Abstract
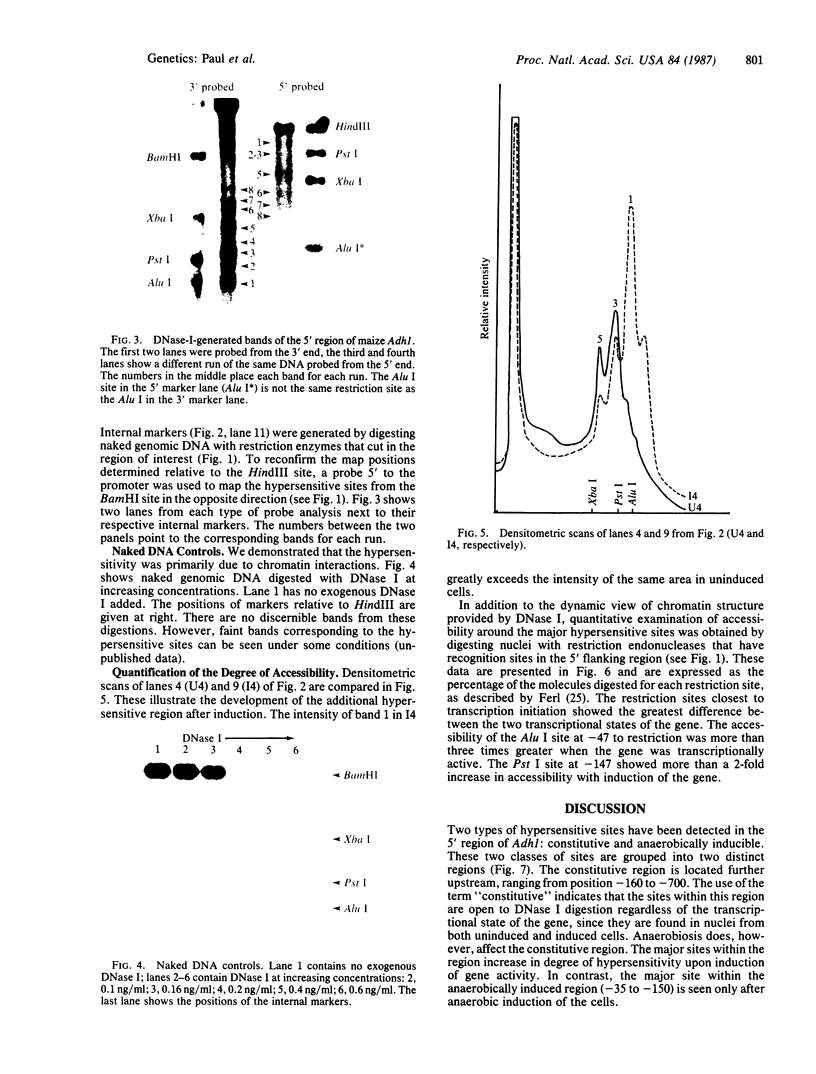
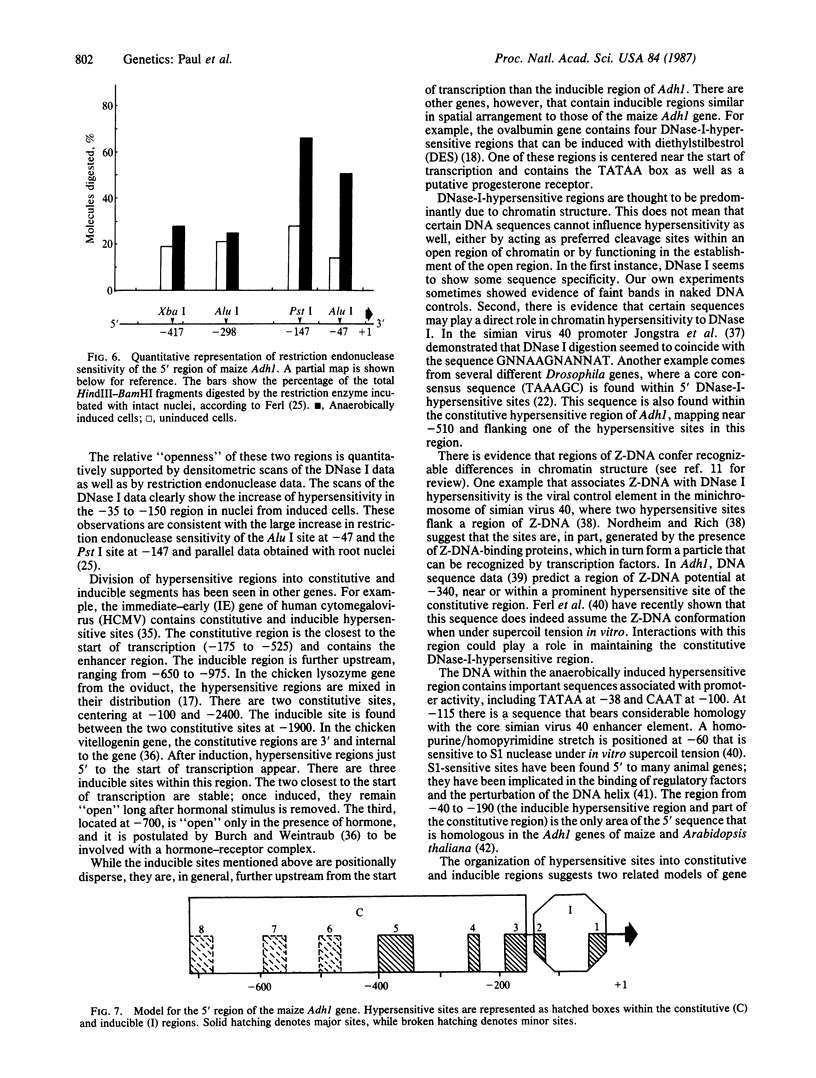
DNase-I-hypersensitive sites have been characterized in a plant gene, maize Adh1 (which encodes alcohol dehydrogenase 1). It has been generally recognized in animal genes that the chromatin of the 5′ flanking region can be characterized by the accessibility of its DNA to the nuclease DNase I (EC 3.1.21.1), indicating which areas in the promoter are “open” to nuclear factors. The 5′ region of the maize Adh1 gene contains two distinct DNase-I-hypersensitive regions, one constitutively present from position -160 to -700 and one that is anaerobically induced from position -35 to -150. The constitutive region contains three major hypersensitive sites, one of which corresponds in part to a region of potential Z-DNA. The induced hypersensitive region includes TATAA at -38 and CAAT at -100 as well as other potential regulatory sequences.
Keywords: chromatin, promoter, regulation, plant
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich H. C., Ferl R. J., Hils M. H., Akin D. E. Ultrastructural correlates of anaerobic stress in corn roots. Tissue Cell. 1985;17(3):341–348. doi: 10.1016/0040-8166(85)90053-9. [DOI] [PubMed] [Google Scholar]
- Burch J. B., Weintraub H. Temporal order of chromatin structural changes associated with activation of the major chicken vitellogenin gene. Cell. 1983 May;33(1):65–76. doi: 10.1016/0092-8674(83)90335-5. [DOI] [PubMed] [Google Scholar]
- Chang C., Meyerowitz E. M. Molecular cloning and DNA sequence of the Arabidopsis thaliana alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison B. L., Egly J. M., Mulvihill E. R., Chambon P. Formation of stable preinitiation complexes between eukaryotic class B transcription factors and promoter sequences. Nature. 1983 Feb 24;301(5902):680–686. doi: 10.1038/301680a0. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Mulvihill E. R., Egly J. M., Chambon P. Interaction of eukaryotic class-B transcription factors and chick progesterone-receptor complex with conalbumin promoter sequences. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):965–975. doi: 10.1101/sqb.1983.047.01.110. [DOI] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Pryor A. J., Bennetzen J. L., Inglis A., Llewellyn D., Sachs M. M., Ferl R. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984 May 11;12(9):3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Sachs M. M., Gerlach W. L., Finnegan E. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase 2 (Adh2) gene of maize. Nucleic Acids Res. 1985 Feb 11;13(3):727–743. doi: 10.1093/nar/13.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Elgin S. C. Anatomy of hypersensitive sites. Nature. 1984 May 17;309(5965):213–214. doi: 10.1038/309213a0. [DOI] [PubMed] [Google Scholar]
- Elgin S. C. DNAase I-hypersensitive sites of chromatin. Cell. 1981 Dec;27(3 Pt 2):413–415. doi: 10.1016/0092-8674(81)90381-0. [DOI] [PubMed] [Google Scholar]
- Emerson B. M., Felsenfeld G. Specific factor conferring nuclease hypersensitivity at the 5' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):95–99. doi: 10.1073/pnas.81.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferl R. J., Brennan M. D., Schwartz D. In vitro translation of maize ADH: evidence for the anaerobic induction of mRNA. Biochem Genet. 1980 Aug;18(7-8):681–691. doi: 10.1007/BF00484585. [DOI] [PubMed] [Google Scholar]
- Freeling M. Simultaneous induction by anaerobiosis or 2,4-D of multiple enzymes specificed by two unlinked genes: differential Adh1-Adh2 expression in maize. Mol Gen Genet. 1973 Dec 31;127(3):215–227. doi: 10.1007/BF00333761. [DOI] [PubMed] [Google Scholar]
- Fritton H. P., Igo-Kemenes T., Nowock J., Strech-Jurk U., Theisen M., Sippel A. E. Alternative sets of DNase I-hypersensitive sites characterize the various functional states of the chicken lysozyme gene. Nature. 1984 Sep 13;311(5982):163–165. doi: 10.1038/311163a0. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack R. S., Gehring W. J., Brack C. Protein component from Drosophila larval nuclei showing sequence specificity for a short region near a major heat-shock protein gene. Cell. 1981 May;24(2):321–331. doi: 10.1016/0092-8674(81)90322-6. [DOI] [PubMed] [Google Scholar]
