Abstract
Since their discovery 50 years ago, the aflatoxins have become recognized as ubiquitous contaminants of the human food supply throughout the economically developing world. The adverse toxicological consequences of these compounds in populations are quite varied because of a wide range of exposures leading to acute effects, including rapid death, and chronic outcomes such as hepatocellular carcinoma. Furthermore, emerging studies describe a variety of general adverse health effects associated with aflatoxin, such as impaired growth in children. Aflatoxin exposures have also been demonstrated to multiplicatively increase the risk of liver cancer in people chronically infected with hepatitis B virus (HBV) illustrating the deleterious impact that even low toxin levels in the diet can pose for human health. The public health impact of aflatoxin exposure is pervasive. Aflatoxin biomarkers of internal and biologically effective doses have been integral to the establishment of the etiologic role of this toxin in human disease through better estimates of exposure, expanded knowledge of the mechanisms of disease pathogenesis, and as tools for implementing and evaluating preventive interventions.
Keywords: aflatoxin, biomarkers, molecular epidemiology, chemoprevention
HISTORICAL PERSPECTIVE
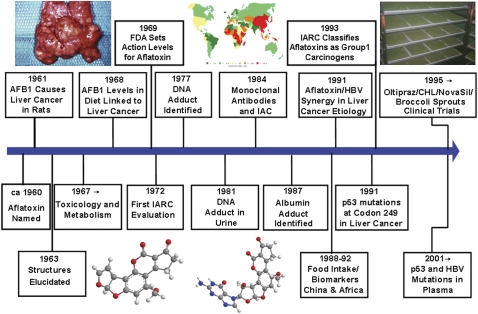
The aflatoxins were discovered in the late 1950s and early 1960s, when they were identified as causative agents of “turkey X” disease, an epidemic involving deaths of numerous turkey poults, ducklings, and chicks fed diets containing certain lots of peanut meal originating in South America (Blount 1961). Investigations revealed that toxicity was associated with the presence of Aspergillus flavus, and further that extracts of cultures of the fungus isolated from the meal were capable of inducing the “turkey X” syndrome. The name “aflatoxin” (A. flavus toxin) was accordingly assigned to the toxic agents. Subsequent studies of A. flavus–contaminated groundnut extracts confirmed that these agents were capable of inducing acute liver disease in ducklings and liver cancer in rats (Lancaster et al. 1961; Sargeant et al. 1961). Detection of aflatoxins in extracts of contaminated peanut meal was facilitated by their intense fluorescence in ultraviolet light, and soon thereafter purified metabolites with identical physical and chemical properties were isolated from A. flavus cultures (Nesbitt et al. 1962; Van der Zijden et al. 1962). These findings stimulated extensive research efforts, which continue to the present, to assess potential health hazards resulting from contamination of the human food supply and to minimize exposure. It is instructive to review certain aspects of the history of the science and policy evolving from the aflatoxins to identify lessons that are applicable to other emerging environmental health issues. A time line highlighting the key milestones in the discovery, toxicological characterization, and regulation of aflatoxins is presented in Figure 1.
FIG. 1.
Time line for key events in the discovery, toxicological evaluation, molecular epidemiology, and regulation of aflatoxins. FDA, Food and Drug Administration; IARC, International Agency for Research on Cancer; IAC, immunoaffinity chromatography; CHL, chlorophyllin.
Of great significance was the early development of analytical methods capable of detecting and quantifying aflatoxins in extracts of foods and food crops. Assay development involved extensive international collaborations among governmental, industrial, and academic research groups, and the resulting methodology greatly enhanced the ability of regulatory agencies and food producers to monitor the food supply and minimize contamination. This methodology also enabled observational epidemiologic studies conducted during 1968–1985 to evaluate the association of aflatoxin ingestion and incidence of hepatocellular carcinoma (HCC) in human populations. Structural characterization and synthesis of the major aflatoxins, accomplished in 1963, facilitated mechanistic studies of their toxicology and metabolism, leading to identification of the major aflatoxin B1-DNA adduct in 1977 and demonstration of its excretion in urine in 1981. Development of monoclonal antibodies specifically recognizing aflatoxin B1 provided a basis for an immunoaffinity methodology not only for analysis of food extracts but also for quantitation of urinary excretion of the DNA adduct and other metabolites. This DNA adduct biomarker, together with measurement of an aflatoxin-serum albumin adduct, provided the tools to evaluate the molecular epidemiology of aflatoxin exposure of individuals within human populations (Fig. 2). Results of such studies in China and sub-Saharan Africa, together with accumulated experimental evidence comprised the basis for classification of aflatoxin as a human carcinogen by the International Agency for Research on Cancer in 1994. These lines of investigation are still being pursued and extended in current efforts to develop effective intervention methodologies to mitigate the health impacts of unavoidable aflatoxin exposures.
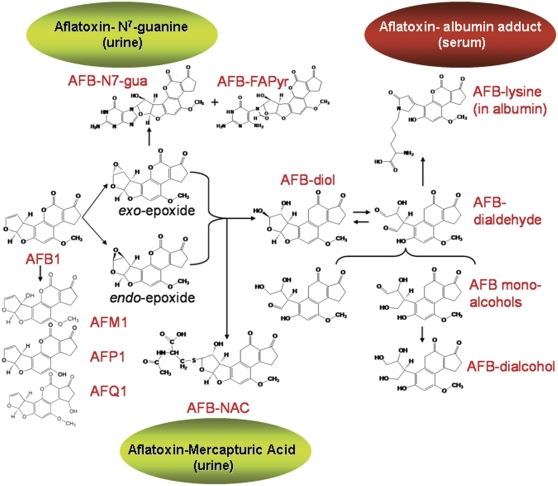
FIG. 2.
Biotransformation pathways for aflatoxin B1. Products measured in biofluids for use as biomarkers in epidemiological and intervention studies are highlighted in green (urine) and red (serum).
Key elements of a strategy to define health risks from environmental agents and to devise strategies to obviate their effects have depended upon: availability of an animal model mimicking the human disease, observational epidemiology associating exposure with disease incidence, development of mechanism-based molecular biomarkers in animal models, validation of these biomarkers in animals by dose-response and mitigation studies, validation of biomarkers in transitional studies in exposed humans, and association of biomarkers with risk in prospective studies of exposed humans. Collectively, the data on aflatoxin and human liver cancer exemplify the importance of these elements and provide a model for the design of future studies for risk assessment of exposure to other environmental agents. The results from molecular epidemiology investigations of aflatoxins and HCC represent one of the most extensive data sets in the field for environmental toxicology.
AFLATOXINS: CHEMISTRY AND OCCURRENCE
The landmark accomplishment of structural elucidation of aflatoxin B1 was confirmed by its total synthesis in 1963 (Asao et al., 1963). Chemically, the aflatoxins are highly substituted coumarins containing a fused dihydrofurofuran moiety (Fig. 2). Four aflatoxins occur naturally: B1, B2, G1, and G2. Members of the blue fluorescent (B) series are characterized by fusion of a cyclopentenone ring to the lactone ring of the coumarin moiety, whereas the green fluorescent (G) toxins contain a fused lactone ring. Aflatoxins B1 and B2 (AFB1 and AFB2) were so named because of their strong blue fluorescence in ultraviolet light, whereas aflatoxins G1 and G2 (AFG1 and AFG2) fluoresced greenish yellow. These properties facilitated the very rapid development in the early 1960s of methods for monitoring grains and other food commodities for the presence of the toxins. AFB1 and AFG1 possess an unsaturated bond at the 8,9 position on the terminal furan ring, and subsequent studies demonstrated that epoxidation at this position was critical for their carcinogenic potency (Groopman and Kensler, 2005). AFB2 and AFG2 are relatively nontoxic unless they are first metabolically oxidized to AFB1 and AFG1 in vivo.
Humans are exposed to aflatoxins by consumption of commodities contaminated by strains of A. flavus or Aspergillus paraciticus during growth, harvest, or storage. In general, diets may contain AFB1 and AFB2 in concentration ratios of 1.0 to 0.1, and when all four aflatoxins occur, AFB1, AFB2, AFG1, and AFG2 proportions of 1.0:0.1:0.3:0.03 exist. However, these ratios can be variable. Grains and foodstuffs contaminated with aflatoxins include corn, peanuts, milo, sorghum, copra, and rice (Busby and Wogan, 1984). Although contamination by the molds may be universal, the levels or final concentrations of aflatoxins in the grain product can vary from less than 1 μg/kg (1 ppb) to greater than 12,000 μg/kg (12 ppm). Indeed, in a recent outbreak of aflatoxin-induced death of people in Kenya, individual daily exposure of AFB1 was estimated to be 50 mg/day (Probst et al., 2007). Exposure estimates based on aflatoxin levels found in individual grain or oilseed samples are confounded by the variation of toxin levels among different samples within a lot of grain. For example, in many peanut lots, only a single nut out of 10,000 may contain aflatoxin, but the level within that nut may be up to several hundred micrograms. Consequently, contamination of an entire shipment to levels exceeding the regulatory level can result when the commodity has been ground, blended, and processed (Campbell et al., 1986). Indeed, heterogeneity of toxin distribution has been confirmed as the major source of error of mycotoxin determination in foods and feeds. For these reasons, estimates of human consumption of aflatoxin based on analysis of market samples of foods and foodstuffs are very imprecise (Richard et al., 1993).
Widespread concern about the potential deleterious effects of aflatoxins in humans and animals as well as possible transfer of residues into edible animal tissues and milk has led to regulatory actions governing U.S. interstate as well as international commerce involving food and feed commodities that may be contaminated with aflatoxins. The U.S. Food and Drug Administration has set action levels for aflatoxin in various foods and feedstuffs. For feeding mature nonlactating animals, the action level is 100–300 ppb depending on the feed type and the species; for commodities destined for human consumption and interstate commerce, 20 ppb; and for milk at 0.5 ppb (Eaton and Groopman, 1994). Products exceeding these levels are subject to seizure. The European Union has promulgated much stricter standards for aflatoxin content in foods (Henry et al., 1999). Regulatory standards for aflatoxin originally promulgated in the 1960s were driven by the analytical methods of the day.
HUMAN TOXICOLOGY OF AFLATOXINS
HCC is a major cause of cancer morbidity and mortality in many parts of the world, including Asia and sub-Saharan Africa (Ferlay et al., 2010; Kew, 2002; Wang et al., 2002). For example, in the People's Republic of China alone there are upwards of 700,000 new cases each year and over 300,000 deaths annually. In contrast to most common cancers in the economically developed world where over 90% of cases are diagnosed after the age of 45, in high-risk regions for liver cancer, onset begins in both men and women by 20 years of age, peaking between 40 and 49 years of age in men and 50 and 59 years in women (Chen et al., 2006; Parkin et al., 2005; Vatanasapt et al., 1995). Gender differences in liver cancer incidence have also been described; the worldwide annual age-standardized incidence rate among men is 15.8 per 100,000 and 5.8 per 100,000 among women (Ferlay et al., 2010). Because the occurrence of HCC is coincident with regions where aflatoxin exposure is high, efforts began in the 1960s to investigate a possible etiologic association. As in all ecologic investigations, the work was hindered by the lack of adequate data on aflatoxin intake, excretion, and metabolism in people, underlying susceptibility factors such as diet and viral exposure, as well as by the incomplete statistics on worldwide cancer morbidity and mortality. Despite these deficiencies, early studies provided data showing that increasing HCC rates corresponded to increasing levels of dietary aflatoxin exposure (Bosch and Munoz, 1988).
There has been an extensive and continuing focus on the role of aflatoxin exposure in HCC, as will be discussed in more detail later. Over the years, outbreaks of acute aflatoxicosis in humans have been reported in regions of several economically developing countries (Shank et al., 1971). Clinical manifestations of aflatoxicosis were vomiting, abdominal pain, pulmonary edema, and fatty infiltration and necrosis of the liver. In the 1970s, a putative aflatoxin poisoning in western India resulted from consumption of heavily molded corn. There were at least 97 fatalities and these deaths occurred only in households where the contaminated corn was eaten. Histopathology of liver specimens revealed extensive bile duct proliferation, a lesion often noted in experimental animals after acute aflatoxin exposure (Bhat and Krishnamachari, 1977; Krishnamachari et al., 1975). An early 1980 incident of acute aflatoxicosis in Kenya in which there were 20 hospital admissions and 20% mortality was also associated with consumption of maize highly contaminated with aflatoxin (Ngindu et al., 1982). As reported more recently (Lye et al., 1995), the consumption of aflatoxin-contaminated noodles resulted in acute hepatic encephalopathy in children in Malaysia; up to 3 mg of aflatoxin was suspected to be present in a single serving of contaminated noodles.
Consecutive outbreaks of acute aflatoxicosis in Kenya in 2004 and 2005 caused more than 150 deaths. In April 2004, one of the largest documented aflatoxicosis outbreaks occurred in rural Kenya, resulting in 317 cases and 125 deaths. Aflatoxin-contaminated maize grown and eaten on family farms was the major source of the outbreak. In a survey of 65 markets and 243 maize vendors, 350 maize products were collected from the most affected districts. Fifty-five percent of maize products had aflatoxin levels greater than the Kenyan regulatory limit of 20 ppb, 35% had levels > 100 ppb, and 7% had levels > 1000 ppb. In addition to the market survey for aflatoxin exposure, this outbreak marked the first time that biomarkers, namely aflatoxin-albumin adducts, were used to independently confirm the exposure in individuals (Anonymous, 2004; Azziz-Baumgartner et al., 2005; Lewis et al., 2005; Probst et al., 2007; Strosnider et al., 2006). Reports of mortality from ingesting aflatoxin-contaminated maize have again been in the news in 2010 (http://www.bbc.co.uk/news/10219505).
