Abstract
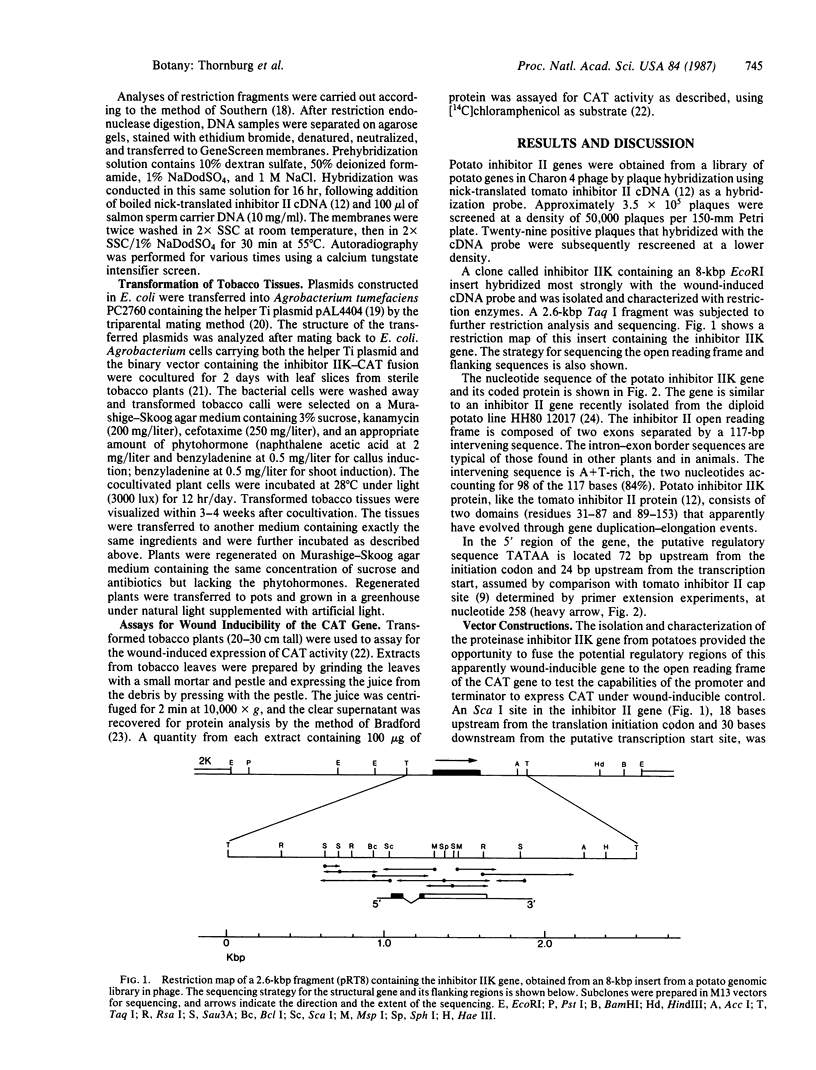
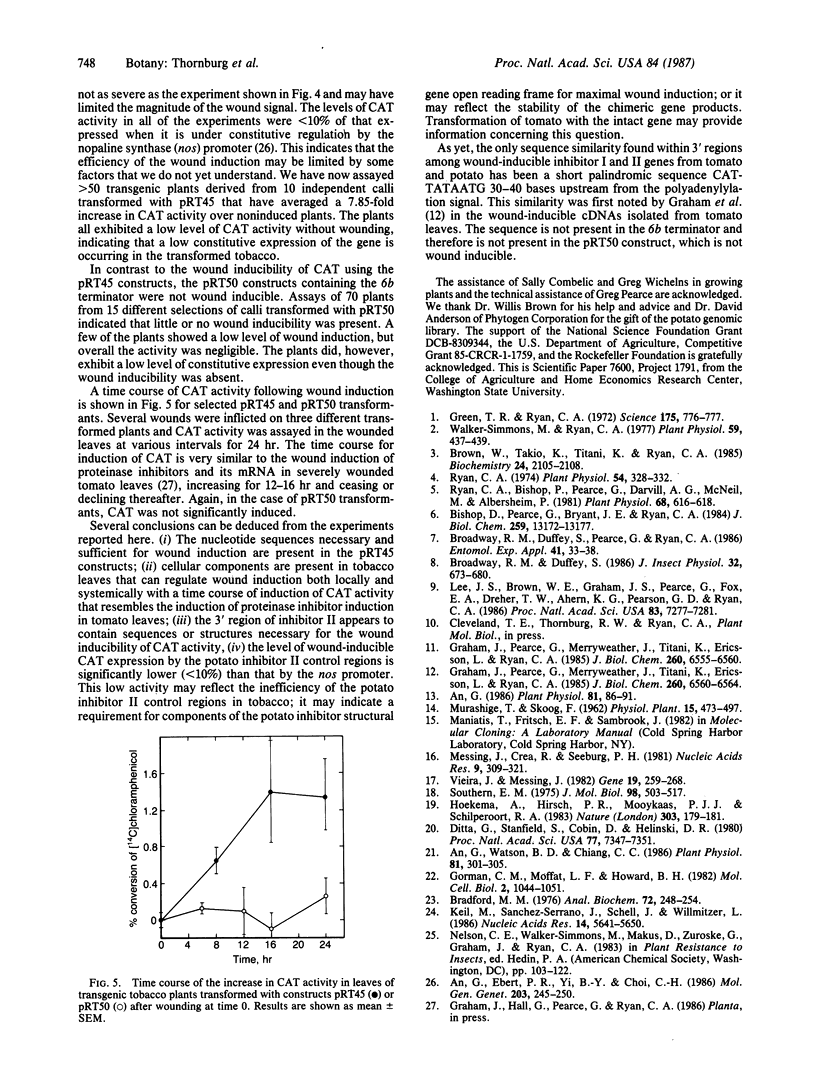
A potato inhibitor II gene (IIK) was isolated from a library of potato genes in λ bacteriophage. An 8-kilobase-pair (kbp) insert was identified using a tomato inhibitor II cDNA as a hybridization probe, and a 2.6-kbp fragment containing the gene was subcloned into the plasmid pUC13 and characterized. The nucleotide sequence of the isolated gene exhibited 87% identity with the wound-inducible tomato inhibitor II cDNA sequence. The amino acid sequence of inhibitor IIK, deduced from the potato gene, exhibited 84% identity with the tomato inhibitor II protein. A 1000-bp restriction fragment from the 5′ flanking region of the gene was fused to the open reading frame of the chloramphenicol acetyltransferase (CAT) gene. This fusion was terminated in two ways: (i) with a terminator sequence from the potato inhibitor II gene and (ii) with a terminator from the 6b gene of Ti plasmid pTiA6. These chimeric genes were transferred into tobacco cells via a binary Ti vector system, and transgenic plants were regenerated. The CAT gene was expressed in leaves of transformed plants in response to wounding when fused with the inhibitor IIK promoter and terminator regions. The chimeric gene containing the 6b terminator did not express CAT in response to wounding. The wound-inducible expression of CAT activity was systemic and was induced in tissues distal to the wounded tissues. The time course of wound induction of CAT activity in transgenic tobacco leaves is similar to that found for wound-inducible inhibitor I and II mRNAs in tomato leaves. These results demonstrate that sequences necessary and sufficient for wound inducibility are present within ≈1000 bp of the control regions of the inhibitor IIK genes and that wound-inducible components of tobacco leaf cells can regulate these sequences.
Keywords: tomatoes, proteinase inhibitors, transformation
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G. Development of plant promoter expression vectors and their use for analysis of differential activity of nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 1986 May;81(1):86–91. doi: 10.1104/pp.81.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Watson B. D., Chiang C. C. Transformation of Tobacco, Tomato, Potato, and Arabidopsis thaliana Using a Binary Ti Vector System. Plant Physiol. 1986 May;81(1):301–305. doi: 10.1104/pp.81.