Abstract
Anaphylaxis is a severe, acute and potentially life-threatening condition, often in response to an allergen. Patients experiencing anaphylaxis can present with cutaneous, respiratory, cardiovascular or gastrointestinal manifestations. Epinephrine given intramuscularly remains the mainstay of treatment for this condition. Other second-line therapies, such as inhaled beta-2 agonists, H1 and H2 receptor antagonists and corticosteroids, may play a role in resolving respiratory and cutaneous signs and symptoms. Biphasic reactions may occur during the resolution phase of symptoms and, thus, all patients should be observed for a minimum of 4 h to 6 h before discharge from hospital. On discharge, all patients should be prescribed epinephrine autoinjectors, and referred to an allergist or immunologist for further evaluation and education.
Keywords: Anaphylaxis, Children, Emergency, Infant, Paediatric, Treatment
Abstract
L’anaphylaxie est un état grave et aigu au potentiel fatal, qui se manifeste souvent en réponse à un allergène. Les patients qui subissent une anaphylaxie peuvent présenter des manifestations cutanées, respiratoires, cardiovasculaires ou gastro-intestinales. L’adrénaline administrée par voie intramusculaire demeure le pilier du traitement. D’autres thérapies de deuxième choix, comme les béta2-agonistes, les antagonistes des récepteurs H1 et H2 et les corticoïdes, peuvent contribuer à résoudre les signes et symptômes cutanés et respiratoires. Des réactions biphasiques peuvent se produire pendant la phase de résolution des symptômes, et par conséquent, tous les patients doivent être maintenus en observation pendant au moins quatre à six heures avant leur congé de l’hôpital. Au congé, il faudrait prescrire des auto-injecteurs d’adrénaline à tous les patients et les orienter vers un allergologue ou un immunologiste pour leur faire subir une évaluation et les éduquer.
Anaphylaxis is a severe, acute and potentially life-threatening medical condition caused by the systemic release of mediators from mast cells and basophils, often in response to an allergen (1,2). The incidence of patients with anaphylaxis presenting to emergency departments (EDs) is estimated to be approximately one to four per 1000 ED visits (0.1% to 0.4%) (3–5). Of these presentations, only one-third end up having an identifiable trigger for the anaphylactic reaction. Food is the most common associated trigger, followed closely by hymenoptera (bee/wasp) stings and medications (6). When food is identified as the trigger, peanuts, tree nuts, fish, milk, eggs and shellfish (eg, shrimp, lobster, crab, scallops and oysters) are the products most often implicated in fatal or near-fatal reactions (7,8).
Although clinical symptoms and signs can involve multiple organ systems (Table 1), cutaneous manifestations such as urticaria, pruritus, angioedema and flushing tend to occur in the majority of children (80% to 90%) with anaphylaxis. Of the more concerning symptoms, respiratory involvement seems to predominate, with 60% to 70% of anaphylactic children being affected. Cardiovascular involvement is less frequent, with 10% to 30% of anaphylactic children developing signs of cardiovascular compromise including dizziness, hypotension and syncope (2,3).
TABLE 1.
Signs and symptoms of anaphylaxis
| System | Signs and symptoms |
|---|---|
| General/CNS | Fussiness, irritability, drowsiness, lethargy, reduced level of consciousness, somnolence |
| Skin | Urticaria, pruritus, angioedema, flushing |
| Upper airway | Stridor, hoarseness, oropharyngeal or laryngeal edema, uvular edema, swollen lips/tongue, sneezing, rhinorrhea, upper airway obstruction |
| Lower airway | Coughing, dyspnea, bronchospasm, tachypnea, respiratory arrest |
| Cardiovascular | Tachycardia, hypotension, dizziness, syncope, arrhythmias, diaphoresis, pallor, cyanosis, cardiac arrest |
| Gastrointestinal | Nausea, vomiting, diarrhea, abdominal pain |
CNS Central nervous system
DEFINITION
In July 2005, a panel of allergy and immunology experts convened at the Second Symposium on the Definition and Management of Anaphylaxis (1). They defined anaphylaxis as, “A serious allergic reaction that is rapid in onset and may cause death”. This group of experts also published a set of three clinical criteria for diagnosing anaphylaxis, as outlined in Table 2. The first clinical criterion, describing acute onset of illness with involvement of cutaneous manifestations, should be applicable to the majority of anaphylaxis presentations because up to 80% to 90% of children experience some degree of skin involvement. Although cutaneous involvement is usually the first and most common manifestation of anaphylaxis, the absence of skin signs at presentation does not rule out a diagnosis of anaphylaxis. The remaining two criteria address clinical features for patients with a known allergic history, and exposure to a likely or known allergen.
