Abstract
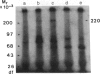
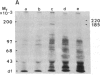
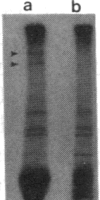
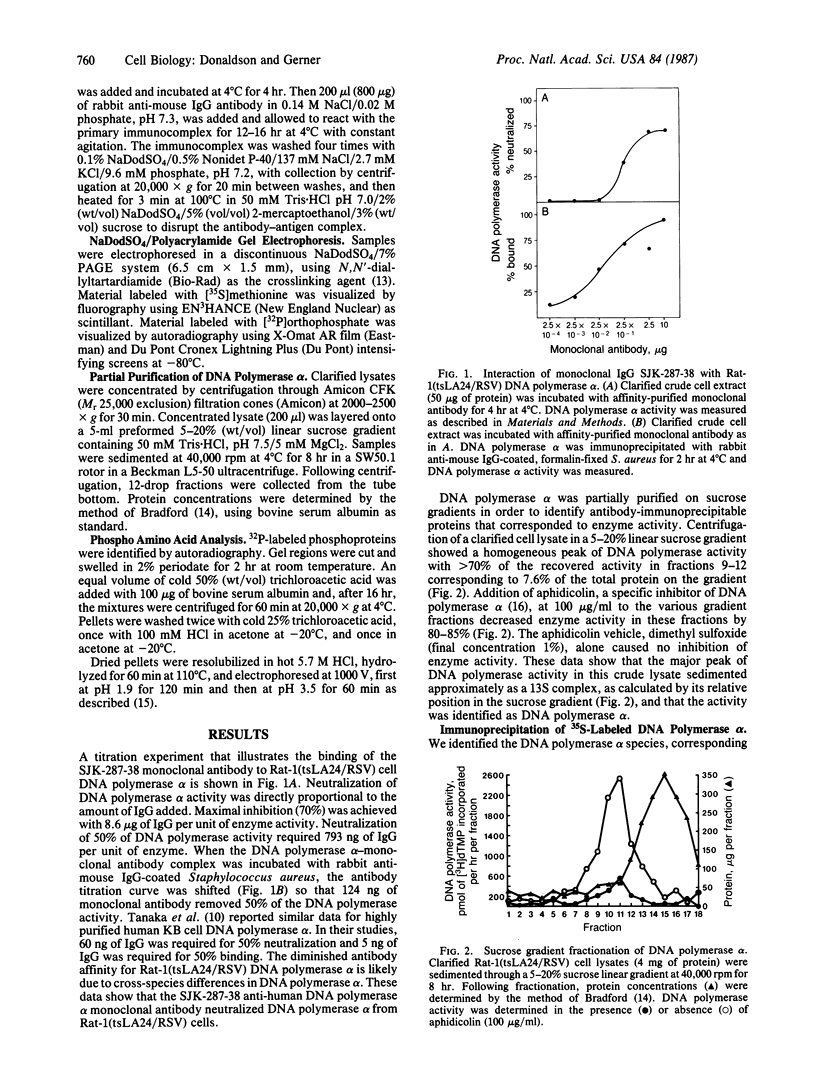
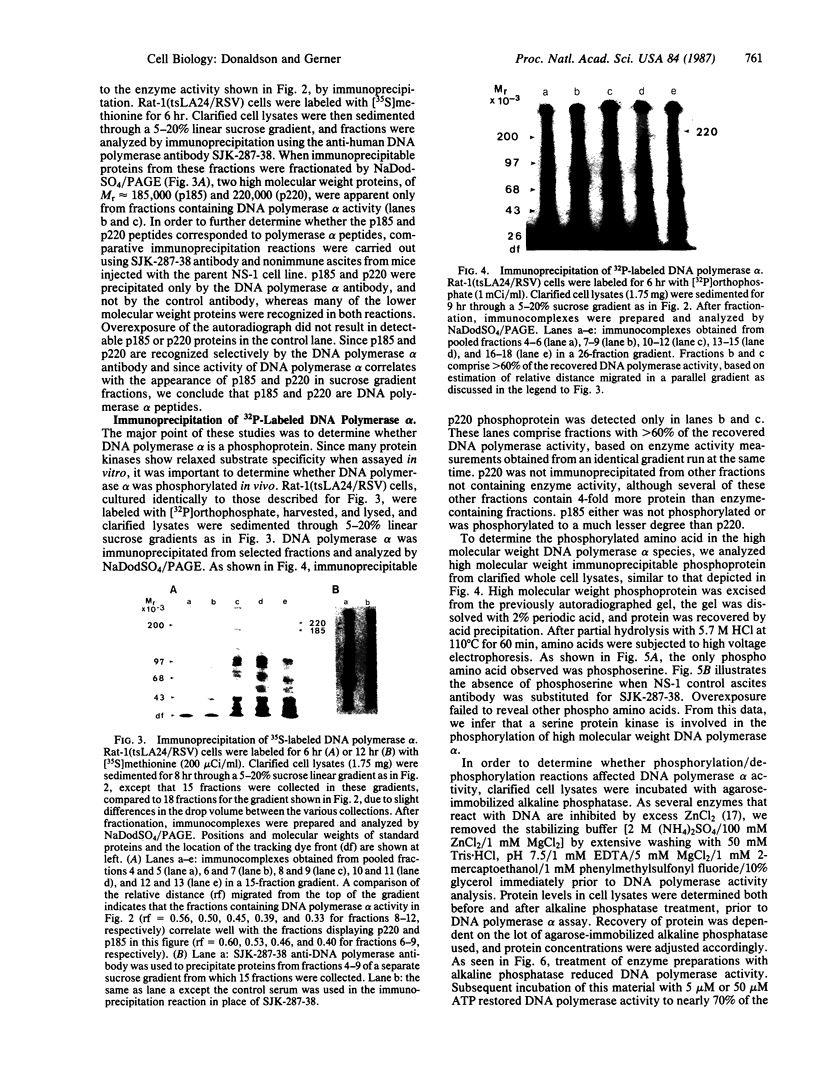
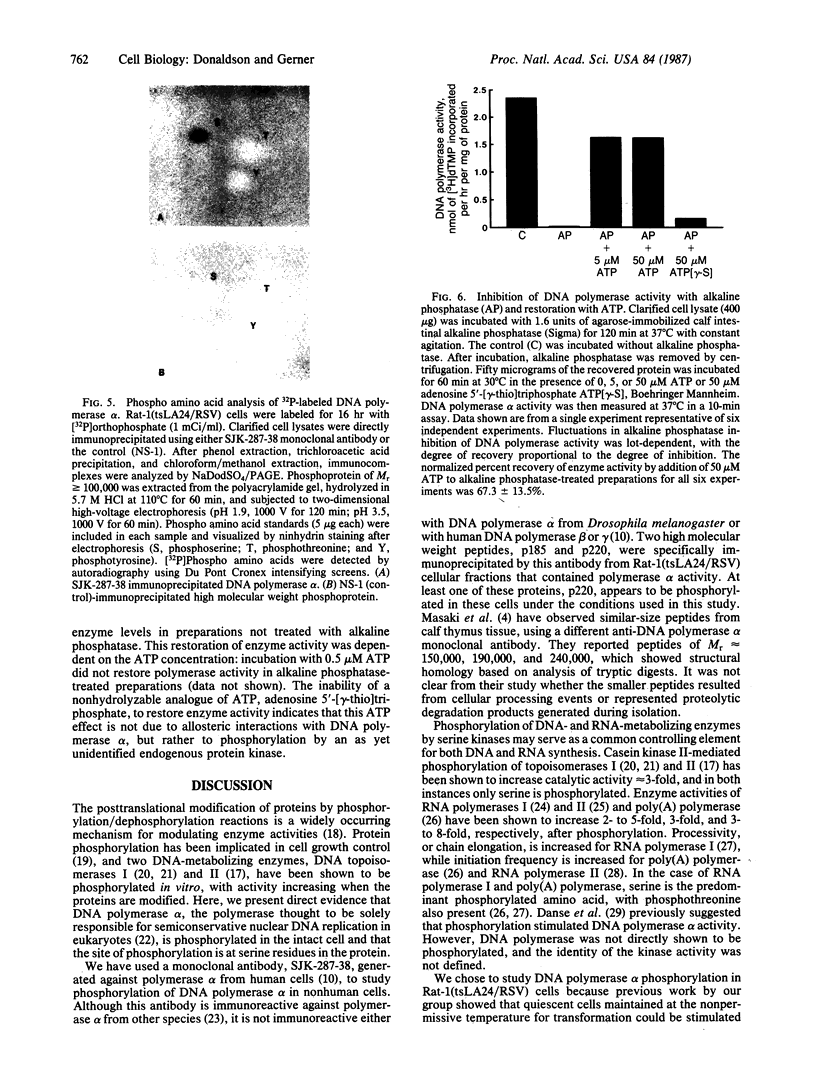
Anti-human DNA polymerase alpha murine IgG SJK-287-38 [Tanaka, S., Hu, S.-Z., Wang, T. S.-F. & Korn, D. (1982) J. Biol. Chem. 257, 8386-8390] neutralized DNA polymerase alpha activity from rat embryonic fibroblasts infected with a temperature-sensitive transformation mutant of Rous sarcoma virus (tsLA24). After centrifugation of a crude cytosol fraction from log-phase cells in a 5-20% linear sucrose gradient, polypeptides of Mr approximately equal to 185,000 and 220,000 were immunoprecipitated only from gradient fractions containing DNA polymerase alpha activity. When similar cultures were incubated in medium containing [32P]orthophosphate, it was found that the Mr 220,000 protein was phosphorylated but that the other peptides specific for polymerase alpha activity did not contain detectable amounts of phosphate. Phospho amino acid analysis of the high molecular weight immunoprecipitable proteins indicated that the labeled amino acid was phosphoserine. Incubation of 2.5 units of crude DNA polymerase alpha with 4 units of agarose-immobilized alkaline phosphatase resulted in a nearly complete inhibition of DNA polymerase alpha activity. Subsequent incubation of this preparation with 5 or 50 microM ATP, but not the nonhydrolyzable analog adenosine 5'-[gamma-thio]triphosphate, restored the in vitro DNA polymerizing activity. These results demonstrate that a high molecular weight DNA polymerase alpha (Mr approximately equal to 220,000) is phosphorylated in cultured cells and that this protein is a substrate for a serine kinase rather than the tyrosine-specific protein kinase of Rous sarcoma virus. The results suggest that phosphorylation/dephosphorylation reactions modulate the activity of this polymerase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Glover C. V., Osheroff N. Phosphorylation of DNA topoisomerase II by casein kinase II: modulation of eukaryotic topoisomerase II activity in vitro. Proc Natl Acad Sci U S A. 1985 May;82(10):3164–3168. doi: 10.1073/pnas.82.10.