Abstract
Protein multi-functionality is an emerging explanation for the complexity of higher organisms. In this regard, while aminoacyl tRNA synthetases catalyze amino acid activation for protein synthesis, some also act in pathways for inflammation, angiogenesis, and apoptosis. How multiple functions evolved and their relationship to the active site is not clear. Here structural modeling analysis, mutagenesis, and cell-based functional studies show that the potent angiostatic, natural fragment of human TrpRS associates via Trp side chains that protrude from the cognate cellular receptor VE-cadherin. Modeling indicates that (I prefer the way it was because the conclusion was reached not only by modeling, but more so by experimental studies.)VE-cadherin Trp side chains fit into the Trp-specific active site of the synthetase. Thus, specific side chains of the receptor mimic (?) amino acid substrates and expand the functionality of the active site of the synthetase. We propose that orthogonal use of the same active site may be a general way to develop multi-functionality of human tRNA synthetases and other proteins.
Along with alternative splicing, post-translational modifications, and regulatory RNAs, protein multi-functionality is an emerging explanation for the complexity of higher organisms. Multi-functionality reduces the need for more protein-coding genes. In addition, expanded functions provide a way to integrate diverse biological systems, where one pathway is connected to another. Prominent among the various examples of multifunctional proteins are members of the aminoacyl tRNA synthetase (AARS) family 1,2. While AARSs are well known for activating amino acids for protein synthesis 3, they also have a broad spectrum of expanded activities in pathways including splicing 4,5, translational and transcriptional control 6–8, pro- and anti-angiogenesis 9–14, inflammation 15, signaling of immune responses 16, apoptosis 17, and viral assembly 18. It is these activities that may explain why there are many disease-associated tRNA synthetase mutations, especially in instances where the change has no effect on aminoacylation activity 19–23. In most cases, however, how and why tRNA synthetases were expropriated for other purposes is still not understood.
Upon stimulation with the anti-proliferative cytokine interferon-γ, the 471 amino acid human tryptophanyl-tRNA synthetase (TrpRS) is up-regulated and secreted from various cell types including endothelial cells 24,25. Natural alternative forms of human TrpRS have potent anti-angiogenic activity 9–11,14. This activity is blocked until the N-terminal domain (N-domain) is removed, either by alternative splicing to give mini-TrpRS, or by proteolysis, to give the closely similar T2-TrpRS (comprised of residues 94–471 of TrpRS). T2-TrpRS binds to the endothelial cell adherens junction molecule—vascular endothelial-cadherin (VE-cadherin) 26—and inhibits activation of genes associated with angiogenesis such as Akt and endothelial cell NO synthase 27. Because it inhibits new blood vessel formation, with no disruption of existing blood vessels 11,14, T2-TrpRS has appeal for therapeutic applications, including macular degeneration and oncology 28.
VE-cadherin, as the major constituent of the adherens junctions of endothelial cells, plays a key role in angiogenesis and vascular permeability. The 784 amino acid molecule has a modular structure consisting of five extracellular cadherin (EC) domains, a single-pass trans-membrane α-helix, and a cytoplasmic tail that links to the cytoskeleton via other protein factors 29. Endothelial cell-cell adhesion depends on the homophilic interaction between VE-cadherin molecules from neighboring cells, and this homophilic interaction depends on the N-terminal EC domain EC1 30 (Fig. 1A). As a type II cadherin, VE-cadherin has two conserved tryptophans (Trp2 and Trp4) at positions 2 and 4 from the N-terminus. Crystal structures of three different type II cadherin molecules showed that Trp2 and Trp4 are the key determinants for interactions between EC1 domains 31. Particularly, Trp2 and Trp4 from one EC1 bind to a large hydrophobic pocket of the other EC1, and vice-versa, to lock together the two cadherin molecules (Fig. 1A) 31. Not surprisingly, mutation of either Trp2 or Trp4 disrupts endothelial cell adhesion 32.
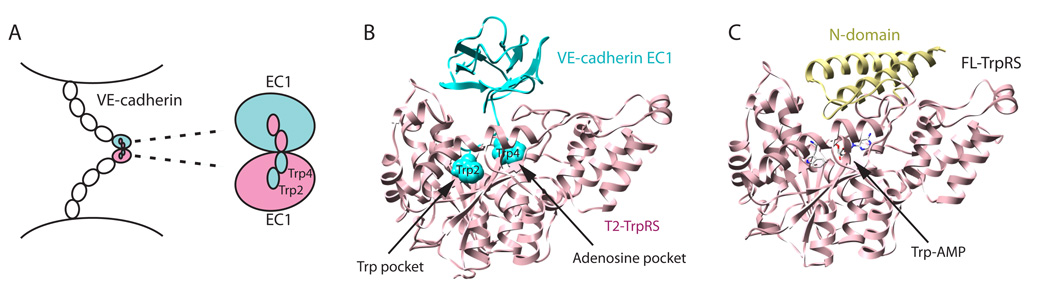
Figure 1.