TABLE 2.
Clinical criteria for diagnosing anaphylaxis
| Anaphylaxis is highly likely when any one of the following three criteria are fulfilled: |
|---|
|
Adapted with permission from reference 1.
Low systolic blood pressure (BP) for children is defined as less than 70 mmHg from one month to one year, less than (70 mmHg + [2 × age]) from one to 10 years, and less than 90 mmHg from 11 to 17 years. PEF Peak expiratory flow
FIRST AID TREATMENT IN THE COMMUNITY
When available, self-injectable epinephrine should be immediately administered as an intramuscular (IM) dose to all children with signs and symptoms suspicious of anaphylaxis before arrival to hospital. Regardless of whether epinephrine is administered, parents should urgently seek medical attention at the nearest ED if they are concerned about anaphylaxis. Currently, self-injectable epinephrine is available in only two doses, from two different manufacturers: 0.15 mg (EpiPen Jr; King Pharmaceuticals Canada Inc) and 0.3 mg (EpiPen), or Twinject (Paladin Labs Inc, Canada), which is available in either 0.15 mg or 0.3 mg doses, and provides two of the same dose in one device (one automatic dose and one manual dose). Based on the recommended epinephrine dose of 0.01 mg/kg, these two doses are most applicable to children weighing 15 kg or 30 kg.
The current recommendations are that patients weighing 10 kg to 25 kg should be prescribed EpiPen Jr or the lower-dose Twinject (0.15 mg), while those weighing more than 25 kg should be prescribed EpiPen or the higher-dose Twinject (0.3 mg) (9,10). For patients weighing less than 10 kg, physicians and families will need to weigh the benefits and risks of administering epinephrine via syringes after being drawn up by a family member from small ampules. This method has been shown to be both error and delay prone, and family members must be fully competent before choosing this method of administration (11).
On prescription of self-injectable epinephrine, parents and children must be educated to administer epinephrine when symptoms occur after known exposure to a trigger that previously caused anaphylaxis. This includes, for example, administration of epinephrine for isolated urticaria in a child who previously suffered anaphylaxis after exposure to the same allergen. Prompt administration is also indicated for treatment of respiratory or cardiovascular symptoms of anaphylaxis, although determining the need for administration under these circumstances may be difficult for parents. In general, physicians should err on the side of caution by recommending that parents and patients inject epinephrine early, rather than to wait for symptoms to progress and worsen (11).
ACUTE MANAGEMENT OF ANAPHYLAXIS IN HOSPITAL
Initial management of the paediatric patient with suspected anaphylaxis should include a rapid, thorough assessment of the airway, breathing and circulation, with immediate and concurrent administration of IM epinephrine. In patients with signs of upper airway obstruction (stridor, swollen tongue or uvular edema) or severe respiratory distress, early preparation for definitive airway management is critical (12). Because intubation may be challenging with a swollen, obstructed airway, additional support from a respiratory therapy, anesthesia, or ear, nose and throat specialist should be requested if available. Careful consideration of the benefits and risks of rapid sequence intubation should be discussed among team members, and equipment for emergency surgical airway placement should ideally be at the bedside and ready for use if required.
