Abstract
Objective To systematically review interventional studies of the effects of alcohol consumption on 21 biological markers associated with risk of coronary heart disease in adults without known cardiovascular disease.
Design Systematic review and meta-analysis.
Data sources Medline (1950 to October 2009) and Embase (1980 to October 2009) without limits.
Study selection Two reviewers independently selected studies that examined adults without known cardiovascular disease and that compared fasting levels of specific biological markers associated with coronary heart disease after alcohol use with those after a period of no alcohol use (controls). 4690 articles were screened for eligibility, the full texts of 124 studies reviewed, and 63 relevant articles selected.
Results Of 63 eligible studies, 44 on 13 biomarkers were meta-analysed in fixed or random effects models. Quality was assessed by sensitivity analysis of studies grouped by design. Analyses were stratified by type of beverage (wine, beer, spirits). Alcohol significantly increased levels of high density lipoprotein cholesterol (pooled mean difference 0.094 mmol/L, 95% confidence interval 0.064 to 0.123), apolipoprotein A1 (0.101 g/L, 0.073 to 0.129), and adiponectin (0.56 mg/L, 0.39 to 0.72). Alcohol showed a dose-response relation with high density lipoprotein cholesterol (test for trend P=0.013). Alcohol decreased fibrinogen levels (−0.20 g/L, −0.29 to −0.11) but did not affect triglyceride levels. Results were similar for crossover and before and after studies, and across beverage types.
Conclusions Favourable changes in several cardiovascular biomarkers (higher levels of high density lipoprotein cholesterol and adiponectin and lower levels of fibrinogen) provide indirect pathophysiological support for a protective effect of moderate alcohol use on coronary heart disease.
Introduction
Moderate alcohol consumption (up to one drink a day for women and up to two for men) has been associated with a decreased risk for certain cardiovascular diseases, particularly coronary heart disease, in several studies of diverse populations.1 2 Most of these studies, however, used an observational design, raising concerns about potential confounding.
Feeding studies (where alcohol is experimentally administered) free of concerns about confounding may help to elucidate the mechanisms by which alcohol affects cardiovascular disease. In 1999, a systematic review of experimental studies of alcohol consumption and changes in lipid levels and haemostatic factors asserted that the protective association of alcohol on certain cardiovascular diseases seemed to be mediated by some of these effects.3 Since that systematic review was published the breadth of research on this topic has expanded substantially. Atherosclerosis, the underlying cause of coronary heart disease and ischaemic stroke, is increasingly understood to be a chronic, low grade inflammatory disease of the arterial wall.4 Increased levels of inflammatory markers have been associated with risk of cardiovascular disease.5 6 New studies have examined not only the effect of alcohol on lipid levels and haemostatic factors but also on other measures of inflammation and endothelial cell function as well as levels of adipocyte hormones. Furthermore, in addition to haemostatic factors, increased levels of other molecules, such as cellular adhesion molecules and adipocyte hormones, are believed to contribute to the development of the systemic inflammatory response associated with increased risk of cardiovascular disease.4 7 8
A synthesis of the evidence from experimental research in this area may inform clinicians trying to interpret the plausibility of the protective effects of alcohol on certain aspects of cardiovascular disease (coronary heart disease) from observational studies. We therefore systematically reviewed the effect of experimentally manipulated alcohol consumption (alcohol use versus a period of no alcohol use) on the circulating concentrations of selected cellular and molecular biological markers of atherothrombotic conditions associated with increased coronary heart disease risk in adults without pre-existing cardiovascular disease. This review offers complementary, indirect mechanistic evidence to that obtained from the expanding epidemiological research on the apparent protective effect of alcohol on certain aspects of cardiovascular disease.9 10
Methods
The systematic review was carried out using a predetermined protocol and in accordance with published guidelines for reporting of systematic reviews of randomised controlled trials (PRISMA).
Data sources and searches
We searched for alcohol intervention studies in adults without pre-existing cardiovascular disease in whom circulating levels of specific biomarkers were measured after a specified amount of alcohol had been consumed within a defined timeframe compared with a period of no alcohol use. We searched Medline (1950 to October 2009) and Embase (1980 to October 2009) without language restrictions for potentially relevant articles.
We used a strategy recommended for searching electronic databases for controlled interventional studies.11 Our search focused on the exposure of interest, relevant outcomes, and study designs. The exposure of interest was alcohol consumption. The relevant outcomes were circulating atherothrombotic biological markers associated with coronary heart disease. These included lipids (triglycerides, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, Lp(a) lipoprotein, and apolipoprotein A1), inflammatory markers (C reactive protein, leucocytes, interleukin 6, tumour necrosis factor α, and haemostatic factors plasminogen activator inhibitor 1, von Willebrand factor, tissue plasminogen activator, fibrinogen, and e-selectin), endothelial cell function markers (intracellular adhesion molecule 1 and vascular cell adhesion molecule), and adipocyte hormones (leptin and adiponectin). Our study designs of interest were experimental studies involving an intentional alcohol intervention to modify levels of biological markers with a no alcohol control. We included randomised controlled trials with two arms, before and after studies, and crossover studies. Using the Boolean operator “and” in varying combinations we then combined the three comprehensive search themes. See web extra appendix 1 for the complete Medline search strategy.
In addition to searching the electronic databases we consulted the bibliography of the only pre-existing systematic review on this subject.3 One of the authors (KJM) served as our content expert and provided us with input on captured literature and direction on pertinent studies in the grey literature.
Study selection
Relevant articles were selected using a two phase process. Two researchers (SEB and PER) independently reviewed all identified abstracts for eligibility. All abstracts reporting on the effect of alcohol consumption and relevant biomarkers in participants without pre-existing cardiovascular disease were selected for full text review. This initial stage was intentionally liberal; we discarded only abstracts that clearly did not meet the aforementioned criteria. The inter-rater agreement for this stage was high (κ=0.80, 95% confidence interval 0.65 to 0.94). Disagreements were resolved by consensus. Secondly, full text articles assessed by one reviewer (SEB) were verified by a second reviewer (PER) to determine if the study met the specified intervention, study population, and design criteria. Specifically, we included studies if they evaluated the circulating blood levels of the specified biomarkers during a period of intentional, prescribed alcohol feeding versus a period of no alcohol use. We excluded studies if participants had pre-existing cardiovascular disease or continued to drink “usual alcohol” in addition to the amounts of intervention alcohol. Both published and unpublished studies were eligible for inclusion.