Aflatoxins cause growth suppression in animals. Limited evidence suggests that such effects may also occur in humans. A longitudinal study over an 8-month period in Benin assessed the effects of aflatoxin exposure on growth (Gong et al., 2004). Two hundred children (16–37 months of age) were recruited from four villages, two with high and two with low aflatoxin exposure. Children who were fully weaned at recruitment had higher levels of aflatoxin-albumin adducts than did those still partially breast-fed; the major weaning food was a maize-based porridge. There was a strong negative correlation between aflatoxin-albumin adducts and height increase over the 8-month follow-up after adjustment for age, sex, height at recruitment, socioeconomic status, village, and weaning status. The highest quartile of biomarker was associated with a mean 1.7 cm reduction in growth over 8 months compared with the lowest quartile. A follow-up study in The Gambia demonstrated a strong effect of maternal aflatoxin exposure during pregnancy on growth in the first year of life, thus extending the earlier observations of an association between aflatoxin exposure during infancy and faltering growth (Turner et al., 2007). The underlying mechanisms remain unclear, but findings certainly imply value in targeting intervention strategies at early life exposures, as underweight is an important contributory risk factor to the burden of disease worldwide.
EXPERIMENTAL AFLATOXIN CARCINOGENESIS
The carcinogenic potency of AFB1 has been well established in many species of animals, including rodents, nonhuman primates, and fish (Busby and Wogan, 1984; Eaton and Groopman, 1994). The liver is consistently the primary target organ and the toxin induces a high incidence of HCC. However, under certain circumstances, depending on animal species and strain, dose, route of administration and dietary factors, significant numbers of tumors have been found at other sites, such as kidney and colon. Indeed, very few animal species have been found to be resistant to aflatoxin-initiated carcinogenesis. Wide cross-species potency, including sensitivity of primates, provided justification for suspecting that this agent could contribute to human cancer.
Much of the published information on AFB1 carcinogenicity has been obtained from studies in rats, which are highly susceptible to the toxin. Such experiments have often examined dose-response characteristics and the influences of such parameters as route of administration, level and frequency of dose, and the sex, age, and strain of the test animal. A seminal study in which aflatoxin was chronically fed to rats at levels of 1, 5, 15, 50, and 100 ppb induced liver tumors at incidences of 9, 4.5, 19, 80, and 100%, respectively (Wogan et al., 1974). Therefore, even microgram per day doses of aflatoxin could induce liver cancer, a finding that provided a context for human cancer investigations exploring the linkage between aflatoxin and HCC. Adult mice are remarkably resistant to aflatoxin hepatocarcinogenesis, although administration to neonatal mice produces liver tumors. In recent years, there has been an increasing literature pertaining to the carcinogenic responses of the rainbow trout (Oncorhynchus mykiss), an even more sensitive species than the rat, and the monkey, possibly a more appropriate model for human risk estimation (Bailey et al., 1994; Williams et al., 2009). As with the rats, these experiments have examined dose-response characteristics and the influences of such parameters as route of administration, level and frequency of dose, and the sex and age of the test animal.
Early studies found that rhesus monkeys were susceptible to AFB1 carcinogenicity. In three reports each on single animals, two cases of HCC (Adamson et al., 1976; Gopalan et al., 1972; Sieber et al., 1979) and one of cholangiocarcinoma (Tilak, 1975) were observed in animals treated for up to 6 years with an oral or a combined oral and im dosing regimen. More recent data on 47 monkeys, representing three species (rhesus, cynomolgus, and African green) that had received AFB1 by ip and/or oral routes for periods greater than 2 months, have been published (Thorgeirsson et al., 1994). Primary liver tumor incidence was 19% (5/26) in animals surviving for longer than 6 months, and total tumor incidence in these animals was 50% (13/26). Although out of necessity these investigations involve small numbers of animals given the cost and long-term nature of the studies, the findings support species extrapolation used in risk assessments for humans.
One of the major controversies in risk assessment is the linearity of the dose-cancer response curve. The high sensitivity of the rainbow trout to aflatoxin-induced HCC, along with low spontaneous tumor incidence and cost, made possible design of a recent study to define an effective tumor-inducing dose in 1% of animals (ED01) that would be impractical in rodents. In this study involving over 42,000 trout, AFB1 elicited a linear dose-response for liver cancer (Williams et al., 2009). These findings complement the earlier observations of a linear dose-response for DNA adduct formation by AFB1 over an 8-log range (Lutz, 1987).
AFLATOXIN BIOMARKERS: DEVELOPMENT, VALIDATION, AND APPLICATION
Molecular biomarkers are typically used as indicators of exposure, effect or susceptibility. A biomarker of exposure refers to measurement of the specific agent of interest, its metabolites, or its specific interactive products in a body compartment or fluid, which indicates the presence and magnitude of current and past exposure. A biomarker of effect indicates the presence and magnitude of a biological response to exposure to an environmental agent. Such a biomarker may be an endogenous component, a measure of the functional capacity of the system, or an altered state recognized as impairment or disease. A biomarker of susceptibility is an indicator of a measure of an inherent or acquired ability of an organism to respond to the challenge of exposure. Measures of these biomarkers have great utility in addressing the relationships between exposure to environmental agents and development of clinical diseases and in identifying those individuals at high risk for the disease. In this context, aflatoxin biomarkers of internal and biologically effective doses have been integral to establishing the etiologic role of this toxin in human HCC and other toxicologic outcomes.
Development of Molecular Biomarkers
Aflatoxin metabolism and mechanisms of aflatoxin-induced hepatocarcinogenesis are well documented. This knowledge provides the basis for rigorous estimates of exposure to aflatoxins, as well as means to assess the modulation of aflatoxin disposition by chemopreventive agents. AFB1 requires metabolic activation to its ultimate carcinogenic form, primarily by the cytochrome P450 (CYP) monooxygenase system. As shown in Figure 2, AFB1 is metabolized to a reactive epoxide (aflatoxin-8,9-epoxide). Epoxidation is catalyzed by CYP1A2 and CYP3A4 in humans (Gallagher et al., 1994; Ueng et al., 1995). Many other oxidation products, including aflatoxin M1, are also formed. The epoxide can react further by interacting with DNA to produce a promutagenic aflatoxin-N7-guanine adduct. This adduct is unstable in DNA, rapidly undergoes depurination, and is excreted in urine (Bennett et al., 1981). The epoxide can also form products that react with serum albumin to form long-lived lysine adducts (Sabbioni et al., 1987). In addition, the epoxide can be conjugated by certain glutathione S-transferases (GSTs), which are further metabolized to form aflatoxin-mercapturic acid detoxification products that can be excreted in urine (Scholl et al., 1997). The initial concentrations of aflatoxin-DNA binding in tissues reflects the rate of formation of the 8,9-epoxide as well as the competing pathways of aflatoxin-8,9-epoxide reactions with DNA versus glutathione, other macromolecular targets such as RNA and water (leading to diol formation and subsequently protein adducts). Urinary measures of aflatoxin M1, aflatoxin-mercapturic acid, and the aflatoxin-albumin adduct are used as biomarkers of internal dose. Aflatoxin-N7-guanine in urine serves as an elegant biomarker of biologically effective dose because it is clear that formation of this adduct lies on the causal pathway to aflatoxin-induced HCC.
Validation of Aflatoxin Biomarkers
There is a marked difference between the ability to measure a particular biomarker in a human biological sample employing high-quality analytical approaches and the ability to interpret that information based on thorough validation of the biomarker. The validation step involves the careful characterization of the relationship between the biomarker and, for example, environmental exposure to the agent of interest or to the consequent progression of disease. Longitudinal studies in animals have served to define the kinetics of the biomarkers, their tracking (intraclass correlations), as well as the predictive value of their modulation, both in the context of risk to individuals and populations. This process of biomarker validation is well served by parallel experimental and human studies (Groopman et al., 1992a; Groopman and Kensler, 1999). Toward this end, these aflatoxin biomarkers can be used to evaluate the impact of modulation of aflatoxin activation and detoxication. For example, levels of both aflatoxin-albumin adducts (measured in blood) and aflatoxin N7-guanine adducts (measured in urine) have been shown to correlate with aflatoxin exposure (Groopman et al., 1992a, 1992c; Wild et al., 1992). In animals, decreases in hepatic aflatoxin-N7-guanine adduct formation are associated with the degree of chemoprotection as measured by reduction in preneoplastic foci and cancers (Kensler et al., 1985; Yates et al., 2006). In addition, the levels of aflatoxin M1 excreted in urine have been shown to reflect human exposure (Groopman et al., 1985) and may predict chemopreventive efficacy and liver cancer risk (Qian et al., 1994; Scholl et al., 1996; Wang et al., 1999). Furthermore, increased formation of aflatoxin-mercapturic acid metabolites can be measured in the urine of laboratory animals as well as humans and is inversely associated with levels of aflatoxin DNA adducts formed in liver and excreted into urine (Wang et al., 1999). These biomarkers have become critical tools for evaluation of chemopreventive agents in animal models and clinical interventions.
Role of Animal Models
Analytical methods have been developed for quantitation of aflatoxin metabolites, aflatoxin-DNA adducts, and aflatoxin-serum albumin adducts in biological samples (Poirier et al., 2000; Santella, 1999; Gan et al., 1988; Groopman et al., 1985). Each methodology has unique specificity and sensitivity, offering the opportunity to choose appropriate methods for specific applications. For example, to measure a single aflatoxin metabolite, a chromatographic method can resolve mixtures of aflatoxins into individual compounds, providing that the extraction procedure does not introduce large amounts of interfering chemicals. Antibody-based methods are often more sensitive than chromatography, but immunoassays are less selective because the antibody may cross-react with multiple metabolites. An immunoaffinity-HPLC procedure was developed to isolate and measure aflatoxin metabolites in biological samples (Groopman et al., 1984, 1985). Recent studies using isotope dilution mass spectrometry with liquid chromatography separation have demonstrated an increase in sensitivity of at least 1000-fold over technologies used for the detection of aflatoxin biomarkers 15 years ago (Egner et al., 2006; Scholl et al., 2006a, 2006b). Excellent correlations between ELISA, HPLC with fluorescence detection, and HPLC coupled with isotope dilution mass spectrometry have been reported (McCoy et al., 2008).
Using these analytic tools, initial validation studies were performed for dose-dependent excretion of urinary aflatoxin biomarkers in rats after a single exposure to AFB1 (Groopman et al., 1992b). A linear relationship was found between AFB1 dose and excretion of the aflatoxin-N7-guanine adduct in urine over the 24-h period after exposure. Subsequent studies that were based on quantification of aflatoxin macromolecular adducts after chronic AFB1 administration to rodents further validated use of DNA and protein adducts as molecular measures of exposure (Egner et al., 1995; Kensler et al., 1986). Controlled exposure levels offer an important advantage in animal studies over the wide variations in exposure usually encountered in humans. Further, unless the method is extremely insensitive, all samples will contain detectable biomarker levels. Unfortunately, extrapolation of the data from experimental models to humans has often neglected to take into account the large day-to-day variations that occur in human exposures. Statistical assumptions of normal distribution used in animal models, where there are few nondetectable values, often do not apply to human studies, where > 50% of the values may be nondetectable. Thus, early studies in rodents with aflatoxin biomarkers did not predict the complexity of future investigations.
Coincident with the development of analytical methods to measure the aflatoxin biomarkers, studies were underway to identify effective chemoprevention strategies for aflatoxin carcinogenesis. Highly quantitative models of aflatoxin tumorigenesis were established using stereologic, morphometric techniques to estimate the burden of presumptive preneoplastic cells, that is, GGT-positive and GST-P–positive foci in rat liver (Roebuck et al., 1991). The hypothesis tested in these investigations was that reduction of aflatoxin-DNA adduct levels by chemopreventive agents would be predictive of cancer preventive efficacy. Initial tests of the efficacy of a variety of established chemopreventive agents demonstrated reduction of DNA adduct levels induced by a single dose of aflatoxin (Kensler et al., 1985). A more comprehensive study using multiple doses of aflatoxin and the chemopreventive agent ethoxyquin reduced the observed area and volume (tumor burden) of liver occupied by presumptive preneoplastic foci by > 95% and dramatically reduced binding of AFB1 to hepatic DNA (Kensler et al., 1986). An earlier lifetime study using a similar dosing protocol had established that ethoxyquin prevented HCC development in rats (Cabral and Neal, 1983). This experimental approach has been repeated for the discovery of novel chemopreventive agents, and in all cases, aflatoxin-derived DNA adducts were reduced (Liby et al., 2008; Roebuck et al., 1991; Yates et al., 2006). However, even under optimal conditions, the reduction in the macromolecular adducts has consistently underrepresented the effect on tumor burden (Kensler et al., 1999). Therefore, these macromolecular adducts can track with disease outcome on a population level, but in the multistage process of cancer the absolute level of adduct provides only a necessary but insufficient measure of tumor risk.
Studies with the chemopreventive agent oltipraz (Roebuck et al., 1991) established correlations between reductions in levels of aflatoxin-N7-guanine excreted in urine and incidence of HCC in aflatoxin-exposed rats. Although overall reduction in biomarker levels reflected protection against carcinogenesis, these studies did not address the quantitative relationship between biomarker levels and individual risk. Thus, in a follow-up study, rats dosed with AFB1 daily for 5 weeks were randomized into three groups: no intervention, delayed-transient intervention with oltipraz during weeks 2 and 3 of exposure, and persistent intervention with oltipraz for all 5 weeks of dosing (Kensler et al., 1997). Serial blood samples were collected from each animal at weekly intervals throughout aflatoxin exposure for measurement of aflatoxin-albumin adducts. The integrated level of aflatoxin-albumin adducts over the exposure period decreased 20 and 39% in the delayed-transient and persistent oltipraz intervention groups, respectively, as compared with no intervention. Similarly, the total incidence of HCC dropped significantly from 83 to 60% and 48% in these groups. Overall, integrated biomarker level and risk of HCC were significantly associated. However, when the predictive value of aflatoxin-serum albumin adducts was assessed within treatment groups, there was no association between integrated biomarker levels and risk of HCC. These data clearly demonstrated that levels of the aflatoxin-albumin adducts could predict population-based changes in disease risk, but they did not have the power to identify individual rats destined to develop HCC. Such limitations in the utility of these biomarkers must be recognized in human biomonitoring.