Hypothesized mode for binding of T2-TrpRS to VE-cadherin. A. Homophilic interaction between type II cadherins for cell-cell adhesion involves the N-terminal extracellular domain EC1. Trp2 and Trp4 of EC1 from one cadherin and a large hydrophobic pocket of EC1 from the other cadherin provide the mutual lock-and-key interaction. B. Structural model of T2-TrpRS and VE-cadherin EC1 locked together, with Trp2 and Trp4 of VE-cadherin binding to the Trp and adenosine pockets in the active site of T2-TrpRS, respectively. The initial structure of T2-TrpRS is from PDB 1O5T35. C. Representation of the co-crystal structure of Trp-AMP bound to full-length human TrpRS (PDB 1R6T)34, showing that the N-domain occupies a space that may block the binding of EC1 to the active site.
Because a tRNA synthetase specific for tryptophan binds to VE-cadherin, whose function, in turn, depends on two specific Trp side chains, we speculated that tryptophan itself is the connection between TrpRS and VE-cadherin. TrpRS, like all 20 members of the tRNA synthetase family, has developed a highly specific amino acid binding pocket during evolution to prevent misaminoacylation and mistranslation. The active site of TrpRS, that binds Trp specifically, may be used to bind the Trp2 or Trp4 residue of VE-cadherin and, for that reason, TrpRS was selected to function in angiogenesis through the VE-cadherin receptor. Using structural modeling, mutagenesis, binding and cell based functional analyses, we set out to extensively test this hypothesis using TrpRS and VE-cadherin proteins from human.
RESULTS
Structural modeling of TrpRS and VE-cadherin interaction
The previously solved co-crystal structure of human TrpRS bound with tryptophanyl adenylate (Trp-AMP, the reaction intermediate formed in the amino acid activation step) showed in clear detail the Trp binding pocket and the adjacent pocket that holds the AMP moiety 33,34. The structure of the EC1 of VE-cadherin was built by the homology modeling program Modeller (http://salilab.org/modeller/), using the structures of EC1 domains of 3 type II cadherins 31 (MN-cadherin, cadherin-8, cadherin-11) that share 41—77% sequence identity with EC1 of VE-cadherin. We have modeled the interaction between human T2-TrpRS35and VE-cadherin (EC1) as described in METHODS. In the modeled complex, Trp2 of VE-cadherin bound in the tryptophan binding pocket of T2-TrpRS, and Trp4 fit well in the pocket for the adenosine moiety of AMP (Fig. 1B). Because the Trp binding pocket is deeper than and inside of the AMP binding pocket, the orientation of the two Trps on VE-cadherin with regard to the two pockets in T2-TrpRS is specific. The rest of the EC1 domain sits on top of, and caps, the active site of T2-TrpRS. Interestingly, the space that the EC1 domain occupied overlaps with the location of the N-domain in the crystal structure of full-length (FL) TrpRS (Fig. 1C). Therefore, EC1 cannot fit into FL-TrpRS because of steric hindrance with the N-domain of TrpRS, which blocks access of Trp2 and Trp4 to the active site. Importantly, this model is consistent with experimental observations that FL-TrpRS is inactive in angiogenesis 9,11,14, and suggests that the inactivation is attributed to the blocking of the interaction by the N-domain.
Binding and functional studies support inhibitory role of N-domain
To test this model, we first used co-immunoprecipitation to detect binding of FL-TrpRS to VE-cadherin, and compared the binding with that of T2-TrpRS. For this purpose, we used a fusion of VE-cadherin (extracellular portion) with Fc that, in turn, binds to Protein G beads. FL-TrpRS or T2-TrpRS was incubated with beads bound with VE-cadherin. In these experiments, with the same amount of input, the amount of bound FL-TrpRS was about 5% of that of bound T2-TrpRS (Fig. 2A). This result suggested strong binding of T2-TrpRS to VE-cadherin and, as expected, a substantially diminished interaction of FL-TrpRS.
