Abstract
Determining the activity of a regulatory T-cell population in vitro is often the first step in analyzing its function. To obtain reliable and reproducible results, it is critical to follow the protocol that is most applicable to your experimental question. We have outlined below a basic in vitro suppression assay as well as a variety of alternative/additional protocols that can be utilized alone or in combination as desired.
Keywords: Treg, In vitro, Suppression, Foxp3
1. Introduction
The first in vitro assays to measure regulatory T-cell (Treg) function were described by two groups over a decade ago (1, 2). The observation that a CD25+ T-cell population possessed regulatory activity enabled isolation of natural Tregs cells from mice and humans. With this knowledge, it was shown that CD4+CD25+ T cells could potently suppress the proliferation of activated CD4+CD25− and CD8+ T cells when the populations were cocultured in vitro. In vitro suppression assays are now widely used to determine the suppressive capacity of Tregs. The benefits of this assay include ease and simplicity of setup and reliability. In addition, few reagents are needed to perform the basic protocol, making it an appropriate initial test of suppressive capacity. Given that conventional T cells (Tconv) and Tregs can be purified from genetically deficient mice, the role that individual molecules play in suppression can easily be determined. In addition, ex vivo suppressive capacity of Tregs obtained from normal or diseased patients can provide information regarding immunocompetance. Lastly, due to the simplicity of assay setup, numerous variables including type of activation, cell number, and degree of proliferation can be manipulated within a single experiment. The primary weakness of in vitro Treg suppression assays is that they do not necessarily recapitulate in vivo processes. In vivo, Tregs are strongly proliferative, yet in vitro Tregs are hypoproliferative in response to antigenic stimulation (1, 2). Another limitation is that antigen-specific assays are limited due to reduced numbers of antigen-specific Tregs that can be purified following immunologic response to a specific pathogen or disease state. For this reason, polyclonal Tregs are typically assayed for their ability to suppress Tconv cell proliferation. Finally, the use of in vitro suppression assays lead to the conclusion that Tregs suppress in a cytokine-dependent manner, yet the role of soluble factors in Treg-mediated suppression in vivo is clear (3-9). Fortunately, however, a new variation of the standard in vitro Treg suppression assay has been developed that demonstrates the importance of soluble factors in Treg-mediated suppression (10).
2. Materials
2.1. Basic Protocol
Murine cell culture media: RPMI (Mediatech) supplemented with 10% FBS (optimal manufacturer and lot to be determined empirically), 2 mM L-glutamine (Mediatech), 1 mM sodium pyruvate (Mediatech), 100 mM non-essential amino acids (Mediatech), 5 mM HEPES free acid (Mediatech), 10 ml of 5.5 × 10−2 2-mercaptoethanol (Invitrogen), and 100 U/ml Penicillin/Streptomycin (Mediatech) (see Note 1).
Human cell culture media: X-VIVO™ 15 Chemically Defined Medium, with gentamicin and phenol red (Lonza) supplemented with 15% male human serum (Lonza) and 10% L-glutamine (Mediatech) (see Note 1).
Gey’s solution for red blood cell lysis: 12 mM potassium bicarbonate (KHCO3), 156 mM ammonium chloride (NH4Cl), diluted in water. Filter sterilize through a 0.2 μm filter to maintain sterility.
Murine anti-CD3 Ab, clone 2c11 (NALE/functional grade) and murine anti-CD28, clone 37.51 (NALE/functional grade).
Human anti-CD3 Ab, clone OKT3 (NALE/functional grade) and human anti-CD28, clone CD28.6 (NALE/functional grade).
Round bottom 96 well tissue culture plate (Nunc).
Purified, azide-free, endotoxin-free anti-CD3 and anti-CD28 antibodies.
5 μM Sulfate latex beads (4% solid) (Molecular Probes).
[3H]-Thymidine (Amersham Biosciences).
70 μM Nylon cell strainer (BD).
50 ml Conical tubes (BD).
Normal mouse serum (Gibco).
Phosphate buffered saline (PBS) (Mediatech).
Hanks Balanced Salt Solution (Mediatech).
1 ml Syringes, use plunger for homogenization.
Fluorescently tagged antibodies (CD4, CD25, CD45RB).
40 μM Nylon cell strainer (BD).
Recombinant human IL-2 (R&D Systems).
Ficoll Paque Plus (GE Healthcare).
Plasma transfer set (Charter Medical).
Phosphate buffer (4.82 g/l monohydrate, monosodium phosphate, pH 6.5).
