Abstract
OBJECTIVE:
To examine the relationship between serum vitamin D concentrations, dietary intake and body mass index among healthy children living in Calgary, Alberta.
METHODS:
The present cross-sectional study included healthy children two to 13 years of age who presented to the Alberta Children’s Hospital for elective surgery during a 12-month period. Data including the child’s weight, height, age, sex, ethnicity, dietary intake, use of vitamin supplements, physical activity and time spent outdoors were collected. Serum concentrations of 25-hydroxyvitamin D (25[OH]D) were measured using commercial immunoradiometric assay kits.
RESULTS:
Serum 25(OH)D concentrations were available for 1442 of 1862 participants, of whom 862 (59.8%) were boys. The mean (± SD) serum 25(OH)D concentration was 86.1±35.1 nmol/L (range 10 nmol/L to 323 nmol/L). Five hundred thirty-nine (37.4%) participants had insufficient vitamin D status (25[OH]D between 25 nmol/L and lower than 75 nmol/L), and vitamin D deficiency (25[OH]D 25 nmol/L or lower) was present in 29 subjects (2.0%). Children in the older age group (nine to 13 years) were more likely to have suboptimal vitamin D (P<0.001). Other risk factors significantly associated with suboptimal vitamin D status included overweight or obesity, nonwhite ethnicity, winter months, dietary vitamin D intake of less than 200 IU/day and less time spent outdoors.
CONCLUSION:
A high rate of suboptimal vitamin D concentrations was observed among the participants. Beyond promoting a vitamin D-enriched diet, physicians should also consider the body mass index and other risk factors to determine the optimal vitamin D intake for children living in the area studied.
Keywords: BMI, Healthy children, Nutrition, Vitamin D
Abstract
OBJECTIF :
Examiner le lien entre les concentrations sériques de vitamine D, la consommation alimentaire et l’indice de masse corporelle chez des enfants en santé de Calgary, en Alberta.
MÉTHODOLOGIE :
La présente étude transversale portait sur les enfants en santé de deux à 13 ans qui se sont présentés à l’Alberta Children’s Hospital afin de subir une opération non urgente sur une période de 12 mois. Les chercheurs ont colligé les données suivantes : le poids, la taille, l’âge, le sexe, l’ethnie, l’apport alimentaire, la prise de suppléments de vitamines, l’activité physique et le temps passé à l’extérieur. Ils ont mesuré les concentrations sériques de 25-hydroxyvitamine D (25[OH]D) au moyen de dosages immunométriques commerciaux.
RÉSULTATS :
On connaissait les concentrations sériques de 25(OH)D chez 1 442 des 1 862 participants, dont 862 (59,8 %) étaient des garçons. La concentration sérique moyenne (±ÉT) de 25(OH)D était de 86,1±35,1 nmol/L (plage de 10 nmol/L à 323 nmol/L). Cinq cent trente-neuf (37,4 %) participants avaient un taux de vitamine D insuffisant (25[OH]D de 25 nmol/L et de moins de 75 nmol/L), et 29 (2,0 %), une carence en vitamine D (25[OH]D de 25 nmol/L ou moins). Les enfants du groupe plus âgé (neuf à 13 ans) étaient plus susceptibles d’avoir une concentration sous-optimale de vitamine D (P<0,001). D’autres facteurs de risque s’associaient de manière significative à un taux sous-optimal de vitamine D, soit l’embonpoint ou l’obésité, l’ethnie non blanche, les mois d’hiver, un apport alimentaire de vitamine D inférieur à 200 UI/jour et moins de temps passé à l’extérieur.
CONCLUSION :
Les chercheurs ont observé un taux élevé de concentrations sous-optimales de vitamine D chez les participants. À part promouvoir un régime enrichi de vitamine D, les médecins devraient également tenir compte de l’indice de masse corporelle et d’autres facteurs de risque pour déterminer l’apport de vitamine D optimal chez les enfants qui vivent dans la région à l’étude.