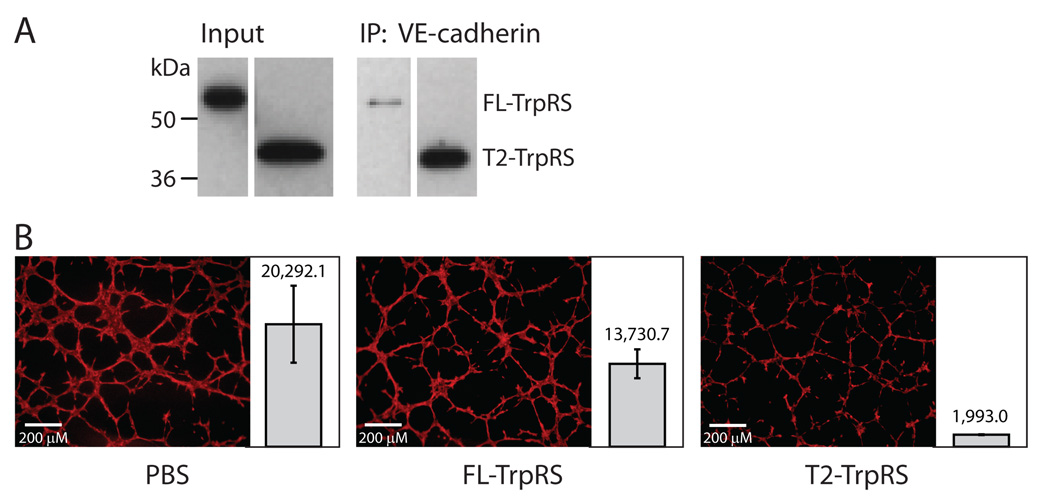
Figure 2.
Comparison of FL- and T2-TrpRS for ability to bind to VE-cadherin and for angiostatic activity. A. Co-immunoprecipitation experiment showing the substantially diminished VE-cadherin interaction with FL- compared with T2-TrpRS. B. Endothelial tube formation assay showing that T2-TrpRS (2 µM) inhibits tube formation while FL-TrpRS (2 µM) has only a small effect. For each experiment, multiple repetitions were performed (11, 13 and 22 repetitions for PBS control, FL- and T2-TrpRS, respectively.) The total area that is covered by vasculature in each experiment was calculated and the average number +/− SEM (standard error of the mean) for each group is shown side-by-side with a representative image.
We further compared the activity of T2- and FL-TrpRS in a tube formation assay using 3B11 mouse tumor endothelial cells36. In this assay, endothelial cells form vessel-like tubes. The formation of these tubes can be blocked by angiostatic factors like T2-TrpRS. As expected, considering the total area that is covered by the vessels, treatment with 2 µM T2-TrpRS for 8 hours gave more than 90% inhibition of tube formation. In contrast, the effect of FL-TrpRS was substantially diminished (Fig. 2B). The result was consistent with previous studies showing that removal of the N-domain was required for activation of the anti-angiogenic activity of TrpRS 9,11,14.
TrpRS active site and Trp pocket are involved in receptor binding
TrpRS catalyzes the condensation of Trp with ATP to give Trp-AMP (?)the adenylate is highly labile, but is sequestered in the active site to protect it from hydrolysis before it is condensed with the 3’-end of tRNA to give the aminoacyl tRNA). A non-hydrolyzable Trp-AMP analog Trp-SA (5’-O-[N-(L-tryptophanyl)sulfamoyl]adenosine) is a potent inhibitor of the enzyme, and binds tightly to the active site. Thus, we imagined that, if T2-TrpRS recognized the exposed Trp2 and Trp4 of VE-cadherin, through the active site, then either Trp or Trp-SA would block the ability of T2-TrpRS to bind to VE-cadherin. The inhibition capacity of Trp-SA would be more potent because Trp-SA binds more tightly to the active site than does tryptophan, and because Trp-SA can block both Trp and AMP binding sites, while tryptophan can only block that for Trp. We found that tryptophan attenuated the binding of T2-TrpRS to VE-cadherin. Moreover, Trp-SA strongly inhibited binding of T2-TrpRS to VE-cadherin in a concentration dependent manner. In contrast, a non-cognate adenylate analog (Met-SA) had no effect (Fig. 3A). Interestingly, using antibodies against the His6 tag of the recombinant T2-TrpRS, we were able to pull down the endogenous VE-cadherin from lysates of bovine aortic endothelial cells. However, no VE-cadherin can be pulled down if Trp-SA was added into the cell lysate, while Met-SA had no effect on the interaction (Supplementary Fig. 1). These results provided clear support for the Trp binding pocket of T2-TrpRS being essential for binding to VE-cadherin.
