Abstract
Inducible nitric oxide synthase (NOS2) is an inflammation responsive enzyme (EC 1.14.13.39) that is induced during acute and chronic inflammation and tissue injury as part of the host defense and wound healing process. NOS2 upregulation leads to increased nitric oxide (NO) production, the means by which this enzyme can initiate NO-dependent signal transduction, influence the redox state of cells and induce modifications of proteins, lipids and DNA. Aberrant expression of NOS2 has been observed in many types of human tumors. In breast cancer, increased NOS2 is associated with markers of poor outcome and decreased survival. Growth factor and cytokine signaling, tissue remodeling, NFκB activation and hypoxia are candidate mechanisms that induce NOS2 in tumor epithelial and tumor-infiltrating cells. NOS2 induction will trigger the release of variable amounts of NO into the tumor microenvironment and can activate oncogenic pathways, including the Akt, epidermal growth factor receptor and c-Myc signaling pathways, and stimulate tumor microvascularization. Constitutively increased NO levels may also select for mutant p53 cells to overcome the tumor suppressor function of NO-activated wild-type p53. More recent findings suggest that NO induces stem cell-like tumor characteristics in breast cancer. In this review, we will discuss the effects of NO in tumor biology and disease progression with an emphasis on breast cancer, and will examine the mechanisms that link increased NO to a basal-like transcription pattern in human breast tumors and poor disease outcome.
Key words: nitric oxide, inducible nitric oxide synthase, breast cancer, basal-like, stem cell, survival
Introduction
Stromal gene signatures predict resistance to therapy and clinical outcome in breast cancer.1,2 Other studies revealed that transcriptional signatures reminiscent of the host wound healing response are induced in breast tumors and other epithelial cancers, and are associated with cancer progression and poor outcome.3,4 Together, these findings highlight the importance of stromal biology and the process of tumor-associated wound healing in cancer biology, disease aggressiveness and therapy response. One of the genes with a key function in wound healing is NOS2.5 In tumor biology, aberrant NOS2 induction may occur to facilitate tissue remodeling and to stimulate neovascularization.6–9 In this function, NOS2 may synergize with the endothelial isoform, eNOS.10,11 NO regulates metalloproteinase activity,9,12 crucial players in tissue remodeling and oncogenic pathways,13–15 and induces angiogenesis by mechanisms that involve activation of vascular endothelial growth factor (VEGF),7,8,16 and inhibition of thrombospondin-1.17 Moreover, recent findings indicate that NO induces stem cell-like tumor characteristics, including upregulation of c-Myc and CD44, and a basal-like phenotype in breast cancer15 which are unfavorable prognostic markers for the disease.18–21 Together, these functions of NO suggest that NOS2 expression in cancer biology could be disease promoting and upregulation of the enzyme in breast cancer and other cancer types could cause a poor outcome phenotype.
NOS2 in Human Tumors
Aberrant expression of NOS2 has been commonly observed in human tumors including human breast,22,23 colon,24 stomach,25 lung,26 head and neck cancer,27 among others. In the two breast cancer studies, increased NOS2 correlated with dedifferentiation as indicated by a high tumor grade22,23 and increased tumor angiogenesis.23 The correlation between increased NOS2 and a high tumor grade was also observed in two other studies15,28 and one of them corroborated the finding that NOS2 upregulation is associated with an increased tumor vascularization.15 In most breast cancer studies, NOS2 was found to be expressed by the cancer cells themselves. Several reports examined the relationship between NOS2 and patient outcome in breast cancer.15,28,29 The two earlier studies detected NOS2 expression in a majority of the analyzed tumors (61 and 78%) and observed borderline associations between increased NOS2 and inferior breast cancer survival in two patient groups that consisted mainly of estrogen receptor (ER)-positive patients.28,29 The third and largest study detected a moderate to strong NOS2 expression in 70% of the analyzed tumors.15 This study found a significant association of NOS2 with poor survival only in the ER-negative patients. From these results it appears that tumor NOS2 expression may have a limited prognostic value in ER-positive breast cancer but is a predictor of outcome in the ER-negative disease.
