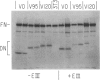
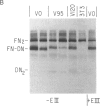
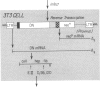
Abstract
We describe retroviral expression vectors containing cDNAs encoding part of fibronectin preceded by the signal and "pro" sequences of parathyroid hormone. The recombinant retroviruses were used to generate NIH 3T3 cell lines stably producing functionally active fragments of fibronectin. The recombinant fibronectins (deminectins) are processed and secreted by the cells and form disulfide-bonded dimers with themselves and with endogenous fibronectin subunits. The fibronectin-deminectin heterodimers are incorporated into the extracellular matrix. We describe cell lines producing six variant forms of deminectin corresponding to variant forms of fibronectin produced by alternative splicing. In constructing fibronectin cDNAs encoding the six variant forms, we also made use of the ability of retroviral vectors to generate cDNAs by accurate splicing of cloned genomic segments. These constructs should be valuable in analyses of the structure-function relationships of fibronectins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Choi M. G., Hynes R. O. Biosynthesis and processing of fibronectin in NIL.8 hamster cells. J Biol Chem. 1979 Dec 10;254(23):12050–12055. [PubMed] [Google Scholar]
- Davies J., Jimenez A. A new selective agent for eukaryotic cloning vectors. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1089–1092. doi: 10.4269/ajtmh.1980.29.1089. [DOI] [PubMed] [Google Scholar]
- Fan H., Jaenisch R., MacIsaac P. Low-multiplicity infection of Moloney murine leukemia virus in mouse cells: effect on number of viral DNA copies and virus production in producer cells. J Virol. 1978 Dec;28(3):802–809. doi: 10.1128/jvi.28.3.802-809.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hellerman J. G., Cone R. C., Potts J. T., Jr, Rich A., Mulligan R. C., Kronenberg H. M. Secretion of human parathyroid hormone from rat pituitary cells infected with a recombinant retrovirus encoding preproparathyroid hormone. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5340–5344. doi: 10.1073/pnas.81.17.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. Extensive disulfide bonding at the mammalian cell surface. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2855–2859. doi: 10.1073/pnas.74.7.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: molecular cloning evidence for two mRNA species differing by an internal segment coding for a structural domain. EMBO J. 1984 Jan;3(1):221–226. doi: 10.1002/j.1460-2075.1984.tb01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Mautner V., Hynes R. O. Surface distribution of LETS protein in relation to the cytoskeleton of normal and transformed cells. J Cell Biol. 1977 Dec;75(3):743–768. doi: 10.1083/jcb.75.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Mosher D. F. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985 Feb;100(2):364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt E., Tamkun J. W., Hynes R. O. Repeating modular structure of the fibronectin gene: relationship to protein structure and subunit variation. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6571–6575. doi: 10.1073/pnas.82.19.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. I., Schwarzbauer J. E., Tamkun J. W., Hynes R. O. Cell-type-specific fibronectin subunits generated by alternative splicing. J Biol Chem. 1986 Sep 15;261(26):12258–12265. [PubMed] [Google Scholar]
- Schwarzbauer J. E., Paul J. I., Hynes R. O. On the origin of species of fibronectin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1424–1428. doi: 10.1073/pnas.82.5.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Shimotohno K., Temin H. M. Loss of intervening sequences in genomic mouse alpha-globin DNA inserted in an infectious retrovirus vector. Nature. 1982 Sep 16;299(5880):265–268. doi: 10.1038/299265a0. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., Hynes R. O. Plasma fibronectin is synthesized and secreted by hepatocytes. J Biol Chem. 1983 Apr 10;258(7):4641–4647. [PubMed] [Google Scholar]
- Tamkun J. W., Schwarzbauer J. E., Hynes R. O. A single rat fibronectin gene generates three different mRNAs by alternative splicing of a complex exon. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5140–5144. doi: 10.1073/pnas.81.16.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibe-Pedersen K., Kornblihtt A. R., Baralle F. E. Expression of a human alpha-globin/fibronectin gene hybrid generates two mRNAs by alternative splicing. EMBO J. 1984 Nov;3(11):2511–2516. doi: 10.1002/j.1460-2075.1984.tb02165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]