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch K. G., Tanaka S., Hu S. Z., Wang T. S., Korn D. Intracellular localization of human DNA polymerase alpha with monoclonal antibodies. J Biol Chem. 1982 Jul 25;257(14):8391–8396. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Bohn E. W., Planck S. R., Wilson S. H. Mouse DNA polymerase alpha. Subunit structure and identification of a species with associated exonuclease. J Biol Chem. 1979 Nov 25;254(22):11678–11687. [PubMed] [Google Scholar]
- Chiu R. W., Baril E. F. Nuclear DNA polymerases and the HeLa cell cycle. J Biol Chem. 1975 Oct 10;250(19):7951–7957. [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Costa M., Gerner E. W., Russell D. H. Cell cycle-specific activity of type I and type II cyclic adenosine 3':5'-monophosphate-dependent protein kinases in Chinese hamster ovary cells. J Biol Chem. 1976 Jun 10;251(11):3313–3319. [PubMed] [Google Scholar]
- Costa M., Gerner E. W., Russell D. H. Cyclic AMP levels and types I and II cyclic AMP-dependent protein kinase activity in synchronized cells and in quiescent cultures stimulated to proliferate. Biochim Biophys Acta. 1978 Jan 3;538(1):1–10. doi: 10.1016/0304-4165(78)90246-5. [DOI] [PubMed] [Google Scholar]
- Danse J. M., Egly J. M., Kempf J. In vitro activation of DNA polymerase-alpha by a protein kinase in chick embryo. FEBS Lett. 1981 Feb 9;124(1):84–88. doi: 10.1016/0014-5793(81)80059-2. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Duceman B. W., Jacob S. T. Transcriptionally active RNA polymerases from Morris hepatomas and rat liver. Elucidation of the mechanism for the preferential increase in the tumour RNA polymerase I. Biochem J. 1980 Sep 15;190(3):781–789. doi: 10.1042/bj1900781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duceman B. W., Rose K. M., Jacob S. T. Activation of purified hepatoma RNA polymerase I by homologous protein kinase NII. J Biol Chem. 1981 Nov 10;256(21):10755–10758. [PubMed] [Google Scholar]