As highlighted in Figure 2, aflatoxin-albumin adducts, like the aflatoxin-N7-guanine adduct, reflect the fate of aflatoxin epoxides. However, they may be subject to distinct metabolic influences as indicated by two recent reports using genetically modified rodents. Studies were undertaken in transgenic rats to examine the role of one highly inducible enzyme, AKR7A1, for protection against acute and chronic actions of AFB1 by enhancing detoxication of a reactive metabolite, AFB1 dialdehyde, by reduction to alcohols (Roebuck et al., 2009). The AFB1 dialdehyde forms adducts with protein amino groups by a Schiff base mechanism, and these adducts have been theorized to be at least one cause of the acute toxicity of AFB1 and to enhance carcinogenesis. A liver-specific AKR7A1 transgenic rat was constructed in the Sprague-Dawley strain and two lines, AKR7A1(Tg2) and AKR7A1(Tg5), were found to overexpress AKR7A1 by 18- and 8-fold, respectively. Rates of formation of AFB1 alcohols, both in hepatic cytosols and as urinary excretion products, dramatically increased in the transgenic lines with AKR7A1(Tg2) being the highest. Surprisingly, neither line offered protection against acute AFB1-induced bile duct proliferation, a functional assessment of acute hepatotoxicity by AFB1, nor did they protect against the formation of GST-P–positive putative preneoplastic foci as a result of chronic exposure to AFB1. Levels of aflatoxin-albumin adducts were reduced in the two transgenic lines; however, no alteration in excretion rates of aflatoxin-N7-guanine was observed. These results imply that the prevention of protein adducts mediated by AKR7A1 are not critical to protection against AFB1 tumorigenicity. Pretreatment of rats with 1,2-dithiole-3-thione, an anticarcinogen known to induce the expression of GST as well as AKR7A1, leads to diminished levels of both aflatoxin albumin and DNA adduct biomarkers. In the mouse, GSTA3 has a uniquely high catalytic activity toward aflatoxin epoxides (Van Ness et al., 1998). Ilic et al. (2010) have observed that mGSTA3 knockout mice have more than 100-fold more aflatoxin-N7-guanine adducts in their livers than do similarly treated wild-type mice. In addition, the mGSTA3 knockout mice die of massive hepatic necrosis, at AFB1 doses that have minimal toxic effects in wild-type mice. Influences of disruption of this GST on the aflatoxin biomarkers have not been undertaken to date. Yet, collectively, these observations indicate that the aflatoxin-DNA adduct biomarker and the albumin adduct biomarker do not always follow in lockstep to perturbations in aflatoxin metabolic pathways. This feature also compromises their utilities in individualizing risk assessments.
Application of Biomarkers to Human Biomonitoring
Environmental carcinogen exposures in people are generally first explored by cross-sectional surveys in which samples are collected from potentially exposed populations. Although these surveys are very valuable for testing the sensitivity and specificity of analytical methods for studying biomarkers, they rarely include a comprehensive examination of exposure, making it difficult to determine dose-response characteristics of individuals. Because health outcomes are not assessed, interpretation of the findings must also be conservative. Nonetheless, these surveys are critical first steps in translating information from experimental studies to an assessment of exposure and risk in humans. Studies of aflatoxin biomarkers in human populations began in the Philippines, where investigators demonstrated that an oxidative metabolite of AFB1, aflatoxin M1, could be measured in urine as an internal dose marker (Campbell et al., 1970). Subsequent work conducted in the People's Republic of China and The Gambia, West Africa, both areas with high incidences of HCC, determined that the levels of urinary aflatoxin biomarkers followed a dose-dependent relationship with aflatoxin intake (Groopman et al., 1992c). However, as in the earlier experimental studies, this relationship was dependent on the specific urinary marker under study; for example, aflatoxin-N7-guanine and aflatoxin M1 showed strong correlations with intake, whereas urinary aflatoxin P1, a different oxidative metabolite, showed no such link. Similarly, highly significant associations between intake of aflatoxin and levels of aflatoxin-albumin adducts have been observed (Gan et al., 1988; Wild et al., 1992). This type of study, that is the measurement of dietary aflatoxin intake and biomarkers at the individual level, is crucial to validate a biomarker for exposure assessment and is often overlooked in molecular epidemiology. Of particular interest in The Gambia was the observation that urinary aflatoxin metabolites reflected day-to-day variations in aflatoxin intake, whereas the aflatoxin-albumin adducts integrated exposure over the week-long study (Groopman et al., 1992c; Wild et al., 1992). This outcome undoubtedly reflects the short whole-body half-life of the aflatoxin-DNA adduct (∼8 h) compared with that of the aflatoxin-albumin adduct (∼3 weeks). Data from these initial cross-sectional biomarker studies supported the validity of these exposure biomarkers for use in epidemiological studies seeking to define associations with risk of HCC, studies of the mechanisms underlying susceptibility, as well as short-term end points for rapid assessment of possible intervention strategies. However, two key attributes, one biological (tracking) and the other chemical (stability), need to be confirmed to successfully use biomarkers for these purposes.
An objective in development of aflatoxin biomarkers is to use them as predictors of past and future exposure status in people. This concept is embodied in the principle of tracking, which is an index of how well an individual's biomarker remains positioned over time in a rank-order relative to other individuals in a group. Tracking within a group of individuals is expressed by the intraclass correlation coefficient, and when this coefficient is 1.0, a person's relative position, independent of exposure, within the group does not change over time. If the intraclass correlation coefficient is 0.0, there is random positioning of the individual's biomarker level relative to the others in the group throughout the time period. The tracking concept is central to interpreting data related to exposure and biomarker levels and requires acquisition of repeated samples from subjects.
Very few multiple sampling or tracking studies for biomarkers of exposure to aflatoxin or indeed any other carcinogen have been conducted in humans. One of the most extensive investigations was conducted in Qidong, People's Republic of China, where the temporal characteristics of aflatoxin-albumin adduct levels over multiple lifetimes of serum albumin in both hepatitis B virus (HBV)–positive and -negative subjects was examined (Wang et al., 1996a). During a 12-week monitoring period and a subsequent follow-up 6 months later for an additional 12 weeks, levels of aflatoxin-albumin adducts were found not to track from one time point to the next (i.e., intraclass correlation coefficient = 0.0). In contrast, in a rat model, the intraclass correlation coefficient was 0.29 (Kensler et al., 1997). There were two possible explanations for the disparity between the human and rat data sets, namely variance in exposure and difference in the experimental method of analysis. In the rat model, exposure to aflatoxin was constant throughout the study. In people, the short-term variation in exposure could be so large that it could mask tracking from one point to the next, even in a long-lived biomarker. Thus, inherent differences in exposure could explain the interclass correlation coefficients. If this were found to be true, the utility of using aflatoxin-albumin adducts as biomarkers of exposure in individuals would be further diminished.
In order to carry out longitudinal studies, it was also important to assess the stability of aflatoxin biomarkers during prolonged storage. In a Shanghai cohort study, aflatoxin biomarker stability was monitored by supplementing urine samples with purified aflatoxins at the time the samples were collected, and analyses carried out over the course of 8 years showed them to be stable (Qian et al., 1994; Ross et al., 1992). Similarly, aflatoxin-albumin adducts in human sera collected in Guangxi, People's Republic of China, were found to be stable for at least 25 years when stored at −20°C (Scholl and Groopman, 2008). Therefore, for at least some of the aflatoxin biomarkers, degradation over time was not a major problem, but similar studies are required to assess this variable for all chemical-specific biomarkers.
Case-Control Studies
Many published case-control studies have examined the relation of aflatoxin exposure and HCC. Compared with cohort studies, case-control studies are both cost and time effective. Unfortunately, case-control studies are initiated long after exposure has occurred, and with specific biomarkers, it cannot be assumed that exposure has been constant over time. Also, such studies involve assumptions in the selection of controls, including that the disease state does not alter metabolism of aflatoxin. Thus, matching of cases and controls in a specific biomarker study is much more difficult than in a case-control study involving genetic markers. Presumably, these inherent problems would bias the results to the no-effect conclusion, and a positive finding likely represents an underestimation of a true effect.
In an early case-control study, Bulatao-Jayme et al. (1982) compared the dietary intake of aflatoxin in cases of HCC in the Philippines and in age- and sex-matched controls. They found that the mean aflatoxin exposure per day in cases of HCC was 4.5 times higher than in the controls; however, alcohol consumption may have enhanced this effect. Van Rensburg and his collaborators (Van Rensburg et al., 1985) and Peers and Linsell (1977) used a similar design for studies in Mozambique and Swaziland, respectively. Again the mean dietary aflatoxin intakes were positively correlated with HCC rates.
In the Guangxi Autonomous Region of China, Yeh and colleagues (Yeh et al., 1985, 1989) examined the interaction between HBV infection and dietary aflatoxin exposure dichotomized for heavy and light levels of contamination. Individuals whose serum was positive for hepatitis B virus surface antigen (HBsAg) and who experienced heavy aflatoxin exposure had a l0-fold higher incidence of HCC than did people living in areas with light aflatoxin contamination (Yeh et al., 1989). In a case-control study in Taiwan, two biomarkers, aflatoxin-albumin adducts and aflatoxin-DNA adducts in liver tissue samples, were measured (Lunn et al., 1997). The proportion of subjects with a detectable level of aflatoxin-albumin adducts was higher for cases of HCC than for matched controls (odds ratio 1.5).
In a more recent study, 145 men with chronic HBV infection were followed for 10 years to determine whether exposure to aflatoxin increased the risk of developing HCC. Eight monthly urine samples collected before the initiation of follow-up were pooled to analyze for aflatoxin M1. Aflatoxin M1 was detected in 54% of the subjects and the risk of HCC was increased 3.3-fold in those with detectable aflatoxin M1. The attributable risk from aflatoxin exposure, defined as the presence of detectable aflatoxin M1, was 0.55. Thus, aflatoxin exposure detected by the presence of the biomarker in urine can account for a substantial portion of HCC risk in men with chronic HBV hepatitis (Sun et al., 1999).
Gene-environment interactions with aflatoxins have also been reported in case-control studies. Although human GSTM1 has low, but measureable catalytic activity toward aflatoxin epoxide relative to mouse GSTA3 or rat GSTA5, the low activity does appear to be protective against aflatoxin-DNA adduct formation, as human hepatocytes from GSTM1-null individuals had threefold higher levels of DNA adducts with the same exposure compared with human hepatocytes that expressed a functional GSTM1 allele (Gross-Steinmeyer et al., 2010). In an at-risk population in Haimen, People’s Republic of China, McGlynn et al. (1995) observed that individuals with mutant genotypes at epoxide hydrolase and GSTMl may be at greater risk of developing aflatoxin adducts, p53 mutations, and HCC when exposed to AFB1. These findings support the existence of genetic susceptibility to AFB1-induced damage in humans and suggest that such susceptibility may interact with HBV infection to enhance HCC risk.
Cohort Studies
Data obtained in cohort studies have the greatest power to establish a valid relationship between exposure and disease outcome because the study is initiated in a cohort of healthy people, includes collection of appropriate samples for biomarker analysis, then involves follow-up of the cohort until significant numbers of disease cases occur. A nested study within the cohort can then be designed to match cases and controls. An advantage of this method is that controls and cases are truly matched because both were recruited at the same time and were healthy at the beginning of the study. A major disadvantage is the time needed for follow-up (often years) to accrue sufficient numbers of cases to fulfill statistical requirements. This disadvantage can be overcome in part by enrolling large numbers of people (often tens of thousands) to ensure case accrual at a rate commensurate with decreased cost.
To date, two major cohort studies incorporating aflatoxin biomarkers have clearly demonstrated the etiologic role of this carcinogen in HCC. The first study, comprising more than 18,000 men in Shanghai, examined the interaction of HBV and aflatoxin biomarkers as independent and interactive risk factors for HCC. The nested case-control data revealed a statistically significant increase in the relative risk of 3.4 for those HCC cases in whom a urinary aflatoxin biomarker (aflatoxin-N7-guanine) was detected. For men whose serum was HBsAg positive but whose urine did not indicate aflatoxin exposure, the relative risk was 7, and in individuals exhibiting both urinary aflatoxin marker and positive HBsAg status, the relative risk was 59 (Qian et al., 1994; Ross et al., 1992). These results strongly support a causal relationship between the presence of chemical carcinogens and viral-specific biomarkers and the risk of HCC.
Subsequent cohort studies in Taiwan have substantially confirmed the results from the Shanghai investigation. Wang et al. (1996b) examined HCC cases and controls nested within a cohort and found that in HBV-infected people there was an adjusted odds ratio of 2.8 for detectable compared with nondetectable aflatoxin-albumin adducts and 5.5 for high compared with low levels of aflatoxin metabolites in urine. In a follow-up study, there was a dose-response relationship between urinary aflatoxin M1 levels and risk of HCC in chronic HBV carriers (Yu et al., 1997). As in the Shanghai cohort, HCC risk associated with AFB1 exposure was most striking among HBV carriers with detectable aflatoxin-N7-guanine in urine.
Thus, these cohort data from two different populations demonstrate the power of validated aflatoxin biomarkers to define a previously unrecognized chemical-viral interaction in the induction of human HCC (Harris, 1994). These findings have significant public health implications. First, vaccination to prevent HBV infection would substantially ameliorate a major risk factor for HCC. Unfortunately, in most parts of the world, HBV infection is acquired before 3 years of age; consequently, worldwide elimination of HBV infection by vaccination will require much of the 21st century to accomplish. Second, minimizing aflatoxin exposure would also significantly reduce HCC risk. Third, and much more broadly, these findings established an environmental health paradigm of complex disease processes involving interactions among multiple risk factors including toxin exposure, pathogens, and susceptibility factors (Feingold et al., 2010).