Data extraction and quality assessment
From relevant studies we extracted information on sample size, population demographics (age, number of men and women, and mean age or age range, or both), inclusion and exclusion criteria (pre-existing health conditions, smoking status, drugs), study design (crossover, randomised crossover, randomised two arm, and before and after), characteristics of the alcohol intervention (amount, frequency, type, duration), use of a concomitant diet intervention, biomarkers sampled, and the mean concentration and error measurements (standard deviation, standard error, or confidence intervals) of specific biomarkers sampled after the alcohol intervention and after no alcohol use. When available we extracted information on amount of alcohol consumed, using grams of alcohol per day as the common unit of measure. When a study did not specifically report the grams of alcohol per unit, we used 12.5 g alcohol per drink for analysis.2 12 For example, if a study indicated that the intervention was 30 g of alcohol a day, we estimated this as 30 g alcohol a day divided by 12.5 g alcohol a drink equals about 2.5 drinks a day. We standardised portions as a 12 oz (355 mL) bottle or can of beer, a 5 oz (148 mL) glass of wine, and 1.5 oz (44 mL) of 80 proof (40% alcohol) distilled spirits.1 We categorised the volume of alcohol intake as <2.5 g/day (<0.5 drink), 2.5-14.9 g/day (about 0.5-1 drink), 15-29.9 g/day (about 1-2.5 drinks), 30-60 g/day (about 2.5-5 drinks), and >60 g/day (≥5 drinks).
We assessed study quality using a previously outlined component approach.13 Study design was considered the most important measure of quality, with randomised studies (crossover and prospective two arm controlled studies) judged to have a higher quality than before and after studies. We carried out sensitivity analyses based on study design to evaluate the effect of this aspect of study quality.13 We also reported several aspects of the quality of studies in meta-analysis: randomisation, assessment of compliance with alcohol consumption or abstinence, losses owing to attrition, and relevant confounders, such as smoking, diabetes, overweight or obesity or dietary controls. We did not assess blinding of participants to the intervention because it was uncertain if blinding alcohol use was effective. We report these study characteristics based on the quality assessment proposed by Jadad et al.14
Data synthesis and analysis
The common unit of measurement across all studies was the mean change (standard error) in the level of each biomarker after alcohol consumption compared with the no alcohol control. This was calculated as (mean concentration of biomarker during alcohol consumption)−(mean concentration of biomarker during no alcohol consumption). To determine the standard error of the mean change, we used the calculation: standard error mean change=square root[(standard deviationno alcohol2/sample sizeno alcohol)+(standard deviationalcohol2/sample sizealcohol)]. Not all studies included in the systematic review reported results in this manner. We converted error measurements reported as standard errors to standard deviations using the formula standard deviation=(standard error)×square root (sample size).15 For studies that did not report mean concentrations of biomarkers either before or after the intervention or that reported results in graphical format only, we contacted the study authors to obtain data suitable for inclusion in our study. In all cases the authors failed to supply the necessary data to determine the mean change in biomarker level, and we therefore excluded these studies from meta-analyses.
When at least two studies reported the mean change in level of a specific biomarker we carried out meta-analyses of the effect of alcohol consumption on biomarker concentrations. All analyses were done using Stata 10.0. To assess heterogeneity of the mean change in biomarker concentrations across studies, we calculated the Q statistic (significance level P≤0.10) and the I2 statistic.16 17 This criterion was used to determine whether to use a fixed or random effects model to pool studies. Where appropriate we pooled data according to the dose of alcohol consumed: 12.5-29.9 g/day (about 1-2.5 drinks), 30-60 g/day (about 2.5-5 drinks), and >60 g/day (≥5 drinks). To visually assess the mean change estimates and corresponding 95% confidence intervals across studies, we generated forest plots and grouped studies by dose of alcohol.
We carried out sensitivity analyses based on study quality for levels of high density lipoprotein cholesterol, low density lipoprotein cholesterol, total cholesterol, triglycerides, and fibrinogen. For these biomarkers we pooled the results from crossover studies separately from before and after studies. We also pooled results for these biomarkers stratified by beverage type (wine, beer, spirits). Data were pooled by the methods described previously.
Finally, we used the Begg test and visual inspection of funnel plots to assess for evidence of publication bias.18 19 We limited this analysis to biomarkers where a statistically significant effect of alcohol was observed and five or more studies were meta-analysed.
Results
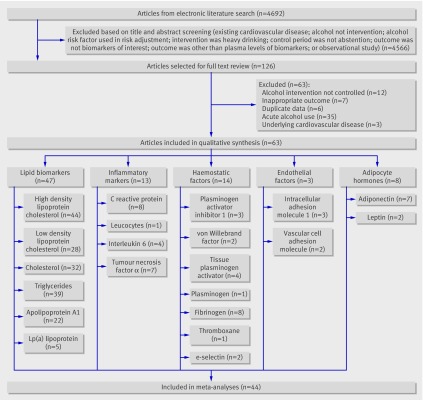
The literature search identified 4690 articles pertaining to the relevant exposure, outcomes, and study designs (fig 1). Two additional articles were added from the bibliographic search. No additional articles were suggested by the content expert (KJM). After the final review, 63 articles were deemed eligible for analysis. Selected studies examined the effect of alcohol consumption on: lipid biomarkers (47 studies), inflammatory markers (13), haemostatic factors (14), endothelial factors (3), and adipocyte hormones (8). Many of the studies examined several of the biomarkers (fig 1).