2.2. Variations of Basic Protocol
Frosted glass microscope slides (Fisher).
1,500 U/ml Collagenase Type III, High specific activity (Worthington).
300 U/ml DNase I, 2,000 U/vial (Sigma).
Anti-CD11c antibody (eBioscience).
Peptides (e.g., Ova3326–339, PCC88–104, or HA110–120 as desired).
PMA and Ionomycin (Calbiochem).
Bovine serum albumin (BSA) (Sigma).
CFSE (carboxyfluorescein succinimidyl ester) or SNARF-1 (Seminaphtharhodafluor) (Molecular Probes).
MTT cell proliferation assay kit (Cayman Chemical).
Transwell: Millicell 96 well receiver plate (Millipore).
Transwell: Millicell 96 cell culture insert plate (0.4 μM) (Millipore).
Foxp3 staining kit and fluorescently conjugated anti-Foxp3 antibody (eBioscience).
3. Methods
3.1. Basic Protocol
The following protocol describes a basic type of in vitro Treg suppression assay where Treg function is measured in the absence of antigen-presenting cells (APCs). In this protocol, activation is mediated by anti-CD3 + anti-CD28 coated beads and, therefore, includes only two cell types, the target Tconv and test Tregs. In this protocol, the experiment is setup in a 96-well round-bottom plate in a total volume of 200 μl. All reagents are prepared at four times their desired final concentration and added to assay in 50 μl such that in the total volume of 200 μl, their concentration will be correct. See Fig. 1a for a 96-well plate layout (see Note 2).
Fig. 1.
Plate diagram for Treg assay. Tregs are titrated into a Tconv cell proliferation assay starting at a 2:1 Tconv:Treg ratio.
Purify Tregs and Tconv from desired source (see Subheading 3.8).
Count Tregs and Tconv and adjust in T-cell culture medium (see Subheading 2.1) to 2.5 × 105/ml and 5 × 105/ml, respectively.
In round-bottom 96-well plate, add 50 μl culture media to wells 1–11 (see Fig. 1b).
Add 100 μl Treg to well 12.
Mix Tregs thoroughly with a pipet and titrate 50 μl of Tregs into well 11 to generate a twofold dilution. For multiple Treg populations, use a multichannel pipet to titrate multiple wells at the same time.
Repeat mixing and titration into successive wells, 50 μl at a time, leaving the well 6 with no Treg to determine maximum proliferation of Tconv.
Add 50 μl Tconv cells to all wells.
Add 100 μl anti-CD3/CD28-coated sulfate latex beads to all wells (see Subheading 3.9).
Incubate plate at 37°C, 5% CO2 for 72 h.
Pulse plates with 0.1 μCi [3H]-thymidine (<!> – Caution: Radioactive material. Institutional approval to handle radioactive materials is required) per well 8 h prior to completion of experiment.
Harvest cultures with a commercial cell harvester and determine counts per minute (cpm) with a direct beta counter (see Notes 3 and 4).
3.2. Variations of Basic Protocol: Antigen Presenting Cell Activation
1. Murine APC activation of Tconv cell proliferation
Irradiated splenocytes or purified dendritic cells combined with soluble anti-CD3 or peptide may be substituted for anti-CD3 + anti-CD28 coated beads to stimulate Tconv cell proliferation. The benefit of using APCs is the more physiological activation of Tconv cells. However, these cells add an additional variable to the assay in that APCs may also mediate/modulate both Tconv and Treg cell function and must be considered when interpreting results. It is important to ensure that only Tconv cell proliferation is measured and that irradiated splenocytes and Tregs do not contribute to the proliferation observed. To this end, control wells containing (a) APCs alone + antigen (or antibody) and (b) Tregs + antigen (or antibody) must be included in all experiments (see Note 5).
Splenocytes as APCs: Make a single cell suspension of splenocytes by homogenizing spleen with a 1 ml syringe through a 0.7 μM filter into a 50 ml conical tube. Alternatively, splenocytes may be homogenized between two frosted glass microscope slides. Following homogenization, lyse red blood cells using commercial lysis solution or Gey’s solution (see Subheading 2.1). Quench lysis reaction with 10 ml HBSS. Irradiate splenocytes using 3,000 rads (<!> – Caution: Institutional approval to irradiate materials is required).