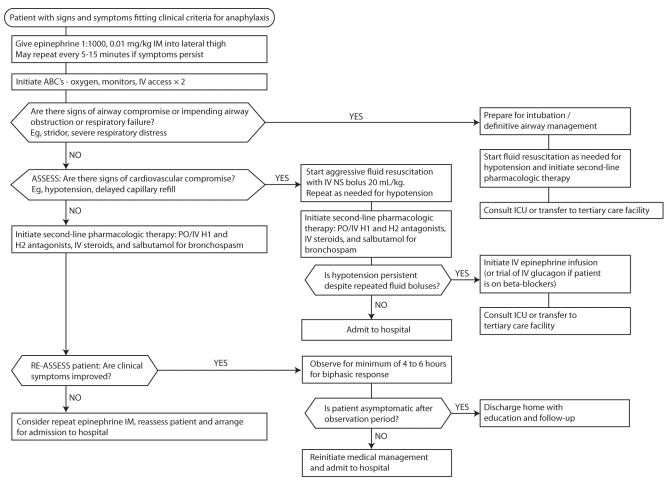
All patients with signs and symptoms of anaphylaxis should receive rapid administration of IM epinephrine. Administration of IM epinephrine should not be delayed while attempting to establish intravenous (IV) access. Patients with suspected anaphylaxis should receive supplemental oxygen and full cardio-respiratory monitoring. Those with respiratory symptoms should have their oxygen delivery titrated to optimize oxygen saturation. Due to the increased vascular permeability associated with anaphylaxis, up to 35% of circulating blood volume may be lost in the first 10 min (1). Thus, two large-bore IV lines should be inserted in all patients experiencing anaphylaxis. An intraosseous needle should be placed if IV access is unobtainable, and the patient is poorly perfused and hypotensive. Patients with cardiovascular involvement (tachycardia, hypotension or delayed capillary refill) should receive aggressive fluid resuscitation with 20 mL/kg boluses of normal saline. This should be repeated as required to maintain cardiovascular stability. Ideally, patients should be placed supine or in the Trendelenburg position, which optimizes venous return to the heart and prevents pooling of blood in the lower extremities (13). Continuous reassessment of vital signs and patient condition during management will help to determine further need for intubation, more fluids or, perhaps, initiation of inotropic support. See Figure 1 for the approach to medical management of anaphylaxis.
Figure 1.
Approach to medical management of anaphylaxis. ABC Airway, breathing and circulation; ICU Intensive care unit; IM Intramuscular; IV Intravenous; NS Normal saline; PO Oral
PHARMACOLOGICAL MANAGEMENT
Although there are several medications available for use in the treatment of anaphylaxis, epinephrine remains the first-line agent, and should be given immediately to any patient who meets the clinical criteria for anaphylaxis. The other medications, consisting of H1 and H2 antihistamines, corticosteroids and inhaled medications, play a less important role and are considered to be second-line agents for the management of anaphylaxis (Table 3).
TABLE 3.
Pharmacological management of anaphylaxis
| Drug and route of administration | Frequency of administration | Paediatric dosing (maximum dose) |
|---|---|---|
| Epinephrine (1:1000) IM | Immediately, then every 5–15 min as required | 0.01 mg/kg (0.5 mg) |
| Cetirizine PO | Single daily dose | 6 months to <2 years: 2.5 mg OD 2–5 years: 2.5–5 mg OD >5 years: 5–10 mg OD |
| Diphenhydramine IM/IV | Every 4–6 h as required for cutaneous manifestations | 1 mg/kg/dose (50 mg) |
| Ranitidine PO/IV | Every 8 h as required for cutaneous manifestations | 1 mg/kg/dose (50 mg) |
| Corticosteroids: prednisone PO or methylprednisolone IV | Every 6 h as required | 1 mg/kg PO (75 mg) or 1 mg/kg IV (125 mg) |
| Salbutamol | Every 20 min or continuous for respiratory symptoms (wheezing or shortness of breath) | 5–10 puffs using MDI or 2.5–5 mg by nebulization |
| Nebulized epinephrine (1:1000) | Every 20 min to 1 h for symptoms of upper airway obstruction (stridor) | 2.5–5 mL by nebulization |
| Epinephrine IV (infusion) | Continuous infusion for hypotension – titrate to effect | 0.1–1 μg/kg/min (maximum 10 μg/min) |
| Glucagon IV | Bolus followed by continuous infusion – titrate to effect | 20–30 μg/kg bolus (maximum 1 mg), then infusion at 5–15 μg/min |
IM Intramuscular; IV Intravenous; MDI Metered dose inhaler; OD Once daily; PO Oral
Epinephrine
Epinephrine is a direct-acting sympathomimetic agent with various properties that help to reverse the pathophysiological effects of anaphylaxis. The alpha-adrenergic actions of epinephrine work to increase peripheral vascular resistance and reverse peripheral vasodilation while also decreasing angioedema and urticaria. The beta-1 adrenergic effects have positive chronotropic and inotropic effects on the heart, while the beta-2 adrenergic effects cause bronchodilation and reduction of inflammatory mediator release from mast cells and basophils (12). In combination, these effects help to reverse the anaphylactic process and, in turn, improve the cutaneous, respiratory and cardiovascular effects of the condition.