INTERVENTION TRIALS FOR REDUCING AFLATOXIN EXPOSURE AND DOSE
Protective interventions against aflatoxin exposures in economically developing countries can take many forms. It is axiomatic that as economic development increases, the ability of a population to afford a more diverse diet also increases. This dietary diversity usually leads to a reduction in aflatoxin exposures because the episodic events, such as those described in Kenya, become less frequent. Obviously, the economic development of a country and regions within countries can proceed at a very slow rate and even regress; therefore, intervention strategies are needed during the interim times to lower risks associated with unavoidable aflatoxin exposures. Any intervention strategies should be rigorously tested and validated using clinical trial designs with biomarkers serving as objective end points. Clinical trials and other interventions are needed and designed to translate findings from human and experimental investigations to public health prevention.
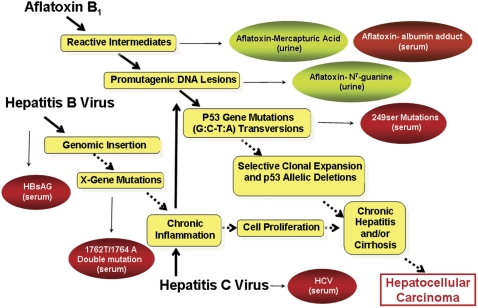
In a primary prevention trial, the goal is to reduce exposure to aflatoxins in the diet. A range of interventions includes planting pest-resistant varieties of staple crops, attempting to lower mold growth in harvested crops, improving storage methods following harvest, and using trapping agents that block the uptake of unavoidably ingested aflatoxins. In secondary prevention trials, one goal is to modulate the metabolism of ingested aflatoxins to enhance detoxification processes, thereby reducing internal doses and subsequent risk. There is active research in the development of mold-resistant strains of grains and in the genetic manipulation of molds to lower aflatoxin production (Menkir et al., 2006; Yu et al., 2005). Detailed descriptions of these types of biocontrol interventions are beyond the scope of this review; the following sections will focus on the use of aflatoxin biomarkers to develop and validate other primary as well as secondary prevention strategies. Figure 3 highlights the key steps in the development of HCC caused by HBV infection and aflatoxin exposure that may serve as targets for preventive interventions. Table 1 summarizes approaches and results from clinical intervention trials seeking to alter aflatoxin exposure and disposition in populations at high risk for aflatoxin-induced disease.
FIG. 3.
Key steps in the development of HCC by viral (HBV and HCV) and chemical (aflatoxin) factors. Blood- and urine-based biomarkers used in etiological studies are indicated.
TABLE I.
Summary of Randomized Clinical Intervention Trials Using Aflatoxin Biomarkers as Intermediate End Points
| Agent | Dose and schedule | Size (duration) | Mode of action | Biomarker modulation | References |
| Postharvest storage methods | Implementation during harvest and postharvest | 20 Villages (5 months) | Reduced exposure | 60% Decrease in AFB-AA levels in intervention villages; increased frequency of nondetect values from 2 to 20% | Turner et al. (2005) |
| NovaSil | Placebo q.d. | 177 (3 months) | Trapping | Significant reduction in AFB-AA at both doses; 58% decrease in urinary AFM1 at 3 months (3.0 g) | Wang et al. (2008) |
| 1.5 g q.d. | |||||
| 3.0 g q.d | |||||
| Chlorophyllin | Placebo q.d. | 180 (4 months) | Trapping | 55% Decrease in urinary excretion of AFB-N7-gua DNA adducts at 3 months. | Egner et al. (2001) |
| 100 mg q.d. × 3 | |||||
| Oltipraz | Placebo q.d. | 234 (2 months) | Enhanced detoxication | 2.6-Fold increase in urinary excretion of AFB-NAC at 1 month (125 mg); 51% decrease in AFM1 at 1 month (500 mg) 6% decrease in AFB-AA at 2 months (500 mg) | Wang et al. (1999) |
| 125 mg q.d. | |||||
| 500 mg q.w. | |||||
| Broccoli sprouts tea (glucoraphanin) | Placebo q.d. | 200 (14 days) | Enhanced detoxication | 9% Decrease in urinary excretion of AFB-N7-gua adducts at 10 days | Kensler et al. (2005) |
| 400 μmol q.d. | |||||
| Green tea polyphenols | Placebo q.d. | 124 (3 months) | Enhanced detoxication | 17- And 14-fold increase in urinary excretion of AFB-NAC at 3 months; 42 and 43% decrease in AFM1. | Tang et al. (2008) |
| 500 mg q.d. | |||||
| 1000 mg q.d. |
Note. AFB-NAC, aflatoxin-mercapturic acid; AFB-AA, aflatoxin albumin adduct; AFB-N7-gua, aflatoxin-N7-guanine.
Postharvest Intervention
The use of aflatoxin biomarkers as efficacy end points in primary prevention trials has been recently reported (Turner et al., 2005). This work was built on earlier research that documented extensive aflatoxin exposure in Guinea, West Africa, because of consumption of groundnuts as a dietary staple (Diallo et al., 1995; Sylla et al., 1999) and revealed that postharvest storage was correlated with increases in exposure. On the basis of these observations, a community-based intervention study was conducted among subsistence farmers in the Kindia region of Guinea, West Africa. The intervention comprised measures to limit postharvest aflatoxin contamination of the groundnut crop (hand sorting, drying on mats, sun drying, storage in natural fiber bags and on wooden pallets, and use of insecticides) (Turner et al., 2005). Farms from 20 villages were included in the design; 10 were assigned to each of the intervention and control arms of the study. The intervention practices were all common in the region but were not systematically applied by the rural farmers. In the control villages, farmers were left to follow their usual postharvest practices, which therefore occasionally included one or more of the elements of the intervention strategy. Altered trajectories of aflatoxin-albumin adduct levels were used to assess the success of the intervention, and this biomarker was measured in 600 subjects over a 5-month period. In the control villages, the aflatoxin-albumin adduct level increased postharvest, whereas, in the intervention villages, the level after 5 months of storage was similar to that immediately post-harvest. Mean levels at this time were 60% lower in intervention compared with control villages. The number of subjects with nondetectable aflatoxin-albumin at the time of harvest was approximately 30%, but this number decreased to 2% 5 months later in control villages. In contrast, almost 20% of individuals in the intervention group had nondetectable levels at the same time. The mean level of AFB1 in groundnuts in household stores in intervention and control villages mirrored the pattern seen for aflatoxin-albumin adducts. The effectiveness of this intervention suggests that significant reductions in exposure can be achieved by using low-technological approaches at the subsistence farm level in sub-Saharan Africa in a setting of limited complexity to the diet. Unfortunately, voluntary adoption of the postharvest storage techniques among farmers in these villages was quite limited in years following the conduct of the trial.
Trapping Agents
For many years, sodium calcium aluminosilicate marketed as NovaSil clay, a common anticaking agent in animal feeds, has worked to adsorb aflatoxins in the gastrointestinal tract of animals and diminish the bioavailability and adverse effects of these toxins (Phillips et al., 2002). Animal model studies using male and female Sprague-Dawley rats fed diets containing up to 2.0% (wt/wt) levels of NovaSil for 28 weeks found no adverse health effects. These experimental findings, in combination with a larger body of literature (Phillips et al., 2002), supported the exploration of clay-based enterosorption for intervention studies in human populations at high risk for aflatoxicosis (Afriyie-Gyawu et al., 2005). An initial study reported on the safety and tolerance of NovaSil in humans and established baseline protocols for long-term efficacy studies. A randomized, double-blinded phase I clinical trial was conducted with 50 volunteers who were randomly divided into 2 groups: the low-dose group received 9 capsules containing 1.5 g NovaSil/day and the high-dose group received 9 capsules containing 3.0 g/day for a period of 2 weeks. Compliance was outstanding, and at the end of the study, there were no statistically significant differences between the two groups for adverse health complaints nor were there differences in any of the clinical chemistries or any other health parameters measured (Wang et al., 2005).
Subsequently, the efficacy of NovaSil clay to reduce aflatoxin biomarkers of exposure was evaluated in blood and urine samples collected from study participants during a 3-month phase IIa clinical intervention trial in Ghana. NovaSil was delivered before meals via capsules. Levels of aflatoxin-albumin adducts in serum samples collected at baseline and at 1 month were similar among the placebo, low-dose (1.5 g NovaSil per day), and high-dose (3.0 g NovaSil per day) groups. However, the levels of aflatoxin-albumin adducts at 3 months were significantly decreased in both intervention groups compared with levels in the placebo group. Levels of aflatoxin M1 (AFM1) in urine samples collected at baseline and at 1 month were not statistically different among the three study groups. However, a significant decrease in the median level of AFM1 in samples collected at 3 months was found in the high-dose group when compared with the median level in the placebo group. The results suggest that, based on a reduction of biomarkers, capsules containing NovaSil clay can be used to reduce the bioavailability of dietary aflatoxins (Wang et al., 2008). It should be noted that clay is a dietary staple in central Africa and is common in the marketplace. However, the forms of clay local to that region do not absorb aflatoxins.
Chlorophyllin
The anticarcinogenic properties of chlorophyllin, a water-soluble derivative of chlorophyll, have been demonstrated in a number of animal models (Breinholt et al., 1995a; Dashwood et al., 1998). The initial characterization of chlorophyllin as an anticarcinogen arose from inhibition of HCC development in aflatoxin-treated trout (Breinholt et al., 1995a). Although the primary mode of action is thought to be the sequestration of aflatoxin by chlorophyllin in a 1:1 ratio (Breinholt et al., 1995b), experimental data have characterized enzyme-inducing properties that may also contribute to its mechanism of action (Fahey et al., 2005). In a recent study, chlorophyll was shown to be protective against aflatoxin-induced cancer in the rat; moreover, the aflatoxin biomarkers tracked with the protective efficacy (Simonich et al., 2007). In a randomized, double-blind, placebo-controlled chemoprevention trial conducted in Qidong, chlorophyllin was determined to alter the disposition of aflatoxin (Egner et al., 2001). One hundred and eighty healthy adults were randomly assigned to ingest 100 mg of chlorophyllin or a placebo three times a day prior to each meal for 4 months. The primary end point was modulation of levels of urinary aflatoxin-N7-guanine adducts collected 3 months into the intervention. Adherence to the study protocol was outstanding, and no adverse events were reported. Aflatoxin-N7-guanine could be detected in 105 of 169 available samples. Chlorophyllin consumption at each meal led to an overall 55% reduction in median urinary levels of this aflatoxin biomarker compared with those subjects taking placebo. A recent phase 0 study was reported by Jubert et al. (2009) which utilized accelerator mass spectrometry to investigate the absorption and pharmacokinetics of AFB1 and its metabolites in four human volunteers, using doses of 14C and AFB1 that each fall within an allowable range of safety. Based on total 14C equivalents, AFB1 was rapidly absorbed into the plasma in all volunteers with first-order kinetics. Interventions with chlorophyllin or naturally occurring chlorophyll a reduced AFB1 uptake and distribution among all individuals. In all individuals, the chlorophyll a intervention produced a significant 40–60% reduction in excretion of urinary aflatoxin equivalents. These results affirm that chlorophyllin (and chlorophyll a) mediate reductions in systemic uptake of aflatoxin in humans, as seen in preclinical models and surmised from the clinical trial in Qidong. Thus, prophylactic interventions with chlorophyllin or supplementation of diets with foods rich in chlorophylls may represent practical means to prevent the development of HCC—or other environmentally induced cancers in which readily adsorbed carcinogens (e.g., polycyclic aromatic hydrocarbons, heterocyclic amines) may be important etiologic factors.
Dithiolethiones (oltipraz)
Cancer chemoprevention is a key strategy for the secondary prevention of cancer. This approach entails the use of drugs, dietary supplements or functional foods to retard, block, or even reverse the carcinogenic process. These strategies serve to alter cell fate, by either preventing cells from acquiring genetic damage or by impeding the proliferation of preneoplastic cells or, alternatively, accelerating their death by apoptosis. One successful strategy for cancer chemoprevention is modulation of drug-metabolizing enzymes, leading to a facilitated elimination of endogenous and environmental carcinogens. Inducers of conjugating enzymes such as dithiolethiones and sulforaphane inhibit tumorigenesis of environmental carcinogens in various animal models (Roebuck et al., 1991; Zhang et al., 1992). Increasing lines of evidence show that the Keap1-Nrf2 complex is a key molecular target of these chemopreventive enzyme inducers. The transcription factor Nrf2 is a member of the basic leucine-zipper NF-E2 family and interacts with the antioxidant response element (ARE) in the promoter region of detoxifying enzymes. A cytoplasmic actin-binding protein, Keap1, is an inhibitor of Nrf2 that sequesters it in the cytoplasm and facilitates its ubiquitination and subsequent degradation. Inducers disrupt this process, allowing Nrf2 to accumulate and translocate to the nucleus (Kensler et al., 2007). Disruption of the Nrf2 gene in mice leads to enhanced sensitivity to carcinogens and the loss of chemopreventive efficacy by inducers (Fahey et al., 2002; Ramos-Gomez et al., 2001).