Dendritic cells as APCs: Make 10× digestion mix by dissolving 2 vials of Collagenase and 5 vials of DNase in 32 ml PBS, filter sterilize, and freeze in 4 ml aliquots (−20°C). Cut spleen into small pieces using sterile scissors. Digest spleen with 4 ml/spleen of RPMI medium containing 5% Fetal Bovine Serum and 10% digestion mix. Incubate 1 h in 37°C shaking water bath. Homogenize through a 0.7 μM filter into a 50 ml conical tube. Lyse red blood cells with commercial lysis solution or Gey’s solution (see Subheading 2.1) and stain cells with a fluorescently conjugated anti-CD11c antibody for purification by FACS.
Resuspend splenocytes (for protocol 1) at 1 × 106/ml or DCs (for protocol 2) at 1 × 105/ml and add anti-CD3 antibody at 1 μg/ml.
Omit anti-CD3 + anti-CD28 beads in basic protocol and replace with 50 μl each APCs and soluble anti-CD3 antibody in all wells.
Add Tconv and titrations of Treg cells to wells as described in Subheading 2.1 (see Note 6).
2. Human APC activation of Tconv cell proliferation
For assays with human cord blood or PBMC derived Tconv, irradiated PBMCs can be used as antigen-presenting cells in a standard mixed lymphocyte reaction. Assays are to be performed in a 96-well round-bottom plate in a final volume of 200 μl of complete medium.
Add Tconv and titrations of Treg cells to wells as described in Subheading 2.1.
Irradiate allogeneic PBMCs or unmanipulated cord blood cells with 2,500 rads (<!> – Caution: Institutional approval to irradiate materials is required) and suspend at 1 × 106/ml.
-
Add 50 μl PBMCs or cord blood cells per well to serve as APCs.
Alternatively, irradiated syngeneic PBMCs can be cultured with anti-CD3 (OKT3) peptide to activate Tconv cells.
3. Murine antigen-specific suppression assays
Suppression of antigen-specific responses can be determined by utilizing murine TCR transgenic Tconv and Treg cells instead of a polyclonal T-cell population. The benefit of this variation to the basic protocol is that monoclonal or polyclonal Tregs as well as Tconv cells and Tregs with a variety of specificities can be utilized in suppression assays. However, many TCR transgenic mice have limited numbers of clonotype positive Tregs, which has to be considered when designing these experiments (e.g., on a Rag1−/− background, OTII transgenic mice have none, while AND transgenic mice have ~10% of normal Treg numbers (11). However, the use of endogenous TCR chains often endows Tregs from TCR transgenic mice with potent peptide specific regulatory capacity.
Prepare irradiated splenocytes for culture as described above.
Dilute cognate antigen in media at desired concentration (0.1–10 μg/ml). For example, T cells from OTII, AND, or 6.5 TCR transgenic mice are cultured with their cognate antigen: Ova326–339, PCC88–104, or HA110–120, respectively (see Note 7).
Omit anti-CD3 + anti-CD28 beads in basic protocol and replace with 50 μl each APCs and cognate antigen in all wells.
3.3. Variations of Basic Protocol: Treg Activation State
Recent studies using pre-activated Treg have contributed to our understanding of the characteristics and conditions required for Treg to suppress Tconv proliferation (1, 12). Reports indicate that previously activated Tregs do not require restimulation through their TCR to suppress Tconv proliferation (12). Freshly isolated Tregs can be utilized for a number of protocols; however, activated or expanded Tregs are sometimes desired. Pre-activated murine Tregs have been shown to have superior suppressive capacity when compared to naïve, freshly purified Tregs. Moreover, human cord blood Tregs are naïve and require activation to suppress Tconv cell proliferation effectively. For this reason, it is sometimes advisable to activate Tregs prior to assaying. In addition, when Tregs numbers are limiting, they can be expanded in vitro to obtain greater numbers of cells.
Freshly isolated Tregs can be directly assayed for regulatory capacity as described in Subheading 2.1.
Alternatively: “Pre-Activated” Tregs can be generated and used in assays by activating purified Tregs for 24 h at 5 × 105/ml in a 96-well round-bottom plate containing anti-CD3 (1 μg/ml) and anti-CD28 (2 μg/ml). Following activation, Tregs should be washed and adjusted to 2.5 × 105/ml for use in suppression assays (as described in Subheading 2.1).
Murine Treg expansion: Several murine Treg in vitro expansion protocols have been described. This could be useful when the number of purified Tregs is very limited, such as when isolated from sites of infection, tumors, or autoimmune lesions (see Chapter 9).
