Abstract
Background. Indoxyl sulphate (IS) and p-cresyl sulphate (PCS) are uraemic toxins that have similar protein binding, dialytic clearance and proinflammatory features. However, only a few prospective studies have evaluated possible associations between these two retained solutes and renal disease progression in chronic kidney disease (CKD) patients.
Methods. This prospective observational study evaluated independent associations between serum total IS and PCS with renal progression in a selected cohort of patients having different stages of CKD. Baseline PCS and IS were correlated with renal progression [defined as decrements in estimated glomerular filtration rate (eGFR) > 50% from baseline or progression to end-stage renal disease (ESRD)] and death during a follow-up period of 24 months.
Results. Of 268 patients, 35 (13.1%) had renal progression and 14 (5.2%) died after a mean follow-up of 21 ± 3 months. Univariate Cox regression analysis followed by multivariate analysis showed that high-serum PCS levels were associated with renal progression and all-cause mortality independent of age, gender, diabetes status, albumin levels, serum IS, serum creatinine, Ca × P product, intact parathyroid hormone, haemoglobin or high-sensitivity C-reactive protein level. Serum IS was only associated with renal progression; however, the predictive power of serum IS was weakened when serum PCS was also present in the analytical model.
Conclusions. In addition to traditional and uraemia-related risk factors such as renal function, serum IS and PCS levels may help in predicting the risk of renal progression in patients having different stages of CKD.
Keywords: chronic kidney disease, indoxyl sulphate, p-cresyl sulphate, protein-bound toxins, proximal tubule
Introduction
Despite a better understanding of disease mechanisms and improved control of important modifiable risk factors, declines in renal function are still inevitable in a substantial proportion of chronic kidney disease (CKD) patients. Traditional and uraemia-related risk factors are not sufficient in explaining renal outcomes in CKD patients.
p-Cresyl sulphate (PCS) and indoxyl sulphate (IS) are prototypic protein-bound uraemic toxin molecules. These two retained solutes are not only biomarkers for renal function but also actively participate in the development of diseases [1]. They share various similarities, including their production by gut bacteria [2], a strong albumin binding at Sudlow II site [3], significant renal metabolism, low dialytic clearance [4,5] and an emerging role in cardiovascular disease and mortality in renal patients [6,7]. The overloading of IS in CKD rats results in glomerular sclerosis and interstitial fibrosis [8] via aberrant genetic expression of TGF-β1, TIMP-1 and Pro-α1 collagen [9, 10], as well as complex redox alterations [11]. IS may also cause endothelial and vascular dysfunction by promoting vascular smooth muscle cell proliferation [12] through activation of platelet-derived growth factor receptors [12] and mitogen-activated protein kinase pathways [13]. Clinically, IS is associated with increased aortic calcification and vascular stiffness [7]. In contrast, possible deleterious effects of PCS on renal cells have been rarely studied. Previous studies revealed that p-cresol induces endothelial dysfunction [14] and decreases mRNA expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 [15]. However, it is now well known that p-cresol is an artifact that results from PCS sample preparation [16] and both p-cresol and PCS exert different behavioural patterns on the respiratory burst activity of leucocytes [17]. Exposure of human umbilical endothelial cells to PCS results in increased shedding of endothelial microparticles via a Rho kinase-dependent pathway [6]. A high total PCS level is associated with aortic calcification and mortality in both CKD [18] and haemodialysis patients [19,20]. However, despite the similarities of these two molecules, studies that compare in parallel the contribution of serum PCS and IS levels to renal progression in patients having different stages of CKD are currently lacking.
In the present study, we prospectively evaluated associations between both serum PCS and IS levels and renal progression and all-cause mortality in CKD patients.
Materials and methods
Patient selection and study population
Prevalent pre-dialysis CKD patients who attended an outpatient clinic in the Nephrology Department of Chang Gung Memorial Hospital at Keelung from November 2006 to October 2007 were recruited into the study. The inclusion criteria were adults aged > 18 but < 80 years old and showed no spontaneous improvement or progression of renal disease in the past 3 months. Patients were excluded from the study if they had cardiovascular disease (coronary artery disease, myocardial ischaemia, cerebrovascular disease or peripheral artery disease) in the past 3 months, infections requiring admission in the past 3 months, uncontrolled hypertension, serum albumin level < 2.5 mg/dL or unwillingness to participate in the trial. CKD was defined as having a persistent proteinuria or a decreased estimated glomerular filtration rate (eGFR) < 90 mL/min per 1.73 m2 (determined by abbreviated Modification of Diet in Renal Disease equation) in two separate measurements within an interval of 3 months. In accordance with the NKF/DOQI classification system, these patients were classified into stages I, II, III, IV or V for descriptive purposes. A total of 268 patients were enrolled into the study and gave their informed written consent. This study was in adherence with the Declaration of Helsinki and was approved by the Ethics Committee of the Institutional Review Board at Chang Gung Memorial Hospital.