Fig 1 Flow of studies through review
Table 1 outlines the characteristics of the 63 included studies, totalling 1686 participants (1049 men and 625 women; one study of 12 participants did not indicate sex).20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 Thirty six of the 63 (57%) studies included only men, eight (12.7%) included only women, and 19 (30%) included both. Thirty six studies used a crossover design and 24 used a before and after design, whereas three were parallel arm controlled trials. The control beverage was generally water, fruit juice, or a de-alcoholised drink (wine or beer), and most studies included a washout period of no alcohol use that was of similar duration to the period of alcohol intervention. Several studies included participants whose clinical characteristics may have influenced some of the biomarkers (for example, patients with diabetes, smokers, or those who were overweight or obese). Twenty eight (44%) studies controlled for an increase in caloric intake from alcohol consumption with isocaloric or controlled diets, whereas others maintained their usual diet. Six studies included people who were overweight or obese and three studies specifically examined inactive people compared with regular runners.
Table 1.
Characteristics of included studies examining the impact of alcohol interventions (1 week or greater in duration) on fasting plasma concentrations of biomarkers associated with cardiovascular disease
| Source | Participants | Study design | Characteristics of participants | Alcohol intervention and diet | Biomarkers sampled | Included in meta-analysis | Reasons for exclusion from meta-analysis |
|---|---|---|---|---|---|---|---|
| Baer 200220 | 51; all women; mean age 60 | Random crossover | Postmenopausal; no hyperlipidaemia, no diabetes, and no peripheral vascular disease | 8 weeks of 15 g/day and 30 g/day 95% ethanol (1 or 2 drinks a day); controlled diet | Triglycerides, total cholesterol, high and low density lipoprotein cholesterol, and apolipoprotein A1 | No | Data reported as least mean squares |
| Bantle 200821 | 17; 7 men; age ≥40 | Random crossover | Type 2 diabetes, no hypertension, no heart failure, and not receiving insulin* | 1 month (12 g/day women, 24 g/day men) red or white wine (1-2 drinks a day); usual diet | Triglycerides, total cholesterol, low and high density lipoprotein cholesterol, C reactive protein, and plasminogen | Yes | — |
| Belfrage 197322 | 8; all men; age 22-26 | Before and after | Healthy (includes some smokers) | 5 weeks of 63 g/day beer (5 drinks a day); usual diet | Triglycerides, total cholesterol, and low and high density lipoprotein cholesterol | No | Error measurements not provided |
| Belfrage 197723 | 9; all men; age 22-29 | Before and after | Healthy (includes some smokers) | 4 weeks of 75 g/day beer or ethanol (6 drinks a day)† | Triglycerides and high density lipoprotein cholesterol | No | Data provided in graph format only |
| Bertiere 198624 | 10; all men; age 18-21 | Before and after | Healthy (includes some smokers) | 4 weeks of 30 g/day red wine (2.5 drinks a day); usual diet | Cholesterol, triglycerides, low and high density lipoprotein cholesterol, and apolipoprotein A1 | Yes except for apolipoprotein A1 | Data reported as density fraction |
| Beulens 200827 | 20; all men; age 18-25 | Random crossover | All healthy, lean, or overweight, non-smokers | 3 weeks of 40 g/day beer (3 drinks a day); controlled diet | Cholesterol, triglycerides, low and high density lipoprotein cholesterol, and C reactive protein | Yes | — |
| Beulens 200828 | 19; all men; age 18-25 | Random crossover | Healthy, lean, or overweight | 3 weeks of 40 g/day beer (3 drinks a day); usual diet | Adiponectin | Yes | — |
| Beulens 200726 | 19; all men; age 18-40 | Random crossover | Healthy, lean or overweight | 4 weeks of 32 g/day whisky (2.5 drinks a day); partially controlled diet | Adiponectin | Yes | — |
| Beulens 200625 | 34; all men; age 35-70 | Random crossover | Abdominal obesity, no cardiovascular disease, no diabetes, non-smokers | 4 weeks of 40 g/day red wine (3 drinks a day); usual diet | High density lipoprotein cholesterol and adiponectin | Yes, except for high density lipoprotein cholesterol | Error measurements not provided |
| Burr 198629 | 100; 48 men (age 20-56). 52 women (age 19-60) | Random crossover | No diabetes and not taking antihypertensive drugs* | 4 weeks (19 g/day men, 17.8 g/day women) beer, wine, or spirits (1.5 drinks a day)† | Triglycerides, total cholesterol, low and high density lipoprotein cholesterol, and fibrinogen | Yes | — |
| Cartron 200330 | 18; all men; age 20-45 | Random crossover | Normal cholesterol and triglyceride levels, no drugs or vitamins, non-smokers | 3 weeks of 26 g/day (250 ml/day) white wine, champagne, or red wine (2 drinks a day); controlled diet | Total cholesterol, triglycerides, and apolipoprotein A1 | Yes | — |
| Clevidence 199531 | 34; all women; age 21-40 | Random crossover | Premenopausal women, healthy, non-smokers | 3 months of 30 g/day grain alcohol (2.5 drinks a day); controlled diet | Triglycerides, total cholesterol, low and high density lipoprotein cholesterol, apolipoprotein A1, and Lp(a) lipoprotein | Yes | — |
| Contaldo 198932 | 8; all men; age 30-47 | Crossover | Healthy, non-smoker or light smoker | 2 weeks of 75 g/day (750 ml wine) (6 drinks/day); isocaloric diet | Triglycerides, total cholesterol, low and high density lipoprotein cholesterol, and apolipoprotein A1 | Yes | — |