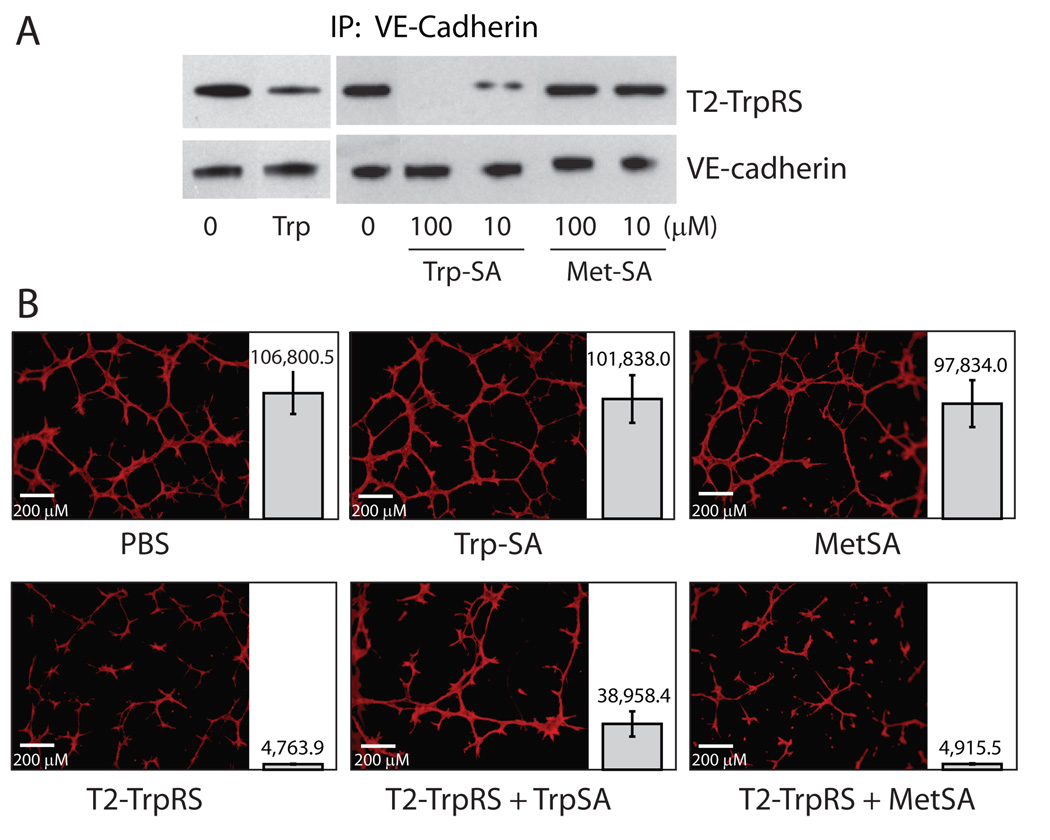
Figure 3.
Inhibition by Trp-SA of T2-TrpRS/VE-cadherin interaction and of angiostatic activity of T2-TrpRS. A. Co-immunoprecipitation experiment showing that Trp (1 mM) and Trp-SA, but not Met-SA, inhibit T2-TrpRS/VE-cadherin interaction. B. Endothelial tube formation assay showing that Trp-SA (50 µM), but not Met-SA (50 µM), inhibits the angiostatic activity of T2-TrpRS (2 µM). For each experiment, multiple repetitions were performed. The total area that is covered by vasculature in each experiment was calculated and the average number +/− SEM for each group is shown side-by-side with a representative image.
To further support these results, we also tested the effect of Trp-SA, the strongest inhibitor of T2-TrpRS binding to VE-cadherin, on the ability of T2-TrpRS to prevent endothelial cell tube formation. Consistent with the previous experiments, T2-TrpRS inhibited tube formation more than 90 %. Adding Trp-SA to T2-TrpRS reduced the inhibition from 96 % to 64 %, while adding the non-cognate analog Met-SA had no effect. Furthermore, adding the same concentrations of Trp-SA or Met-SA alone did not affect tube formation (Fig. 3B).