Study design
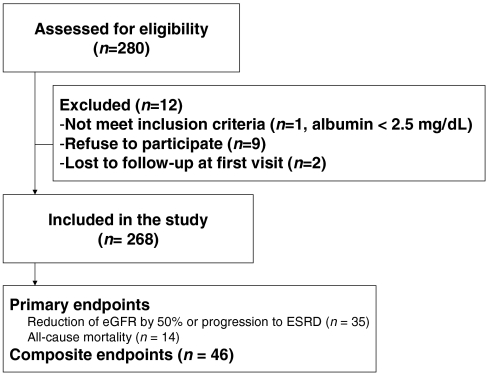
All eligible patients were carefully interviewed to identify medical disease and concomitant medications. Twelve-hour fasting blood samples were obtained for determination of serum levels of total PCS, IS, and for standard laboratory parameters. Medical visits and renal function measurements were followed-up prospectively at 3-month, 6-month, 12-month and 24-month intervals, until commencement of dialysis therapy or death. All eligible patients were followed-up until 15 April 2010 for recording of renal progression [defined as reduction of eGFR by 50% or end-stage renal disease (ESRD) requiring dialysis] or death. Composite endpoints of renal progression and/or death were also evaluated (Figure 1). Diabetes mellitus (DM) was defined as a fasting glucose level ≥ 126 mg/dL or use of any hypoglycaemic medication. Hypertension was considered present if the patient received medical therapy for such a condition or if blood pressure was > 140/90 mm Hg.
Fig. 1.
Flow chart indicates patient enrollment.
Baseline measurements
Serum samples were deproteinized by addition of 3 parts methanol to 1 part serum for determination of total IS and PCS. All analyses were performed on a Waters Acquity Ultra Performance Liquid Chromatography (UPLC) system (Milford, MA, USA), including binary solvent manager, sampler manager, column compartment and photo diode array detector, connected with Waters Empower 2 software. IS and PCS were detected at 280 and 260 nm. Buffer flow was 0.4 mL/min using 10 mM NH4H2PO4 (pH = 4.0) (A) and 100% acetonitrile (B) with a gradient from 82.5%A/17.5%B to 55%A/45%B, over 9 min. Under these conditions, IS and PCS appeared at 1.4 and 1.7 min, respectively [21]. The limits of detection of this assay were 0.225 mg/L for IS and 1 mg/L for PCS. Calibration curves were constructed by plotting the peak areas versus the concentrations of each analysate and had average r2 values of 0.999 ± 0.001. Quantitative results were obtained and calculated as concentrations (mg/L). Intra- and inter-assay coefficients of variation relative standard deviation were 0.4 and 0.05% for IS and 5.50 and 7.48% for PCS, respectively. We spiked different concentrations of IS, PC and PCS in serum of healthy individuals (n = 5). The recovery rate was calculated as [(final concentration − initial concentration) / added concentration]. Recoveries were 100.99 and 108.73% for IS and PCS, respectively. Furthermore, parallel comparisons of serum total PCS and IS levels obtained from UPLC and mass spectrometry (MS) in 10 random selected patients did not reveal significant disagreement from Bland–Altman plots (for serum IS, Pitman’s test of difference in variance showed r = − 0.263, P = 0.493 and for serum PCS, r = − 0.765, P = 0.124).
In addition to the demographic and clinical data, calcium (Ca), phosphate (P), intact parathyroid hormone (iPTH), total cholesterol, haemoglobin, high-sensitivity C-reactive protein (hs-CRP), uric acid and albumin were also measured at baseline. Serum creatinine (sCr) was assessed at the above-mentioned time points by spectrophotometric analysis using a modified kinetic Jaffe reaction.
Statistical methods
Descriptive statistics are expressed as means ± standard deviation, median, range or percentage frequency, as appropriate. All variables were tested for normal distribution by the Kolmogorov–Smirnov test. Student’s t-tests or Mann–Whitney U-tests were applied to compare means of continuous variables. Categorical data were tested using the Chi-square test. Pearson or Spearman correlation coefficients were used to test correlations between PCS and IS with other variables. Data were log-transformed to approximate normal distributions. Variance Inflation Factor (VIF) calculation was performed to address the issue of collinearity by using principle component analysis. Kaplan–Meier curves were performed to assess renal and overall survival in patients with serum PCS and IS levels above and below the median. Adjusted risk estimates for endpoints were calculated using univariate, followed by multivariate, Cox proportional hazard regression analysis. Hierarchical selection procedures were employed to construct a powerful covariate set for adjustment in the subsequent major hypothesized variables, such as PCS and IS. The inclusion criteria for model selection in a covariate set were predetermined as P < 0.2 according to major statistical packages. The set of covariates with P-values < 0.2 for predicting renal progression included albumin, Ca × P product, eGFR, DM and gender. For analysis of all-cause mortality, the hierarchical selected covariates included age, albumin, haemoglobin and hs-CRP. The assumption of proportionality was checked graphically using the complementary log–log plot and was found to be acceptable for the risk factors of interest. Bland–Altman plots were used to test agreement between UPLC and MS. All statistical tests were two-tailed, and a P-value of < 0.05 was considered statistically significant. Data were analysed using the SPSS 13.0 software for Windows XP (SPSS Inc., Chicago, IL).
Results
Baseline characteristics of study population
Table 1 shows the baseline characteristics of the study population. Serum total PCS levels were significantly higher compared with those of the healthy controls (7.16 [< 1.0–42.06] vs 1.93 [1–3.8] mg/L, P < 0.001), as were serum total IS levels (4.63 [< 0.225–53.58] vs 0.88 [0.59–1.26] mg/L, P < 0.001). Of all patients, 35 (13.1%) had renal progression and 14 (5.2%) patients died (7 patients from cardiovascular cause, 6 from infection and 1 from liver cirrhosis) during a mean follow-up of 21 ± 3 months. Table 2 shows correlations between serum levels of PCS and IS with eGFR and other important risk factors of renal progression.
Table 1.