| Couzigou 198433 | 7; all men; age 28-31; mean age 29.6 | Before and after | Healthy, no drugs, usual smoking habits | 1 week 23 g/day, then 4 weeks 31 g/day red wine (1.5, 2.5 drinks a day); usual diet | Low and high density lipoprotein cholesterol and apolipoprotein A1 | Yes | — |
| Crouse 198434 | 12; all men; age 22-62 | Before and after | No liver dysfunction, no metabolic disorders, no diabetes; 3 had hyperglycaemia, 2 atherosclerosis * | 4 weeks 90 g/day‡ (7 drinks a day); controlled diet | Triglycerides, total cholesterol, and low and high density lipoprotein cholesterol | Yes | — |
| Davies 200235 | 51; all women; mean age 59.5 | Random crossover | Post-menopausal, healthy | 8 weeks of 15 g/day or 30 g/day‡ (1 or 2.5 drinks a day); controlled diet | Triglycerides | Yes | — |
| De Oliveira e Silva 200036 | 14; 9 men; age 21-70; mean age 53.3 | Crossover | Without significant disease, non-smokers | 2 weeks of 1mL/kg/day vodka (1.5-2 drinks a day); controlled diet | Total cholesterol, triglycerides, and low and high density lipoprotein cholesterol | Yes | — |
| Djurovic 200737 | 87; 30 men; age 35-70 | Random crossover | Healthy, non-smokers | 3 weeks of 16 g/day (150 mL) red wine (1 drink a day)† | C reactive protein, tumour necrosis factor α, interleukin 6, intracellular adhesion molecule 1, vascular cellular adhesion molecule, and leptin | Yes, except for C reactive protein, tumour necrosis factor α, intracellular adhesion molecule 1, vascular cellular adhesion molecule, and leptin | C reactive protein: data reported as correlation with leptin; tumour necrosis factor α: non-detectable change reported; leptin, intracellular adhesion molecule 1, vascular cellular adhesion molecule: only one study reporting usable data |
| Estruch 200438 | 40; all men; age 30-50; mean age 37.6 | Random crossover | Excludes those with hypertension, diabetes, high low density lipoprotein cholesterol, low high density lipoprotein cholesterol, coronary heart disease, cerebrovascular disease, peripheral vascular disease; non-smokers | 28 days of 33 g/day (320 mL) red wine or (100 mL) gin (2.5 drinks a day); isocaloric diet | C reactive protein, fibrinogen, e-selectin, tumour necrosis factor α, intracellular adhesion molecule 1, and vascular cellular adhesion molecule | Yes except for e-selectin intracellular adhesion molecule 1, and vascular cellular adhesion molecule | e-selectin: only study reporting suitable data; intracellular adhesion molecule 1, vascular cellular adhesion molecule: reports on other cellular adhesion molecules |
| Fraser 198339 | 10; all men | Crossover | Generally healthy | 3 weeks of 10-74 g/day beer or whisky (1-5 drinks a day); controlled diet | High density lipoprotein cholesterol and apolipoprotein A1 | Yes | — |
| Frimpong 198940 | 8; all men; age 21-35 | Before and after | Healthy, with normal lipid levels, non-smokers | 6 weeks of 40 g/day beer (3 drinks a day); controlled diet | Triglycerides, total cholesterol, and low and high density lipoprotein cholesterol | Yes | — |
| Glueck 198041 | 6; all men; 18-19 | Before and after | Healthy, normal lipid profile | 1week of 35 g/day, 1 week 53 g/day vodka (2 then 3.5 drinks a day); controlled diet | Triglycerides, total cholesterol, and high and low density lipoprotein cholesterol | Yes | — |
| Goldberg 199642 | 24; all men; age 26-45 | Crossover | Healthy | 4 weeks of 40 g/day red wine or white wine (3 drinks a day); usual diet | Triglycerides, total cholesterol, high density lipoprotein cholesterol, and apolipoprotein A1 | Yes | — |
| Gottrand 199943 | 5; all men; mean age 22.8 | Random crossover | Healthy, non-smokers, no drugs | 4 weeks of 50 g/day red wine (4 drinks a day); controlled diet | Triglycerides, total cholesterol, high density lipoprotein cholesterol, apolipoprotein A1, and Lp(a) lipoprotein | Yes | — |
| Hagiage 199244 | 14; all men; mean age 28 | Before and after | Healthy, light or non-smokers, without history of chronic illness; 7 were normal weight, 7 were obese | 2 weeks of 30 g/day red wine (2.5 drinks a day); usual diet | Triglycerides, total cholesterol, high and low density lipoprotein cholesterol, apolipoprotein A1, and Lp(a) lipoprotein | Yes | — |
| Hansen 200545 | 19; 9 men; age 38-75; mean age 50 | Random crossover | No lipid lowering drugs or antihypertensives, includes some smokers | 4 weeks (38.3 g/day men, 25.5 g/day women) red wine (1.5 or 2.5 drinks a day); controlled diet | Total cholesterol, high and low density lipoprotein cholesterol, and fibrinogen | Yes | — |
| Hartung 198346 | 44; all men; age 27-59 | Before and after | 16 marathoners, 15 joggers and 13 inactive; all healthy* | 3 weeks of 37.5 g/day beer (3 drinks a day); usual diet | Triglycerides, total cholesterol, and high and low density lipoprotein cholesterol | Yes | — |
| Hartung 198647 | 32; all women; age 30-49 | Before and after | Premenopausal, half were habitual runners, half inactive; some smokers in inactive group | 3 weeks of 35 g/day wine (3 drinks a day); usual diet | Triglycerides, total cholesterol, high and low density lipoprotein cholesterol, and apolipoprotein A1 | Yes | — |
| Hartung 199048 | 49; all men; age 30-54 | Before and after | 26 habitual runners, 23 inactive; all healthy; some smokers in inactive group | 3 weeks of 12.5 g/day or 37.5 g/day beer (1 or 3 drinks a day)† | High density lipoprotein cholesterol and apolipoprotein A1 | Yes | — |