1,2-Dithiole-3-thiones were reported in the 1950s to be constituents of cruciferous vegetables in Czechoslovakia (Jirousek, 1958), although a more recent study failed to find the unsubstituted 3H-1,2-dithiole-3-thione in cabbage in the United States (Marks, 1991). Oltipraz, a substituted 1,2-dithiole-3-thione, was originally developed by the pharmaceutical industry as a possible treatment for schistosomiasis and was extensively evaluated in clinical trials in the early 1980s. Field trials in Mali, Gaboon, and France, using short courses of 1–5 days with total doses of 1.25–7.5 g, achieved cure rates of greater than 90%. Although studying mechanisms of antischistosomiasis by oltipraz, Bueding and colleagues initially noted that giving the drug to mice infected with Schistosoma mansoni caused a dramatic reduction in the glutathione stores of the parasite while paradoxically markedly elevating glutathione levels in many tissues of the host (Bueding et al., 1982). Subsequent studies demonstrated that oltipraz and some structurally related 1,2-dithiole-3-thiones were potent inducers of enzymes concerned with the maintenance of reduced glutathione pools as well as enzymes important to carcinogen detoxication in multiple tissues of rats and mice (Ansher et al., 1983, 1986). These results prompted Bueding to predict that oltipraz might have cancer chemopreventive properties. This prediction held true, as upon extensive preclinical evaluation by the National Cancer Institute and others, oltipraz was found to be effective as an anticarcinogen in nearly a score of animal models (Kensler et al., 1999).
Aflatoxin biomarkers were used as intermediate end points in a phase IIa chemoprevention trial of oltipraz in Qidong, People's Republic of China (Kensler et al., 1998; Wang et al., 1999). This was a placebo-controlled, double-blind study in which participants were randomized to receive placebo or 125 mg oltipraz daily or 500 mg oltipraz weekly. Urinary aflatoxin M1 levels were reduced by 51% compared with the placebo group in persons receiving the 500 mg weekly dose. No significant differences were seen in urinary aflatoxin M1 levels in the 125-mg group compared with placebo. This effect was thought to be because of inhibition of cytochrome P450 1A2 activity. Median levels of aflatoxin-mercapturic acid (a glutathione conjugate derivative) were elevated sixfold in the 125-mg group but were unchanged in the 500-mg group. Increased aflatoxin-mercapturic acid reflects induction of aflatoxin conjugation through the actions of GSTs. The apparent lack of induction in the 500-mg group probably reflects masking caused by diminished aflatoxin-8,9-epoxide formation for conjugation through the inhibition of CYPlA2 seen in this group. This study demonstrated for the first time that aflatoxin biomarkers could be modulated in humans in a manner that would predict decreased disease risk.
Sulforaphane
Although the oltipraz clinical trial demonstrated the proof of principle for inducing pathways leading to aflatoxin detoxication in humans, the practicality of using a drug-based method for prevention in the economically developing world is limited. Not only is there a potential for adverse health effects from any long-term exposure to a drug, the expense of this type of intervention may make the intervention cost prohibitive for these populations. There may also be culture-based aversion to the use of drugs. Fortunately, oltipraz is not the only agent that affects enzyme changes through the Nrf2-Keap1 pathway. Many foods have high levels of these enzyme inducers (Fahey and Kensler, 2007; Talalay and Fahey, 2001). Sulforaphane has been extensively examined for its chemopreventive properties and is a potent activator of the Nrf2-Keap1 pathway leading to increased expression of carcinogen detoxifying enzymes (Dinkova-Kostova et al., 2007; Fahey et al., 2002). A beverage formed from hot water infusions of 3-day-old broccoli sprouts, containing defined concentrations of glucosinolates as a stable precursor of the anticarcinogen sulforaphane, was recently evaluated for its ability to alter the disposition of aflatoxin. In this study, 200 healthy adults drank infusions containing either 400 or < 3 μmole glucoraphanin nightly for 2 weeks. Urinary levels of aflatoxin-N7-guanine were similar between the two intervention arms. However, measurement of urinary levels of dithiocarbamates (sulforaphane metabolites) indicated striking interindividual differences in bioavailability. This outcome may reflect individual differences in the rates of hydrolysis of glucoraphanin to sulforaphane by the intestinal microflora of the study participants. Accounting for this variability, a significant inverse association was observed for excretion of dithiocarbamates and aflatoxin-N7-guanine adducts in individuals receiving broccoli sprout glucosinolates (Kensler et al., 2005). This preliminary study illustrates the potential use of an inexpensive, easily implemented, food-based method for secondary prevention in a population at high risk for aflatoxin exposures. A follow-up intervention seeking to minimize the interindividual variability in the pharmacokinetics of the glucoraphanin precursor is currently in progress.
Green Tea Polyphenols
Many studies have demonstrated that green tea polyphenols (GTPs) inhibit various chemically induced cancers in experimental animals and epidemiological studies also point to the potential benefit of these compounds (Moyers and Kumar, 2004; Yang et al., 2006). Qin et al. (1997) studied the effects of GTP in drinking water for 2 or 4 weeks to protect against the development of AFB1-induced hepatocarcinogenesis in the rat. Data from this investigation revealed that aflatoxin-DNA binding in the liver was significantly inhibited by 20–30% in animals pretreated with green tea, and that the burden of preneoplastic lesions was significantly inhibited by 60–70%. The experimental data on GTP provided the impetus to translate this strategy to human clinical trials. In an initial study, in an aflatoxin-exposed high-risk group in Guangxi, People's Republic of China, the effects of GTP were assessed in urine samples collected from a randomized, double-blinded, placebo-controlled phase IIa chemoprevention trial (Luo et al., 2006). All participants tested positive for aflatoxin-albumin adducts and took GTP capsules daily at doses of 500, 1000 mg, or a placebo for 3 months. Analyses were done in blood and urine samples collected during this clinical trial (Tang et al., 2008). Levels of albumin adducts at baseline were comparable for all three dose groups, and no significant differences were observed in adduct levels in the placebo group over the 3-month period. However, reductions in albumin adduct levels were observed in both groups receiving GTPs over the 3-month intervention period. An analysis using a mixed-effects model indicated that the reduction in aflatoxin-albumin adduct levels over time was dose and time dependent. Reductions in median aflatoxin M1 levels, as compared with the placebo, were found in both GTP groups at 3 months of the intervention, whereas significant elevations in median aflatoxin mercapturic acid levels were observed in both GTP groups compared with the placebo group at 1 and 3 months of GTPs intervention. These results indicate that GTPs effectively modulate aflatoxin metabolism and metabolic activation, as had been previously observed with oltipraz in Qidong (Wang et al., 1999).
FUTURE PROSPECTS
Though many nations suffering from both high aflatoxin exposures and high HBV prevalence have nominally established maximum allowable aflatoxin standards in food, there is little if any evaluation or enforcement of these standards in many rural areas. Indeed, the food of subsistence farmers and in local food markets is rarely formally inspected. When food is traded with other nations, strict aflatoxin standards can even lead to large economic losses for poor food-exporting nations (Phillips et al., 2008; Wu, 2004). Subsistence farmers and local food traders sometimes have the luxury of discarding obviously moldy maize and groundnuts, but in seasons of climatic stress, oftentimes people have no choice but to eat moldy food or starve (Phillips et al., 2008). Currently, over 5 billion people worldwide experience uncontrolled exposure to aflatoxin (Strosnider et al., 2006). What remains unknown is how many liver cancer cases can be attributed to this aflatoxin exposure worldwide. Recently Liu and Wu (2010) have developed a risk assessment for the contribution of aflatoxin to the global burden of HCC. Of the 550,000–600,000 new HCC cases worldwide per year, they estimate about 25,200–155,000 (4.6–28.2%) may be attributable to aflatoxin exposure alone. Most cases occur in sub-Saharan Africa, Southeast Asia, and China, where populations suffer from both high HBV prevalence and largely uncontrolled aflatoxin exposure in food. The broad range in the estimate reflects limitations in determining levels of aflatoxin exposures, uncertainties in the nature of the dose-response curve, uncertainties in the mode of interaction between aflatoxins and viruses, and incomplete data on the prevalence of HBV in different regions of the world. Data-driven estimates of the noncarcinogenic health effects of aflatoxins in humans have not been undertaken.
The next few years portend tremendous opportunities for applying deep sequencing technologies and other strategies to discover the spectrum of mutational changes, chromosomal aberrations, and epigenetic modifications in human HCCs and to link them to their underlying etiological factors, including aflatoxin and/or exposure to hepatitis viruses. Sequencing tumors will provide the capacity to develop new biomarkers that can be used for prevention and early screening interventions. Similarly an understanding of these genetic alterations in human liver tumors will refine understanding of etiology and facilitate the development of new experimental models that comprehensively recapitulate the biology of HCC, thereby enabling the development of new therapeutic and preventive investigations. All this future work builds upon the foundation of the biomarker strategies that have been described and promises new opportunities for amelioration of HCC.
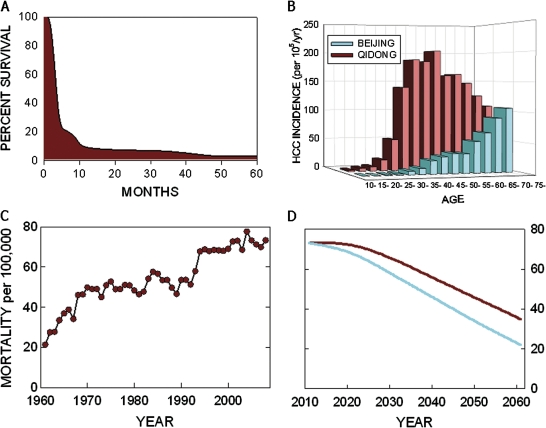
The total impact of HCC is reflected by the overall incidence of the disease and very poor prognosis following diagnosis. In Qidong, People's Republic of China, median survival following diagnosis of HCC is less than 3 months (Chen et al., 2003) (Fig. 4A), a time frame much earlier than typically seen in Western countries for HCC. In these very high-risk regions of the world, exposure to aflatoxin and HBV and their resultant deleterious effects occur across the lifespan from birth to adult hood. Thus, the median age of diagnosis and death from HCC in Qidong ranges between 45 and 50 years of age (Fig. 4B). Recent data assessing the 20-year follow-up of the implementation of early childhood HBV vaccination in Taiwan reveals up to a 70% reduction in HCC in the 6-to 29-year age group (Chang et al., 2009). Although this reduction should be viewed within the context of a low age-specific incidence rate of HCC in this birth cohort, it nonetheless points to a probable lowering of HCC incidence in other populations, such as Qidong, where a vaccination program was implemented about 20 years ago. Figure 4C depicts the mortality rate of HCC in Qidong over the past 50 years. Mortality from HCC has remained static over the past decade at a rate of about 75/100,000 per year after tripling during the antecedent four decades. The projected impact of HBV vaccination in Qidong, assuming the Taiwan success rate, for the next 50 years is illustrated in Figure 4D. By 2061, the incidence of HCC is likely to be reduced by about 50% as a result of the total implementation of HBV vaccination at birth. Experimental studies from our laboratories have demonstrated that chemopreventive interventions can reduce HCC incidence in rats by 50% despite aflatoxin exposures that induce HCC in over 80% of animals in the absence of chemoprotective interventions (Kensler et al., 1997). Because aflatoxin exposure alone is probably responsible for 20% of the risk of HCC in Qidong, then chemoprotection could reduce HCC incidence by an additional 10% by 2061 (Fig. 4D). Further, because chemoprotective interventions have been shown to delay the development of HCC, an earlier decline in HCC in residents of Qidong could also be inferred. Finally, because epidemiologic investigations have shown that there exists a multiplicative interaction between aflatoxin and HBV in HCC development (Qian et al., 1994), strategies employing both vaccination against HBV and chemoprotection or primary prevention against aflatoxin could result in a 40–50% reduction in HCC in Qidong by 2041, a 15-year foreshortening compared with vaccination alone (Fig. 4D).
FIG. 4.
Mortality from HCC in Qidong, People's Republic of China. (A) Survival curve following diagnosis of HCC in Qidong. Median survival is < 3 months. Adapted from Chen et al. (2003). (B) Age-specific incidence of HCC in Qidong and Beijing, China. The rate of infection with HBV is the same in both Qidong and Beijing, China. Additional environmental factors, such as exposure to aflatoxin (which is more prevalent in Qidong), could underlie the elevated risk of HCC in this region. This regional dichotomy highlights the opportunity to reduce the incidence of HCC simply by reducing the effects of aflatoxin. Adapted from Kensler et al. (2003). (C) Mortality rates of HCC by year in Qidong. Data provided by Dr Jian-Guo Chen, Qidong Liver Cancer Institute. (D) Projected mortality rates from HCC over the next 50 years on the basis of existing vaccination programs against HBV infection (red line) and coupled with possible reductions in aflatoxin exposures (blue line). The impact of vaccination is modeled on the basis of the current experience in Taiwan, where near universal vaccination was implemented in 1984 (Chang et al., 2009). The effect of attenuated aflatoxin exposure is modeled from the results of animal intervention studies, where reduction and delay of incidence of HCC have been observed (Kensler et al., 1997). See text for further discussion.
There is a compelling need to develop and implement preventive strategies for people at risk for liver cancer and other manifestations of aflatoxicoses. Clearly, for HCC, the longer term public health solutions will arise out of universal vaccination programs against HBV, the development of vaccines against HCV or other means to prevent its transmission, and overall economic improvement for the populations that bear the largest burden of liver cancer. Collectively, these outcomes will dramatically reduce exposures of future generations to the major risk factors for HCC. At present though, there are nearly 400 million individuals infected with HBV for whom vaccination programs hold no promise and many millions ingesting foods with levels of aflatoxins deemed unacceptable to the developed world. Preventive modalities targeted at these populations should be a priority. A major challenge lies in identifying effective interventions, be it primary prevention of aflatoxin contamination of dietary staples through biocontrol or removal and secondary chemopreventive interventions or some combinations of approaches. Ongoing developments in the use of molecular biomarkers to gain rapid insights into the efficacy of interventions are accelerating the discovery process. However, key roadblocks lie in application of what is known already. The biggest challenge remains to deliver these interventions to places of the world where they are most needed. Wu and Khlangwiset (2010) have identified key questions to be addressed regarding the evaluation of aflatoxin interventions:
Are there any countervailing health or ecological risks associated with each of these interventions?
What would be the delivery mechanism of the intervention?
Can the intervention be manufactured locally, or must it be imported?