Induction of NOS2
Constitutive expression of NOS2 is rarely observed and most cells do not express the enzyme without a stimulus. NOS2 expression occurs in normal breast tissue where weaning induces this enzyme in the lactating gland.30 Estrogen has been found to alter NOS2 expression in murine macrophages, splenocytes and vascular smooth muscle cells by mechanisms involving ER,31,32 but because of the known differences in promoter regulation of murine and human NOS2,33,34 we cannot be sure that estrogen would have similar effects on human NOS2. NOS2 is an inflammation responsive enzyme and bacterial lipopolysaccharide and cytokines have been shown to induce NOS2 in a variety of cell types through mechanisms that involve NFκB.33–35 Hormones may interact with cytokines in NOS2 induction and progesterone has been shown to enhance cytokine-stimulated NOS2 expression in MCF7 human breast cancer cells.36 However, the level of NOS2 induction can be different between murine and human cell lines, with human cell lines commonly showing a lower expression of NOS2 following cytokine exposure. This observation is explained, at least partly, by promoter differences that regulate NOS2 expression in response to inflammatory stimuli. These findings suggest that the induction of NOS2 in murine cells can lead to a phenotype that is somewhat different to that observed in their human counterparts because the effects of NO are strictly concentration-dependent with high concentrations causing cytostasis and apoptosis while lower concentrations may activate oncogenic signaling pathways, promote proliferation and invasion and inhibit apoptosis.14,17,37–39 Thus, NOS2 effects in murine models may not apply to humans, and the use of the recently established human NOS2 transgenic mouse with a more human-specific release of NO may prove advantageous in experimental settings studying NOS2 effects in mouse models of cancer.40 Other cancer-related signaling pathways that stimulate NOS2 expression include the Wnt adenomatous polyposis coli pathway, hypoxia, colony-stimulating factor-1 and epidermal growth factor (EGF) signaling. In human colon and liver cancer cell lines, Wnt signaling induces NOS2 in a β-catenin-dependent manner.41 Hypoxia synergizes with interferon, resulting in a more than additive induction of NOS2 in murine macrophages.42 This mechanism could be important for NOS2 induction associated with tumor hypoxia and the tumor-associated wound healing response. Colony-stimulating factor-1 released by human breast carcinoma cells was found to induce NOS2 in macrophages, leading to increased NO-induced invasiveness of the breast cancer cells.43 Lastly, EGF was shown to induce NOS2 in MDA-MB-468 human breast cancer cells. This cell line has basal-like breast cancer characteristics and EGF enhanced both NO production and vascular endothelial growth factor expression in these cells by a mechanism that involves the nuclear interaction of the EGF receptor and STAT3.44
Effects of NO in Tumor Biology
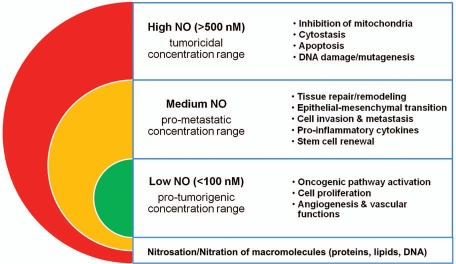
NO can influence tumor biology in various and sometimes dichotomous ways (Fig. 1). Genetic ablation of NOS2 increases mammary tumor latency and inhibits lung tumor development in mouse models of cancer.45,46 These genetic findings demonstrate that NOS2 can have tumor promoting activities. However, NOS2 expression or other forms of NO exposure are not always tumor promoting, and there are many examples where exposure to NO either delayed or inhibited tumor growth and metastasis.47–49 In some studies, tumor cell NO inhibited primary tumor growth but stimulated experimental metastasis,50 while others found that metastasis was suppressed by NO.49 Because the effects of NO are strictly concentration-dependent with high concentrations causing cytostasis and apoptosis, at least some of the observed differences are likely explained by a difference in levels of NO exposure. If a NOS2 transgene is used to experimentally study the effect of NO on tumor growth, the use of a weak promoter, when compared to the CMV promoter, may reveal cancer promoting activities of the enzyme,6,8 while CMV-driven expression of the transgene may yield high NOS2 expression and cytotoxic effects of NO.48,49 Another factor that influences the effect of NO is the tumor microenvironment. For example, it was found that the ablation of NOS2 in cancer-prone p53 knockout mice can either suppress or enhance cancer development depending on the presence of chronic inflammation.51,52 This dichotomy of NO function is well documented, and NO can cause both DNA damage and protect from cytotoxicity, and either inhibit or stimulate cell proliferation and migration, and apoptosis.14,37,39,53–56 The NO effect will also dependent on tissue oxygen tension and local superoxide concentrations,55 and are therefore difficult to predict in human tumors. Thus, NO effects in human cancer are perhaps best estimated from the expression analysis of nitric oxide synthases in the tumors, adjacent non-tumor tissues, and in the involved lymph nodes, and examining their association with tumor markers and survival.