Vitamin D is essential for bone mineralization and maintenance of bone strength. Vitamin D deficiency results in rickets in children and osteomalacia in adults. There have been increasing numbers of reports regarding the prevalence of hypovitaminosis D in North America (1,2). Furthermore, vitamin D-deficiency rickets is an ongoing concern among children living in Canada (3). Beyond its effects on bone health, vitamin D may also protect against type I diabetes, multiple sclerosis, hypertension and cancer (4).
Vitamin D formed in the skin and from dietary sources is transported to the liver, where it is converted to 25-hydroxyvitamin D (25[OH]D). Serum 25(OH)D is a reliable biomarker of vitamin D status, and it is used to define vitamin D deficiency or intoxication (5–8). Laboratory reference ranges traditionally have set the lower normal limits for serum 25(OH)D concentrations at 37.5 nmol/L to 40 nmol/L. However, it has been suggested that at concentrations between 50 nmol/L and 100 nmol/L, the body store of vitamin D is already depleted, with compensatory elevation of parathyroid hormone (PTH) indicative of hypovitaminosis D (9). In adult populations, it has been shown that intestinal calcium absorption increases as serum 25(OH)D concentration rises, up to approximately 80 nmol/L, where it reaches a plateau (10). While it is generally agreed that 25(OH)D concentrations of 50 nmol/L can prevent rickets, higher concentrations may be necessary to affect the prevalence of diabetes, multiple sclerosis, fractures and various types of cancer.
Many authors have focused on the assessment of vitamin D status in children suffering from various chronic diseases (11–15) or severe burns (16). There are only a few studies on vitamin D status among healthy children (17–22). Therefore, the objectives of the present study were to determine the 25(OH)D concentrations in a healthy paediatric population living in Calgary, Alberta, and to identify risk factors associated with suboptimal vitamin D.
METHODS
Study population and design
The data were prospectively collected from otherwise healthy children two to 13 years of age who presented to the Alberta Children’s Hospital (Calgary) operating room for elective surgery during a one-year time period, from January to December 2006, inclusively. Only children in class I (healthy and normal) of the American Society of Anesthesiologists’ physical status classification system were eligible for inclusion in the study. Children in American Society of Anesthesiologists class II (mild systemic disease) or higher (III to V, severe or life-threatening systemic disease) were not eligible. Other exclusion criteria included children who did not need an intravenous access for their operating room procedures; children with a history of chronic illnesses, autoimmune diseases or recurrent fractures; children undergoing diagnostic biopsies; children undergoing bronchoscopy or endoscopy; children on medications known to affect serum 25(OH)D; pregnant teenagers or those on oral contraceptive pills; and children whose parents were unable or unwilling to provide informed consent.
The parents or legal guardians of all eligible participants provided written informed consent, and the study was approved by the institutional review board. Personal data, including the child’s weight, height, age, sex, parent/guardian age, self-declared ethnicity and educational level, were collected. The parents were asked to complete a questionnaire related to their child’s use of vitamin supplements, sunscreen, physical activity, time spent outdoors, medical history, medications used on a regular basis, and holiday travel at a latitude below 40°N for at least one week in the previous six months. Each child’s dietary intake during the past month was assessed using the Harvard Service Food Frequency Questionnaire (23,24). Height and weight were measured using a stadiometer and balance scale, respectively, with children dressed in light clothing and without their shoes. Body mass index (BMI) was calculated as the weight (in kilograms) divided by the square of height (in metres). Children were classified as underweight, healthy weight, overweight or obese based on their BMI percentile according to the Centers for Disease Control and Prevention (USA) definitions (25).
One blood sample (6 mL to 8 mL) was obtained for each participant once intravenous access had been obtained under general anesthesia. Serum 25(OH)D concentrations, intact PTH, phosphate, total calcium, albumin and creatinine were measured in the Calgary Laboratory Services Diagnostic and Scientific Centre. Serum 25(OH)D concentrations were measured using commercial immunoradiometric assay kits (DiaSorin Inc, USA). The DiaSorin 25(OH)D assay consisted of a two-way procedure. The first procedure involved a rapid extraction of 25(OH)D and other hydroxylated metabolites from serum or plasma with acetonitrile. Following extraction, the treated sample was then assayed using an equilibrium radioimmunoassay (RIA) procedure. The RIA method was based on an antibody with specificity to 25(OH)D. The intra- and interassay coefficients of variation for the RIA were 10% and 16%, respectively, and external quality control was provided by the Vitamin D External Quality Assessment Scheme. Intact serum PTH concentrations were measured using a one-step commercial immunoradiometric assay (DiaSorin Inc) in which the patient sample was incubated with two different antibodies simultaneously. Serum concentrations of phosphate, total calcium, albumin and creatinine were measured using a multichannel autoanalyzer (Roche Hitachi model, Roche Diagnostics, Canada).