| Imhof 200949 | 72; 36 men; age 22-56 | Random crossover | Healthy, non-smokers | 3 weeks (30 g/day men, 20 g/day women) of beer, wine, or ethanol (2-2.5 drinks a day) | Adiponectin | No | Data presented as percentage change |
| Jensen 200650 | 80; 28 men; age 35-70 | Random crossover | Healthy, non-smokers | 3 weeks 15 g/day red wine (1 drink a day)† | Fibrinogen | Yes | — |
| Joosten 200851 | 36; all women; mean age 56.5 | Crossover | Postmenopausal, healthy* | 6 weeks of 20 g/day white wine (1.5 drinks a day); usual diet | Triglycerides, total cholesterol, high and low density lipoprotein cholesterol, and adiponectin | Yes | — |
| Karlsen 200752 | 49; 15 men; age 35-70 | Random prospective two arm control | Healthy, non-smokers, no cardiovascular disease, no diabetes, no liver disease, no lipid lowering drugs, no aspirin | 3 weeks of 15 g/day red wine (1 drink a day)† | Interleukin 6 and tumour necrosis factor α | No | Data presented as median difference |
| Malmendier 198553 | 9; all men; age 23-39 | Before and after | Healthy, no drugs, no history of cardiovascular disease, no diabetes, no hyperlipoproteinaemia; 7 normal weight, 2 obese | 2 weeks of 60 g/day (normal weight) or 70 g/day (obese) gin or vodka (5-6 drinks a day); isocaloric diet | Triglycerides, total cholesterol, high and low density lipoprotein cholesterol, and apolipoprotein A1 | Yes | — |
| McConnell 199754 | 20; 11 men; age 23-51 | Before and after | Healthy, non-smokers, no hyperlipidaemia, no coronary disease, no vascular disease, no hypertension, no diabetes | 6 weeks of 16.5 g/day beer (1.5 drinks a day); usual diet | Triglycerides, high and low density lipoprotein cholesterol, apolipoprotein A1, Lp(a) lipoprotein, tissue plasminogen activator, plasminogen activator inhibitor 1, and von Willebrand factor | Yes except for Lp(a) lipoprotein and von Willebrand factor | Lp(a) lipoprotein: incongruent units of analysis; von Willebrand factor: data presented as percentage normal |
| Mezzano 200356 | 42; all men; mean age 22 years | Before and after | Healthy, non-smokers | 30 days of 23.2 g/day red wine (2 drinks a day); specialised diets | von Willebrand factor | No | Too few studies for meta-analysis |
| Mezzano 200155 | 42; all men; mean age 22 | Before and after | Healthy, non-smokers | 30 days of 23.2 g/day red wine (2 drinks a day); specialised diets | C reactive protein, fibrinogen, tissue plasminogen activator, and plasminogen activator inhibitor 1 | Yes | — |
| Naissides 200657 | 19; all women; age 50-70 (mean age 58.4) | Random prospective two arm control | Postmenopausal, moderately hypercholesterolaemic, excludes obese, physically active, smokers, poor diet, or those receiving lipid lowering drugs | 6 weeks of 40 g/day red wine (3 drinks a day); controlled diet | Triglycerides, total cholesterol, and high and low density lipoprotein cholesterol | No | Data in graph format |
| Nishiwaki 199458 | 25; all men; mean age 31.4 | Before and after | Healthy, no diabetes | 4 weeks of 30-49 g/day (0.5 g/kg for 3 hours after dinner) alcohol‡ (2-4 drinks a day); controlled diet | Triglycerides, total cholesterol, high density lipoprotein cholesterol, and apolipoprotein A1 | No | Data in graph format |
| Pace-Asciak 199659 | 24; all men; age 26-45 | Crossover | Healthy | 4 weeks of 40 g/day red or white wine (2.5 drinks a day); usual diet | Thromboxane | No | Only study reporting on this biomarker |
| Pikaar 198760 | 12; all men; age 21-29 | Random crossover | Healthy, non-smokers | 5 weeks of 25 g/day or 50 g/day wine (2-4 drinks a day); usual diet | Triglycerides, total cholesterol, high density lipoprotein cholesterol, plasminogen, and tissue plasminogen activator | No | Error measurements not provided |
| Retterstol 200561 | 87; 30 men; age 35-70 | Random crossover | Healthy, non-smokers | 3 weeks of 15 g/day red wine (1 drink a day)† | Triglycerides, total cholesterol, high density lipoprotein cholesterol, C reactive protein, and fibrinogen | Yes | — |
| Romeo 200763 | 57; 30 men; age 25-50 | Before and after | “Medically healthy,” no chronic conditions involving immune system | 1 month (22 g/day men, 11 g/day women) beer (1-2 drinks a day); usual diet | Leucocytes | No | Only study reporting on this biomarker |
| Romeo 200762 | 57; 30 men; age 25-50 | Before and after | “Medically healthy,” no chronic conditions involving immune system | 1 month (22 g/day men, 11 g/day women) of beer (1-2 drinks a day); usual diet | Interleukin 6, tumour necrosis factor α | Yes | — |
| Roth 200364 | 53; all women; age ≥49; mean age 59.7 | Random crossover | Healthy, non-smokers, postmenopausal | 8 weeks of 15 g/day or 30 g/day alcohol‡ (1 or 2.5 drinks a day); controlled diet | Leptin | No | Reported as percentage change and geometric mean |
| Schneider 198565 | 6; 4 men; age 27-33 | Before and after | Healthy | 4 weeks of 70-80 g/day white wine (5-6 drinks a day); controlled diet | Triglycerides, total cholesterol, and high and low density lipoprotein cholesterol | No | Data in graph format |
| Senault 200066 | 56; all men; age 18-35 | Random crossover | Healthy, no drugs | 2 weeks of 30 g/day red wine or hydroalcohol (2.5 drinks a day); usual diet | Trigylcerides, total cholesterol, high and low density lipoprotein cholesterol, apolipoprotein A1, and Lp(a) lipoprotein | Yes | — |
| Sharpe 199567 | 20; 11 men; age 25-60; mean age 37.2 | Before and after | Healthy | 10 days of 21 g/day red or white wine (1.5 drinks a day); usual diet | Total cholesterol, triglycerides, high and low density lipoprotein cholesterol, apolipoprotein A1, and Lp(a) lipoprotein | Not Lp(a) lipoprotein | Error measurements not provided |