Figure 1.
Concentration-dependent effects of NO in tumor biology.
Cancer cell lines can adapt to high NO concentrations and thereby change their phenotype.57 NO targets DNA repair processes and can induce genetic instability and cell transformation.58–61 DNA damage can trigger activation of the tumor suppressor p53. We and others have reported the existence of a negative feedback loop in which NO-induced p53 activation results in p53-mediated growth arrest and apoptosis, and in the trans-repression of NOS2.8,62–64 NO may promote carcinogenesis through the inactivation of wild-type p53 function, either by causing loss of DNA-binding activity65 and/or selecting for mutant p53. The latter hypothesis is supported by our observations that the p53 mutation frequency in colon and breast tumors is increased in tumors with high NOS2.15,66 Other candidate oncogenic functions of NO include the activation of the cyclooxygenase-2 (COX2) pathway by NO and the induction of the chemokine receptor, CXCR4, which has a critical function in the homing of cancer cells to specific metastatic sites.67–69 NOS2 and COX2 are both inflammation responsive enzymes that are commonly expressed in breast tumors and their co-expression could have more deleterious effects than the expression of only one of these two markers. The activation of COX2 by NO may also enhance the anti-apoptotic and pro-angiogenic effects of NO that are observed when local NO concentrations remain below a 300 to 500 nmol/l threshold. Here, NO can suppress apoptosis through the inhibition of caspases by S-nitrosylation.39
Other studies discovered that NOS2 expression correlates with increased Akt phosphorylation in breast tumors, and that NO induces Akt phosphorylation and activation of the oncogenic Akt pathway in breast cancer cells in culture.13,14 Akt activation is a pro-survival signal that suppresses the activation of key factors like BAD and caspase-9 in the apoptosis pathway, but also activates eNOS and eNOS-mediated tumor maintenance.11,70 We found that NOS2 expression was associated with Akt activation in both ER-positive and ER-negative tumors.13 However, NOS2 expression was a predictor of survival only in the ER-negative disease.15 It is likely that both the difference in ER expression and also intrinsic differences between ER-negative and ER-positive breast tumors independent of the tumor ER status contribute to the increased responsiveness to NO in ER-negative breast cancer. We observed that interleukin-8 (IL-8) is significantly upregulated in ER-negative breast tumors, but not ER-positive tumors, with high NOS2. IL-8 is induced by NO.15,71–73 This chemokine is preferentially expressed by ER-negative breast tumors and cancer cell lines and its expression is low or absent in most ER-positive breast cancer cells.74 IL-8 is of particular importance in human breast cancer biology and is associated with increased cancer cell invasion and microvessel density.75 IL-8 mediates metastasis in breast cancer and other cancers and its secretion correlates with early disease dissemination and poor survival.76 We hypothesize that NOS2 may lead to poor survival among ER-negative patients partly because it induces IL-8 selectively in tumors of these patients, leading to increased angiogenesis and early metastasis. ER-negative tumors also tend to have more tumor-associated macrophages than ER-positive tumors,77 and many pro-inflammatory cytokines are more highly expressed in ER-negative tumors than ER-positive tumors.78 Both macrophages and cytokines will alter the tumor microenvironment and may lead to a proinflammatory state and increased oxygen radical formation. As already mentioned at beginning of this section, the presence of chronic inflammation can lead to an opposite effect of NO in a mouse model of cancer.51,52 Thus, NO biochemistry and signaling, which is greatly influenced by reactive oxygen species availability, may be different in ER-negative and ER-positive breast cancer.
NOS2 and Basal-Like Breast Cancer
Recent results from large-scale gene expression profiling studies showed that that ER-negative and ER-positive breast tumors should be further subdivided based on their gene expression profiles.79 Distinct molecular signatures characterize three luminal subtypes among the ER-positive tumors and basal-like, HER2-positive and normal-like subtypes among the ER-negative tumors. Among all subtypes, basal-like and HER2-positive, ER-negative tumors have been recognized as the most aggressive subtypes.21,80 They are not treatable by endocrine-targeted therapy such as tamoxifen and aromatase inhibitors and have a worse prognosis than ER-positive breast tumors independent of therapy, particularly in the first five years following diagnosis.81,82 There is an urgent need to identify novel targets for the treatment of ER-negative breast cancer in general, and even more for the basal-like subtype and the so called triple-negative tumors (ER/PR/HER2-negative) which mostly represent basal-like tumors but also some other ones.