Baseline characteristics of all patients
| All patients | Progressor | Non-progressor | P-value | |
|---|---|---|---|---|
| n = 268 | n = 35 | n = 233 | ||
| Age, years | 66.9 ± 12 | 67 ± 14 | 66.8 ± 12 | 0.929a |
| Male, n, % | 154 (57.5%) | 13 (37.1%) | 141 (60.5%) | 0.009b |
| BMI, kg/m2 | 25.8 ± 3.5 | 26.9 ± 5.5 | 25.7 ± 3.1 | 0.200a |
| Diabetes, n, % | 126 (47%) | 24 (68.6%) | 102 (43.8%) | 0.006b |
| SBP, mm Hg | 135 ± 15 | 142 ± 18 | 138 ± 14 | 0.093a |
| DBP, mm Hg | 71 ± 8 | 72 ± 10 | 72 ± 7 | 0.709a |
| eGFR, mL/min/1.73 m2 | 44.8 ± 32 | 25.8 ± 22 | 47.58 ± 32 | < 0.001a |
| Initial CKD stage | < 0.001b | |||
| I, n, % | 25 (9.3%) | 2 (5.8%) | 23 (9.9%) | |
| II, n, % | 42 (15.7%) | 1 (2.8%) | 41 (17.6%) | |
| IIIa, n, % | 37 (13.8%) | 1 (2.8%) | 36 (15.5%) | |
| IIIb, n, % | 54 (20.1%) | 5 (14.3%) | 49 (21%) | |
| IV, n, % | 76 (28.4%) | 9 (25.7%) | 67 (28.7%) | |
| V, n, % | 34 (12.7%) | 17 (48.6%) | 17 (7.3%) | |
| sCr, mg/dL | 1.9 ± 1.4 | 3.6 ± 2.3 | 1.7 ± 1.0 | < 0.001a |
| Ca, mg/dL | 9.2 ± 0.5 | 8.8 ± 0.8 | 9.3 ± 0.4 | < 0.001a |
| P, mg/dL | 3.8 ± 0.9 | 4.7 ± 1.4 | 3.8 ± 0.6 | < 0.001a |
| Ca × P, mg2/dL2 | 35.5 ± 7.1 | 40.8 ± 10.5 | 34.8 ± 6.1 | < 0.001a |
| iPTH, pmol/L | 89.9 (1–692) | 144 (25.8–333) | 52.3 (1–692) | < 0.001c |
| Cholesterol, mg/dL | 193 ± 60 | 211 ± 87 | 191 ± 56 | 0.069a |
| haemoglobin, g/dL | 12.6 ± 2.1 | 11.1 ± 2.0 | 12.8 ± 2.0 | < 0.001a |
| hs-CRP, mg/L | 3.2 (0.2–48.4) | 2.1 (0.4–19.6) | 1.63 (0.2–48.4) | 0.564 |
| Uric acid, mg/dL | 6.9 ± 1.8 | 7.8 ± 1.8 | 6.8 ± 1.8 | 0.002a |
| Albumin, g/dL | 3.9 ± 0.4 | 3.6 ± 0.5 | 4.0 ± 0.4 | < 0.001a |
| Microalbumin, mg/day | 61.4 (2–16 900) | 173 (2.5–3159) | 41 (2–16 900) | 0.07 |
| Total PCS, mg/L | 7.16 (< 1–42.06) | 10.26 (1.69–36.24) | 3.97 (< 1–42.06) | < 0.001c |
| Total IS, mg/L | 4.63 (< 0.225–53.58) | 7.62 (< 0.225–53.58) | 1.94 (0.29–39.09) | < 0.001c |
Abbreviation: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; sCr, serum creatinine; Ca, calcium; P, phosphate; iPTH, intact parathyroid hormone; hs-CRP, high-sensitivity C-reactive protein; PCS, p-cresyl sulphate; IS, indoxyl sulphate.
Student’s t-test P-value, progressor vs non-progressor.
Chi-square test P-value, progressor vs non-progressor.
Mann–Whiney U-test P-value, progressor vs non-progressor.
Table 2.
Correlations between log-transformed serum total PCS, IS and selected risk factors
| Log PCS |
Log IS |
|||
|---|---|---|---|---|
| r | P-value | r | P-value | |
| − log-eGFR | 0.642 | < 0.001 | 0.720 | < 0.001 |
| Potassium | 0.269 | < 0.001 | 0.194 | < 0.001 |
| Ca × P | 0.233 | < 0.001 | 0.184 | < 0.001 |
| Haemoglobin | − 0.513 | < 0.001 | − 0.546 | < 0.001 |
| Albumin | − 0.317 | < 0.001 | − 0.394 | < 0.001 |
| Log IS | 0.655 | < 0.001 | – | – |
Abbreviation: eGFR, estimated glomerular filtration rate; Ca, calcium; P, phosphate; PCS, p-cresyl sulphate; IS, indoxyl sulphate.
Serum PCS/IS and stage of CKD
Baseline serum PCS and IS levels were significantly higher in patients who had renal progression during follow-up compared with non-progressors [serum PCS levels were 10.26 (1.69–36.24) mg/L in progressor patients and 3.97 (< 1–42.06) mg/L in non-progressor patients, P < 0.001; serum IS level, 7.62 (< 0.225–53.58) mg/L vs 1.94 (0.29–39.09) mg/L, P < 0.001, respectively].
Table 3 summarizes the hazard ratios (HR) for renal progression and all-cause mortality in the entire study population and in patient subsets divided according to baseline eGFR level as function of serum PCS and IS levels. Higher serum PCS levels were significantly associated with renal progression [HR, 1.092; 95% confidence interval (CI), 1.060–1.126; P < 0.001] and all-cause mortality (HR, 1.099; 95% CI, 1.053–1.148; P < 0.001) in all patients. A higher serum IS level was associated with renal progression (HR, 1.063; 95% CI, 1.041–1.085; P < 0.001) but not with all-cause mortality. In the subset analysis of patients with different baseline renal functions, these associations remained significant in patients with eGFR < 45 mL/min. However, there was no association between either serum PCS or IS with the risk of renal progression or all-cause mortality in patients with eGFR > 45 mL/min.