Epinephrine 1:1000 should be administered IM into the anterolateral thigh at a dose of 0.01 mg/kg (maximum total dose 0.5 mg), and can be repeated every 5 min to 15 min depending on the patient’s response to previous doses (2). IM administration of epinephrine into the thigh results in higher peak plasma concentrations compared with IM or subcutaneous (SC) injection into the upper arm (14). Additionally, peak plasma concentrations are achieved significantly faster after IM injection into the thigh compared with SC administration into the deltoid region (15). The local vasoconstriction caused by SC injection may inhibit absorption from the injection site. Thus, IM injection of epinephrine into the anterior lateral thigh is the preferred route of delivery for anaphylaxis. Some patients with persistent symptoms may require repeat doses of epinephrine. The decision to administer a repeat dose of epinephrine should be made on an individual basis, and response to therapy should be carefully monitored with frequent reassessment of vital signs and the patient’s clinical condition.
H1 and H2 antihistamines
Although oral antihistamines are considered to be the mainstay of treatment for minor allergic reactions, their slow onset of action and limited effect on symptoms makes them a second- line agent for anaphylaxis (16–18). These agents are not appropriate for first-line treatment of anaphylaxis, and should never be used in place of IM epinephrine. Unfortunately, there are no randomized, placebo-controlled clinical trials of antihistamines for use in anaphylaxis. However, given their proven benefit with localized allergic reactions such as urticaria, H1 antagonists such as cetirizine or diphenhydramine can be given to relieve the cutaneous symptoms of anaphylaxis (eg, urticaria, pruritus and angioedema). H1 antagonists have no effect on the respiratory, gastrointestinal or cardiovascular symptoms of anaphylaxis. If the patient is not vomiting, cetirizine should be used because it is faster in onset than diphenhydramine, and much less sedating. H2 antagonists, such as raniditine, can be given in combination with H1 antagonists because their combined effect is superior in treating cutaneous manifestations compared with the use of H1 antagonists alone (19,20). Cetirizine should be given orally at a weight-appropriate dose (Table 3). Diphenhydramine for the vomiting child can be given as an IV or IM dose of 1 mg/kg/dose, with a maximum dose of 50 mg. Ranitidine should be given as an oral or IV dose of 1 mg/kg/dose, also with a maximum dose of 50 mg.
Corticosteroids
Corticosteroids play an integral role in the treatment of several allergy-related diseases including asthma and allergic rhinitis. However, no randomized controlled trials have demonstrated a proven benefit of steroids in the treatment of anaphylaxis. Despite this, most experts would still recommend treatment with corticosteroids, with the knowledge that their onset of action is slow (4 h to 6 h), and that there will likely be little benefit in the acute phase of management (1,2). When ordered, oral prednisone can be given at a dose of 1 mg/kg (maximum single dose 75 mg) or, for more severe reactions, methylprednisolone at a dose of 1 mg/kg IV (maximum single dose 125 mg).
Inhaled medications
Children who present with bronchospasm and wheezing, or who have a history of asthma may benefit from inhaled salbutamol as part of their anaphylaxis treatment. Salbutamol should be given at a dose of five to 10 puffs using a metered dose inhaler, and administered every 20 min or continuously until symptoms of wheezing or respiratory distress improve (1,2,6). Infants and children unable to effectively use the metered dose inhaler may be given 2.5 mg to 5 mg of salbutamol per dose via nebulization. Children who present with stridor may find some relief from inhaled epinephrine, although no studies have documented the clinical efficacy of epinephrine delivered by this route for the treatment of upper airway obstruction induced by anaphylaxis. Certainly, IM epinephrine remains the first-line treatment for symptoms of upper or lower airway obstruction due to anaphylaxis, with inhaled salbutamol and epinephrine playing more supportive roles.
POSTRESUSCITATIVE CARE
IV epinephrine
Some patients who experience severe anaphylactic shock may have persistent hypotension despite aggressive fluid resuscitation and repeated doses of IM epinephrine. In fact, repeated administration of IM epinephrine has no demonstrated benefit for improving persistent hypotension related to anaphylaxis (13). Instead, these patients should be started on an epinephrine infusion at a dose of 0.1 μg/kg/min to 1 μg/kg/min (maximum 10 μg/min), with gradual titration of the infusion to produce a normal blood pressure.