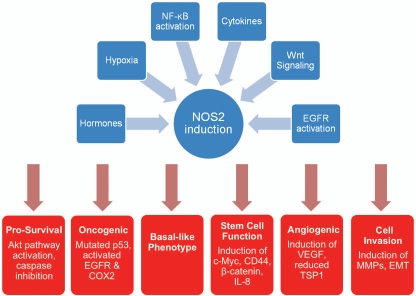
We think that NOS2 and downstream targets of NOS2 are candidate new targets for therapeutic intervention in both the basal-like and triple-negative disease (Fig. 2). Recent research by our laboratory led to the novel and clinically significant observation that NOS2 expression is associated with a prognostic basal-like transcription pattern and is an independent predictor of poor survival in women with ER-negative breast tumors.15 NOS2 remained a predictor of poor survival when the analysis was restricted to only those patients with basal-like breast tumors. This result suggests that in addition to inducing a basal-like signature in ER-negative tumors, NOS2 may further enhance disease aggressiveness in the presence of this signature. Increased NOS2 in breast tumors also correlated with other poor outcome markers, such as an increased tumor vascularization and p53 mutation frequency, and activated EGFR. Both p53 mutations and EGFR overexpression occur more commonly in basal-like breast tumors than other breast cancer subtypes.21,79 Additional work showed that NO activates EGFR, consistent with previous findings in lung cancer,83 and induces proteins, such as CD44 and c-Myc, in ER-negative human breast cancer cells. These markers have been linked to an embryonic stem cell-like phenotype in breast cancer and disease outcome.19,84,85 Recently, an increased expression of stem cell markers in basal-like tumors has been reported.86,87 Among them was CD44, which is a receptor for hyaluronan and osteopontin.88 CD44 is a poor outcome marker in breast cancer18 and CD44-positive breast cancer cells have an increased invasive activity,89 increased resistance to radiation therapy and chemotherapeutics.90,91 Together, these novel observations link NOS2 to the development of a poorly differentiated breast cancer phenotype with stem cell-like characteristics. NO may induce this phenotype by activation of c-Myc or by inducing the release of stem cell renewal factors like IL-8, a property that NO has, as we have shown.
Figure 2.
Candidate pro-tumorigenic properties of NOS2 in ER-negative breast tumors. EMT = epithelial to mesenchymal transition.
Concluding Remarks
In conclusion, NOS2 was found to be a predictor of survival and determinant of disease aggressiveness associated with ER-negative breast cancer. The underlying mechanisms that lead to an NO-induced poor outcome phenotype in breast cancer may include a combination of events, such as the induction of a basal-like phenotype, activation of the EGFR pathway, increased IL-8 and tumor angiogenesis and selection for mutant p53 cells. We propose that NOS2 and downstream targets of NOS2 signaling are novel therapeutic targets for ER-negative breast cancer in general and more specifically for basal-like breast cancer and the triple-negative disease.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, USA.