Table 3.
Univariate Cox proportional hazard regression analysis in subsets of patients according to eGFR level
| Variables | All patients |
eGFR < 45 mL/min |
eGFR > 45 mL/min |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| For renal progression | n = 35/268 | n = 31/164 | n = 4/104 | |||
| Serum total IS, mg/L | 1.063 (1.041–1.085) | < 0.001 | 1.051 (1.027–1.075) | < 0.001 | 0.553 (0.09–3.405) | 0.523 |
| Serum total PCS, mg/L | 1.092 (1.060–1.126) | < 0.001 | 1.074 (1.038–1.111) | < 0.001 | 1.049 (0.821–1.340) | 0.702 |
| For all-cause mortality | n = 14/164 | n = 11/164 | n = 3/104 | |||
| Serum total IS, mg/L | 1.014 (0.956–1.075) | 0.647 | 1 (0.934–1.071) | 0.993 | 0.248 (0.015–4.2) | 0.334 |
| Serum total PCS, mg/L | 1.099 (1.053–1.148) | < 0.001 | 1.104 (1.049–1.160) | < 0.001 | 0.883 (0.468–1.668) | 0.702 |
Abbreviation: PCS, p-cresyl sulphate; IS, indoxyl sulphate.
Serum PCS/IS and progression of CKD
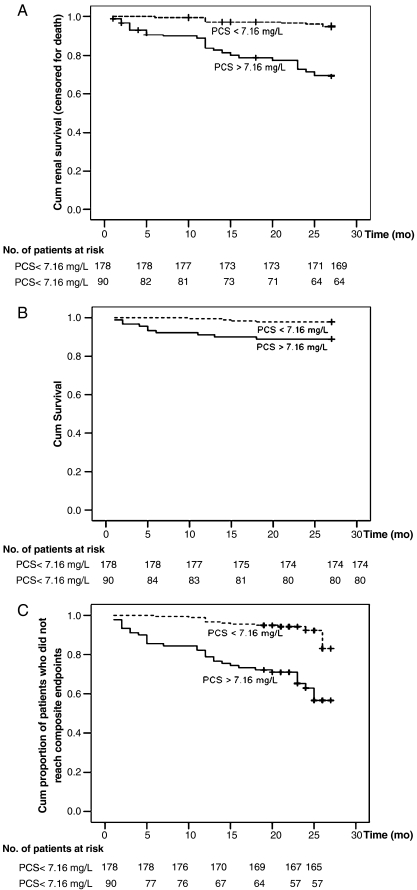
A crude analysis found that a serum total PCS level > 7.16 mg/L (the median) and a serum total IS level > 4.63 mg/L (the median) were associated with renal progression (log-rank P < 0.001; Figures 2A and 3A). Univariate analysis (Table 4) identified that higher serum total IS (HR, 1.063; 95% CI, 1.041–1.085; P < 0.001) and PCS (HR, 1.092; 95% CI, 1.060–1.126; P < 0.001) levels were significantly associated with progression of CKD. Other significant risk factors included presence of DM (HR, 2.618; 95% CI, 1.282–5.344; P = 0.008), eGFR (HR, 0.96; 95% CI, 0.94–0.981; P < 0.001), calcium (HR, 0.183; 95% CI, 0.110–0.306; P < 0.001), phosphate (HR, 2.899; 95% CI, 2.136–3.934; P < 0.001), Ca/P product (HR, 1.109; 95% CI, 1.067–1.154; P < 0.001), iPTH (HR, 1.003; 95% CI, 1.001–1.005; P < 0.001), haemoglobin (HR, 0.678; 95% CI, 0.572–0.802; P < 0.001), uric acid (HR, 1.255; 95% CI, 1.094–1.493; P < 0.001) and albumin (HR, 0.236; 95% CI, 0.141–0.392; P < 0.001). Multivariate Cox regression analyses were constructed with different adjustments for important risk factors for CKD progression (Table 5). Serum PCS, analysed as a continuous variable, was independently associated with CKD progression after adjustment for patient demographic characteristics (age, gender and DM, model 1). The predictive role of serum PCS remained independently significant after adjustment for its binding protein (albumin, model 2), baseline renal function (eGFR, model 3), IS (model 4a) and other common risk factors for CKD progression (Ca × P product, iPTH, haemoglobin and hs-CRP, model 5). To overcome the possible effects of residual confounding between individual cofactors of various models, a final model of adjustment with a set of covariates (albumin, Ca × P product, eGFR, DM and gender) predetermined by hierarchical selection procedure was used. The high-serum total PCS (HR, 1.036; 95% CI, 1.003–1.083; P = 0.037) was an independent risk factor for renal progression after adjustment of these hierarchical selected covariates (model 6).
Fig. 2.
Kaplan–Meier survival curves in all patients according to serum PCS level (above and below the median of 7.16 mg/L); (A) cumulative renal survival (censored for death), log-rank, P < 0.001; (B) cumulative survival, log-rank, P = 0.002; (C) cumulative proportion of patients who did not reach composite endpoints, log-rank, P < 0.001.