Study definitions
The definitions of vitamin D status used in the study were based on the recent Canadian Paediatric Society guidelines (26). Furthermore, suboptimal vitamin D was defined as serum 25(OH)D concentrations of lower than 75 nmol/L, and optimal vitamin D as serum 25(OH)D concentrations of 75 nmol/L or greater. A participant was considered to be underweight if his/her BMI was lower than the fifth percentile; healthy weight if BMI was between the fifth and less than the 85th percentile; overweight if his/her BMI was between the 85th and less than the 95th percentile; and obese if his/her BMI was on the 95th percentile or greater based on age and sex (25).
Previous research has found that the amount of sunlight in Edmonton (latitude 53°34′N) does not promote the conversion of the vitamin D precursors in the skin from October through March (27). Because the latitude of Calgary (51°05′N) is similar to that of Edmonton, winter was defined as the months of October through March.
Sample size justification
The National Committee for Clinical Laboratory Standards (28) recommends that a minimum of 120 observations (ie, subjects) be tested to determine a reference interval. Therefore, it was planned that a minimum of 120 boys and girls in each of the three age groups (ie, two to four years, five to eight years, and nine to 12 years) would be recruited during the winter (October to March) and summer (April to September) over a one-year period.
Statistical analysis
The statistical analysis was conducted using STATA version 9.0 (StataCorp, USA). Descriptive statistics were used to present the clinical characteristics of the participants. Mean serum 25(OH)D concentrations and their corresponding 95% CIs were calculated. Continuous variables were described using the means and medians. Bivariate associations between serum 25(OH)D concentrations, suboptimal vitamin D and individual predictor variables were assessed using the χ2 statistics and Student’s t tests. The potential predictive role of the independent variables on suboptimal vitamin D status was assessed through a stepwise multivariate logistic regression model. The results of the logistic regression modelling are presented as ORs. All P values were two sided, and P<0.05 was considered to be statistically significant.
RESULTS
Study population
A total of 1862 children two to 13 years of age were enrolled. Serum 25(OH)D concentrations were available for 1442 (77.4%) study participants, of whom 862 (59.8%) were boys and 580 (40.2%) were girls. Their mean (± SD) age was 6.1±2.9 years. Two hundred eight children (12.4%) were underweight, 1129 (67.4%) were healthy weight, 183 (10.9%) were overweight, and 156 (9.3%) were obese. (Body mass index data were not available for all participants.) The other characteristics of the study participants are summarized in Table 1.
TABLE 1.