Titrated IV infusions of epinephrine seem to produce a more sustained improvement in blood pressure, whereas intermittent IV boluses of epinephrine may have an immediate effect that is often short lived, accompanied by coexisting concerns for induced cardiac arrhythmias when administered too rapidly (13,21).
Glucagon
Patients regularly taking beta-blockers who present with anaphylactic shock may have persistent hypotension despite epinephrine administration. In this situation, glucagon, which activates adenylate cyclase independent of the beta-receptor, may be given in an attempt to reverse the cardiovascular effects of anaphylaxis (1). Glucagon should be given at a dose of 20 μg/kg to 30 μg/kg IV over 5 min (maximum dose 1 mg), followed by the initiation of a glucagon infusion at a rate of 5 μg/min to 15 μg/min, which is then titrated to effect.
OBSERVATION PERIOD AND DISPOSITION
Biphasic reactions, defined as a recurrence of anaphylactic symptoms after initial resolution, can occur anywhere from 1 h to 72 h after the first onset of symptoms (22–25). Approximately 5% to 20% of patients with anaphylaxis experience a biphasic reaction, with 3% of children having a significant reaction requiring oxygen, vasopressors, intubation, repeat epinephrine administration or unscheduled bronchodilator treatments (21). Although no validated clinical predictors of biphasic reactions have been verified, some studies suggest that biphasic reactions are more likely to occur in patients who had delayed administration of epinephrine, who needed more than one dose of epinephrine or who initially presented with more severe symptoms (22–25).
Because most biphasic reactions occur within the first 4 h to 6 h after initial onset of symptoms, a reasonable length of time for observation of an anaphylactic patient would be 4 h to 6 h. However, the physician must be aware that symptoms may still recur up to 72 h after initial presentation, and counsel parents accordingly to monitor for such a recurrence (23). In rural environments, where larger distances of travel are required to reach medical care, it may be reasonable to observe patients for a longer period of time (eg, 12 h) or to admit them to hospital overnight. Patients who require repeated doses of epinephrine, who initially presented with more severe symptoms (eg, hypotension, severe respiratory distress) or who experience a biphasic reaction should be admitted to hospital for observation. Other patients who have high-risk features, such as peanut allergy, asthma or use of beta-blockers, should also be strongly considered for overnight observation or admission (12). Patients presenting with severe respiratory symptoms requiring definitive airway management or those who have persistent hypotension requiring IV epinephrine or glucagon infusions should be admitted to the intensive care unit.
DISCHARGE MANAGEMENT
The decision to discharge a patient should be individualized to take into account initial presentation, responsiveness to therapy, persistence of symptoms and accessibility to an urgent care facility. On discharge, patients suffering from anaphylaxis should be given a prescription for a self-injectable form of epinephrine (eg, EpiPen, EpiPen Jr or Twinject). If possible, patients should leave the emergency room with an epinephrine autoinjector because a biphasic reaction could occur on the way home. Parents, caretakers, older children and adolescents should be carefully educated about how to administer epinephrine, and counselled to err on the side of caution and administer the drug when symptoms occur after exposure to the individual’s known trigger. An epinephrine autoinjector should be kept with the child at all times (at school and with the parent or child). Ideally, two doses should be available for administration at each location (eg, two EpiPen autoinjectors or one Twinject). Children who present with less severe allergic reactions and risk factors for anaphylaxis should also be prescribed self-injectable epinephrine (Table 4) (9). The emphasis in educating parents should be placed on responding promptly to anaphylactic symptoms, rather than delaying treatment due to confusion attributed to an unknown allergen.
TABLE 4.
Risk factors that may indicate the need to prescribe self-injectable epinephrine
Reaction history
|
Adapted with permission from reference 9
In addition to epinephrine, a three-day course of oral H1 and H2 antihistamines (cetirizine and ranitidine) and oral corticosteroids may be prescribed on discharge. Most experts recommend this additional therapy despite limited data to support its use because these drugs are unlikely to cause harm, and may have some added benefit in the resolution of symptoms (12). All patients suffering from anaphylaxis should be provided with strict guidelines for avoidance of the precipitating trigger, and education about prevention of allergic reactions. Patient information resources available online, such as Anaphylaxis Canada (www.anaphylaxis.ca) or the Allergy/Asthma Information Association (www.aaia.ca), should also be passed on to the family. Finally, MedicAlert bracelets (Canadian MedicAlert Foundation) should be recommended, and referral to an allergist or immunologist who can provide additional testing, information and therapy should be initiated.