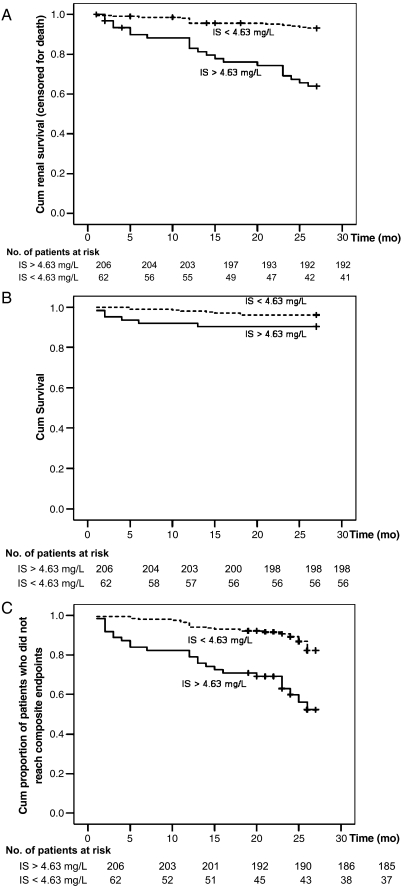
Fig. 3.
Kaplan–Meier survival curves in all patients according to serum IS level (above and below the median of 4.63 mg/L); (A) cumulative renal survival (censored for death), log-rank, P < 0.001; (B) cumulative survival, log-rank, P = 0.062; (C) cumulative proportion of patients who did not reach composite endpoints, log-rank, P < 0.001.
Table 4.
Unadjusted HR for different endpoints
| Baseline variable | Units of increase | Renal progression
(event/total = 35/268) |
All-cause mortality
(event/total = 14/268) |
Composite endpoints
(event/total = 46/268) |
|||
|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | P | Unadjusted HR (95% CI) | P | Unadjusted HR (95% CI) | P | ||
| Age, years | 1 year | 1.003 (0.976–1.031) | 0.828 | 1.102 (1.036–1.173) | 0.002 | 1.018 (0.992–1.044) | 0.184 |
| Male (vs female) | – | 0.439 (0.221–0.872) | 0.019 | 1.369 (0.459–4.086) | 0.573 | 0.560 (0.312–1.003) | 0.051 |
| Diabetes (yes vs no) | – | 2.618 (1.282–5.344) | 0.008 | 1.128 (0.396–3.216) | 0.822 | 1.792 (0.991–3.240) | 0.054 |
| eGFR, mL/min/1.73 m2 | 1 mL/min/1.73 m2 | 0.96 (0.940–0.981) | < 0.001 | 0.98 (0.956–1.004) | 0.101 | 0.971 (0.955–0.987) | < 0.001 |
| Ca, mg/dL | 1 mg/dL | 0.183 (0.110–0.306) | < 0.001 | 0.564 (0.256–1.240) | 0.154 | 0.239 (0.154–0.373) | < 0.001 |
| P, mg/dL | 1 mg/dL | 2.899 (2.136–3.934) | < 0.001 | 1.132 (0.661–1.939) | 0.651 | 2.211 (1.704–2.870) | < 0.001 |
| Ca × P, mg2/dL2 | 1 mg2/dL2 | 1.109 (1.067–1.154) | < 0.001 | 1.002 (0.931–1.078) | 0.96 | 1.076 (1.037–1.116) | < 0.001 |
| iPTH, pmol/L | 1 pmol/L | 1.003 (1.001–1.005) | 0.001 | 1.001 (0.997–1.006) | 0.527 | 1.003 (1.001–1.005) | 0.008 |
| haemoglobin, g/dL | 1 g/dL | 0.678 (0.572–0.802) | < 0.001 | 0.70 (0.538–0.910) | 0.008 | 0.687 (0.593–0.797) | < 0.001 |
| Uric acid, mg/dL | 1 mg/dL | 1.255 (1.094–1.439) | 0.001 | 1.194 (0.942–1.514) | 0.143 | 1.188 (1.042–1.354) | 0.01 |
| Albumin, g/dL | 1 g/dL | 0.236 (0.141–0.392) | < 0.001 | 0.277 (0.118–0.665) | 0.003 | 0.270 (0.173–0.420) | < 0.001 |
| Total IS, mg/L | 1 mg/L | 1.063 (1.041–1.085) | < 0.001 | 1.014 (0.956–1.075) | 0.647 | 1.050 (1.028–1.071) | < 0.001 |
| Total PCS, mg/L | 1 mg/L | 1.092 (1.060–1.126) | < 0.001 | 1.099 (1.053–1.148) | < 0.001 | 1.090 (1.062–1.118) | < 0.001 |
Abbreviation: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; Ca, calcium; P, phosphate; iPTH, intact parathyroid hormone; PCS, p-cresyl sulphate; IS, indoxyl sulphate.
Table 5.