Characteristics of study participants
| Characteristic | n (%)* |
|---|---|
| Sex | |
| Male | 1092 (58.6) |
| Female | 770 (41.4) |
| Age, years, mean ± SD | 6.1±2.9 |
| Age group, years | |
| 2–4.99 | 788 (42.3) |
| 5–8.99 | 727 (39.1) |
| 9–13 | 347 (18.6) |
| Ethnicity† | |
| White – Caucasian | 1413 (76.7) |
| African American | 26 (1.4) |
| Hispanic | 25 (1.4) |
| Asian | 163 (8.8) |
| Other‡ | 216 (11.7) |
| Weight category (based on body mass index)† | |
| Healthy weight (5th to <85th percentile) | 1129 (67.4) |
| Underweight (<5th percentile) | 208 (12.4) |
| Overweight (85th to <95th percentile) | 183 (10.9) |
| Obese (≥95th percentile) | 156 (9.3) |
| Season of data collection | |
| October to March | 978 (52.5) |
| April to September | 884 (47.5) |
| Vitamin D status (serum 25[OH]D concentrations [nmol/L]) | |
| Deficient (<25 nmol/L) | 29 (2.0) |
| Insufficient (25 nmol/L to <75 nmol/L) | 539 (37.4) |
| Optimal (75 nmol/L to 225 nmol/L) | 867 (60.1) |
| Potential adverse effects (>225 nmol/L) | 7 (0.5) |
| Stayed in same city†,§ | |
| Yes | 1705 (96.8) |
| No | 56 (3.2) |
| Parental education† | |
| High school completion | 415 (23.4) |
| College certificate/diploma | 599 (33.8) |
| University undergraduate | 208 (11.7) |
| University graduate | 384 (21.7) |
| Other | 165 (9.4) |
| Daily vitamin D intake¶, IU | |
| Mean ± SD | 229.3±121.2 |
| <200 | 637 (40.2) |
| ≥200 | 949 (59.8) |
| <400 | 1441 (90.9) |
| ≥400 | 145 (9.1) |
| Daily calcium intake, mg, mean ± SD | 960.9±441.1 |
| Used multivitamin regularly† | |
| Yes | 10 (0.5) |
| No | 1837 (99.5) |
| Hours spent outdoors in past 30 days, mean ± SD | 55.6±58.2 |
| Sunscreen use† | |
| No | 654 (38.1) |
| Yes | 1065 (61.9) |
| Skin colour†,** | |
| Fair/light | 1485 (82.2) |
| Medium brown | 284 (15.7) |
| Dark | 38 (2.1) |
Unless otherwise indicated;
In some categories, data were not available for all participants;
Of the 216 participants who answered ‘Other’, 36 (16.7%) were Aboriginal, 54 (25.0%) were mixed (ie, parents of different ethnicities), 43 (19.9%) declared themselves as Lebanese, Middle Eastern, Sudanese, Métis or Arab, and 83 (38.4%) did not specify;
Stayed in the same city in the past six months;
Refers to daily intake of vitamin D from dietary sources in the one month before data collection (assessed using the Harvard Service Food Frequency Questionnaire);
The skin colour was subjectively recorded by the research assistant. 25(OH)D 25-hydroxyvitamin D
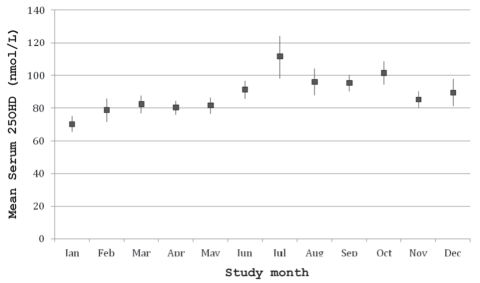
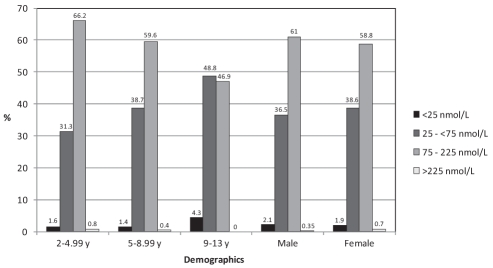
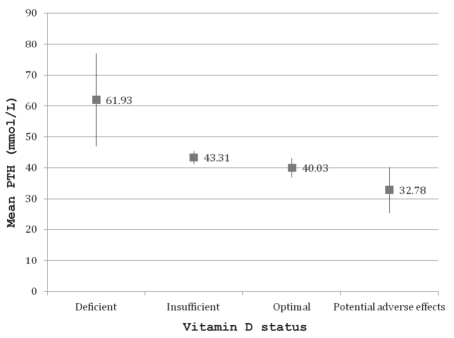
The mean serum 25(OH)D concentration was 86.1±35.1 nmol/L (range 10 nmol/L to 323 nmol/L), and the distribution by month of data collection is shown in Figure 1. The mean serum concentrations for PTH, calcium, phosphate, albumin and creatinine were within the normal range. Twenty-nine children (2.0%) were vitamin D deficient, 539 (37.4%) were vitamin D insufficient, 867 (60.1%) had optimal vitamin D and seven (0.5%) had serum 25(OH)D concentrations above the conventional reference range. Suboptimal vitamin D status (25[OH]D concentration of lower than 75 nmol/L) was present in 568 subjects (39.4%). The prevalence of each category of vitamin D status in the different age and sex groups is displayed in Figure 2. Among the children with serum 25(OH)D concentrations of greater than 225 nmol/L, only one had a dietary vitamin D intake of 400 IU/day or greater, and this child’s sunscreen use and time spent outdoors were similar to those of the other participants. The concentrations from October through March (83.2±36.6 nmol/L) were significantly lower than those from April through September (89.23±33.1 nmol/L, P=0.0011). The mean daily vitamin D intake from dietary sources was 229.3±121.2 IU; only 145 children (9.1%) received the daily recommended amount of vitamin D (400 IU or greater) from their diet. Ten children (0.5%) reported taking a daily multivitamin, but the exact amount of vitamin D in the supplement was unspecified. The mean serum PTH concentrations by vitamin D status are displayed in Figure 3. The vitamin D-deficient group had a significantly higher mean serum PTH concentration than the other groups (P=0.0179).