References
- 1.Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74. doi: 10.1038/nm.1908. [DOI] [PubMed] [Google Scholar]
- 2.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 3.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Troester MA, Lee MH, Carter M, Fan C, Cowan DW, Perez ER, et al. Activation of host wound responses in breast cancer microenvironment. Clin Cancer Res. 2009;15:7020–7028. doi: 10.1158/1078-0432.CCR-09-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamasaki K, Edington HD, McClosky C, Tzeng E, Lizonova A, Kovesdi I, et al. Reversal of impaired wound repair in iNOS-deficient mice by topical adenoviral-mediated iNOS gene transfer. J Clin Invest. 1998;101:967–971. doi: 10.1172/JCI2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins DC, Charles IG, Thomsen LL, Moss DW, Holmes LS, Baylis SA, et al. Roles of nitric oxide in tumor growth. Proc Natl Acad Sci USA. 1995;92:4392–4396. doi: 10.1073/pnas.92.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambs S, Merriam WG, Ogunfusika MO, Bennett WP, Ishibe N, Hussain SP, et al. p53 and vascular endothelial growth factor regulate tumour growth of NOS2-expressing human carcinoma cells. Nature Med. 1998;4:1371–1376. doi: 10.1038/3957. [DOI] [PubMed] [Google Scholar]
- 9.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, et al. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylylcyclase-dependent and -independent pathways. Proc Natl Acad Sci USA. 2007;104:16898–16903. doi: 10.1073/pnas.0702761104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–156. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 11.Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mei JM, Borchert GL, Donald SP, Phang JM. Matrix metalloproteinase(s) mediate(s) NO-induced dissociation of beta-catenin from membrane bound E-cadherin and formation of nuclear beta-catenin/LEF-1 complex. Carcinogenesis. 2002;23:2119–2122. doi: 10.1093/carcin/23.12.2119. [DOI] [PubMed] [Google Scholar]
- 13.Prueitt RL, Boersma BJ, Howe TM, Goodman JE, Thomas DD, Ying L, et al. Inflammation and IGF-I activate the Akt pathway in breast cancer. Int J Cancer. 2007;120:796–805. doi: 10.1002/ijc.22336. [DOI] [PubMed] [Google Scholar]
- 14.Pervin S, Singh R, Hernandez E, Wu G, Chaudhuri G. Nitric oxide in physiologic concentrations targets the translational machinery to increase the proliferation of human breast cancer cells: involvement of mammalian target of rapamycin/eIF4E pathway. Cancer Res. 2007;67:289–299. doi: 10.1158/0008-5472.CAN-05-4623. [DOI] [PubMed] [Google Scholar]
- 15.Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, et al. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest. 2010;120:3843–3854. doi: 10.1172/JCI42059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D'Acquisto F, et al. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–197. [PubMed] [Google Scholar]
- 17.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA. 2005;102:13147–13152. doi: 10.1073/pnas.0502979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Jiang Y, Yan T, Di G, Shen Z, Shao Z, Lu J. The prognostic role of cancer stem cells in breast cancer: a meta-analysis of published literatures. Breast Cancer Res Treat. 2010;122:795–801. doi: 10.1007/s10549-010-0999-4. [DOI] [PubMed] [Google Scholar]
- 19.Ben Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 22.Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72:41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vakkala M, Kahlos K, Lakari E, Paakko P, Kinnula V, Soini Y. Inducible nitric oxide synthase expression, apoptosis and angiogenesis in in situ and invasive breast carcinomas. Clin Cancer Res. 2000;6:2408–2416. [PubMed] [Google Scholar]
- 24.Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- 25.Chen CN, Hsieh FJ, Cheng YM, Chang KJ, Lee PH. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in angiogenesis and clinical outcome of human gastric cancer. J Surg Oncol. 2006;94:226–233. doi: 10.1002/jso.20372. [DOI] [PubMed] [Google Scholar]
- 26.Marrogi AJ, Travis WD, Welsh JA, Khan MA, Rahim H, Tazelaar H, et al. Nitric oxide synthase, cyclooxygenase 2 and vascular endothelial growth factor in the angiogenesis of non-small cell lung carcinoma. Clin Cancer Res. 2000;6:4739–4744. [PubMed] [Google Scholar]