Mutational study confirms the role of active site and Trp pocket
Having established that the active site of T2 is important for VE-cadherin interaction and for anti-angiogenic activity (as monitored by the tube formation assay), the active site of T2 was dissected by mutagenesis. As suggested by modeling, Trp2 of VE-cadherin binds to the Trp-binding pocket, while Trp4 binds to the AMP pocket. Thus, guided by our co-crystal structure of TrpRS bound with Trp-AMP (Fig. 4A), we made 4 mutants of T2-TrpRS that should disrupt either the Trp or the AMP binding pocket. Particularly, Y159A/Q194A TrpRS was created to disrupt specific recognition of the indole nitrogen of tryptophan by Y159 and Q194. In addition, G161W TrpRS was created to sterically block the Trp pocket by replacing G161 with the bulky indole side chain of W (Fig. 4A). Using isothermal titration calorimetry (ITC), while wild-type T2-TrpRS bound to Trp with Kd = 11 µM (similar to that of FL-TrpRS), Y159A/Q194A T2-TrpRS had no detectable association with tryptophan, and G161W T2-TrpRS had a more than 20-fold decreased affinity for tryptophan. Similarly, two single mutations—A310W and G172M—were created to block the AMP pocket (Fig. 4A). The weakened binding of these two mutant proteins with AMP was confirmed by equilibrium dialysis (see METHODS for detail).
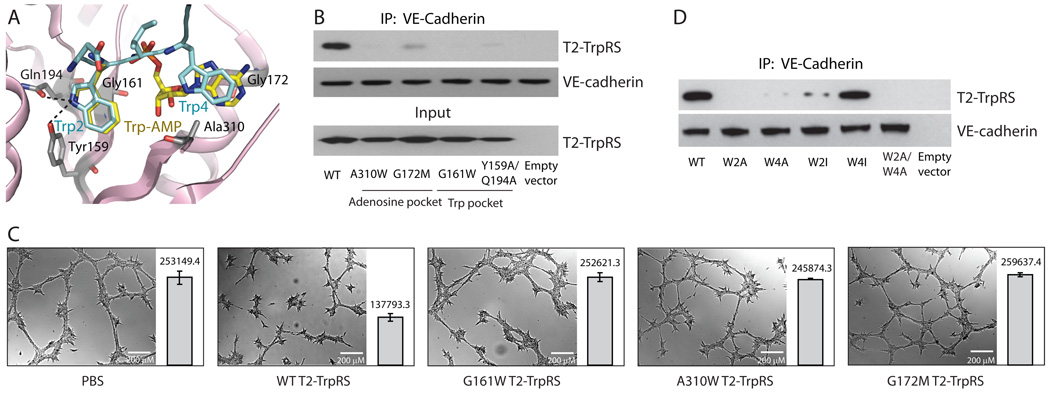
Figure 4.
Mutagenesis studies confirming that the mechanism for T2-TrpRS’s angiostatic activity involves the Trp and adenosine pockets of T2-TrpRS, and Trp2 and Trp4 of VE-cadherin. A. The active site of T2-TrpRS bound with Trp-AMP (yellow, from crystal structure) and with D-W2-I-W4 region of VE-cadherin (cyan, from modeling). B. Disruption of T2-TrpRS/VE-cadherin interaction with mutations that disrupt the Trp or the adenosine pocket of T2-TrpRS. Lysates of E. coli cells transformed with genes expressing WT or mutant T2-TrpRS, or with an “empty vector” control, were used for co-immunoprecipitations to detect interactions with purified human VE-cadherin-Fc. C. Tube formation assay showing that the mutant T2-TrpRS proteins are no longer angiostatic. For each experiment, multiple images were taken. The total area that is covered by vasculature in each image was calculated and the average number +/− SEM for each group is shown side-by-side with a representative image. D. The effect of Trp2 and Trp4 mutations of VE-cadherin on the T2-TrpRS interaction. For the co-immuniprecipitation experiments, T2-TrpRS was incubated with lysates of 293T cells stably transfected with genes expressing WT or mutant VE-cadherin-Fc fusion proteins, or with lysates from an empty vector control.
Next, the T2-TrpRS mutants were tested for binding to VE-cadherin by co-immunoprecipitation. Strikingly, compared to the wild-type protein, all four mutant T2-TrpRSs had substantially diminished binding to VE-cadherin (Fig. 4B), consistent with the Trp and AMP pockets of T2-TrpRS being essential for interaction with VE-cadherin.
We also investigated the functional consequence of the mutations in the endothelial cell tube-formation assay. The wild-type and three mutant proteins (G161W, G172M and A310W) were freshly prepared side-by-side to ensure that any differences in activities did not come from sample variability. Although wild-type T2-TrpRS in this set of experiments was not as robust as in some of the earlier experiments, its angiostatic activity was clear (Fig. 4C). However, this activity was completely abolished for all three mutant proteins (Fig. 4C).