Figure 1).
Mean serum 25-hydroxyvitamin D (25[OH]D) concentrations and corresponding 95% CIs by study month
Figure 2).
The prevalence of each category of vitamin D status by the different age and sex groups. y Years
Figure 3).
Mean serum intact parathyroid hormone (PTH) concentrations by vitamin D status
Factors associated with suboptimal vitamin D status
Risk factors found to significantly predict suboptimal vitamin D as determined through the multivariate logistic regression included the child’s age, ethnicity, season of data collection, weight, daily dietary intake of vitamin D and number of hours spent outdoors (Table 2). Daily dietary calcium intake, sunscreen use, travel south in the previous six months, and parental education were not predictive of suboptimal vitamin D and not included in the final model.
TABLE 2.
Risk factors for suboptimal vitamin D (serum 25-hydroxyvitamin D of lower than 75 nmol/l) as determined from the multivariate logistic regression model*
| Risk factor† | OR | 95% CI | P |
|---|---|---|---|
| Sex (male) | |||
| Female | 1.15 | 0.89–1.50 | 0.283 |
| Age group, years (2–4.99) | |||
| 5–8.99 | 1.53 | 1.15–2.05 | 0.004 |
| 9–13 | 2.49 | 1.72–3.59 | <0.001 |
| Ethnicity (white – Caucasian) | |||
| Nonwhite | 1.52 | 1.10–2.08 | 0.010 |
| Season (April to September) | |||
| October to March | 1.47 | 1.12–1.93 | 0.005 |
| Weight (healthy weight) | |||
| Underweight | 0.93 | 0.62–1.38 | 0.712 |
| Overweight/obese | 1.77 | 1.28–2.45 | 0.001 |
| Vitamin D intake, IU/day (≥200) | |||
| <200 | 1.34 | 1.05–1.76 | 0.021 |
| Hours spent outdoors | 0.99 | 0.991–0.997 | <0.001 |
Factors that were not predictive of vitamin D inadequacy included daily calcium intake, sunscreen use, travel to southern areas in the past six months and parental education;
Baseline categories are shown in parentheses
DISCUSSION
The present study was the first investigation aimed at determining vitamin D status in a large sample of healthy children living in Calgary. We found a high prevalence (568 subjects, 39.4%) of suboptimal vitamin D status among the paediatric participants. Only 29 children (2.0%) were vitamin D deficient, which was lower than previous reports (21,29). In 2005, Roth et al (29) recruited 90 children two to 16 years of age from Edmonton, Alberta; the mean serum 25(OH)D was 47.2 nmol/L and 6% had 25(OH)D concentrations of lower than 25 nmol/L. The difference could be related to the substantially larger sample size in the present study. Because Roth et al recruited children who presented to the emergency department, it is conceivable that they might have had associated medical conditions that interfered with vitamin D status. Another explanation may be interlaboratory variations in the 25(OH)D assays. In 2003, using the same definition for vitamin D deficiency as in our study, Cheng et al (21) found that 32% of 193 Finnish girls 10 to 12 years of age were vitamin D deficient. The girls in the vitamin D-deficient group also had significantly higher intact PTH concentrations.