- Durban E., Goodenough M., Mills J., Busch H. Topoisomerase I phosphorylation in vitro and in rapidly growing Novikoff hepatoma cells. EMBO J. 1985 Nov;4(11):2921–2926. doi: 10.1002/j.1460-2075.1985.tb04024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durban E., Mills J. S., Roll D., Busch H. Phosphorylation of purified Novikoff hepatoma topoisomerase I. Biochem Biophys Res Commun. 1983 Mar 29;111(3):897–905. doi: 10.1016/0006-291x(83)91384-0. [DOI] [PubMed] [Google Scholar]
- Haddox M. K., Magun B. E., Russell D. H. Differential expression of type I and type II cyclic AMP-dependent protein kinases during cell cycle and cyclic AMP-induced growth arrest. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3445–3449. doi: 10.1073/pnas.77.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Karawya E., Swack J., Albert W., Fedorko J., Minna J. D., Wilson S. H. Identification of a higher molecular weight DNA polymerase alpha catalytic polypeptide in monkey cells by monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7777–7781. doi: 10.1073/pnas.81.24.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N. N., Brown N. C. Purification and characterization of DNA polymerase alpha of Chinese hamster ovary cells. Mol Cell Biochem. 1985 Oct;68(2):169–179. doi: 10.1007/BF00219381. [DOI] [PubMed] [Google Scholar]
- Kranias E. G., Schweppe J. S., Jungmann R. A. Phosphorylative and functional modifications of nucleoplasmic RNA polymerase II by homologous adenosine 3':5'-monophosphate-dependent protein kinase from calf thymus and by heterologous phosphatase. J Biol Chem. 1977 Oct 10;252(19):6750–6758. [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Magun B. E., Thompson R. L., Gerner E. W. Regulation of DNA replication by serum and the transforming function in cultured rat fibroblasts transformed by Rous sarcoma virus. J Cell Physiol. 1979 May;99(2):207–216. doi: 10.1002/jcp.1040990207. [DOI] [PubMed] [Google Scholar]