Human Treg expansion: Human Tregs are activated at a density of 5 × 105 cells/ml in a 24-well plate in complete X-VIVO 15 media supplemented with anti-CD3/anti-CD28 coated beads at a 3:1 (bead:cell) ratio and 500 IU of IL-2. Cells are passaged to maintain cell density of 5 × 105cells/ml. Following 10 days culture, Treg expansion is approximately 20-fold. Expanded Tregs maintain FoxP3 expression and suppressive capacity.
3.4. Variations of Basic Protocol: MTT Assay as a Readout of Suppression
Suppression of proliferation can be monitored without the use of radioisotopes or fluorescence chemistries by using Cayman Chemical’s MTT Cell Prolilferation Assay Kit. This method utilizes the reduction of MTT reagent by intracellular NAD(P)H oxidoreductases as a measure of cellular proliferation.
Reagent Preparation: Dissolve the Cell Based Assay Buffer tablet in 100 ml of distilled water. Prepare MTT reagent by dissolving the 25 mg vial of reagent in 5 ml Assay Buffer. Store at 4°C.
Approximately 4 h prior to completion of assay: Add 20 μl MTT reagent to each well, mix gently, and return to incubator.
Allow cells to reduce MTT reagent for 4 h. Formazan produced by the cells will appear as purple/black dots in the wells.
Centrifuge the plate at 400 × g for 10 min to pellet the cells. Aspirate supernatant.
Add 100 μl of Crystal Dissolving Detergent Solution to the wells and pipet to mix.
Measure the absorbance of the samples at 570 nm using a microplate reader.
3.5. Variations of Basic Protocol: CFSE as a Readout of Suppression
Suppression of proliferation can be monitored without the use of radioisotopes by monitoring the dilution of a green fluorochrome ester CFSE, or the red alternative, SNARF-1 (Seminaphtharhodafluor) by flow cytometry. In addition, CFSE analysis allows for the determination of the number of cell divisions with or without Treg suppression.
Reagent Preparation: Prepare solution of sterile PBS + 0.1% BSA to use as a diluent. Prepare single use aliquots of CFSE and store at −20°C.
Wash Tconv cells once with PBS.
Resuspend cells at 2–3 × 106/ml in PBS + 0.1% BSA and keep on ice.
Prepare 8 μM CFSE in PBS + 0.1% BSA. Discard unused CFSE solution.
While vortexing cells, add volume of CFSE solution equivalent to volume of cells (i.e., for 2 × 106 cells, resuspend in 1 ml PB and add 1 ml CFSE solution).
Incubate at room temperature without agitation for 10 min.
While vortexing cells, quench reaction as quickly as possible with three times the staining volume of ice-cold FBS (i.e., 2 ml staining volume, add 6 ml FBS).
Put on ice immediately for 2 min.
Wash cells two times with 10 ml T-cell culture medium, centrifuging at 300 × g for 10 min in between washes.
Count CFSE labeled Tconv cells, resuspend at 5 × 105/ml, and add to suppression assay as described in Subheading 2.1.
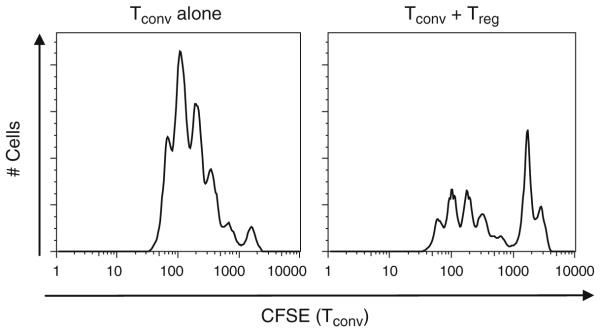
Analyze proliferation as determined by CFSE dilution on a cytometer. See Fig. 2 for a representative flow cytometric histogram of CFSE dilution of Tconv in the presence and absence of Tregs (see Notes 8 and 9).
Fig. 2.
Treg-mediated suppression as measured by carboxyfluorescein succinimidyl ester (CFSE) dilution. Tconv were isolated from C57BL/6 mice and labeled with 5 μM CFSE. Cells were activated with anti-CD3 + anti-CD28 coated beads and cultured either alone or in the presence of Tregs at a 4:1 Tconv:Treg ratio. After 72 h, proliferation was determined by CFSE dilution and flow cytometric analysis.