Multivariate Cox regression analysis for primary and composite endpoints
| Models | Renal progression |
All-cause mortality |
Composite endpoints |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Serum p-cresyl sulphate—continuous variable | |||||||||
| Unadjusted | 1.092 | 1.060–1.126 | < 0.001 | 1.099 | 1.053–1.148 | < 0.001 | 1.09 | 1.062–1.118 | < 0.001 |
| Model 1 | 1.086 | 1.52–1.121 | < 0.001 | 1.134 | 1.076–1.196 | < 0.001 | 1.089 | 1.059–1.119 | < 0.001 |
| Model 2 | 1.076 | 1.042–1.110 | < 0.001 | 1.083 | 1.033–1.136 | 0.001 | 1.075 | 1.047–1.104 | < 0.001 |
| Model 3 | 1.061 | 1.020–1.103 | 0.003 | 1.101 | 1.044–1.162 | < 0.001 | 1.074 | 1.040–1.109 | < 0.001 |
| Model 4a | 1.066 | 1.016–1.119 | 0.009 | 1.162 | 1.099–1.229 | < 0.001 | 1.094 | 1.053–1.137 | < 0.001 |
| Model 5 | 1.057 | 1.019–1.098 | 0.003 | 1.119 | 1.058–1.184 | < 0.001 | 1.075 | 1.040–1.111 | < 0.001 |
| Model 6 | 1.042 | 1.003–1.083 | 0.037 | – | – | – | 1.061 | 1.027–1.095 | < 0.001 |
| Model 7 | – | – | – | 1.136 | 1.069–1.207 | < 0.001 | 1.083 | 0.952–1.066 | < 0.001 |
| Serum indoxyl sulphate—continuous variable | |||||||||
| Unadjusted | 1.063 | 1.041–1.085 | < 0.001 | 1.014 | 0.956–1.075 | 0.647 | 1.05 | 1.028–1.071 | < 0.001 |
| Model 1 | 1.058 | 1.035–1.081 | < 0.001 | 1.022 | 0.954–1.094 | 0.536 | 1.048 | 1.025–1.071 | < 0.001 |
| Model 2 | 1.06 | 1.037–1.085 | < 0.001 | 0.997 | 0.929–1.070 | 0.932 | 1.045 | 1.022–1.069 | < 0.001 |
| Model 3 | 1.04 | 1.012–1.068 | 0.004 | 0.981 | 0.904–1.065 | 0.651 | 1.03 | 1.004–1.057 | 0.026 |
| Model 4b | 1.025 | 0.988–1.062 | 0.188 | 0.903 | 0.812–1.004 | 0.059 | 0.995 | 0.964–1.028 | 0.769 |
| Model 5 | 1.034 | 1.004–1.064 | 0.028 | 0.97 | 0.876–1.074 | 0.558 | 1.025 | 0.995–1.056 | 0.104 |
| Model 6 | 1.033 | 1.004–1.064 | 0.027 | – | – | – | 1.023 | 0.994–1.053 | 0.119 |
| Model 7 | – | – | – | 0.984 | 0.895–1.083 | 0.748 | 1.007 | 0.983–1.032 | 0.576 |
Model 1 was adjusted for age (1-year increment), male gender and diabetes status.
Model 2 was adjusted for serum albumin (1 g/L increments).
Model 3 was adjusted for eGFR (1 mL/min increments).
Model 4a was adjusted for indoxyl sulphate (1 mg/L increments).
Model 4b was adjusted for p-cresyl sulphate (1 mg/L increments).
Model 5 was adjusted for Ca × P product (1 mg2/dL2 increments), intact parathyroid hormone (log 1 pmol/L increments), haemoglobin (1 g/dL increments) and hs-CRP (log 1 mg/L increments).
Model 6 was adjusted by hierarchically selected covariates of albumin, Ca × P product, eGFR, diabetes status and gender.
Model 7 was adjusted for hierarchically selected covariates of age, hs-CRP, albumin and haemoglobin.
The analysis of serum IS (as continuous variable) resulted in significant association with CKD progression in the above-mentioned models (model 1, 2, 3, 5 and 6), except for the adjustment of serum PCS (model 4b, Table 5).
Collinearity was checked by using principle component analysis with the variables: Ca × P, log iPTH, haemoglobin, log hs-CRP, log PCS and log IS. The multiple correlation coefficient was 0.6 with these selected variables in the model. According to VIF calculation, the value was found to be 2.5 less than a preset critical point for a potential collinearity problem (VIF > 10). A further examination of our presented models showed that many of the independent variables were chosen from some of these six variables, which showed no collinearity problem after the analyses. Therefore, there was no significant impact of collinearity phenomena on the instability of the regression model.
Serum PCS/IS and all-cause mortality
Baseline serum PCS and IS levels were also significantly increased in patients that died [serum PCS levels were 12.07 (< 1–42.06) mg/L in deaths and 4.1 (< 1–36.24) mg/L in survivors, P = 0.002; serum IS levels, 4.78 (0.7–12.54) mg/L vs 2.07 (< 0.225–53.58), P = 0.05, respectively]. Univariate analysis showed that higher serum total PCS (HR, 1.099; 95% CI, 1.053–1.148; P < 0.001), age (HR, 1.102; 95% CI, 1.036–1.173; P = 0.002), haemoglobin (HR, 0.7; 95% CI, 0.538–0.910; P = 0.008) and albumin (HR, 0.277; 95% CI, 0.118–0.665; P = 0.003) were significantly associated with all-cause mortality in CKD patients. The serum total IS level was not associated with all-cause mortality. Serum PCS, analysed as a continuous variable, remained independently associated with all-cause mortality in multivariate Cox regression analysis with different adjustment models (Table 5, models 1–5 and 7). Figures 2 and 3 show Kaplan–Meier estimates of all-cause mortality as a function of total PCS and IS levels relative to the median.
Discussion
In the present study, we evaluated associations between total PCS and IS with renal progression and all-cause mortality in patients having different stages of CKD. We found that serum total PCS was associated with renal progression and that this was independent of baseline renal function and other modifiable and non-modifiable risk factors, such as age, diabetes, calcification, anaemia, malnutrition-inflammation and IS. Serum total IS was associated with renal progression; however, this association was lost when serum PCS was present in the analytical model.
Renal disease progression constitutes a troublesome dilemma in clinical practice. Despite proper control of ‘classical’ and uraemia-related risk factors, a deterioration of renal function is still inevitable in a substantial proportion of patients. The impact of known risk factors has not been adequate for prediction of renal progression. Our study demonstrated for the first time that both PCS and IS may not be only markers for renal function but may also predict disease progression. Baseline renal function and proteinuria are important predictors of subsequent renal progression in both diabetic and non-diabetic CKD patients [22,23]. In the present study, we prospectively followed-up patients having different stages of CKD and included a diversity of common measurable risk factors. Our findings suggest that serum IS and PCS levels are novel predictors of renal progression and that they may provide additional information beyond baseline renal function as well as other traditional and uraemia-related predictors.