The role of Trp2 and Trp4 of VE-cadherin
To investigate the interaction from the VE-cadherin side, we substituted Trp2 and Trp4 of VE-cadherin to Ala, to create W2A and W4A VE-cadherin, and thus test the role of Trp2 and Trp4. For this purpose, we used VE-cadherin expressed in HeLa cells that had been transfected with a gene encoding either a wild-type or a mutant VE-cadherin-Fc fusion protein (HeLa cells do not express VE-cadherin endogenously). The transfected HeLa cell lysates were incubated with T2-TrpRS and, as expected, T2-TrpRS was co-immunoprecipitated with wild-type VE-cadherin. However, W2A, W4A, and W2A/W4A VE-cadherin pulled down little or no T2-TrpRS (Fig. 4D), suggesting that both Trp2 and Trp4 are critical for T2-TrpRS interaction, as suggested by our structural model.
To further test the role of Trp4, which is proposed to fit with the adenosine pocket of T2-TrpRS that is adjacent to the tryptophan-binding site (Fig. 4A), we attempted to create a VE-cadherin mutation of Trp4 that would still accommodate binding of T2-TrpRS. Because the interaction of Trp4 with T2-TrpRS is based on the mutual hydrophobicity of the bulky indole ring of tryptophan and the adenosine pocket of T2-TrpRS, we reasoned that substitution of Trp4 with a bulky hydrophobic residue such as isoleucine would allow interaction of VE-cadherin with T2-TrpRS. (We noted that the 4th position of a type I cadherin is usually an Ile 29,31.) Indeed, W4I VE-cadherin clearly bound to T2-TrpRS (Fig. 4D). In contrast, the exact fit of Trp2 with the tryptophan binding pocket showed little tolerance for a W2I substitution (Fig. 4D). The specificity of Trp2 reinforced the idea that the T2-TrpRS/VE-cadherin interaction is primarily determined by the precise complementarity between a protruding Trp residue of VE-cadherin and a specific Trp binding pocket of T2-TrpRS.
DISCUSSION
As stated above, T2-TrpRS does not affect developed blood vessels, but instead blocks development of neovasculature 11,14. An interaction between T2-TrpRS and Trp2 and Trp4 of EC1 of VE-cadherin can explain this observation. In particular, because Trp2 and Trp4 are buried in homophilic interactions between the N-terminal EC1s of VE-cadherin in normal, existing blood vessels, they are not exposed in a way where they could interact with T2-TrpRS. In contrast, T2-TrpRS can bind to the neovasculature, where VE-cadherin has not yet engaged in trans-adhesion so that its Trp2 and Trp4 epitopes are fully exposed. As a result, new blood vessel formation cannot occur when T2-TrpRS binds and blocks the critical Trp2 and Trp4 of VE-cadherin. In support of this concept, May et al showed that a monoclonal anti-VE-cadherin antibody can specifically target the neovasculature due to its binding to Trp2 and Trp4 32.
There are two types of cadherins—type I and type II. VE-cadherin is type II, which has two Trps (Trp2 and Trp4) near the N-terminus. Typically, Trp4 of type II cadherins is replaced by an Ile in type I cadherins, while Trp2 is conserved in all cadherin molecules 29,31. Our mutational study showed that substitution of Trp4 with Ile in VE-cadherin did not affect T2-TrpRS binding, suggesting that T2-TrpRS may have a broader specificity beyond VE-cadherin. We tested N-cadherin, a type I cadherin that is mostly found in neurons. Indeed, T2-TrpRS can bind to N-cadherin-Fc (data not shown), suggesting a potentially broad specificity of T2-TrpRS towards the cadherin family, but only towards free cadherin molecules that are not yet involved in cell-cell junctions. Therefore, T2-TrpRS may function as a general inhibitor for cadherin junction-dependent growth.
The catalytic domains of tRNA synthetases have only two known architectures—the Rossmann fold of the class I synthetases, and the central β-sheet with flanking α-helices of the class II synthetases. In spite of there being only two general architectures, the known expanded functions of tRNA synthetases mostly are idiosyncratic and specific to each synthetase. Thus, those characteristics that are idiosyncratic (i.e. unique) to each synthetase may explain the different novel activities of the different synthetases. One way that idiosyncratic functions can be developed is through acquisitions of appended domains that are specific to the synthetase 6,12. Alternatively, new functions may arise from expanded uses of motifs—such as the site for aminoacyl adenylate synthesis—originally developed for the ancient aminoacylation function. This work demonstrates how the angiostatic function of TrpRS is connected to VE-cadherin through a completely different use of the synthetase’s unique local architecture for engaging Trp-AMP. More generally, during evolution and development of the tree of life, where tRNA synthetases sit at the base, the serendipitous capture of protruding side chains (as substrate mimics) of other proteins may have been the first step in connecting tRNA synthetases with other cellular functions and pathways.