Our study showed a lower prevalence of suboptimal vitamin D compared with a recent study (30) in the United States, which reported 53% of children one to five years of age and 73% of children six to 11 years of age with serum 25(OH)D concentrations of lower than 75 nmol/L. Aside from interlaboratory variation, the better vitamin D status of the Calgary children may also be due to socioeconomic and racial differences between the two populations.
Consistent with previous research, we found that the serum 25(OH)D concentrations were lower in the winter than in the summer. In 2004, Gordon et al (19) assessed the vitamin D status among healthy adolescents (11 to 18 years of age) who presented consecutively to the Children’s Hospital Boston (Massachusetts, USA) outpatient clinic for routine bloodwork. They reported that 24.1% had serum 25(OH)D concentrations of lower than 37.5 nmol/L. During the winter, the serum 25(OH)D concentrations were significantly lower (50.3±24.7 nmol/L) than values obtained during the summer (65.5±28.0 nmol/L). We found that even in the summer, 231 children (33.2%) had suboptimal vitamin D concentrations, which suggests that more sun exposure and/or higher vitamin D intake may be necessary.
Older children in our study were more likely to have suboptimal vitamin D status than younger children. The differences were even more striking during the winter. A study from Quebec showed similar findings. The prevalence of vitamin D deficiency (serum 25[OH]D 27.5 nmol/L or lower) and hypovitaminosis (25[OH]D 37.5 nmol/L or lower) increased significantly with age (31).
Consistent with other studies (22,31,32), we found an inverse relationship between vitamin D concentrations and increased adiposity. Overweight or obese children were more likely to have suboptimal vitamin D status than children with healthy weight. This finding remained significant even after controlling for daily vitamin D intake and time spent outdoors. BMI was also shown to be negatively associated with serum 25(OH)D concentrations in adult subjects (33). These results suggest that increased adipose tissue can sequester vitamin D, leading to lower serum concentrations.
Consistent with the results of previous studies, we found that being nonwhite was a significant predictor of suboptimal vitamin D. In 2009, Mansbach et al (30) found a higher prevalence of serum 25(OH)D concentrations of lower than 75 nmol/L in black and Hispanic children compared with non-Hispanic white children. Also in 2009, Winters et al (34) reported that mean 25(OH)D concentrations were significantly lower in African-American women than in white women. In 2007, Ward et al (3) reported that 89% of the children with vitamin D-deficiency rickets had intermediate or darker skin tone. This was possibly due to reduced conversion of vitamin D in individuals with darker skin and reduced skin exposure to the sun due to cultural influences regarding clothing.
Recently, the American Academy of Pediatrics (35) recommended an adequate intake of vitamin D of 400 IU/day to prevent vitamin D deficiency and rickets. We found that only 145 participants (9.1%) received the recommended 400 IU/day of vitamin D from their diet. The question remains whether a daily intake of 400 IU is enough for all children regardless of their sunlight exposure, skin pigmentation and sunscreen use. We determined that 29% of the children who received 400 IU/day of vitamin D had serum 25(OH)D concentrations of lower than 75 nmol/L, which suggests that this recommended amount may not be adequate for maintaining optimal vitamin D status. Because the risk for suboptimal vitamin D increases with age, vitamin D requirements in children may need to be considered on an IU/kg basis. Several experts have advocated for a much higher oral vitamin D intake (1000 IU/day to 4000 IU/day) than what is currently recommended for maintaining adequate circulating 25(OH)D concentrations in the absence of adequate ultraviolet light exposure in both children and adults (9). The Canadian Paediatric Society recommends a vitamin D intake of 400 IU/day from all sources for healthy term infants, with a further increase to 800 IU/day during winter months (November to March) for those who live at latitudes above 55°N or within communities where deficiency is known to be common (26). There are fewer data for older children and adolescents, but the same recommendations are made for these age groups while awaiting further research.