| Sierksma 200472 | 23; all men; age 45-65; mean age 52 | Random crossover | Apparently healthy non-smokers | 17 days of 40 g/day whisky (3.5 drinks a day); controlled diet | Triglycerides, high density lipoprotein cholesterol, tumour necrosis factor α, and adiponectin | No | Error measurements not provided |
| Sierksma 200471 | 18; all women; age 49-65; mean age 57 | Random crossover | Postmenopausal, non-smokers, healthy | 3 weeks of 24 g/day white wine (2 drinks a day); usual diet | Triglycerides, high density lipoprotein cholesterol, total cholesterol, and apolipoprotein A1 | Yes | — |
| Sierksma 200269 | 19; 10 men (age 45-64), 9 women (age 49-62) | Random crossover | Women postmenopausal, healthy, no prescribed drugs | 3 weeks of (men) 40 g/day and (women) 30 g/day (3 and 2.5 drinks a day, respectively) beer; controlled diet | High density lipoprotein cholesterol and apolipoprotein A1 | No | Data reported as percentage change |
| Sierksma 200270 | 19; 10 men (age 45-64), 9 women (age 49-62) | Random crossover | Women postmenopausal, healthy, no prescribed drugs | 3 weeks of (men) 40 g/day and (women) 30 g/day (3 and 2.5 drinks a day, respectively) beer; usual diet | Triglycerides, high density lipoprotein cholesterol, C reactive protein, and fibrinogen | Not C reactive protein | Data presented as median change |
| Sierksma 200168 | 19; 10 men (age 45-64), 9 women (age 49-62) | crossover | Healthy, non-smokers; postmenopausal women | 3 weeks of (men) 40 g/day and (women) 30 g/day (3 and 2.5 drinks a day, respectively) beer; controlled diet | Fibrinogen | No | Data in graph format |
| Suzukawa 199473 | 12; all men; mean age 31.4 | Random prospective two arm control | Healthy, normal anthropometrics | 4 weeks of 0.5 g/kg/day brandy (2 drinks a day); usual diet | Triglycerides, total cholesterol, and high and low density lipoprotein cholesterol | Yes | — |
| Thornton 198374 | 12; 3 men; age 39-57; mean age 47 | Before and after | Healthy, normolipidaemic, non-smokers | 6 weeks 39 g/day wine (3.5 drinks a day); usual diet | Triglycerides, total cholesterol, and high and low density lipoprotein cholesterol | Yes | — |
| Tsang 200575 | 12; unclear No of men; age 23-50 | Random prospective two arm control | Healthy, non-smokers | 2 weeks of 39.7 g/day red wine (3.5 drinks a day); controlled diet | Triglycerides and high and low density lipoprotein cholesterol | Yes | — |
| Valimaki 199177 | 10; all men; age 27-45; mean age 36 | Before and after | Healthy | 3 weeks of 60 g/day wine, whisky, or vodka (5 drinks a day); usual diet | Triglycerides, total cholesterol, high density lipoprotein cholesterol, and apolipoprotein A1 | Yes | — |
| Valimaki 198876 | 10; all men; age 30-43 | Before and after | Healthy | 3 weeks of 30 g/day or 60 g/day wine, whisky, or vodka (2.5 or 5 drinks a day); usual diet | Triglycerides, total cholesterol, high density lipoprotein cholesterol, and apolipoprotein A1 | Not apolipoprotein A1 | Error measurements not provided |
| Van der Gaag 200179 | 11; all men; age 45-60 | Random crossover | Non-smokers, healthy | 3 weeks of 40 g/day red wine, beer, and gin (3.5 drinks a day); controlled diet | Triglycerides, total cholesterol, high and low density lipoprotein cholesterol, and apolipoprotein A1 | Yes | — |
| Van der Gaag 199978 | 11; all men; age 44-59; mean age 51.7 | Random crossover | Healthy, non-smokers | 3 weeks of 40 g/day red wine, beer, or spirits (3.5 drinks a day); controlled diet | High density lipoprotein cholesterol and apolipoprotein A1 | No | Data in graph format |
| Van Golde 200280 | 6; all men; mean age 34 | Before and after | Healthy, non-smokers, normal liver function and lipid profile | 2 weeks of 37.5 g/day wine (3 drinks a day)† | Tissue plasminogen activator and plasminogen activator inhibitor 1 | Yes | — |
| Vazquez-Agell 200781 | 20; all men; age 25-50 | Random crossover | Non-smokers, normal lipids, no hypertension, no diabetes, no cardiovascular disease, no peripheral vascular disease | 28 days of 30 g/day gin or white wine (2.5 drinks a day)† | C reactive protein, interleukin 6, e-selectin, adiponectin, tumour necrosis factor α, and intracellular adhesion molecule 1 | No | Data presented as percentage change |
| Watzl 200482 | 24; all men; mean age 30.6 | Random crossover | Healthy, non-smokers | 2 weeks of 53 g/day red wine or ethanol (4 drinks a day); usual diet | Tumour necrosis factor α | Yes | — |
*Smoking status not specified.
†Diet not specified.
‡Type of alcohol not specified.
Meta-analyses
Of the 63 studies, 44 reported adequate data to permit pooled analyses (see web extra figure). Most commonly, studies could not be included because they did not report data in a format that permitted pooling (for example, graphically or as percentage change), or did not report error calculations. The subsets of identified studies with adequate data dealt with levels of high density lipoprotein cholesterol (33 of 44), total cholesterol (26 of 32), triglycerides (31 of 39), low density lipoprotein cholesterol (24 of 28), apolipoprotein A1 (16 of 22), Lp(a) lipoprotein (3 of 5), C reactive protein (5 of 8), interleukin 6 (2 of 4), tumour necrosis factor α (3 of 7), plasminogen activator inhibitor 1 (3 of 3), tissue plasminogen activator (3 of 4), fibrinogen (7 of 8), and adiponectin (4 of 7; see web extra figure). See web extra appendix 2 for details about exclusion of studies from meta-analysis.