3.6. Reporting Data as cpm Versus Percent Suppression
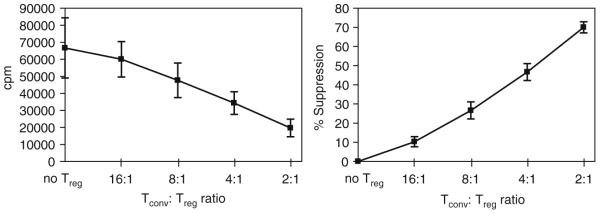
The results of in vitro Treg suppression assays are most commonly reported as cpm when [3H]-thymidine is incorporated into proliferating cells. Wells containing both Tconv and Tregs will have lower cpm than wells that contain Tconv cells alone because coculture of Tregs with Tconv cells reduces the proliferative capacity of Tconv cells. In addition, as the ratio of Tconv cells to Treg increases, the cpm values will increase proportionately. As Tregs proliferate very poorly in vitro, they do not contribute significantly to cpm values. Control wells containing activated Tregs and no Tconv cells should have cpm values of less than 1,000, similar to that seen in wells containing unstimulated Tconv. Due to day to day or sample to sample variability, experimental replicates will often not result in identical cpm values. For this reason, a percent suppression (% supp) calculation assay can be graphed in order to depict many experiments with slightly (or significantly) different cpm values. Percent suppression can be calculated using the following formula: ((cpm of Tconv cells alone – cpm of Tconv cells treated with Treg)/cpm of Tconv cells alone)*100. Alternatively, a representative experiment can be depicted with cpm. The data graphed are the same; however, the graphs will appear differently (see Fig. 3 for examples).
Fig. 3.
Treg-mediated suppression. Treg cells were purified by FACS and mixed at different ratios with naïve wild type Tconv cells and anti-CD3 + anti-CD28 coated beads for 72 h. Proliferation was determined by [3H]-thymidine incorporation.
Statistical Analysis of Results
To determine statistical significance between groups, a variety of different statistical methods can be used. For comparisons of two samples, an unpaired T test can be used. For this analysis, a two-tailed p value with a confidence interval of 95% is recommended. For analyses of three or more samples, one-way ANOVA with a confidence interval of 95% is recommended.
3.7. Variations of Basic Protocol: Transwell Suppression Assay
The importance of cytokines in mediating in vitro Treg suppression has been controversial. Neutralizing IL-10 and TGFβ in a conventional in vitro Treg assays does not inhibit suppression by Treg, suggesting that these cytokines are not required for Treg-mediated suppression in vitro (1, 2, 13, 14). However, cytokines are critical for Treg-mediated suppression in vivo (3-9), making it difficult to reconcile these differential requirements. By using a specialized 96 well plate in which a permeable membrane called a Transwell membrane is inserted, cells can be separated from one another via a membrane that permits exchange of soluble molecules between cells but does not allow cell–cell contact. Addition of Tconv and Treg alone or in combination on either side of the Transwell membrane allows one to permit cell contact between populations as desired (see Fig. 4 for a Transwell plate diagram).
Fig. 4.

Transwell plate setup. Tconv cell proliferation in the lower well of a Transwell plate can be suppressed Treg cells in the top well of a Transwell plate when they are activated in the presence of Tconv cells. Proliferation of lower well Tconv cells is determined by [3H]-thymidine incorporation.
Add freshly purified Tconv cells (5 × 104/well) in 50 μl media in the bottom chamber of a 96 well receiver plate.
Add 50 μl anti-CD3/CD28 coated sulfate latex beads to all bottom wells (see Subheading 3.9).
Add 50 μl T cell culture media to bring all wells to a final volume of 200 μl.
Gently insert 0.4 μM Transwell membrane into bottom chamber of receiver plate.
Add cells that are to be tested for regulatory capacity to the top chamber wells. (ex) Tconv and Treg either alone at 1.25 × 104/well or coculture at a ratio of 4:1 with a total of 2.5 × 104 cells in top chamber.
Add 50 μl anti-CD3/CD28 coated sulfate latex beads to all top wells.
Where necessary, add T-cell culture media to bring top wells to a final volume of 150 μl.
After 64 h in culture, remove top chambers, and add [3H]-thymidine directly to the responder Tconv cells in the bottom chambers of the original receiver plate.
Harvest as described above (see Note 10).