SUMMARY
Anaphylaxis is a serious and potentially life-threatening condition that requires immediate diagnosis and treatment with IM epinephrine to ensure optimal outcome. Adjunctive therapies for treatment of anaphylaxis are available, but epinephrine remains the most important component of the acute management phase. Hypotension should be managed aggressively with repeated boluses of normal saline, with initiation of an IV epinephrine infusion in refractory cases. Patients experiencing resolution of symptoms while in hospital should be observed for a minimum of 4 h to 6 h before discharge to monitor for a biphasic reaction. Patients with severe symptoms at presentation, repeat doses of epinephrine, or who suffer a biphasic reaction should be admitted to hospital. On discharge, parents should be carefully counselled and educated about the signs and symptoms of anaphylaxis, the avoidance of triggers, the use of self-injectable epinephrine, and the importance of follow-up with an allergy or immunology specialist.
ACKNOWLEDGEMENTS
The principal author thanks Dr Janet Roberts and Dr Zave Chad for their expert advice and assistance with the development of this article. This position statement was reviewed by the Canadian Paediatric Society’s Allergy Section and Community Paediatrics Committee.
Footnotes
ACUTE CARE COMMITTEE
Members: Drs Adam Cheng, British Columbia Children’s Hospital, Vancouver, British Columbia; Catherine Farrell, CHU Sainte-Justine, Montreal, Quebec; Jeremy Friedman, The Hospital for Sick Children, Toronto, Ontario; Marie Gauthier, CHU Sainte-Justine, Montreal, Quebec (Board Representative); Angelo Mikrogianakis, Alberta Children’s Hospital, Calgary, Alberta (Chair); Oliva Ortiz-Alvarez, St Martha’s Regional Hospital, Antigonish, Nova Scotia
Liaisons: Drs Claudette Bardin, Montreal Children’s Hospital, Montreal, Quebec (Canadian Paediatric Society, Hospital Paediatrics Section); Laurel Chauvin-Kimoff, Montreal Children’s Hospital, Montreal, Quebec (Canadian Paediatric Society, Paediatric Emergency Medicine Section); Dawn Hartfield, University of Alberta, Edmonton, Alberta (Canadian Paediatric Society, Hospital Paediatrics Section)
Principal author: Dr Adam Cheng, Vancouver, British Columbia
The recommendations in this statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. All Canadian Paediatric Society position statements and practice points are reviewed, revised or retired as needed on a regular basis. Please consult the “Position Statements” section of the CPS website (www.cps.ca/english/publications/statementsindex.htm) for the most current version.
REFERENCES
- 1.Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second Symposium on the Definition and Management of Anaphylaxis: Summary Report – Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J Allergy Clin Immunol. 2006;117:391–7. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 2.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of anaphylaxis: An updated practice parameter. J Allergy Clin Immunol. 2005;115:S483–S523. doi: 10.1016/j.jaci.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Brown AF, McKinnon D, Chu K. Emergency department anaphylaxis: A review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108:861–6. doi: 10.1067/mai.2001.119028. [DOI] [PubMed] [Google Scholar]
- 4.Pastorello EA, Rivolta F, Bianchi M, Mauro M, Pravettoni V. Incidence of anaphylaxis in the emergency department of a general hospital in Milan. J Chromatogr B Biomed Sci Appl. 2001;756:11–7. doi: 10.1016/s0378-4347(01)00067-6. [DOI] [PubMed] [Google Scholar]