- 27.Brennan PA, Dennis S, Poller D, Quintero M, Puxeddu R, Thomas GJ. Inducible nitric oxide synthase: correlation with extracapsular spread and enhancement of tumor cell invasion in head and neck squamous cell carcinoma. Head Neck. 2008;30:208–214. doi: 10.1002/hed.20675. [DOI] [PubMed] [Google Scholar]
- 28.Loibl S, Buck A, Strank C, von Minckwitz G, Roller M, Sinn HP, et al. The role of early expression of inducible nitric oxide synthase in human breast cancer. Eur J Cancer. 2005;41:265–271. doi: 10.1016/j.ejca.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Bulut AS, Erden E, Sak SD, Doruk H, Kursun N, Dincol D. Significance of inducible nitric oxide synthase expression in benign and malignant breast epithelium: an immunohistochemical study of 151 cases. Virchows Arch. 2005;447:24–30. doi: 10.1007/s00428-005-1250-2. [DOI] [PubMed] [Google Scholar]
- 30.Zaragoza R, Miralles VJ, Rus AD, Garcia C, Carmena R, Garcia-Trevijano ER, et al. Weaning induces NOS-2 expression through NFkappaB modulation in the lactating mammary gland: importance of GSH. Biochem J. 2005;391:581–588. doi: 10.1042/BJ20050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You HJ, Kim JY, Jeong HG. 17beta-estradiol increases inducible nitric oxide synthase expression in macrophages. Biochem Biophys Res Commun. 2003;303:1129–1134. doi: 10.1016/s0006-291x(03)00477-7. [DOI] [PubMed] [Google Scholar]
- 32.Dai R, Phillips RA, Karpuzoglu E, Khan D, Ahmed SA. Estrogen regulates transcription factors STAT-1 and NFkappaB to promote inducible nitric oxide synthase and inflammatory responses. J Immunol. 2009;183:6998–7005. doi: 10.4049/jimmunol.0901737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Q, Nathan C. The high-output nitric oxide pathway: role and regulation. J Leukoc Biol. 1994;56:576–582. doi: 10.1002/jlb.56.5.576. [DOI] [PubMed] [Google Scholar]
- 34.de Vera ME, Shapiro RA, Nussler AK, Mudgett JS, Simmons RL, Morris SM, Jr, et al. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. Proc Natl Acad Sci USA. 1996;93:1054–1059. doi: 10.1073/pnas.93.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM, Jr, et al. Multiple NFkappaB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–15156. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 36.Bentrari F, Arnould L, Jackson AP, Jeannin JF, Pance A. Progesterone enhances cytokine-stimulated nitric oxide synthase II expression and cell death in human breast cancer cells. Lab Invest. 2005;85:624–632. doi: 10.1038/labinvest.3700267. [DOI] [PubMed] [Google Scholar]
- 37.Pervin S, Singh R, Chaudhuri G. Nitric oxide-induced cytostasis and cell cycle arrest of a human breast cancer cell line (MDA-MB-231): Potential role of cyclin D1. Proc Natl Acad Sci USA. 2001;98:3583–3588. doi: 10.1073/pnas.041603998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci USA. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim PK, Zamora R, Petrosko P, Billiar TR. The regulatory role of nitric oxide in apoptosis. Int Immunopharmacol. 2001;1:1421–1441. doi: 10.1016/s1567-5769(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 40.Vitek MP, Brown C, Xu Q, Dawson H, Mitsuda N, Colton CA. Characterization of NO and cytokine production in immune-activated microglia and peritoneal macrophages derived from a mouse model expressing the human NOS2 gene on a mouse NOS2 knockout background. Antioxid Redox Signal. 2006;8:893–901. doi: 10.1089/ars.2006.8.893. [DOI] [PubMed] [Google Scholar]
- 41.Du Q, Park KS, Guo Z, He P, Nagashima M, Shao L, et al. Regulation of human nitric oxide synthase 2 expression by Wnt beta-catenin signaling. Cancer Res. 2006;66:7024–7031. doi: 10.1158/0008-5472.CAN-05-4110. [DOI] [PubMed] [Google Scholar]
- 42.Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995;182:1683–1693. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin CW, Shen SC, Ko CH, Lin HY, Chen YC. Reciprocal activation of macrophages and breast carcinoma cells by nitric oxide and colony-stimulating factor-1. Carcinogenesis. 2010;31:2039–2048. doi: 10.1093/carcin/bgq172. [DOI] [PubMed] [Google Scholar]
- 44.Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, et al. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–589. doi: 10.1016/j.ccr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Kisley LR, Barrett BS, Bauer AK, Dwyer-Nield LD, Barthel B, Meyer AM, et al. Genetic ablation of inducible nitric oxide synthase decreases mouse lung tumorigenesis. Cancer Res. 2002;62:6850–6856. [PubMed] [Google Scholar]
