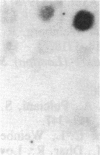
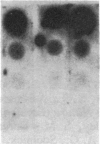
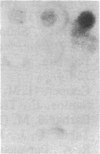
Abstract
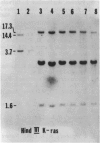
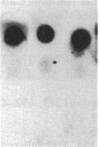
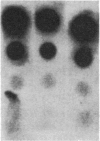
DNAs from hamster embryo cells and mouse C3H/10T1/2 cells transformed in vitro by x-irradiation into malignant cells transmit the radiation transformation phenotype by producing transformed colonies (transfectants) in two mouse recipient lines, the NIH 3T3 and C3H/101/2 cells, and in a rat cell line, the Rat-2 cells. DNAs from unirradiated cells or irradiated and visibly untransformed cells do not produce transformed colonies. The transfectants grow in agar and form tumors in nude mice. Treatment of the DNAs with restriction endonucleases prior to transfection indicates that the same transforming gene (oncogene) is present in each of the transformed mouse cells and is the same in each of the transformed hamster cells. Southern blot analysis of 3T3 or Rat-2 transfectants carrying oncogenes from radiation-transformed C3H/10T1/2 or hamster cells indicates that the oncogenes responsible for the transformation of 3T3 cells are not the Ki-ras, Ha-ras, or N-ras genes, nor are they neu, trk, raf, abl, or fms, although quick blot analysis using 11 oncogene probes detected increased transcripts of c-abl and c-fms in the 3T3 transformants containing oncogenic sequences from the x-ray-transformed C3H/10T1/2 cells. The work demonstrates that DNAs from mammalian cells transformed into malignancy by direct exposure in vitro to radiation contain genetic sequences with detectable transforming activity in three recipient cell lines. The results provide evidence that DNA is the target of radiation carcinogenesis induced at a cellular level in vitro. The experiments indicate that malignant radiogenic transformation in vitro of hamster embryo and mouse C3H/10T1/2 cells involves the activation of unique non-ras transforming genes, which heretofore have not been described.
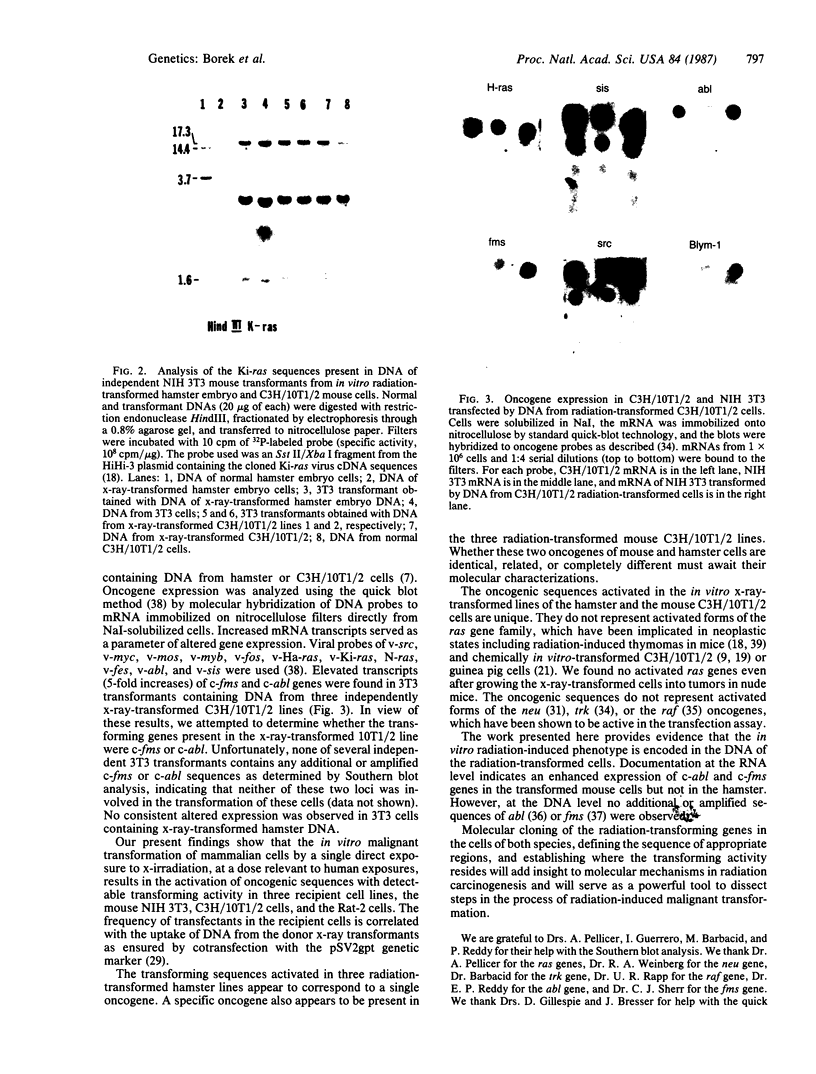
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Pragnell I. B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Cooper C. S., Oskarsson M. K., Eader L. A., Vande Woude G. F. New method for detecting cellular transforming genes. Science. 1982 Dec 10;218(4577):1122–1125. doi: 10.1126/science.6293052. [DOI] [PubMed] [Google Scholar]
- Borek C., Hall E. J. Transformation of mammalian cells in vitro by low doses of X-rays. Nature. 1973 Jun 22;243(5408):450–453. doi: 10.1038/243450a0. [DOI] [PubMed] [Google Scholar]
- Borek C. In vitro cell cultures as tools in the study of free radicals and free radical modifiers in carcinogenesis. Methods Enzymol. 1984;105:464–479. doi: 10.1016/s0076-6879(84)05065-5. [DOI] [PubMed] [Google Scholar]
- Borek C. Radiation oncogenesis in cell culture. Adv Cancer Res. 1982;37:159–232. doi: 10.1016/s0065-230x(08)60884-2. [DOI] [PubMed] [Google Scholar]
- Borek C., Sachs L. In vitro cell transformation by x-irradiation. Nature. 1966 Apr 16;210(5033):276–278. doi: 10.1038/210276a0. [DOI] [PubMed] [Google Scholar]
- Borek C., Sachs L. The difference in contact inhibition of cell replication between normal cells and cells transformed by different carcinogens. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1705–1711. doi: 10.1073/pnas.56.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek C. X-ray induced in vitro neoplastic transformation of human diploid cells. Nature. 1980 Feb 21;283(5749):776–778. doi: 10.1038/283776a0. [DOI] [PubMed] [Google Scholar]