- 5.Braganza SC, Acworth JP, McKinnon DR, Peake JE, Brown AF. Paediatric emergency department anaphylaxis: Different patterns from adults. Arch Dis Child. 2006;91:159–63. doi: 10.1136/adc.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberman DB, Teach SJ. Management of anaphylaxis in children. Pediatr Emerg Care. 2008;24:861–9. doi: 10.1097/PEC.0b013e31818ea116. [DOI] [PubMed] [Google Scholar]
- 7.Sampson HA. Anaphylaxis and emergency treatment. Pediatrics. 2003;111:1601–8. [PubMed] [Google Scholar]
- 8.Peng MM, Jick H. A population-based study of the incidence, cause and severity of anaphylaxis in the United Kingdom. Arch Intern Med. 2004;164:317–9. doi: 10.1001/archinte.164.3.317. [DOI] [PubMed] [Google Scholar]
- 9.Sicherer SH, Simons FE Section on Allergy and American Academy of Pediatrics. Self-injectable epinephrine for first-aid management of anaphylaxis. Pediatrics. 2007;119:638–46. doi: 10.1542/peds.2006-3689. [DOI] [PubMed] [Google Scholar]
- 10.Simons FE. First-aid treatment of anaphylaxis to food: Focus on epinephrine. J Allergy Clin Immunol. 2004;113:837–44. doi: 10.1016/j.jaci.2004.01.769. [DOI] [PubMed] [Google Scholar]
- 11.Sicherer SH. Self-injectable epinephrine: No size fits all! Ann Allergy Asthma Immunol. 2001;86:597–8. doi: 10.1016/S1081-1206(10)62284-3. [DOI] [PubMed] [Google Scholar]
- 12.Davis JE, Norris RL. Allergic emergencies in children: The pivotal role of epinephrine. Pediatric Emergency Medicine Practice. 2007;4:1–28. [Google Scholar]
- 13.Brown SG. Cardiovascular aspects of anaphylaxis: Implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol. 2005;5:359–64. doi: 10.1097/01.all.0000174158.78626.35. [DOI] [PubMed] [Google Scholar]
- 14.Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: Intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001;108:871–3. doi: 10.1067/mai.2001.119409. [DOI] [PubMed] [Google Scholar]
- 15.Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol. 1998;101:33–7. doi: 10.1016/S0091-6749(98)70190-3. [DOI] [PubMed] [Google Scholar]
- 16.Sheikh A, Ten Broek V, Brown SG, Simons FE. H1-antihistamines for the treatment of anaphylaxis: Cochrane systemic review. Allergy. 2007;62:830–7. doi: 10.1111/j.1398-9995.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 17.Andreae D, Andreae M. Should antihistamines be used to treat anaphylaxis? BMJ. 2009;339:b2489. doi: 10.1136/bmj.b2489. [DOI] [PubMed] [Google Scholar]
- 18.Simons FE. Advances in H1-antihistamines. N Engl J Med. 2004;351:2203–17. doi: 10.1056/NEJMra033121. [DOI] [PubMed] [Google Scholar]
- 19.Knight R, Lin RY, Curry A, et al. Clinical effects of combined anti-H1 and anti-H2 treatment in patients presenting with acute allergic syndromes: A randomized controlled trial. Ann Emerg Med. 1999;34:S18–S19. [Google Scholar]
- 20.Lin RY, Curry A, Pesola GR, et al. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000;36:462–8. doi: 10.1067/mem.2000.109445. [DOI] [PubMed] [Google Scholar]
- 21.Mink SN, Simons FE, Simons KJ, Becker AB, Duke K. Constant infusion of epinephrine, but not bolus treatment, improves haemodynamic recovery in anaphylactic shock in dogs. Clin Exp Allergy. 2004;34:1776–83. doi: 10.1111/j.1365-2222.2004.02106.x. [DOI] [PubMed] [Google Scholar]
- 22.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380–4. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 23.Lee JM, Greenes DS. Biphasic anaphylactic reactions in pediatrics. Pediatrics. 2000;106:762–6. doi: 10.1542/peds.106.4.762. [DOI] [PubMed] [Google Scholar]
- 24.Stark BJ, Sullivan TJ. Biphasic and protracted anaphylaxis. J Allergy Clin Immunol. 1986;78:76–83. doi: 10.1016/0091-6749(86)90117-x. [DOI] [PubMed] [Google Scholar]
- 25.Douglas DM, Sukenick E, Andrade WP, Brown JS. Biphasic systemic anaphylaxis: An inpatient and outpatient study. J Allergy Clin Immunol. 1994;93:977–85. doi: 10.1016/s0091-6749(94)70044-3. [DOI] [PubMed] [Google Scholar]