Limitations
Our study had several limitations. The study sample was a convenient sample of children who presented for elective surgery at the Alberta Children’s Hospital and not of randomly selected healthy children in the community. However, the types of surgeries included in the present study were minor in nature and likely did not have a significant impact on the participants’ vitamin D status. Owing to selective surgeries, our study had a larger number of boys than girls, and the older age group (nine to 13 years) was under-represented. The diet questionnaire was largely based on parent self-report, and included food items used in the United States, which could potentially have had different vitamin supplementation than items consumed in Canada. Because the Harvard Service Food Frequency Questionnaire does not ask for specific brands of multivitamins, the reliance on parent self-report can also lead to imprecise estimations of the amount of vitamin D present in the supplement. However, it is reassuring that the mean daily dietary vitamin D intake in our study was similar to other Canadian reports (29,36).
CONCLUSION
Our findings have several important implications for health promotion. To follow vitamin D status longitudinally and to compare values from different geographical regions, it would be helpful to have a standardized method for measuring 25(OH)D concentrations across Canada. Second, to ensure optimal bone health, health care providers should encourage children to consume a diet sufficient in vitamin D content, especially for those with identified risk factors. Given that suboptimal vitamin D is prevalent even in children with dietary vitamin D intake at the current recommended dose, achieving optimal vitamin D concentrations may require a greater amount of vitamin D supplementation, especially for those with a higher BMI.
Evidence-based categorization of vitamin D status, based on functional outcomes, is needed in children and, while results of the present study suggest that Canadian children need more vitamin D, further research is needed to make better recommendations regarding optimal vitamin D intake.
Acknowledgments
The authors thank all the parents and children who participated in this study. They are also grateful to all the paediatric anesthesiologists and nurses in the operating room at the Alberta Children’s Hospital, as well as their research assistants (Edit Goia and Natarie Liu) for their support. This study was funded by the Alberta Children’s Hospital Foundation.
REFERENCES
- 1.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans J. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–7. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 2.Rucker D, Allan JA, Fick GH, Hanley DA. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ. 2002;166:1517–24. [PMC free article] [PubMed] [Google Scholar]
- 3.Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ. 2007;177:161–6. doi: 10.1503/cmaj.061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80(Suppl):1706S–9S. doi: 10.1093/ajcn/80.6.1706S. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 7.Weaver CM, Fleet JC. Vitamin D requirements: Current and future. Am J Clin Nutr. 2004;80(Suppl):1735S–9S. doi: 10.1093/ajcn/80.6.1735S. [DOI] [PubMed] [Google Scholar]
- 8.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: A systematic review. Am J Clin Nutr. 2009;89(Suppl):1997S–2008S. doi: 10.3945/ajcn.2009.27230D. [DOI] [PubMed] [Google Scholar]
- 9.Zittermann A. Vitamin D in preventive medicine: Are we ignoring the evidence? British J Nutr. 2003;89:552–72. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 10.Heaney RP. Vitamin D and calcium interactions: Functional outcomes. Am J Clin Nutr. 2008;88(Suppl):541S–4S. doi: 10.1093/ajcn/88.2.541S. [DOI] [PubMed] [Google Scholar]
- 11.Buison AM, Kawchak DA, Schall J, Ohene-Frempong K, Stallings VA, Zemel BS. Low vitamin D status in children with sickle cell disease. J Pediatr. 2004;145:622–7. doi: 10.1016/j.jpeds.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 12.Sentongo TA, Seamaeo EJ, Stettler N, Piccoli DA, Stallings VA, Zemel BS. Vitamin D status in children, adolescents and young adults with Crohn disease. Am J Clin Nutr. 2002;76:1077–81. doi: 10.1093/ajcn/76.5.1077. [DOI] [PubMed] [Google Scholar]