- Jongstra J., Reudelhuber T. L., Oudet P., Benoist C., Chae C. B., Jeltsch J. M., Mathis D. J., Chambon P. Induction of altered chromatin structures by simian virus 40 enhancer and promoter elements. Nature. 1984 Feb 23;307(5953):708–714. doi: 10.1038/307708a0. [DOI] [PubMed] [Google Scholar]
- Kaye J. S., Pratt-Kaye S., Bellard M., Dretzen G., Bellard F., Chambon P. Steroid hormone dependence of four DNase I-hypersensitive regions located within the 7000-bp 5'-flanking segment of the ovalbumin gene. EMBO J. 1986 Feb;5(2):277–285. doi: 10.1002/j.1460-2075.1986.tb04210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Groudine M. Transcriptional regulation of the human cytomegalovirus major immediate-early gene is associated with induction of DNase I-hypersensitive sites. Mol Cell Biol. 1986 Feb;6(2):452–461. doi: 10.1128/mcb.6.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983 Jun 23;303(5919):674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Ramain P., Bourouis M., Dretzen G., Richards G., Sobkowiak A., Bellard M. Changes in the chromatin structure of Drosophila glue genes accompany developmental cessation of transcription in wild type and transformed strains. Cell. 1986 May 23;45(4):545–553. doi: 10.1016/0092-8674(86)90286-2. [DOI] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Dennis E. S., Gerlach W. L., Peacock W. J. Two Alleles of Maize ALCOHOL DEHYDROGENASE 1 Have 3' Structural and Poly(a) Addition Polymorphisms. Genetics. 1986 Jun;113(2):449–467. doi: 10.1093/genetics/113.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senear A. W., Palmiter R. D. Expression of the mouse metallothionein-I gene alters the nuclease hypersensitivity of its 5' regulatory region. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):539–547. doi: 10.1101/sqb.1983.047.01.063. [DOI] [PubMed] [Google Scholar]
- Shermoen A. W., Beckendorf S. K. A complex of interacting DNAase I-hypersensitive sites near the Drosophila glue protein gene, Sgs4. Cell. 1982 Jun;29(2):601–607. doi: 10.1016/0092-8674(82)90176-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Wu C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature. 1984 May 17;309(5965):229–234. doi: 10.1038/309229a0. [DOI] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]