Supplementary Material
ACKNOWLEDGMENTS
We thank Professors Lawrence Shapiro and Barry Honig for reviewing the manuscript. This work was supported by grants CA92577 from the National Cancer Institute and GM 15539 from the National Institutes of Health, and by a fellowship from the National Foundation for Cancer Research.
Footnotes
AUTHOR CONTRIBUTIONS
Q.Z., M.K., M.G., R.B., L.A.N., P.S. and X.-L.Y. designed research; Q.Z., M.K., M.G, X.X, M.H., C.P., E.A. and M.-H.D. performed research; Q.Z., M.K., M.G, R.B., W.B.K, P.S. and X.-L.Y. analyzed data; Q.Z., M.K. M.G. P.S. and X.-L.Y. wrote the paper.
REFERENCES
- 1.Hausmann CD, Ibba M. Aminoacyl-tRNA synthetase complexes: molecular multitasking revealed. FEMS Microbiol Rev. 2008;32:705–721. doi: 10.1111/j.1574-6976.2008.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SG, Ewalt KL, Kim S. Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem Sci. 2005;30:569–574. doi: 10.1016/j.tibs.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Giege R. Toward a more complete view of tRNA biology. Nat Struct Mol Biol. 2008;15:1007–1014. doi: 10.1038/nsmb.1498. [DOI] [PubMed] [Google Scholar]
- 4.Paukstelis PJ, et al. Structure of a tyrosyl-tRNA synthetase splicing factor bound to a group I intron RNA. Nature. 2008;451:94–97. doi: 10.1038/nature06413. [DOI] [PubMed] [Google Scholar]
- 5.Rho SB, Lincecum TL, Jr, Martinis SA. An inserted region of leucyl-tRNA synthetase plays a critical role in group I intron splicing. EMBO J. 2002;21:6874–6881. doi: 10.1093/emboj/cdf671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia J, Arif A, Ray PS, Fox PL. WHEP domains direct noncanonical function of glutamyl-Prolyl tRNA synthetase in translational control of gene expression. Mol Cell. 2008;29:679–690. doi: 10.1016/j.molcel.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YN, Nechushtan H, Figov N, Razin E. The function of lysyl-tRNA synthetase and Ap4A as signaling regulators of MITF activity in FcepsilonRI-activated mast cells. Immunity. 2004;20:145–151. doi: 10.1016/s1074-7613(04)00020-2. [DOI] [PubMed] [Google Scholar]
- 8.Sampath P, et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell. 2004;119:195–208. doi: 10.1016/j.cell.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Kise Y, et al. A short peptide insertion crucial for angiostatic activity of human tryptophanyl-tRNA synthetase. Nat Struct Mol Biol. 2004;11:149–156. doi: 10.1038/nsmb722. [DOI] [PubMed] [Google Scholar]
- 10.Otani A, et al. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nature Medicine. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- 11.Otani A, et al. A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc Natl Acad Sci U S A. 2002;99:178–183. doi: 10.1073/pnas.012601899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science. 1999;284:147–151. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- 13.Wakasugi K, et al. Induction of angiogenesis by a fragment of human tyrosyl-tRNA synthetase. J Biol Chem. 2002;277:20124–20126. doi: 10.1074/jbc.C200126200. [DOI] [PubMed] [Google Scholar]
- 14.Wakasugi K, et al. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci U S A. 2002;99:173–177. doi: 10.1073/pnas.012602099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SG, et al. Human lysyl-tRNA synthetase is secreted to trigger proinflammatory response. Proc Natl Acad Sci U S A. 2005;102:6356–6361. doi: 10.1073/pnas.0500226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard OM, et al. Histidyl-tRNA synthetase and asparaginyl-tRNA synthetase, autoantigens in myositis, activate chemokine receptors on T lymphocytes and immature dendritic cells. J Exp Med. 2002;196:781–791. doi: 10.1084/jem.20020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko YG, et al. Glutamine-dependent antiapoptotic interaction of human glutaminyl-tRNA synthetase with apoptosis signal-regulating kinase 1. J Biol Chem. 2001;276:6030–6036. doi: 10.1074/jbc.M006189200. [DOI] [PubMed] [Google Scholar]