- Bresser J., Hubbell H. R., Gillespie D. Biological activity of mRNA immobilized on nitrocellulose in NaI. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6523–6527. doi: 10.1073/pnas.80.21.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M. Cellular transforming genes. Science. 1982 Aug 27;217(4562):801–806. doi: 10.1126/science.6285471. [DOI] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Aaronson S. A. Frequent activation of c-kis as a transforming gene in fibrosarcomas induced by methylcholanthrene. Science. 1983 May 27;220(4600):955–956. doi: 10.1126/science.6302839. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Activation of a c-K-ras oncogene by somatic mutation in mouse lymphomas induced by gamma radiation. Science. 1984 Sep 14;225(4667):1159–1162. doi: 10.1126/science.6474169. [DOI] [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Hall A., Marshall C. J., Spurr N. K., Weiss R. A. Identification of transforming gene in two human sarcoma cell lines as a new member of the ras gene family located on chromosome 1. Nature. 1983 Jun 2;303(5916):396–400. doi: 10.1038/303396a0. [DOI] [PubMed] [Google Scholar]
- Hsiao W. L., Gattoni-Celli S., Weinstein I. B. Oncogene-induced transformation of C3H 10T1/2 cells is enhanced by tumor promoters. Science. 1984 Nov 2;226(4674):552–555. doi: 10.1126/science.6436974. [DOI] [PubMed] [Google Scholar]
- Hung M. C., Schechter A. L., Chevray P. Y., Stern D. F., Weinberg R. A. Molecular cloning of the neu gene: absence of gross structural alteration in oncogenic alleles. Proc Natl Acad Sci U S A. 1986 Jan;83(2):261–264. doi: 10.1073/pnas.83.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Haynes S. R. The mammalian Alu family of dispersed repeats. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1123–1130. doi: 10.1101/sqb.1983.047.01.126. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada L. F., Weinberg R. A. Presence of a Kirsten murine sarcoma virus ras oncogene in cells transformed by 3-methylcholanthrene. Mol Cell Biol. 1983 Dec;3(12):2298–2301. doi: 10.1128/mcb.3.12.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Goldsborough M. D., Mark G. E., Bonner T. I., Groffen J., Reynolds F. H., Jr, Stephenson J. R. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Santos E., Tronick S. R., Aaronson S. A., Pulciani S., Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982 Jul 22;298(5872):343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Shilo B. Z., Goldfarb M. P., Dannenberg A., Weinberg R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5714–5718. doi: 10.1073/pnas.76.11.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo B. Z., Weinberg R. A. Unique transforming gene in carcinogen-transformed mouse cells. Nature. 1981 Feb 12;289(5798):607–609. doi: 10.1038/289607a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sukumar S., Pulciani S., Doniger J., DiPaolo J. A., Evans C. H., Zbar B., Barbacid M. A transforming ras gene in tumorigenic guinea pig cell lines initiated by diverse chemical carcinogens. Science. 1984 Mar 16;223(4641):1197–1199. doi: 10.1126/science.6322298. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]