Can farmers or consumers afford the up-front costs, and if not, who will pay for the intervention?
Is the intervention culturally appropriate?
Would people comply with the intervention? Related to this, how often does it need to be implemented, and how many people need to implement it?
How easy or difficult is it to apply the intervention effectively, so as to reduce aflatoxin or its adverse effects? Are there risks associated with applying it improperly?
How can health economics be used to determine the extent to which different intervention strategies are cost effective?
Over the next 50 years, much of the population growth in the world is going to occur in countries that have intrinsically high aflatoxin exposure, in addition to exposures to many other chemical and biological agents. The observation that aflatoxin exposure may affect child growth and susceptibility to infection (Gong et al., 2002), as well HCC risk, serves to emphasize further the public health need for development and implementation of interventions in these countries. Over the past 15 years, aflatoxin biomarkers have permitted the exploration of not just the etiology of aflatoxin-related disease but also the efficacy of various intervention strategies. One element in the appropriate application of prevention strategies is the targeting to various stages of the disease progression, based on knowledge of the underlying mechanisms of action (see Fig. 3). In addition, there is a need to consider the temporal relationship between exposure and disease. For example, it is now well established that aflatoxin exposure begins in utero and increases markedly as a child is weaned onto solid family foods (Wild and Turner, 2002). This early exposure, at the time the child is also first exposed to numerous infections including HBV, suggests that there may be particular merit in intervening to reduce exposure during these critical windows. Finally, different interventions may be appropriate to different exposure scenarios. For example, the aflatoxicosis cases in Kenya occurred on a background of food insufficiency, where avoidance of exposure through primary prevention approaches was not feasible. However, some rapidly deployed secondary prevention may have provided benefits. Alternatively, in subsistence farming communities where dietary staples are frequently subject to aflatoxin contamination year after year, the introduction, through education of primary prevention approaches, may provide longer term solutions of both health and economic benefit (Turner et al., 2007). Overall, the availability of a range of intervention approaches will help combat the adverse health effects of these common environmental contaminants. As the interventions are applied in longer term studies, the impact not only on exposure but also health end points will allow a more precise understanding of their benefits to public health.
In populations of Asia and Africa experiencing relatively high aflatoxin exposures, molecular biomarkers have been essential tools for dissecting the contributions of this carcinogen to the etiology liver cancer. Further, when paired with HBV biomarkers, a multiplicative interaction between the two risk factors has been identified. This body of work, which fulfilled the Bradford Hill criteria for causality (Hill, 1965), has had major implications for public health and control of liver cancer in high-risk and high incidence regions of the world. Aflatoxin exposures in populations of economically developed countries are vastly reduced, but not completely eliminated, through implementation of effective control strategies in agricultural and manufacturing processes. However, possible impacts of unavoidable lower endemic exposures on liver cancer risk have received little systematic study, and application of the biomarker approach in the United States and Europe could be envisaged for this purpose. Given the analytical sensitivity of current technologies, initial studies of aflatoxin exposures could be launched using national or transnational health survey cohorts such as National Health and Nutrition Examination Survey or European Prospective Investigation into Cancer and Nutrition to provide a metric for determining exposure to aflatoxin in people living in economically developed regions of the world. It seems highly likely that specific subpopulations in developed countries are at increased risk, particularly any who may be afflicted with concurrent HBV or HCV infection, and studies need to be designed to acquire data from such groups. Information generated by such studies would further inform the scientific basis for current regulatory policies and practices required for aflatoxin risk assessments. This challenge recognizes that when the aflatoxin problem first arose in the late 1960s, standards for regulating aflatoxin contamination of human and animal food products were necessarily based on extrapolation of data from studies in experimental animals. Action levels justifying seizure of aflatoxin-contaminated products in interstate commerce were not based on risk assessments of health hazard because requisite data did not exist but rather were predicated on attainability of minimized aflatoxin levels.
Thus, it would be productive to address a number of open questions, taking advantage of existing insights into mechanisms underlying the toxicology of aflatoxins coupled with current analytical capabilities for biomarker measurement. Examples of such questions include:
Do current regulatory standards for aflatoxin levels in human diets adequately protect public health?
To what extent do current levels of aflatoxin exposure contribute to HCC risk in the economically developed countries?
Do subpopulations exist within the U.S. or European populations who experience elevated aflatoxin exposures through dietary habits involving consumption of large amounts of food products especially prone to aflatoxin contamination. If so, does this exposure put them at significantly elevated risk of adverse health outcomes?
Implementation of a strategy to provide answers to such questions would mark a truly translational toxicological effort that encompasses the full range of issues from molecular mechanisms to global health and places a cornerstone for further investigations over the next 50 years.
FUNDING
National Institutes of Health (grants P01 ES006052 and R01 CA39416); National Institute of Environmental Health Sciences; National Cancer Institute.
Acknowledgments
We wish to acknowledge the over 40 years of continuous research and training grant support from the National Institute of Environmental Health Sciences and the National Cancer Institute that enabled the career development of all the authors. We are also indebted to our extensive multidisciplinary, international team of colleagues and collaborators who over these years contributed greatly to making the aflatoxin story a model for translational research in toxicology.
References
- Adamson RH, Correa P, Sieber SM, McIntire KR, Dalgard DW. Carcinogenicity of aflatoxin B1 in rhesus monkeys: two additional cases of primary liver cancer. J. Natl Cancer Inst. 1976;57:67–78. doi: 10.1093/jnci/57.1.67. [DOI] [PubMed] [Google Scholar]
- Afriyie-Gyawu E, Mackie J, Dash B, Wiles M, Taylor J, Huebner H, Tang L, Guan H, Wang JS, Phillips T. Chronic toxicological evaluation of dietary NovaSil clay in Sprague-Dawley rats. Food Addit. Contam. 2005;22:259–269. doi: 10.1080/02652030500110758. [DOI] [PubMed] [Google Scholar]
- Anonymous Outbreak of aflatoxin poisoning—eastern and central provinces, Kenya, January–July 2004. MMWR Morb. Mortal Wkly. Rep. 2004;53:790–793. [PubMed] [Google Scholar]
- Ansher SS, Dolan P, Bueding E. Chemoprotective effects of two dithiolthiones and of butylhydroxyanisole against carbon tetrachloride and acetaminophen toxicity. Hepatology. 1983;3:932–935. doi: 10.1002/hep.1840030608. [DOI] [PubMed] [Google Scholar]
- Ansher SS, Dolan P, Bueding E. Biochemical effects of dithiolthiones. Food Chem. Toxicol. 1986;24:405–415. doi: 10.1016/0278-6915(86)90205-x. [DOI] [PubMed] [Google Scholar]
- Asao T, Buchi G, Abdel-Kader M, Chang S, Wick E, Wogan GN. Aflatoxins B and G. J. Am. Chem. Soc. 1963;85:1706–1707. doi: 10.1021/ja01082a031. [DOI] [PubMed] [Google Scholar]
- Azziz-Baumgartner E, Lindblade K, Gieseker K, Rogers HS, Kieszak S, Njapau H, Schleicher R, McCoy LF, Misore A, DeCock K, et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005;113:1779–1783. doi: 10.1289/ehp.8384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GS, Loveland PM, Pereira C, Pierce D, Hendricks JD, Groopman JD. Quantitative carcinogenesis and dosimetry in rainbow trout for aflatoxin B1 and aflatoxicol, two aflatoxins that form the same DNA adduct. Mutat. Res. 1994;313:25–38. doi: 10.1016/0165-1161(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Bennett RA, Essigmann JM, Wogan GN. Excretion of an aflatoxin-guanine adduct in the urine of aflatoxin B1-treated rats. Cancer Res. 1981;41:650–654. [PubMed] [Google Scholar]
- Bhat RV, Krishnamachari KA. Follow-up study of aflatoxic hepatitis in parts of western India. Ind. J. Med. Res. 1977;66:55–58. [PubMed] [Google Scholar]
- Blount WP. Turkey ”X” disease. J. Br. Turkey Fed. 1961;9:55–58. [Google Scholar]
- Bosch FX, Munoz N. Prospects for epidemiological studies on hepatocellular cancer as a model for assessing viral and chemical interactions. IARC Sci. Publ. 1988;89:427–438. [PubMed] [Google Scholar]
- Breinholt V, Hendricks J, Pereira C, Arbogast D, Bailey G. Dietary chlorophyllin is a potent inhibitor of aflatoxin B1 hepatocarcinogenesis in rainbow trout. Cancer Res. 1995a;55:57–62. [PubMed] [Google Scholar]
- Breinholt V, Schimerlik M, Dashwood R, Bailey G. Mechanisms of chlorophyllin anticarcinogenesis against aflatoxin B1: complex formation with the carcinogen. Chem. Res. Toxicol. 1995b;8:506–514. doi: 10.1021/tx00046a004. [DOI] [PubMed] [Google Scholar]
- Bueding E, Dolan P, Leroy JP. The antischistosomal activity of oltipraz. Res. Commun. Chem. Pathol. Pharmacol. 1982;37:293–303. [PubMed] [Google Scholar]
- Bulatao-Jayme J, Almero EM, Castro MC, Jardeleza MT, Salamat LA. A case-control dietary study of primary liver cancer risk from aflatoxin exposure. Int. J. Epidemiol. 1982;11:112–119. doi: 10.1093/ije/11.2.112. [DOI] [PubMed] [Google Scholar]
- Busby WFJ, Wogan GN. Aflatoxins. In: Searle CD, editor. Chemical Carcinogens. Washington, DC: American Chemical Society; 1984. pp. 945–1136. [Google Scholar]
- Cabral JRP, Neal GE. The inhibitory effects of ethoxyquin on the carcinogenic action of aflatoxin B1 in rats. Cancer Lett. 1983;19:125–132. doi: 10.1016/0304-3835(83)90146-5. [DOI] [PubMed] [Google Scholar]
- Campbell AD, Whitaker TB, Pohland AE, Dickens JW, Park DL. Sampling, sample preparation, and sampling plans for foodstuffs for mycotoxin analysis. Pure Appl. Chem. 1986;58:305–314. [Google Scholar]
- Campbell TC, Caedo JP, Jr, Bulatao-Jayme J, Salamat L, Engel RW. Aflatoxin M1 in human urine. Nature. 1970;227:403–404. doi: 10.1038/227403a0. [DOI] [PubMed] [Google Scholar]
- Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccines: a 20-year follow-up study. J. Natl Cancer Inst. 2009;101:1348–1355. doi: 10.1093/jnci/djp288. [DOI] [PubMed] [Google Scholar]
- Chen JG, Parkin DM, Chen QG, Shen QJ, Zhang BC, Zhu YR. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J. Med. Screen. 2003;10:204–209. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- Chen JG, Zhu J, Parkin DM, Zhang YH, Lu JH, Zhu YR, Chen TY. Trends in the incidence of cancer in Qidong, China, 1978–2002. Int. J. Cancer. 2006;119:1447–1454. doi: 10.1002/ijc.21952. [DOI] [PubMed] [Google Scholar]
- Dashwood R, Negishi T, Hayatsu H, Breinholt V, Hendricks J, Bailey G. Chemopreventive properties of chlorophylls towards aflatoxin B1: a review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat. Res. 1998;399:245–253. doi: 10.1016/s0027-5107(97)00259-5. [DOI] [PubMed] [Google Scholar]
- Diallo MS, Sylla A, Sidibe K, Sylla BS, Trepo CR, Wild CP. Prevalence of exposure to aflatoxin and hepatitis B and C viruses in Guinea, West Africa. Nat. Toxins. 1995;3:6–9. doi: 10.1002/nt.2620030103. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Fahey JW, Wade KL, Jenkins SN, Shapiro TA, Fuchs EJ, Kerns ML, Talalay P. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol. Biomarkers Prev. 2007;16:847–851. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Groopman JD. The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance. San Diego, CA: Academic Press, Inc.; 1994. [Google Scholar]
- Egner PA, Gange SJ, Dolan PM, Groopman JD, Munoz A, Kensler TW. Levels of aflatoxin-albumin biomarkers in rat plasma are modulated by both long-term and transient interventions with oltipraz. Carcinogenesis. 1995;16:1769–1773. doi: 10.1093/carcin/16.8.1769. [DOI] [PubMed] [Google Scholar]