- 18.Javanbakht H, et al. The interaction between HIV-1 Gag and human lysyl-tRNA synthetase during viral assembly. J Biol Chem. 2003;278:27644–27651. doi: 10.1074/jbc.M301840200. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443:50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- 20.Nangle LA, et al. Charcot-Marie-Tooth disease-associated mutant tRNA synthetases linked to altered dimer interface and neurite distribution defect. Proc Natl Acad Sci U S A. 2007;104:11239–11244. doi: 10.1073/pnas.0705055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SG, Schimmel P, Kim S. Aminoacyl tRNA synthetases and their connections to disease. Proc Natl Acad Sci U S A. 2008;105:11043–11049. doi: 10.1073/pnas.0802862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seburn KL, et al. An active dominant mutation of glycyl-tRNA synthetase causes neuropathy in a Charcot-Marie-Tooth 2D mouse model. Neuron. 2006;51:715–726. doi: 10.1016/j.neuron.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 23.Yang XL, et al. Gain-of-function mutational activation of human tRNA synthetase procytokine. Chem Biol. 2007;14:1323–1333. doi: 10.1016/j.chembiol.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleckner J, et al. Differential regulation of the human, interferon inducible tryptophanyl-tRNA synthetase by various cytokines in cell lines. Cytokine. 1995;7:70–77. doi: 10.1006/cyto.1995.1009. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor M, et al. Evidence for annexin II-S100A10 complex and plasmin in mobilization of cytokine activity of human TrpRS. J Biol Chem. 2008;283:2070–2077. doi: 10.1074/jbc.M706028200. [DOI] [PubMed] [Google Scholar]
- 26.Tzima E, et al. VE-cadherin links tRNA synthetase cytokine to anti-angiogenic function. J Biol Chem. 2005;280:2405–2408. doi: 10.1074/jbc.C400431200. [DOI] [PubMed] [Google Scholar]
- 27.Tzima E, et al. Biologically active fragment of a human tRNA synthetase inhibits fluid shear stress-activated responses of endothelial cells. Proc Natl Acad Sci U S A. 2003;100:14903–14907. doi: 10.1073/pnas.2436330100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorrell MI, et al. Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc Natl Acad Sci U S A. 2007;104:967–972. doi: 10.1073/pnas.0607542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro L, et al. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 31.Patel SD, et al. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 2006;124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 32.May C, et al. Identification of a transiently exposed VE-cadherin epitope that allows for specific targeting of an antibody to the tumor neovasculature. Blood. 2005;105:4337–4344. doi: 10.1182/blood-2005-01-0010. [DOI] [PubMed] [Google Scholar]
- 33.Yang XL, et al. Functional and crystal structure analysis of active site adaptations of a potent anti-angiogenic human tRNA synthetase. Structure. 2007;15:793–805. doi: 10.1016/j.str.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XL, et al. Crystal structures that suggest late development of genetic code components for differentiating aromatic side chains. Proc Natl Acad Sci U S A. 2003;100:15376–15380. doi: 10.1073/pnas.2136794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Y, et al. Crystal structure of human tryptophanyl-tRNA synthetase catalytic fragment: insights into substrate recognition, tRNA binding, and angiogenesis activity. J Biol Chem. 2004;279:8378–8388. doi: 10.1074/jbc.M311284200. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Q, Kiosses WB, Liu J, Schimmel P. Tumor endothelial cell tube formation model for determining anti-angiogenic activity of a tRNA synthetase cytokine. Methods. 2008;44:190–195. doi: 10.1016/j.ymeth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 38.Laskowski RA, Moss DS, Thornton JM. Main-chain bond lengths and bond angles in protein structures. J Mol Biol. 1993;231:1049–1067. doi: 10.1006/jmbi.1993.1351. [DOI] [PubMed] [Google Scholar]
- 39.Winter MC, Shasby SS, Ries DR, Shasby DM. Histamine selectively interrupts VE-cadherin adhesion independently of capacitive calcium entry. Am J Physiol Lung Cell Mol Physiol. 2004;287:L816–L823. doi: 10.1152/ajplung.00056.2004. [DOI] [PubMed] [Google Scholar]
- 40.Wiseman T, Williston S, Brandts JF, Lin LN. Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem. 1989;179:131–137. doi: 10.1016/0003-2697(89)90213-3. [DOI] [PubMed] [Google Scholar]
- 41.Austin J, First EA. Potassium functionally replaces the second lysine of the KMSKS signature sequence in human tyrosyl-tRNA synthetase. J Biol Chem. 2002;277:20243–20248. doi: 10.1074/jbc.M201923200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.