- 13.Grey V, Lands L, Pall H, Drury D. Monitoring of 25-OH vitamin D levels in children with cystic fibrosis. J Ped Gastroenterol Nutr. 2000;30:314–9. doi: 10.1097/00005176-200003000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Feranchak AP, Sontag MK, Wagener JS, Hammond KB, Accurso FJ, Sokol RJ. Prospective, long-term study of fat-soluble vitamin status in children with cystic fibrosis identified by newborn screen. J Pediatr. 1999;135:601–10. doi: 10.1016/s0022-3476(99)70059-4. [DOI] [PubMed] [Google Scholar]
- 15.Greenway A, Zacharin M. Vitamin D status in chronically ill or disabled children in Victoria. J Pediatr Child Health. 2003;39:543–7. doi: 10.1046/j.1440-1754.2003.00211.x. [DOI] [PubMed] [Google Scholar]
- 16.Gottschlich MM, Mayes T, Khoury J, Warden GD. Hypovitaminosis D in acutely injured pediatric burn patients. J Am Diet Assoc. 2004;104:931–41. doi: 10.1016/j.jada.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Gessner BD, Plotnik J, Muth PT. 25-Hydroxyvitamin D levels among healthy children in Alaska. J Pediatr. 2003;143:434–7. doi: 10.1067/S0022-3476(03)00410-4. [DOI] [PubMed] [Google Scholar]
- 18.Lebrun JB, Moffatt ME, Mundy RJ, et al. Vitamin D deficiency in a Manitoba community. Can J Public Health. 1993;84:394–6. [PubMed] [Google Scholar]
- 19.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans J. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–7. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 20.Green TJ, Skeaff CM, Rockell JE, Taylor JR, Whiting SJ. Serum 25-hydroxyvitamin D status in New Zealand children. Asia Pac J Clin Nutr. 2004;13(Suppl):S46. (Abst) [Google Scholar]
- 21.Cheng S, Tylavsky F, Kroger H, et al. Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr. 2003;78:485–92. doi: 10.1093/ajcn/78.3.485. [DOI] [PubMed] [Google Scholar]
- 22.Kumar J, Munter P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124:e362–70. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockett HR, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc. 1995;95:336–40. doi: 10.1016/S0002-8223(95)00086-0. [DOI] [PubMed] [Google Scholar]
- 24.Rockett HR, Breitenbach M, Frazier AL, et al. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26:808–16. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention Healthy Weight – it’s not a diet, it’s a lifestyle! < http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html> (Accessed on January 21, 2011).
- 26.Canadian Paediatric Society Vitamin D supplementation: Recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12:583–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3; exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards . How to define and determine reference intervals in the clinical laboratory; Approved guideline – second edition. NCCLS document C28–A2. Wayne: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 29.Roth DE, Martz P, Yeo R, Prosser C, Bell M, Jones AB. Are national vitamin D guidelines sufficient to maintain adequate blood levels in children? Can J Public Health. 2005;96:443–9. doi: 10.1007/BF03405185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansbach JM, Ginde AA, Camargo CA. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: Do children need more vitamin D? Pediatrics. 2009;124:1404–10. doi: 10.1542/peds.2008-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mark S, Gray-Donald K, Delvin EE, et al. Low vitamin D status in a representative sample of youth from Quebec, Canada. Clin Chem. 2008;54:1283–9. doi: 10.1373/clinchem.2008.104158. [DOI] [PubMed] [Google Scholar]
- 32.Alemzadeh R. Hypovitaminosis D in obese children and adolescents: Relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57:183–91. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Ramel A, Jonsson PV, Bjornsson S, Thorsdottir I. Vitamin D deficiency and nutritional status in elderly hospitalized subjects in Iceland. Public Health Nutrition. 2009;12:1001–5. doi: 10.1017/S1368980008004527. [DOI] [PubMed] [Google Scholar]
- 34.Winters SJ, Chennubhatla R, Wang C, Miller JJ. Influence of obesity on vitamin D-binding protein and 25-hydroxyvitamin D levels in African American and white women. Metabolism. 2009;58:438–42. doi: 10.1016/j.metabol.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 35.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, on behalf of the Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics. 2008;122:398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 36.Gillis L, Gillis A. Nutrient inadequacy in obese and non-obese youth. Can J Diet Pract Res. 2005;66:237–42. doi: 10.3148/66.4.2005.237. [DOI] [PubMed] [Google Scholar]