Despite a significant association between high-serum PCS and renal progression, the mechanisms of how it promotes disease remain to be elucidated. In vitro, PCS significantly increased the percentage of leucocytes displaying oxidative burst activity at baseline [17]. PCS also induces a dose-dependent shedding of endothelial microparticles in the absence of overt endothelial damage [6]. These findings indicate that PCS exerts a proinflammatory effect and can alter endothelial function. Although the relationship between PCS and cardiovascular disease and mortality has been evaluated in previous investigations [18,20,24], there is no clinical evidence pointing to an association between PCS and renal progression. Further in vivo or in vitro investigations demonstrating an active role of PCS in stimulating renal disease progression are eagerly awaited.
Detrimental effects of IS on renal progression have been extensively evaluated in experimental and in vivo studies [8,25]. The present longitudinal study confirmed an association between serum IS and renal progression in CKD patients. However, the predictive power of IS was reduced at high-serum PCS levels. Serum PCS and IS are competitive binding inhibitors for the same albumin binding site (Sudlow site II) [3]. It is unknown whether high-serum levels of PCS and IS also behave as competitive inhibitors at the cellular level. Our findings offer new insights into the different pathogenic mechanisms that link PCS and IS with the genesis of renal disease progression. Further experimental models capable of clarifying the biological role of PCS (in conjunction with IS) should be constructed to confirm our findings.
Our finding of a significant association between serum PCS and all-cause mortality was similar to that of earlier studies [18,19,26]. Barreto et al. [7] demonstrated that high-serum IS was associated with vascular disease and mortality in CKD patients. However, this later association was not observed in our patients. We speculate that the number of deaths in our study was not sufficient to form a firm conclusion about mortality.
Both the temporal relationship between serum IS/PCS and renal progression and the prospective design of our study suggest that this association is causal. However, causality cannot be inferred from these data for a number of reasons. Although our small scale study suggested a role for serum PCS and IS in CKD progression, there were limitations of generalizability which included different ethnic groups, observation times, single-centre experiences and unavailability of the free form of toxins. Associations between free solute concentrations and mortality [19] and cardiovascular disease [20] have been well established in haemodialysis patients but these are less clear in CKD patients who have not yet started dialysis. Recently, Liabeuf et al. [18] demonstrated that free PCS is a predictor of mortality in patients at different stages of CKD. However, 65.5% of their patients were in stage 4, 5 or 5D, and one-third of the study population were on dialysis. Previous investigations showed that unconjugated p-cresol is not detectable in normal or pre-dialysis CKD human plasma, and almost 99% of circulating toxins are in their sulphated form [16], which is the main culprit of tissue damage [17,18]. Our colleagues recently revealed that the free forms of IS and PCS represent a small fraction (~ 10%) of the total forms found in the blood of peritoneal dialysis patients. In addition, residual kidney function significantly affects the levels of free and total IS [21]. Since all of our participants were pre-dialysis CKD patients with a mean eGFR of 44.8 ± 32 mL/min, the free forms of IS and PCS were below our detection limit in a large proportion of patients. In spite of good agreement between HPLC and MS as demonstrated by Meijers et al. [24] and by our study, it is still unknown whether MS had a greater ability to detect the free forms in our patients.
Several small interventional studies have demonstrated that AST-120, an orally ingested charcoal adsorbent, reduces IS levels [27], slows renal progression [28,29] and delays the initiation of dialysis [30]. Recently, a multi-centre randomized controlled trial having a follow-up time of 1-year found that administration of AST-120 did not delay the occurrence of serious clinical events, such as the doubling of sCr levels, increases in sCr levels > 6.0 mg/dL, the need for dialysis or transplantation or death [31]. However, AST-120 slowed the decrease in estimated creatinine clearance during the 1-year trial period. An effect of AST-120 on slowing renal disease progression remains to be demonstrated.
In conclusion, serum IS and PCS levels may help in predicting the risk of renal progression in patients having different stages of CKD beyond traditional and uraemia-related risk factors including renal function. Additional studies are warranted to elucidate the mechanisms of how IS and PCS affect renal progression and to further develop therapeutic strategies aimed at lowering protein-bound toxins.
Acknowledgments
We thank Conmed Pharmaceutical and Bio-Medical Corporation and Prevision Medical Corporation for excellent technical assistance. Chang Gung Memorial Hospital at Keelung provided grant support for this research (CMRPG260621).
Conflict of interest statement. None declared.
(See related article by Meijers and Evenepoel. The gut–kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol Dial Transplant 2011; 26: 759–761.)