- 46.Ellies LG, Fishman M, Hardison J, Kleeman J, Maglione JE, Manner CK, et al. Mammary tumor latency is increased in mice lacking the inducible nitric oxide synthase. Int J Cancer. 2003;106:1–7. doi: 10.1002/ijc.11178. [DOI] [PubMed] [Google Scholar]
- 47.Stuehr DJ, Nathan CF. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989;169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong Z, Staroselsky AH, Qi X, Xie K, Fidler IJ. Inverse correlation between expression of inducible nitric oxide synthase activity and production of metastasis in K-1735 murine melanoma cells. Cancer Res. 1994;54:789–793. [PubMed] [Google Scholar]
- 49.Xie K, Dong Z, Fidler IJ. Activation of nitric oxide synthase gene for inhibition of cancer metastasis. J Leukoc Biol. 1996;59:797–803. doi: 10.1002/jlb.59.6.797. [DOI] [PubMed] [Google Scholar]
- 50.Edwards P, Cendan JC, Topping DB, Moldawer LL, MacKay S, Copeland EM, III, Lind DS. Tumor cell nitric oxide inhibits cell growth in vitro, but stimulates tumorigenesis and experimental lung metastasis in vivo. J Surg Res. 1996;63:49–52. doi: 10.1006/jsre.1996.0221. [DOI] [PubMed] [Google Scholar]
- 51.Hussain SP, Trivers GE, Hofseth LJ, He P, Shaikh I, Mechanic LE, et al. Nitric oxide, a mediator of inflammation, suppresses tumorigenesis. Cancer Res. 2004;64:6849–6853. doi: 10.1158/0008-5472.CAN-04-2201. [DOI] [PubMed] [Google Scholar]
- 52.Hussain SP, He P, Subleski J, Hofseth LJ, Trivers GE, Mechanic L, et al. Nitric oxide is a key component in inflammation-accelerated tumorigenesis. Cancer Res. 2008;68:7130–7136. doi: 10.1158/0008-5472.CAN-08-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wink DA, Hanbauer I, Krishna MC, DeGraff W, Gamson J, Mitchell JB. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci USA. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamir S, Burney S, Tannenbaum SR. DNA damage by nitric oxide. Chem Res Toxicol. 1996;9:821–827. doi: 10.1021/tx9600311. [DOI] [PubMed] [Google Scholar]
- 55.Wink DA, Mitchell JB. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic and cytoprotective mechanisms of nitric oxide. Free Radic Biol Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 56.Brune B, Von Knethen A, Sandau KB. Nitric oxide (NO): an effector of apoptosis. Cell Death Differ. 1999;6:969–975. doi: 10.1038/sj.cdd.4400582. [DOI] [PubMed] [Google Scholar]
- 57.Vesper BJ, Elseth KM, Tarjan G, Haines GK, III, Radosevich JA. Long-term adaptation of breast tumor cell lines to high concentrations of nitric oxide. Tumour Biol. 2010;31:267–275. doi: 10.1007/s13277-010-0028-6. [DOI] [PubMed] [Google Scholar]
- 58.Laval F, Wink DA. Inhibition by nitric oxide of the repair protein, O6-methylguanine-DNA-methyltransferase. Carcinogenesis. 1994;15:443–447. doi: 10.1093/carcin/15.3.443. [DOI] [PubMed] [Google Scholar]
- 59.Graziewicz M, Wink DA, Laval F. Nitric oxide inhibits DNA ligase activity: potential mechanisms for NO-mediated DNA damage. Carcinogenesis. 1996;17:2501–2505. doi: 10.1093/carcin/17.11.2501. [DOI] [PubMed] [Google Scholar]
- 60.Tamir S, deRojas-Walker T, Gal A, Weller AH, Li X, Fox JG, et al. Nitric oxide production in relation to spontaneous B-cell lymphoma and myositis in SJL mice. Cancer Res. 1995;55:4391–4397. [PubMed] [Google Scholar]
- 61.Gal A, Tamir S, Tannenbaum SR, Wogan GN. Nitric oxide production in SJL mice bearing the RcsX lymphoma: a model for in vivo toxicological evaluation of NO. Proc Natl Acad Sci USA. 1996;93:11499–11503. doi: 10.1073/pnas.93.21.11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Messmer UK, Ankarcrona M, Nicotera P, Brune B. p53 expression in nitric oxide-induced apoptosis. FEBS Lett. 1994;355:23–26. doi: 10.1016/0014-5793(94)01161-3. [DOI] [PubMed] [Google Scholar]
- 63.Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase (NOS2) expression by wild-type p53. Proc Natl Acad Sci USA. 1996;93:2442–2447. doi: 10.1073/pnas.93.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ambs S, Ogunfusika MO, Merriam WG, Bennett WP, Billiar TR, Harris CC. Upregulation of NOS2 expression in cancer-prone p53 knockout mice. Proc Natl Acad Sci USA. 1998;95:8823–8828. doi: 10.1073/pnas.95.15.8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calmels S, Hainaut P, Ohshima H. Nitric oxide induces conformational and functional modifications of wild-type p53 tumor suppressor protein. Cancer Res. 1997;57:3365–3369. [PubMed] [Google Scholar]