- Egner PA, Groopman JD, Wang JS, Kensler TW, Friesen MD. Quantification of aflatoxin-B1-N7-guanine in human urine by high-performance liquid chromatography and isotope dilution tandem mass spectrometry. Chem. Res. Toxicol. 2006;19:1191–1195. doi: 10.1021/tx060108d. [DOI] [PubMed] [Google Scholar]
- Egner PA, Wang JB, Zhu YR, Zhang BC, Wu Y, Zhang QN, Qian GS, Kuang SY, Gange SJ, Jacobson LP, et al. Chlorophyllin intervention reduces aflatoxin-DNA adducts in individuals at high risk for liver cancer. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14601–14606. doi: 10.1073/pnas.251536898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Kensler TW. Role of dietary supplements/nutraceuticals in chemoprevention through induction of cytoprotective enzymes. Chem. Res. Toxicol. 2007;20:572–576. doi: 10.1021/tx7000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, Stephenson KK, Dinkova-Kostova AT, Egner PA, Kensler TW, Talalay P. Chlorophyll, chlorophyllin and related tetrapyrroles are significant inducers of mammalian phase 2 cytoprotective genes. Carcinogenesis. 2005;26:1247–1255. doi: 10.1093/carcin/bgi068. [DOI] [PubMed] [Google Scholar]
- Feingold BJ, Vegosen L, Davis M, Leibler J, Peterson A, Silbergeld EK. A niche for infectious disease in environmental health: rethinking the toxicological paradigm. Environ. Health Perspect. 2010;118:165–172. doi: 10.1289/ehp.0901866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010 doi: 10.1002/ijc.25516. Advance Access published on June 17, 2010; doi: 10.1002/ijc.5516. [DOI] [PubMed] [Google Scholar]
- Gallagher EP, Wienkers LC, Stapleton PL, Kunze KL, Eaton DL. Role of human microsomal and human complementary DNA-expressed cytochromes P4501A2 and P4503A4 in the bioactivation of aflatoxin B1. Cancer Res. 1994;54:101–108. [PubMed] [Google Scholar]
- Gan LS, Skipper PL, Peng X, Groopman JD, Chen JS, Wogan GN, Tannenbaum SR. Serum albumin adducts in the molecular epidemiology of aflatoxin carcinogenesis: correlation with aflatoxin B1 intake and urinary excretion of aflatoxin M1. Carcinogenesis. 1988;9:1323–1325. doi: 10.1093/carcin/9.7.1323. [DOI] [PubMed] [Google Scholar]
- Gong Y, Hounsa A, Egal S, Turner PC, Sutcliffe AE, Hall AJ, Cardwell K, Wild CP. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ. Health Perspect. 2004;112:1334–1338. doi: 10.1289/ehp.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong YY, Cardwell K, Hounsa A, Egal S, Turner PC, Hall AJ, Wild CP. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. Br. Med. J. 2002;325:20–21. doi: 10.1136/bmj.325.7354.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan C, Tulpule PG, Krishnamurthi D. Induction of hepatic carcinoma with aflatoxin in the rhesus monkey. Food Cosmet. Toxicol. 1972;10:519–521. doi: 10.1016/s0015-6264(72)80086-5. [DOI] [PubMed] [Google Scholar]
- Groopman JD, Donahue PR, Zhu J, Chen J, Wogan GN. Aflatoxin metabolism in humans: detection of metabolites and nucleic acid adducts in urine by affinity chromatography. Proc. Natl. Acad. Sci. U.S.A. 1985;82:6492–6496. doi: 10.1073/pnas.82.19.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman JD, Hall AJ, Whittle H, Hudson GJ, Wogan GN, Montesano R, Wild CP. Molecular dosimetry of aflatoxin-N 7-guanine in human urine obtained in The Gambia, West Africa. Cancer Epidemiol. Biomarkers Prev. 1992a;1:221–227. [PubMed] [Google Scholar]
- Groopman JD, Hasler JA, Trudel LJ, Pikul A, Donahue PR, Wogan GN. Molecular dosimetry in rat urine of aflatoxin-N7-guanine and other aflatoxin metabolites by multiple monoclonal antibody affinity chromatography and immunoaffinity/high performance liquid chromatography. Cancer Res. 1992b;52:267–274. [PubMed] [Google Scholar]
- Groopman JD, Jiaqi Z, Donahue PR, Pikul A, Lisheng Z, Jun-shi C, Wogan GN. Molecular dosimetry of urinary aflatoxin-DNA adducts in people living in Guangxi Autonomous Region, People's Republic of China. Cancer Res. 1992c;52:45–52. [PubMed] [Google Scholar]
- Groopman JD, Kensler TW. The light at the end of the tunnel for chemical-specific biomarkers: daylight or headlight? Carcinogenesis. 1999;20:1–11. doi: 10.1093/carcin/20.1.1. [DOI] [PubMed] [Google Scholar]
- Groopman JD, Kensler TW. Role of metabolism and viruses in aflatoxin-induced liver cancer. Toxicol. Appl. Pharmacol. 2005;206:131–137. doi: 10.1016/j.taap.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Groopman JD, Trudel LJ, Donahue PR, Marshak-Rothstein A, Wogan GN. High-affinity monoclonal antibodies for aflatoxins and their application to solid-phase immunoassays. Proc. Natl. Acad. Sci. U.S.A. 1984;81:7728–7731. doi: 10.1073/pnas.81.24.7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Steinmeyer K, Stapleton PL, Tracy JH, Bammler TK, Strom SC, Eaton DL. Sulforaphane- and phenethyl isothiocyanate-induced inhibition of aflatoxin B1-mediated genotoxicity in human hepatocytes: role of GSTM1 genotype and CYP3A4 gene expression. Toxicol. Sci. 2010;116:422–432. doi: 10.1093/toxsci/kfq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CC. Solving the viral-chemical puzzle of human liver carcinogenesis. Cancer Epidemiol. Biomarkers Prev. 1994;3:1–2. [PubMed] [Google Scholar]
- Henry SH, Bosch FX, Troxell TC, Bolger PM. Policy forum: public health. Reducing liver cancer–global control of aflatoxin. Science. 1999;286:2453–2454. doi: 10.1126/science.286.5449.2453. [DOI] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: association or causation? Proc. R. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic Z, Crawford D, Egner PA, Sell S. Glutathione-S-transferase A3 knockout mice are sensitive to acute cytotoxic and genotoxic effects of aflatoxin B1. Toxicol. Appl. Pharmacol. 2010;242:241–246. doi: 10.1016/j.taap.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirousek L. Uber das vorkommen von trithionen (1,2-dithiocyclopent-4-en-3-thione) in Brassicapflanzen. Naturwissenschaften. 1958;45:386–387. [Google Scholar]
- Jubert C, Mata JE, Bench G, Dashwood R, Pereira C, Tracewell W, Turteltaub KW, Williams D, Bailey G. Effects of chlorophyll and chlorophyllin on low-dose aflatoxin B1 pharmacokinetics in human volunteers. Cancer Prev. Res. 2009;2:1015–1022. doi: 10.1158/1940-6207.CAPR-09-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler TW, Chen JG, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye LX, Coady J, Wang JB, Wu Y, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo Township, Qidong, People's Republic of china. Cancer Epidemiol. Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Davidson NE, Roebuck BD, Pikul A, Groopman JD. Modulation of aflatoxin metabolism, aflatoxin-N7-guanine formation, and hepatic tumorigenesis in rats fed ethoxyquin: role of induction of glutathione S -transferases. Cancer Res. 1986;46:3924–3931. [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Trush MA, Bueding E, Groopman JD. Modification of aflatoxin B1 binding to DNA in vivo in rats fed phenolic antioxidants, ethoxyquin and a dithiothione. Carcinogenesis. 1985;6:759–763. doi: 10.1093/carcin/6.5.759. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Gange SJ, Egner PA, Dolan PM, Munoz A, Groopman JD, Rogers AE, Roebuck BD. Predictive value of molecular dosimetry: individual versus group effects of oltipraz on aflatoxin-albumin adducts and risk of liver cancer. Cancer Epidemiol. Biomarkers Prev. 1997;6:603–610. [PubMed] [Google Scholar]
- Kensler TW, Groopman JD, Sutter TR, Curphey TJ, Roebuck BD. Development of cancer chemopreventive agents: oltipraz as a paradigm. Chem. Res. Toxicol. 1999;12:113–126. doi: 10.1021/tx980185b. [DOI] [PubMed] [Google Scholar]
- Kensler TW, He X, Otieno M, Egner PA, Jacobson LP, Chen B, Wang JS, Zhu YR, Zhang BC, Wang JB, Wu Y, et al. Oltipraz chemoprevention trial in Qidong, People's Republic of China: modulation of serum aflatoxin albumin adduct biomarkers. Cancer Epidemiol. Biomarkers Prev. 1998;7:127–134. [PubMed] [Google Scholar]
- Kensler TW, Qian GS, Chen JG, Groopman JD. Translational strategies for cancer prevention in liver. Nat. Rev. Cancer. 2003;3:321–329. doi: 10.1038/nrc1076. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kew MC. Epidemiology of hepatocellular carcinoma. Toxicology. 2002;181–182:35–38. doi: 10.1016/s0300-483x(02)00251-2. [DOI] [PubMed] [Google Scholar]
- Krishnamachari KA, Bhat RV, Nagarajan V, Tilak TB. Hepatitis due to aflatoxicosis. An outbreak in Western India. Lancet. 1975;1:1061–1063. doi: 10.1016/s0140-6736(75)91829-2. [DOI] [PubMed] [Google Scholar]
- Lancaster MC, Jenkins FP, Philp JM. Toxicity associated with certain samples of groundnuts. Nature. 1961;192:1095–1096. [Google Scholar]
- Lewis L, Onsongo M, Njapau H, Schurz-Rogers H, Luber G, Kieszak S, Nyamongo J, Backer L, Dahiye AM, Misore A, et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005;113:1763–1767. doi: 10.1289/ehp.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K, Yore MM, Roebuck BD, Baumgartner KJ, Honda T, Sundararajan C, Yoshizawa H, Gribble GW, Williams CR, Risingsong R, et al. A novel acetylenic tricyclic bis-(cyano enone) potently induces phase 2 cytoprotective pathways and blocks liver carcinogenesis induced by aflatoxin. Cancer Res. 2008;68:6727–6733. doi: 10.1158/0008-5472.CAN-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ. Health Perspect. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn RM, Zhang YJ, Wang LY, Chen CJ, Lee PH, Lee CS, Tsai WY, Santella RM. p53 mutations, chronic hepatitis B virus infection, and aflatoxin exposure in hepatocellular carcinoma in Taiwan. Cancer Res. 1997;57:3471–3477. [PubMed] [Google Scholar]
- Luo H, Tang L, Tang M, Billam M, Huang T, Yu J, Wei Z, Liang Y, Wang K, Zhang ZQ, et al. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–268. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- Lutz WK. Quantitative evaluation of DNA-binding data in vivo for low-dose extrapolations. Arch. Toxicol. 1987;(Suppl. 11):66–74. doi: 10.1007/978-3-642-72558-6_9. [DOI] [PubMed] [Google Scholar]
- Lye MS, Ghazali AA, Mohan J, Alwin N, Nair RC. An outbreak of acute hepatic encephalopathy due to severe aflatoxicosis in Malaysia. Am. J. Trop. Med. Hyg. 1995;53:68–72. [PubMed] [Google Scholar]
- Marks SH. Analysis of a reported organosulfur, carcinogenesis inhibitor: 1,2-dithiole-3-thione in cabbage. J. Agric. Food Chem. 1991;39:893–895. [Google Scholar]
- McCoy LF, Scholl PF, Sutcliffe AE, Kieszak SM, Powers CD, Rogers HS, Gong YY, Groopman JD, Wild CP, Schleicher RL. Human aflatoxin albumin adducts quantitatively compared by ELISA, HPLC with fluorescence detection, and HPLC with isotope dilution mass spectrometry. Cancer Epidemiol. Biomarkers Prev. 2008;17:1653–1657. doi: 10.1158/1055-9965.EPI-07-2780. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Rosvold EA, Lustbader ED, Hu Y, Clapper ML, Zhou T, Wild CP, Xia XL, Baffoe-Bonnie A, Ofori-Adjei D, et al. Susceptibility to hepatocellular carcinoma is associated with genetic variation in the enzymatic detoxification of aflatoxin B1. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2384–2387. doi: 10.1073/pnas.92.6.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkir A, Brown RL, Bandyopadhyay R, Chen ZY, Cleveland TE. A USA-Africa collaborative strategy for identifying, characterizing, and developing maize germplasm with resistance to aflatoxin contamination. Mycopathologia. 2006;162:225–232. doi: 10.1007/s11046-006-0056-3. [DOI] [PubMed] [Google Scholar]
- Moyers SB, Kumar NB. Green tea polyphenols and cancer chemoprevention: multiple mechanisms and endpoints for phase II trials. Nutr. Rev. 2004;62:204–211. doi: 10.1111/j.1753-4887.2004.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Nesbitt BF, O'Kelly J, Sargeant K, Sheridan A. Aspergillus flavus and turkey X disease. Toxic metabolites of Aspergillus flavus. Nature. 1962;195:1062–1063. doi: 10.1038/1951062a0. [DOI] [PubMed] [Google Scholar]
- Ngindu A, Johnson BK, Kenya PR, Ngira JA, Ocheng DM, Nandwa H, Omondi TN, Jansen AJ, Ngare W, Kaviti JN, et al. Outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya. Lancet. 1982;1:1346–1348. doi: 10.1016/s0140-6736(82)92411-4. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Peers FG, Linsell CA. Dietary aflatoxins and human primary liver cancer. Ann Nutr. Aliment. 1977;31:1005–1017. [PubMed] [Google Scholar]
- Phillips TD, Afriyie-Gyawu E, Williams J, Huebner H, Ankrah NA, Ofori-Adjei D, Jolly P, Johnson N, Taylor J, Marroquin-Cardona A, et al. Reducing human exposure to aflatoxin through the use of clay: a review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008;25:134–145. doi: 10.1080/02652030701567467. [DOI] [PubMed] [Google Scholar]
- Phillips TD, Lemke SL, Grant PG. Characterization of clay-based enterosorbents for the prevention of aflatoxicosis. Adv. Exp. Med. Biol. 2002;504:157–171. doi: 10.1007/978-1-4615-0629-4_16. [DOI] [PubMed] [Google Scholar]