3.8. Purification of Tconv Cells and Tregs for Assay
An important difference between murine and human in vitro Treg suppression assays is the source of cells. Murine Tconv and Treg are predominantly purified from spleens and lymph nodes on the basis of CD25 expression. However, human Tconv and Treg can be isolated from peripheral blood (from PBMCs or apheresis rings, depending upon availability) or umbilical cord blood. In human peripheral blood, suppressive capacity is not associated with all CD25+ cells, as it is in the mouse, but instead with the brightest subset of CD25+ cells (termed CD25bright). Another complication with using peripheral blood Tconv and Tregs is that unlike in the mouse, Foxp3 can be expressed in both Treg and activated Tconv, making classification and purity analysis difficult. For this reason, a number of additional cell surface markers have been used to help purify peripheral blood Tconv and Treg, with relative degrees of success. For detailed information regarding purification, subsets of human Tregs, and alternative cell surface markers for identification of Tregs, see refs. (15-18). An alternative source of human Tconv and Treg is umbilical cord blood. Unlike peripheral T cells, cord blood Tconv have not encountered any peripheral antigen; therefore, CD25 expression is a much better marker for Tregs. In addition, both Foxp3 expression and suppressive capacity are exclusively within the CD25+ population. The complications with using umbilical cord blood samples are (1) access to samples (2) both Tconv and Treg are antigen inexperienced as they have never entered peripheral circulation. For this reason, additional manipulation is required; IL-2 supplementation to achieve strong proliferation of Tconv and pre-activation for maximum suppressive capacity of Tregs.
1. Purification of murine Tconv/Treg (CD4, CD45RB, CD25)
Murine Tconv and Treg can be separated using only CD4 and CD25 markers. However, by also staining with CD45RB, naïve Tconv can be separated from memory Tconv and Treg, resulting in better purity of both populations. A similar strategy can be utilized by staining cells with CD44 and CD62L, where CD44low, CD62Lhigh populations represent the naïve Tconv cells. The only disadvantages with this staining is that an additional fluorochrome-conjugated antibody is needed that adds to the expense of purification as well as utilizing another fluorescent channel (thus eliminating this flow cytometer channel for staining for downstream applications such as intracellular staining).
Harvest spleen and lymph nodes from mice.
-
Homogenize tissue with a 1-ml syringe through a 70-μm cell strainer into a 50-ml conical tube. Rinse strainer two times with HBSS to recover all cells.
Alternatively, splenocytes may be homogenized between two frosted glass microscope slides.
Centrifuge homogenate at 300 × g for 10 min.
Resuspend homogenate in 1 ml Gey’s solution (see Subheading 2.1) per spleen. Gently swirl for 2 min and then quench reaction by adding 12 ml of HBSS.
Centrifuge at 300 × g for 10 min.
Resuspend cells in blocking solution at 0.5 ml/spleen (10% mouse serum in PBS + 5% FBS).
Incubate cells for 10 min at 4°C.
Add 0.5 ml/spleen fluorescently conjugated antibodies at final concentration of 1:200 for 20–30 min at 4°C, for example, anti-CD4 Alexa 647 (or APC), anti-CD45RB (PE), and anti-CD25 FITC (see Note 11).
Wash cells with 5 ml PBS + 5% FBS. Centrifuge cells at 300 × g for 10 min.
Resuspend cells in PBS + 5% FBS and strain through 40 μm filter.
Purify cells by FACS according to the profile shown in Fig. 5.
Fig. 5.
Tconv/Treg purification. (a) Murine splenocytes were processed and red blood cells lysed prior to staining with anti-CD4, anti-CD25 and anti-CD45RB antibodies. Tconv and Treg were purified by FACS based on the profile shown. (b) Red blood cell depleted murine splenocytes were stained with anti-CD4 and anti-Foxp3 antibodies. In parallel, purified Tconv (CD4+CD25−CD45RBhi) and Treg (CD4+CD25+CD45RBlo) were stained with anti-CD4 and anti-Foxp3 antibodies and % Foxp3+ cells were determined by flow cytometry.
2. Purification of human PBMC or cord blood Tconv/Treg
Obtain PBMCs or cord blood samples (see Note 12).
In hood, wipe down tip of ring or bag with 70% ethanol.
Ensure that the clamp that comes in the unit is secured tightly.
Attach the plasma transfer set to collect blood.
Clamp the set closed and remove the plastic piercing cover.
Open new port of blood unit and insert piercing pin.
Remove female adaptor, open up clamp and pour blood from female adaptor port into 50 ml conical tube(s).
Pellet blood at 1,800 × g for 15 min at room temperature. Discard supernatant (serum).