- Masaki S., Tanabe K., Yoshida S. Large polypeptides of 10S DNA polymerase alpha from calf thymus: rapid isolation using monoclonal antibody and tryptic peptide mapping analysis. Nucleic Acids Res. 1984 Jun 11;12(11):4455–4467. doi: 10.1093/nar/12.11.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottiger H. P., Hübscher U. Mammalian DNA polymerase alpha holoenzymes with possible functions at the leading and lagging strand of the replication fork. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3993–3997. doi: 10.1073/pnas.81.13.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K. M., Jacob S. T. Phosphorylation of nuclear poly(A) polymerase. Comparison of liver and hepatoma enzymes. J Biol Chem. 1979 Oct 25;254(20):10256–10261. [PubMed] [Google Scholar]
- Sedwick W. D., Wang T. S., Korn D. Purification and properties of nuclear and cytoplasmic deoxyribonucleic acid polymerases from human KB cells. J Biol Chem. 1972 Aug 25;247(16):5026–5033. [PubMed] [Google Scholar]
- Stetler D. A., Rose K. M. Phosphorylation of deoxyribonucleic acid dependent RNA polymerase II by nuclear protein kinase NII: mechanism of enhanced ribonucleic acid synthesis. Biochemistry. 1982 Jul 20;21(15):3721–3728. doi: 10.1021/bi00258a030. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Hu S. Z., Wang T. S., Korn D. Preparation and preliminary characterization of monoclonal antibodies against human DNA polymerase alpha. J Biol Chem. 1982 Jul 25;257(14):8386–8390. [PubMed] [Google Scholar]
- Tas J., de Vries A. C., Berndsen R. G. A method for the quantitative determination of protein incorporated in solubilized polyacrylamide gels. Anal Biochem. 1979 Dec;100(2):264–270. doi: 10.1016/0003-2697(79)90229-x. [DOI] [PubMed] [Google Scholar]
- Wong S. W., Paborsky L. R., Fisher P. A., Wang T. S., Korn D. Structural and enzymological characterization of immunoaffinity-purified DNA polymerase alpha.DNA primase complex from KB cells. J Biol Chem. 1986 Jun 15;261(17):7958–7968. [PubMed] [Google Scholar]