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. D., Pearce G., Bryant J. E., Ryan C. A. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves. Identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984 Nov 10;259(21):13172–13177. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown W. E., Takio K., Titani K., Ryan C. A. Wound-induced trypsin inhibitor in alfalfa leaves: identity as a member of the Bowman-Birk inhibitor family. Biochemistry. 1985 Apr 23;24(9):2105–2108. doi: 10.1021/bi00330a002. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L. H., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. II. The cDNA-deduced primary structure of pre-inhibitor II. J Biol Chem. 1985 Jun 10;260(11):6561–6564. [PubMed] [Google Scholar]
- Graham J. S., Pearce G., Merryweather J., Titani K., Ericsson L., Ryan C. A. Wound-induced proteinase inhibitors from tomato leaves. I. The cDNA-deduced primary structure of pre-inhibitor I and its post-translational processing. J Biol Chem. 1985 Jun 10;260(11):6555–6560. [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Keil M., Sanchez-Serrano J., Schell J., Willmitzer L. Primary structure of a proteinase inhibitor II gene from potato (Solanum tuberosum). Nucleic Acids Res. 1986 Jul 25;14(14):5641–5650. doi: 10.1093/nar/14.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Brown W. E., Graham J. S., Pearce G., Fox E. A., Dreher T. W., Ahern K. G., Pearson G. D., Ryan C. A. Molecular characterization and phylogenetic studies of a wound-inducible proteinase inhibitor I gene in Lycopersicon species. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7277–7281. doi: 10.1073/pnas.83.19.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Assay and Biochemical Properties of the Proteinase Inhibitor-inducing Factor, a Wound Hormone. Plant Physiol. 1974 Sep;54(3):328–332. doi: 10.1104/pp.54.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A., Bishop P., Pearce G. A sycamore cell wall polysaccharide and a chemically related tomato leaf polysaccharide possess similar proteinase inhibitor-inducing activities. Plant Physiol. 1981 Sep;68(3):616–618. doi: 10.1104/pp.68.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Wound-induced Accumulation of Trypsin Inhibitor Activities in Plant Leaves: Survey of Several Plant Genera. Plant Physiol. 1977 Mar;59(3):437–439. doi: 10.1104/pp.59.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]