Resuspend pellet at 1:2.5–3 ratio of pellet volume: PBS.
Overlay 15 ml diluted blood onto 25–30 ml Ficoll. Centrifuge at 1,150 × g for 20 min without brake at room temperature.
After centrifugation, sample will separate into bands (shown in Fig. 6).
Aspirate excess Ficoll into biohazard container.
With 5 ml pipet, slowly remove white lymphocyte layer and put into new 50 ml conical tube.
Fill tube to 50 ml with sterile PBS. Centrifuge at 480 × g for 10 min. Max brake.
Resuspend cells in antibody staining buffer containing anti-CD4 and anti-CD25 at 1:20 dilution.
Incubate on ice for 30 min. Add 5 ml PBS + 5% FBS and centrifuge 480 × g for 10 min with max brake.
Resuspend in PBS + 5% FBS. Filter cells through a 40 μM strainer and purify by FACS (see Note 13).
Fig. 6.

Ficoll gradient for T-cell purification. Depiction of lymphocyte layer following Ficoll separation of cord blood or PBMCs.
3.9. Labeling of Anti-CD3 + Anti-CD28 Coated Latex Beads
-
Make antibody mix in Phosphate Buffer:
Anti-CD3 Ab (NALE/functional grade) – murine 13.3 μg/ml, human 26.6 μg/ml
A nti-CD28 (NALE/functional grade) – murine and human 26.6 μg/ml
Add 750 μl sterile 5 mM phosphate buffer (4.82 g/l monohydrate, monosodium phosphate, pH 6.5).
Incubate 5 μM sulfate latex beads (4% solid) in a 1:4 dilution of antibody mix to make 1% solid. (ex) 250 μl beads + 750 μl antibody mix in a 1.5 ml tube.
Incubate overnight at room temperature either by vortexing or by rotation.
Wash beads three times with Phosphate Buffer, centrifuging at 200 × g to remove buffer between washes.
Count beads with a hemacytometer and resuspend beads at 5 × 107/ml in sterile Phosphate Buffer with 2 mM BSA.
Optimal bead concentration is typically between 3:1 and 10:1 (T cell:bead ratio); however, this must be determined empirically by titrating beads into a proliferation assay prior to suppression assays. Desired Tconv cell proliferation is 40,000–80,000 cpm following 8 h [3H]-thymidine culture for the final 72 h of assay. Alternatively, at least four CFSE peaks should be visible by flow cytometry following 72 h assay (e.g., see Fig. 2).
3.10. Foxp3 Staining to Determine Purity
To ensure purity of isolated Tconv and Tregs, Foxp3 staining of cells before and after purification should be performed (e.g., see Fig. 5). The Foxp3 staining kit manufactured by eBioscience provides all of the reagents needed for optimal Foxp3 staining and is the recommended kit for this purpose.
Add 100 μl of prepared cells (2 × 105/well) to a v-bottom 96 well plate.
Stain surface molecules such as CD4, CD8, CD25, etc. in PBS. Incubate at 4°C for 20 min.
Wash in 50 μl cold PBS, centrifuging at 200 × g for 2 min.
Resuspend cell pellet with pulse vortex and add 100 μl of freshly prepared Fixation/Permeabilization working solution to each sample. Pulse vortex again.
Incubate at 4°C for 30–60 min in the dark.
Wash once by adding 100 μl 1× Permeabilization Buffer (made from 10× Permeabilization Buffer) followed by centrifugation and decanting of supernatant.
Add 100 μl fluorochrome conjugated anti-Foxp3 antibody or isotype control at 1:100 dilution in 1× Permeabilization Buffer and incubate at 4°C for 30 min in the dark.
Wash cells with 200 μl 1× Permeabilization Buffer. Centrifuge and decant supernatant.
Resuspend in appropriate volume of PBS and analyze on cytometer.
4. Notes
The optimal manufacturer and lot number of FBS can vary; therefore, this must be determined empirically. Prior to use in assays, FBS must be heat inactivated for 30 min at 56°C. Following heat inactivation, FBS can be stored at 4°C for up to 1 month.
Sterility during all steps of the protocols is essential. Sterile technique must be followed, and all reagents used including buffers and antibodies must be sterile filtered through a 0.2 μm filter.
Human cord blood Tconv are naïve and require IL-2 supplementation and longer stimulation to obtain optimal proliferation. Therefore, for assays with human cord blood Treg, recombinant human IL-2 is added (10 U/ml) and cultures are harvested after 6 days. Human PBMC derived Tconv are fully capable of responding to stimulation without exogenous IL-2 within the 3 days assay; therefore, no alterations from the basic protocol are needed to perform assays with PBMC derived Tconv.