- Poirier MC, Santella RM, Weston A. Carcinogen macromolecular adducts and their measurement. Carcinogenesis. 2000;21:353–359. doi: 10.1093/carcin/21.3.353. [DOI] [PubMed] [Google Scholar]
- Probst C, Njapau H, Cotty PJ. Outbreak of an acute aflatoxicosis in Kenya in 2004: identification of the causal agent. Appl. Environ. Microbiol. 2007;73:2762–2764. doi: 10.1128/AEM.02370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian GS, Ross RK, Yu MC, Yuan JM, Gao YT, Henderson BE, Wogan GN, Groopman JD. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol. Biomarkers Prev. 1994;3:3–10. [PubMed] [Google Scholar]
- Qin G, Gopalan-Kriczky P, Su J, Ning Y, Lotlikar PD. Inhibition of aflatoxin B1-induced initiation of hepatocarcinogenesis in the rat by green tea. Cancer Lett. 1997;112:149–154. doi: 10.1016/s0304-3835(96)04568-5. [DOI] [PubMed] [Google Scholar]
- Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JL, Bennett GA, Ross PF, Nelson PE. Analysis of naturally occurring mycotoxins in feedstuffs and food. J. Anim. Sci. 1993;71:2563–2574. doi: 10.2527/1993.7192563x. [DOI] [PubMed] [Google Scholar]
- Roebuck BD, Johnson DN, Sutter CH, Egner PA, Scholl PF, Friesen MD, Baumgartner KJ, Ware NM, Bodreddigari S, Groopman JD, et al. Transgenic expression of aflatoxin aldehyde reductase (AKR7A1) modulates aflatoxin B1 metabolism but not hepatic carcinogenesis in the rat. Toxicol. Sci. 2009;109:41–49. doi: 10.1093/toxsci/kfp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebuck BD, Liu YL, Rogers AE, Groopman JD, Kensler TW. Protection against aflatoxin B 1-induced hepatocarcinogenesis in F344 rats by 5-(2-pyrazinyl)-4-methyl-1,2-dithiole-3-thione (oltipraz): predictive role for short-term molecular dosimetry. Cancer Res. 1991;51:5501–5506. [PubMed] [Google Scholar]
- Ross RK, Yuan JM, Yu MC, Qian GS, Tu JT, Gao YT, Henderson BE, Wogan GN, Groopman JD. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–946. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- Sabbioni G, Skipper PL, Buchi G, Tannenbaum SR. Isolation and characterization of the major serum albumin adduct formed by aflatoxin B1 in vivo in rats. Carcinogenesis. 1987;8:819–824. doi: 10.1093/carcin/8.6.819. [DOI] [PubMed] [Google Scholar]
- Santella RM. Immunological methods for detection of carcinogen-DNA damage in humans. Cancer Epidemiol. Biomarkers Prev. 1999;8:733–739. [PubMed] [Google Scholar]
- Sargeant K, Sheridan A, O'Kelly J, Carnaghan RBA. Toxicity associated with certain samples of groundnuts. Nature. 1961;192:1096–1097. [Google Scholar]
- Scholl P, Musser SM, Kensler TW, Groopman JD. Inhibition of aflatoxin Ml excretion in rat urine during dietary intervention with oltipraz. Carcinogenesis. 1996;17:1385–1388. doi: 10.1093/carcin/17.6.1385. [DOI] [PubMed] [Google Scholar]
- Scholl PF, Groopman JD. Long-term stability of human aflatoxin B1 albumin adducts assessed by isotope dilution mass spectrometry and high-performance liquid chromatography-fluorescence. Cancer Epidemiol. Biomarkers Prev. 2008;17:1436–1439. doi: 10.1158/1055-9965.EPI-07-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl PF, McCoy L, Kensler TW, Groopman JD. Quantitative analysis and chronic dosimetry of the aflatoxin B1 plasma albumin adduct Lys-AFB1 in rats by isotope dilution mass spectrometry. Chem. Res. Toxicol. 2006a;19:44–49. doi: 10.1021/tx050251r. [DOI] [PubMed] [Google Scholar]
- Scholl PF, Musser SM, Groopman JD. Synthesis and characterization of aflatoxin B1 mercapturic acids and their identification in rat urine. Chem. Res. Toxicol. 1997;10:1144–1151. doi: 10.1021/tx960161+. [DOI] [PubMed] [Google Scholar]
- Scholl PF, Turner PC, Sutcliffe AE, Sylla A, Diallo MS, Friesen MD, Groopman JD, Wild CP. Quantitative comparison of aflatoxin B1 serum albumin adducts in humans by isotope dilution mass spectrometry and ELISA. Cancer Epidemiol. Biomarkers Prev. 2006b;15:823–826. doi: 10.1158/1055-9965.EPI-05-0890. [DOI] [PubMed] [Google Scholar]
- Shank RC, Bourgeois CH, Keschamras N, Chandavimol P. Aflatoxins in autopsy specimens from Thai children with an acute disease of unknown aetiology. Food Cosmet. Toxicol. 1971;9:501–507. doi: 10.1016/0015-6264(71)90080-0. [DOI] [PubMed] [Google Scholar]
- Sieber SM, Correa P, Dalgard DW, Adamson RH. Induction of osteogenic sarcomas and tumors of the hepatobiliary system in nonhuman primates with aflatoxin B1. Cancer Res. 1979;39:4545–4554. [PubMed] [Google Scholar]
- Simonich MT, Egner P, Roebuck BD, Orner G, Jubert C, Pereira C, Groopman JD, Kensler TW, Dashwood RH, Williams D, et al. Natural chlorophyll inhibits aflatoxin B1 induced multi-organ carcinogenesis in the rat. Carcinogenesis. 2007;28:1294–1302. doi: 10.1093/carcin/bgm027. [DOI] [PubMed] [Google Scholar]
- Strosnider H, Azziz-Baumgartner E, Banziger M, Bhat RV, Breiman R, Brune MN, DeCock K, Dilley A, Groopman J, Hell K, et al. Workgroup report: public health strategies for reducing aflatoxin exposure in developing countries. Environ. Health Perspect. 2006;114:1898–1903. doi: 10.1289/ehp.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Lu P, Gail MH, Pee D, Zhang Q, Ming L, Wang J, Wu Y, Liu G, Wu Y, et al. Increased risk of hepatocellular carcinoma in male hepatitis B surface antigen carriers with chronic hepatitis who have detectable urinary aflatoxin metabolite M1. Hepatology. 1999;30:379–383. doi: 10.1002/hep.510300204. [DOI] [PubMed] [Google Scholar]
- Sylla A, Diallo MS, Castegnaro J, Wild CP. Interactions between hepatitis B virus infection and exposure to aflatoxins in the development of hepatocellular carcinoma: a molecular epidemiological approach. Mutat. Res. 1999;428:187–196. doi: 10.1016/s1383-5742(99)00046-0. [DOI] [PubMed] [Google Scholar]
- Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- Tang L, Tang M, Xu L, Luo H, Huang T, Yu J, Zhang L, Gao W, Cox SB, Wang JS. Modulation of aflatoxin biomarkers in human blood and urine by green tea polyphenols intervention. Carcinogenesis. 2008;29:411–417. doi: 10.1093/carcin/bgn008. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson UP, Dalgard DW, Reeves J, Adamson RH. Tumor incidence in a chemical carcinogenesis study of nonhuman primates. Regul. Toxicol. Pharmacol. 1994;19:130–151. doi: 10.1006/rtph.1994.1013. [DOI] [PubMed] [Google Scholar]
- Tilak TB. Induction of cholangiocarcinoma following treatment of a rhesus monkey with aflatoxin. Food Cosmet. Toxicol. 1975;13:247–249. doi: 10.1016/s0015-6264(75)80011-3. [DOI] [PubMed] [Google Scholar]
- Turner PC, Collinson AC, Cheung YB, Gong Y, Hall AJ, Prentice AM, Wild CP. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int. J. Epidemiol. 2007;36:1119–1125. doi: 10.1093/ije/dym122. [DOI] [PubMed] [Google Scholar]
- Turner PC, Sylla A, Gong YY, Diallo MS, Sutcliffe AE, Hall AJ, Wild CP. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: a community-based intervention study. Lancet. 2005;365:1950–1956. doi: 10.1016/S0140-6736(05)66661-5. [DOI] [PubMed] [Google Scholar]
- Ueng YF, Shimada T, Yamazaki H, Guengerich FP. Oxidation of aflatoxin B1 by bacterial recombinant human cytochrome P450 enzymes. Chem. Res. Toxicol. 1995;8:218–225. doi: 10.1021/tx00044a006. [DOI] [PubMed] [Google Scholar]
- Van der Zijden AS, Blanche Koelensmid WA, Boldingh J, Barrett CB, Ord WO, Philp J. Isolation in crystalline form of a toxin responsible for Turkey X disease. Nature. 1962;195:1060–1062. [Google Scholar]
- Van Ness KP, McHugh TE, Bammler TK, Eaton DL. Identification of amino acid residues essential for high aflatoxin B1-8,9-epoxide conjugation activity in alpha class glutathione S-transferases through site-directed mutagenesis. Toxicol. Appl. Pharmacol. 1998;152:166–174. doi: 10.1006/taap.1998.8493. [DOI] [PubMed] [Google Scholar]
- Van Rensburg SJ, Cook-Mozaffari P, Van Schalkwyk DJ, Van der Watt JJ, Vincent TJ, Purchase IF. Hepatocellular carcinoma and dietary aflatoxin in Mozambique and Transkei. Br. J. Cancer. 1985;51:713–726. doi: 10.1038/bjc.1985.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatanasapt V, Martin N, Sriplung H, Chindavijak K, Sontipong S, Sriamporn S, Parkin DM, Ferlay J. Cancer incidence in Thailand, 1988–1991. Cancer Epidemiol. Biomarkers Prev. 1995;4:475–483. [PubMed] [Google Scholar]
- Wang JS, Luo H, Billam M, Wang Z, Guan H, Tang L, Goldston T, Afriyie-Gyawu E, Lovett C, Griswold J, et al. Short-term safety evaluation of processed calcium montmorillonite clay (NovaSil) in humans. Food Addit. Contam. 2005;22:270–279. doi: 10.1080/02652030500111129. [DOI] [PubMed] [Google Scholar]
- Wang JS, Qian GS, Zarba A, He X, Zhu YR, Zhang BC, Jacobson L, Gange SJ, Munoz A, Kensler TW, et al. Temporal patterns of aflatoxin-albumin adducts in hepatitis B surface antigen-positive and antigen-negative residents of Daxin, Qidong County, People's Republic of China. Cancer Epidemiol. Biomarkers Prev. 1996a;5:253–261. [PubMed] [Google Scholar]
- Wang JS, Shen X, He X, Zhu YR, Zhang BC, Wang JB, Qian GS, Kuang SY, Zarba A, Egner PA, Jacobson LP, et al. Protective alterations in phase 1 and 2 metabolism of aflatoxin B1 by oltipraz in residents of Qidong, People's Republic of China. J. Natl Cancer Inst. 1999;91:347–354. doi: 10.1093/jnci/91.4.347. [DOI] [PubMed] [Google Scholar]
- Wang LY, Hatch M, Chen CJ, Levin B, You SL, Lu SN, Wu MH, Wu WP, Wang LW, Wang Q, et al. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int. J. Cancer. 1996b;67:620–625. doi: 10.1002/(SICI)1097-0215(19960904)67:5<620::AID-IJC5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Wang P, Afriyie-Gyawu E, Tang Y, Johnson NM, Xu L, Tang L, Huebner HJ, Ankrah NA, Ofori-Adjei D, Ellis W, et al. NovaSil clay intervention in Ghanaians at high risk for aflatoxicosis: II. Reduction in biomarkers of aflatoxin exposure in blood and urine. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008;25:622–634. doi: 10.1080/02652030701598694. [DOI] [PubMed] [Google Scholar]
- Wang XW, Hussain SP, Huo TI, Wu CG, Forgues M, Hofseth LJ, Brechot C, Harris CC. Molecular pathogenesis of human hepatocellular carcinoma. Toxicology. 2002;181–182:43–47. doi: 10.1016/s0300-483x(02)00253-6. [DOI] [PubMed] [Google Scholar]
- Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle H, Montesano R, Groopman JD. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiol. Biomarkers Prev. 1992;1:229–234. [PubMed] [Google Scholar]
- Wild CP, Turner PC. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis. 2002;17:471–481. doi: 10.1093/mutage/17.6.471. [DOI] [PubMed] [Google Scholar]
- Williams DE, Orner G, Willard KD, Tilton S, Hendricks JD, Pereira C, Benninghoff AD, Bailey GS. Rainbow trout (Oncorhynchus mykiss) and ultra-low dose cancer studies. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009;149:175–181. doi: 10.1016/j.cbpc.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogan GN, Paglialunga S, Newberne PM. Carcinogenic effects of low dietary levels of aflatoxin B1 in rats. Food Cosmet. Toxicol. 1974;12:681–685. doi: 10.1016/0015-6264(74)90239-9. [DOI] [PubMed] [Google Scholar]
- Wu F. Mycotoxin risk assessment for the purpose of setting international regulatory standards. Environ. Sci. Technol. 2004;38:4049–4055. doi: 10.1021/es035353n. [DOI] [PubMed] [Google Scholar]
- Wu F, Khlangwiset P. Health economic impacts and cost-effectiveness of aflatoxin-reduction strategies in Africa: case studies in biocontrol and post-harvest interventions. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010;27:496–509. doi: 10.1080/19440040903437865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CS, Lambert JD, Hou Z, Ju J, Lu G, Hao X. Molecular targets for the cancer preventive activity of tea polyphenols. Mol. Carcinog. 2006;45:431–435. doi: 10.1002/mc.20228. [DOI] [PubMed] [Google Scholar]
- Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baumgartner KJ, Roebuck BD, Liby KT, Yore MM, et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Mo CC, Ren RC. Risk factors for hepatocellular carcinoma in Guangxi, People's Republic of China. Natl. Cancer Inst. Monogr. 1985;69:47–48. [PubMed] [Google Scholar]
- Yeh FS, Yu MC, Mo CC, Luo S, Tong MJ, Henderson BE. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Res. 1989;49:2506–2509. [PubMed] [Google Scholar]
- Yu J, Cleveland TE, Nierman WC, Bennett JW. Aspergillus flavus genomics: gateway to human and animal health, food safety, and crop resistance to diseases. Rev. Iberoam Micol. 2005;22:194–202. doi: 10.1016/s1130-1406(05)70043-7. [DOI] [PubMed] [Google Scholar]
- Yu MW, Lien JP, Chiu YH, Santella RM, Liaw YF, Chen CJ. Effect of aflatoxin metabolism and DNA adduct formation on hepatocellular carcinoma among chronic hepatitis B carriers in Taiwan. J. Hepatol. 1997;27:320–330. doi: 10.1016/s0168-8278(97)80178-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc. Natl Acad. Sci. U.S.A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]