis. N Engl J Med. 2005;353:9–11. doi: 10.1056/NEJMp058118. [DOI] [PubMed] [Google Scholar]
- 16.Podrez EA, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 17.Podrez EA, et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 18.Sun M, et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J Biol Chem. 2006;281:4222–4230. doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podrez EA, et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Febbraio M, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tandon NN, Lipsky RH, Burgess WH, Jamieson GA. Isolation and characterization of platelet glycoprotein IV (CD36) J Biol Chem. 1989;264:7570–7575. [PubMed] [Google Scholar]
- 23.Hoebe K, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 24.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kieffer N, et al. Developmentally-regulated expression of a 78-kDa erythroblast membrane glycoprotein immunologically related to the platelet thrombospondin receptor. Biochem J. 1989;262:835–842. doi: 10.1042/bj2620835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson AD, et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally-oxidized low-density lipoproteins that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 27.Boullier A, et al. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46:969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Simon DI, et al. Decreased neointimal formation in Mac1−/− mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarma J, et al. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 2002;105:2166–2171. doi: 10.1161/01.cir.0000015700.27754.6f. [DOI] [PubMed] [Google Scholar]
- 30.Huo Y, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 31.Pearce SF, et al. Recombinant glutathione S-transferase/CD36 fusion proteins define an oxidized low-density lipoprotein–binding domain. J Biol Chem. 1998;273:34875–34881. doi: 10.1074/jbc.273.52.34875. [DOI] [PubMed] [Google Scholar]
- 32.Febbraio M, Guy E, Silverstein RL. Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2333–2338. doi: 10.1161/01.ATV.0000148007.06370.68. [DOI] [PubMed] [Google Scholar]
- 33.Eitzman DT, Westrick RJ, Xu Z, Tyson J, Ginsburg D. Hyperlipidemia promotes thrombosis after injury to atherosclerotic vessels in apolipoprotein E–deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:1831–1834. doi: 10.1161/01.atv.20.7.1831. [DOI] [PubMed] [Google Scholar]
- 34.Schafer K, et al. Enhanced thrombosis in atherosclerosis-prone mice is associated with increased arterial expression of plasminogen activator inhibitor-1. Arterioscler Thromb Vasc Biol. 2003;23:2097–2103. doi: 10.1161/01.ATV.0000097766.36623.DF. [DOI] [PubMed] [Google Scholar]
- 35.Barter PJ, et al. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 36.Bodart V, et al. CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circ Res. 2002;90:844–849. doi: 10.1161/01.res.0000016164.02525.b4. [DOI] [PubMed] [Google Scholar]
- 37.Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- 38.Tandon NN, Ockenhouse CF, Greco NJ, Jamieson GA. Adhesive functions of platelets lacking glycoprotein IV (CD36) Blood. 1991;78:2809–2813. [PubMed] [Google Scholar]
- 39.Englyst NA, Taube JM, Aitman TJ, Baglin TP, Byrne CD. A novel role for CD36 in VLDL-enhanced platelet activation. Diabetes. 2003;52:1248–1255. doi: 10.2337/diabetes.52.5.1248. [DOI] [PubMed] [Google Scholar]
- 40.Huang MM, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci USA. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maschberger P, et al. Mildly oxidized low-density lipoprotein rapidly stimulates via activation of the lysophosphatidic acid receptor Src family and Syk tyrosine kinases and Ca2+ influx in human platelets. J Biol Chem. 2000;275:19159–19166. doi: 10.1074/jbc.M910257199. [DOI] [PubMed] [Google Scholar]
- 42.Angelillo-Scherrer A, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 43.Andre P, et al. CD40L stabilizes arterial thrombi by a β3 integrin–dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 44.Prevost N, et al. Eph kinases and ephrins support thrombus growth and stability by regulating integrin outside-in signaling in platelets. Proc Natl Acad Sci USA. 2005;102:9820–9825. doi: 10.1073/pnas.0404065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plow EF, et al. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985;66:724–727. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.