References
- 1.Raff AC, Meyer TW, Hostetter TH. New insights into uremic toxicity. Curr Opin Nephrol Hypertens. 2008;17:560–565. doi: 10.1097/MNH.0b013e32830f45b6. [DOI] [PubMed] [Google Scholar]
- 2.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316–1325. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 3.Meijers BK, De Loor H, Bammens B, et al. p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1932–1938. doi: 10.2215/CJN.02940509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanholder R, Meert N, Schepers E, et al. Review on uraemic solutes II–variability in reported concentrations: causes and consequences. Nephrol Dial Transplant. 2007;22:3115–3121. doi: 10.1093/ndt/gfm151. [DOI] [PubMed] [Google Scholar]
- 5.Martinez AW, Recht NS, Hostetter TH, et al. Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol. 2005;16:3430–3436. doi: 10.1681/ASN.2005030310. [DOI] [PubMed] [Google Scholar]
- 6.Meijers BK, Van Kerckhoven S, Verbeke K, et al. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis. 2009;54:891–901. doi: 10.1053/j.ajkd.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;10:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. J Lab Clin Med. 1994;124:96–104. [PubMed] [Google Scholar]
- 9.Miyazaki T, Ise M, Seo H, et al. Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1 collagen in uremic rat kidneys. Kidney Int. 1997;52:S15–S22. [PubMed] [Google Scholar]
- 10.Niwa T, Nomura T, Sugiyama S, et al. The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int. 1997;52:S23–S28. [PubMed] [Google Scholar]
- 11.Gelasco AK, Raymond JR. Indoxyl sulfate induces complex redox alterations in mesangial cells. Am J Physiol Renal Physiol. 2006;290:F1551–F1558. doi: 10.1152/ajprenal.00281.2004. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu H, Hirose Y, Nishijima F, et al. ROS and PDGF-beta [corrected] receptors are critically involved in indoxyl sulfate actions that promote vascular smooth muscle cell proliferation and migration. Am J Physiol Cell Physiol. 2009;297:C389–C396. doi: 10.1152/ajpcell.00206.2009. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto H, Tsuruoka S, Ioka T, et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006;69:1780–1785. doi: 10.1038/sj.ki.5000340. [DOI] [PubMed] [Google Scholar]
- 14.Dou L, Bertrand E, Cerini C, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 15.Dou L, Cerini C, Brunet P, et al. P-cresol, a uremic toxin, decreases endothelial cell response to inflammatory cytokines. Kidney Int. 2002;62:1999–2009. doi: 10.1046/j.1523-1755.2002.t01-1-00651.x. [DOI] [PubMed] [Google Scholar]
- 16.de Loor H, Bammens B, Evenepoel P, et al. Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin Chem. 2005;51:1535–1538. doi: 10.1373/clinchem.2005.050781. [DOI] [PubMed] [Google Scholar]
- 17.Schepers E, Meert N, Glorieux G, et al. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol Dial Transplant. 2007;22:592–596. doi: 10.1093/ndt/gfl584. [DOI] [PubMed] [Google Scholar]
- 18.Liabeuf S, Barreto DV, Barreto FC, et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant. 2009;25:1183–1191. doi: 10.1093/ndt/gfp592. [DOI] [PubMed] [Google Scholar]
- 19.Bammens B, Evenepoel P, Keuleers H, et al. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006;69:1081–1087. doi: 10.1038/sj.ki.5000115. [DOI] [PubMed] [Google Scholar]
- 20.Meijers BK, Bammens B, De Moor B, et al. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008;73:1174–1180. doi: 10.1038/ki.2008.31. [DOI] [PubMed] [Google Scholar]
- 21.Lee CT, Kuo CC, Chen YM, et al. Factors associated with blood concentrations of indoxyl sulfate and p-cresol in patients undergoing peritoneal dialysis. Perit Dial Int. 2010;30:456–463. doi: 10.3747/pdi.2009.00092. [DOI] [PubMed] [Google Scholar]
- 22.Kent DM, Jafar TH, Hayward RA, et al. Progression risk, urinary protein excretion, and treatment effects of angiotensin-converting enzyme inhibitors in nondiabetic kidney disease. J Am Soc Nephrol. 2007;18:1959–1965. doi: 10.1681/ASN.2006101081. [DOI] [PubMed] [Google Scholar]
- 23.Keane WF, Zhang Z, Lyle PA, et al. Risk scores for predicting outcomes in patients with type 2 diabetes and nephropathy: the RENAAL study. Clin J Am Soc Nephrol. 2006;1:761–767. doi: 10.2215/CJN.01381005. [DOI] [PubMed] [Google Scholar]
- 24.Meijers BKI, Claes K, Bammens B, et al. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol. 2010;5:1182–1189. doi: 10.2215/CJN.07971109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owada S, Goto S, Bannai K, et al. Indoxyl sulfate reduces superoxide scavenging activity in the kidneys of normal and uremic rats. Am J Nephrol. 2008;28:446–454. doi: 10.1159/000112823. [DOI] [PubMed] [Google Scholar]
- 26.Lin CJ, Wu CJ, Pan CF, et al. Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq251. Epub May 13. [DOI] [PubMed] [Google Scholar]
- 27.Schulman G, Agarwal R, Acharya M, et al. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis. 2006;47:565–577. doi: 10.1053/j.ajkd.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu H, Okada S, Shinsuke OI, et al. Kremezin (AST-120) delays the progression of diabetic nephropathy in Japanese type 2 diabetic patients. Diab Care. 2005;28:2590. doi: 10.2337/diacare.28.10.2590. [DOI] [PubMed] [Google Scholar]
- 29.Shoji T, Wada A, Inoue K, et al. Prospective randomized study evaluating the efficacy of the spherical adsorptive carbon AST-120 in chronic kidney disease patients with moderate decrease in renal function. Nephron Clin Pract. 2007;105:c99–c107. doi: 10.1159/000097985. [DOI] [PubMed] [Google Scholar]
- 30.Ueda H, Shibahara N, Takagi S, et al. AST-120, an oral adsorbent, delays the initiation of dialysis in patients with chronic kidney diseases. Ther Apher Dial. 2007;11:189–195. doi: 10.1111/j.1744-9987.2007.00430.x. [DOI] [PubMed] [Google Scholar]
- 31.Akizawa T, Asano Y, Morita S, et al. Effect of a carbonaceous oral adsorbent on the progression of CKD: a multicenter, randomized, controlled trial. Am J Kidney Dis. 2009;54:459–467. doi: 10.1053/j.ajkd.2009.05.011. [DOI] [PubMed] [Google Scholar]