Lipid biomarkers
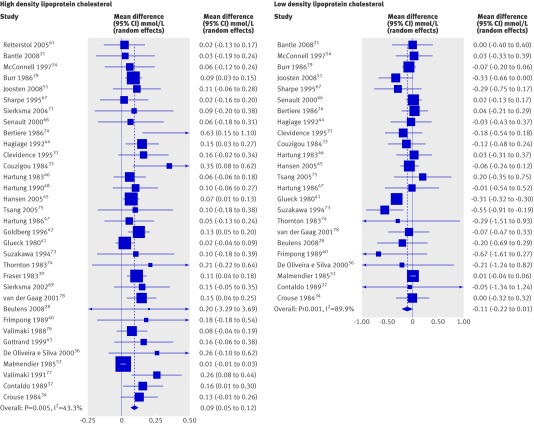
The pooled analysis of the effect of alcohol consumption on mean high density lipoprotein cholesterol showed a consistent increase in these levels but with significant heterogeneity among studies (fig 2 and table 2; P=0.005). Pooling of studies stratified by dose may partially explain this heterogeneity. A significant dose-response was observed between alcohol consumption and high density lipoprotein cholesterol levels (fig 2): 12.5-29.9 g/day (1-2 drinks, n=7), mean difference of 0.072 mmol/L (95% confidence interval: 0.024 to 0.119); 30-60 g/day (2-4 drinks, n=24), mean difference of 0.103 mmol/L (0.065 to 0.141); and >60 g/day (≥5 drinks, n=2), mean difference of 0.141 mmol/L (0.042 to 0.240; P for trend 0.013). Similar to the effect with high density lipoprotein cholesterol, apolipoprotein A1 also significantly increased in a random effects model pooling 16 studies (table 2).
Fig 2 Forest plot of meta-analysis (random effects) of effect of alcohol consumption on levels of high and low density lipoprotein cholesterol
Table 2.
Summary of pooled mean difference in biomarker level after alcohol use
| Biomarker | No of pooled studies | No of pooled participants | Type of model | Pooled mean difference in biomarker level (95% CI) |
|---|---|---|---|---|
| High density lipoprotein cholesterol (mmol/L) | 33 | 796 | Random | 0.094 (0.064 to 0.123)*† |
| Low density lipoprotein cholesterol (mmol/L) | 24 | 513 | Random | −0.11 (−0.22 to 0.006)† |
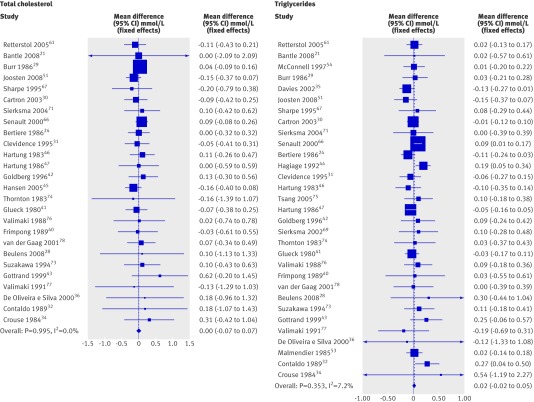
| Total cholesterol (mmol/L) | 26 | 596 | Fixed | 0.00 (−0.066 to 0.067) |
| Triglycerides (mmol/L) | 31 | 752 | Fixed | 0.016 (−0.018 to 0.051) |
| Apolipoprotein A1 (g/L) | 16 | 374 | Random | 0.101 (0.073 to 0.129)*† |
| Lp(a) lipoprotein (mg/dL) | 3 | 114 | Fixed | 0.80 (−4.17 to 5.76) |
| C reactive protein (mg/L) | 5 | 186 | Fixed | −0.11 (−0.31 to 0.10) |
| Interleukin 6 (pg/mL) | 2 | 144 | Fixed | 0.502 (−3.482 to 4.486) |
| Tumour necrosis factor α (pg/mL) | 3 | 121 | Fixed | −0.469 (−32.02 to 31.08) |
| Plasminogen activator inhibitor 1 (ng/mL) | 3 | 67 | Fixed | 3.285 (−0.898 to 7.469) |
| Tissue plasminogen activator (ng/mL) | 3 | 67 | Fixed | 0.754 (−0.132 to 1.641) |
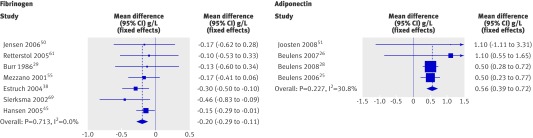
| Fibrinogen (g/L) | 7 | 387 | Fixed | −0.20 (−0.29 to −0.11)* |
| Adiponectin (mg/L) | 4 | 108 | Fixed | 0.56 (0.39 to 0.72)* |
*Indicates significant (P<0.01) change in biomarker level after alcohol use compared with a period of no alcohol use.
†Heterogeneity detected across pooled studies, where Q statistic P<0.10.
In contrast, alcohol consumption did not significantly change levels of total cholesterol, low density lipoprotein cholesterol, triglycerides, or Lp(a) lipoprotein (table 2). The 24 studies reporting on low density lipoprotein cholesterol were pooled using a random effects model because heterogeneity was present. Pooled analyses stratified by dose of alcohol also showed no significant effects of alcohol on low density lipoprotein cholesterol. Pooled analysis of the impact of alcohol by dose on triglycerides showed a significant increase at the highest dose of alcohol (>60 g/day) in the two studies reporting alcohol consumption at this dose: mean difference 0.274 mmol/L (0.043 to 0.505), test for heterogeneity P=0.763 (fig 3).
Fig 3 Forest plot of meta-analysis (fixed effects) of effect of alcohol consumption on levels of total cholesterol and triglycerides
Inflammatory markers
The association of alcohol with levels of C reactive protein, interleukin 6, and tumour necrosis factor α was not significant (table 2). Only one study reported that alcohol (in this case beer) increased leucocyte levels in women (0.51 (SD 0.47)×109/L) but not in men (0.19 (SD 0.31)×109/L).63
Haemostatic factors
Fibrinogen levels significantly decreased after alcohol consumption (fig 4 and table 2). Meta-analyses of the remaining haemostatic biomarkers, however, did not show any significant effect of alcohol, including plasminogen activator inhibitor 1 and tissue plasminogen activator antigens (table 2).