- 66.Ambs S, Bennett WP, Merriam WG, Ogunfusika MO, Oser SM, Harrington AM, et al. Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. J Natl Cancer Inst. 1999;91:86–88. doi: 10.1093/jnci/91.1.86. [DOI] [PubMed] [Google Scholar]
- 67.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marnett LJ, Wright TL, Crews BC, Tannenbaum SR, Morrow JD. Regulation of prostaglandin biosynthesis by nitric oxide Is revealed by targeted deletion of inducible nitric-oxide synthase. J Biol Chem. 2000;275:13427–13430. doi: 10.1074/jbc.275.18.13427. [DOI] [PubMed] [Google Scholar]
- 69.Yasuoka H, Tsujimoto M, Yoshidome K, Nakahara M, Kodama R, Sanke T, Nakamura Y. Cytoplasmic CXCR4 expression in breast cancer: induction by nitric oxide and correlation with lymph node metastasis and poor prognosis. BMC Cancer. 2008;8:340. doi: 10.1186/1471-2407-8-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 71.Xiong Q, Shi Q, Le X, Wang B, Xie K. Regulation of interleukin-8 expression by nitric oxide in human pancreatic adenocarcinoma. J Interferon Cytokine Res. 2001;21:529–537. doi: 10.1089/10799900152434411. [DOI] [PubMed] [Google Scholar]
- 72.Ma P, Cui X, Wang S, Zhang J, Nishanian EV, Wang W, et al. Nitric oxide post-transcriptionally upregulates LPS-induced IL-8 expression through p38 MAPK activation. J Leukoc Biol. 2004;76:278–287. doi: 10.1189/jlb.1203653. [DOI] [PubMed] [Google Scholar]
- 73.Sparkman L, Boggaram V. Nitric oxide increases IL-8 gene transcription and mRNA stability to enhance IL-8 gene expression in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:764–773. doi: 10.1152/ajplung.00165.2004. [DOI] [PubMed] [Google Scholar]
- 74.Freund A, Chauveau C, Brouillet JP, Lucas A, Lacroix M, Licznar A, et al. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. 2003;22:256–265. doi: 10.1038/sj.onc.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, et al. Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells. Int J Cancer. 2007;121:1949–1957. doi: 10.1002/ijc.22930. [DOI] [PubMed] [Google Scholar]
- 76.Benoy IH, Salgado R, van Dam P, Geboers K, Van Marck E, Scharpe S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–7162. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 77.Steele RJ, Eremin O, Brown M, Hawkins RA. Oestrogen receptor concentration and macrophage infiltration in human breast cancer. Eur J Surg Oncol. 1986;12:273–276. [PubMed] [Google Scholar]
- 78.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 81.Bentzon N, During M, Rasmussen BB, Mouridsen H, Kroman N. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer. 2008;122:1089–1094. doi: 10.1002/ijc.22892. [DOI] [PubMed] [Google Scholar]
- 82.Blows FM, Driver KE, Schmidt MK, Broeks A, Van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee HC, An S, Lee H, Woo SH, Jin HO, Seo SK, et al. Activation of epidermal growth factor receptor and its downstream signaling pathway by nitric oxide in response to ionizing radiation. Mol Cancer Res. 2008;6:996–1002. doi: 10.1158/1541-7786.MCR-08-0113. [DOI] [PubMed] [Google Scholar]
- 84.Al-Hajj M, Wicha MS, ito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 86.Rennstam K, McMichael N, Berglund P, Honeth G, Hegardt C, Ryden L, et al. Numb protein expression correlates with a basal-like phenotype and cancer stem cell markers in primary breast cancer. Breast Cancer Res Treat. 2010;122:315–324. doi: 10.1007/s10549-009-0568-x. [DOI] [PubMed] [Google Scholar]
- 87.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, et al. The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1087–1097. doi: 10.1158/1055-9965.EPI-06-1008. [DOI] [PubMed] [Google Scholar]
- 89.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 91.Bourguignon LY, Peyrollier K, Xia W, Gilad E. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression and ankyrin-regulated multidrug eff lux in breast and ovarian tumor cells. J Biol Chem. 2008;283:17635–17651. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]