For large scale isolation of Tregs, or if purification by FACS is not possible, magnetic-based cell separations provide an alternative means of Treg isolation. For a detailed protocol describing purification by MACS of human Tregs, see ref. (19).
When performing APC driven Treg suppression assays, it is imperative to use mice of the same genetic background and sex.
With the addition of APCs and anti-CD3 or peptide, the volume will be 200 μl (50 μl Tconv, 50 μl Tregs, 50 μl APCs, and 50 μl anti-CD3 or peptide); therefore, no additional media should be added to culture wells.
TCR specific T cells are optimally stimulated by different concentrations of peptides. A titration must be done to determine optimal antigen concentration.
To obtain clear CFSE peaks, it is critical that CFSE is intercalated into all cells at the same time, hence the reason for vortexing cells while adding CFSE solution. Furthermore, CFSE quenching must occur quickly, completely, and while vortexing. Deviation from this protocol will yield less clear results.
Tregs are not labeled with CFSE and can, therefore, easily be distinguished from proliferating Tconv cells as a CFSE negative population. The use of Tconv and Tregs with different congenic markers (i.e., Thy1.1 Tconv and Thy1.2 Tregs) can help to distinguish Tconv and Tregs by flow cytometry.
If so desired, Tconv in the top well can be fixed with 4% formaldehyde (<!> Caution: Irritant and suspected carcinogen) in media for 10 min, at room temperature in order to eliminate any contribution of Tconv-derived soluble factors. Tconv should be fixed at 1 × 106 cells/ml and washed four times with 10 ml of fresh media prior to assay. Care must be taken to thoroughly wash cells to eliminate formaldehyde carryover into culture.
Antibodies used can be altered depending upon lasers available, and optimal antibody concentrations must be determined empirically.
Institutional Review Board (IRB) approval must be obtained prior to use unless samples are purchased from commercial sources.
Additional cell surface molecules such as CD127, HLA-DR, etc. may be used in addition to CD4 and CD25, as desired (15-18).
Acknowledgments
We wish to thank members of the Vignali lab for many discussions regarding these methods. We are particularly grateful to Andrea Szymczak-Workman (for advice on anti-CD3/CD28 bead conjugation), Creg Workman and Andrea Szymczak-Workman (set up of murine antigen specific suppression assays), Janice Riberdy (human suppression assay setup), and Sam Connell (CFSE labeling). LWC is supported by an Individual NIH NRSA (F32 AI072816). DAAV is supported by the National Institutes of Health (NIH) (AI39480, AI52199, AI072239), Juvenile Diabetes Research Foundation International (1-2004-141 [The Robert and Janice Compton Research Grant, In Honor of Elizabeth S. Compton] and 1-2006-847), a Cancer Center Support CORE grant (CA21765), and the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 2.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Bandeira A. On the ontogeny and physiology of regulatory T cells. Immunol Rev. 2001;182:5–17. doi: 10.1034/j.1600-065x.2001.1820101.x. [DOI] [PubMed] [Google Scholar]
- 4.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 6.Cavinato RA, Casiraghi F, Azzollini N, Mister M, Pezzotta A, Cassis P, Cugini D, Perico N, Remuzzi G, Noris M. Role of thymic- and graft-dependent mechanisms in tolerance induction to rat kidney transplant by donor PBMC infusion. Kidney Int. 2007;71:1132–1141. doi: 10.1038/sj.ki.5002202. [DOI] [PubMed] [Google Scholar]
- 7.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of allo-responses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 8.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 9.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szymczak-Workman AL, Workman CJ, Vignali DA. Cutting edge: regulatory T cells do not require stimulation through their TCR to suppress. J Immunol. 2009;182:5188–5192. doi: 10.4049/jimmunol.0803123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+) CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 16.Baecher-Allan C, Hafler DA. Suppressor T cells in human diseases. J Exp Med. 2004;200:273–276. doi: 10.1084/jem.20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wichlan DG, Roddam PL, Eldridge P, Handgretinger R, Riberdy JM. Efficient and reproducible large-scale isolation of human CD4+ CD25+ regulatory T cells with potent suppressor activity. J Immunol Methods. 2006;315:27–36. doi: 10.1016/j.jim.2006.06.014. [DOI] [PubMed] [Google Scholar]