Fig 4 Forest plot of meta-analysis of effect of alcohol consumption on levels of fibrinogen and adiponectin
Data were insufficient to permit meta-analysis for plasminogen, thromboxane, von Willebrand factor, and e-selectin levels. One study reported a significant increase in plasminogen levels after red wine consumption in 12 men.60 Another study reported a significant decrease in thromboxane levels after both white and red wine consumption.59 The two studies reporting on the effect of alcohol on von Willebrand factor found no significant change in these biomarker levels.54 56 For e-selectin, one study found a significant increase after alcohol consumption,81 whereas another study found no change.38
Endothelial factors
Three studies reported on the impact of alcohol on intracellular adhesion molecule 1 and two reported on vascular cellular adhesion molecule levels. However, only one study reported data suitable for pooling for each biomarker. Two studies showed no impact of alcohol on intracellular adhesion molecule,37 81 whereas one showed a significant decrease in intracellular adhesion molecule 1 after consumption of red wine but not after gin.38 One study reported no change in vascular cellular adhesion molecule after alcohol consumption,37 whereas another found a significant decrease after consumption of red wine but not after gin.38
Adipocyte hormones
Adiponectin levels were consistently significantly increased after alcohol consumption (fig 4). Only two studies reported on the effect of alcohol on levels of leptin; one study found a significant increase after alcohol consumption64 and the other no effect.37
Sensitivity analyses
Study quality
Study quality was analysed using the component approach,13 focusing primarily on study design as the most important quality factor. Sensitivity analyses were stratified by the two major study designs in selected studies (24 crossover studies, 18 before and after studies), with crossover studies being considered the more robust study design. The findings of these stratified sensitivity analyses are in web extra appendix 3 and show generally similar results for both types of study design from analyses that include a sufficient number of studies to yield stable pooled estimates—that is, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, and fibrinogen. This sensitivity analysis suggests that, regardless of the study design, alcohol had consistent effects on biomarker levels.
See web extra appendix 4 for additional study quality characteristics. Of the 44 studies meta-analysed, 17 randomised the participants into treatment groups but only one study described the randomisation process. Forty three of the 44 studies described the presence of relevant covariates, such as diet, smoking, and physical activity. Twenty of the studies measured compliance with alcohol consumption, whereas 24 described losses to attrition.
Beverage type
Analyses were also stratified by beverage type (wine, beer, spirits). The results were similar to the combined analyses of all beverage types (see web extra appendix 3).
Publication bias
Evidence of publication bias was assessed for high density lipoprotein cholesterol, apolipoprotein A1, and fibrinogen. No asymmetry was found on visual inspection of the funnel plot for each biomarker, suggesting that significant publication bias was unlikely. This was further confirmed by a non-significant Begg test for each outcome of interest (high density lipoprotein cholesterol P=0.12, apolipoprotein A1 P=0.064, and fibrinogen P=0.88).
Discussion
This meta-analysis shows that moderate consumption of alcohol (up to one drink or 15 g alcohol a day for women and up to two drinks or 30 g alcohol a day for men) has beneficial effects on a variety of biomarkers linked to the risk of coronary heart disease. The experimental interventional studies showed that alcohol consumption significantly increased circulating levels of high density lipoprotein cholesterol, apolipoprotein A1, and adiponectin and significantly decreased fibrinogen levels, all changes reported to be cardioprotective.4 5 6 7 8
Comparison with other studies
The findings from our review expand on those reported a decade ago by Rimm et al,3 which focused only on alcohol’s effect on lipids and haemostatic factors. Since that review substantial growth has occurred in research on the effect of alcohol on the more traditional biomarkers associated with cardiovascular risk as well as on newer biomarkers such as inflammatory markers, endothelial factors, and adipocyte hormones. To identify eligible studies we followed a similar protocol to that used by the previous reviewers, but we also required that eligible studies had to compare fasting levels of biomarkers after alcohol consumption with those from a period of no alcohol use.
Similar to the review by Rimm et al, our updated meta-analyses found increases in levels of high density lipoprotein cholesterol and apolipoprotein A1. For 30 g alcohol consumed a day (about two drinks) Rimm et al reported an increase in high density lipoprotein cholesterol level of 3.99 mg/dL (0.103 mmol/L) (95% confidence interval 3.25 to 4.73) and an increase in apolipoprotein A1 of 8.82 mg/dL (7.79 to 9.86). For a similar dose of alcohol (30 g a day was the standard dose used by Rimm et al), our results are nearly the same: an increase in high density lipoprotein cholesterol level of 3.66 mg/dL (95% confidence interval: 2.22 to 5.13) and an increase in apolipoprotein A1 of 8.67 mg/dL (6.81 to 10.32). In contrast with the study by Rimm et al, however, we observed that alcohol consumption significantly decreased fibrinogen concentrations. Evidence that alcohol decreases fibrinogen levels from experimental studies supports an important postulated mechanism by which alcohol consumption protects against certain aspects of cardiovascular disease, such as coronary heart disease.3 83
Another finding that varied from the earlier review was that alcohol consumption did not increase triglyceride levels aside from pooled results from two studies of heavy alcohol consumption (>60 g/day, or >4 drinks a day). Although the results of the two studies should be viewed cautiously as they pool two different study designs (a crossover study32and a before and after study34), they do indicate an adverse effect of heavy alcohol consumption on triglyceride levels. At low levels of alcohol consumption, our findings do not support the previously reported association of alcohol consumption and raised triglyceride levels. Furthermore, we also determined that different types of alcoholic beverage (wine, beer, and spirits) have similar effects on biomarkers. Inferences on beverage type should be viewed with some caution, however, as most of the studies used wine as the alcohol intervention. This preference for using wine, and in most cases red wine, as the type of alcohol for intervention may be related to the other chemical components of red wine, such as polyphenols, which are believed to have cardioprotective effects.30 38 42 45 50 However, it is interesting that in many of these studies, comparisons were made either with a non-red wine alcohol intervention or with de-alcoholised red wine and it was concluded that the effect observed was most likely due to alcohol rather than to the other components in red wine.30 42 45
This review also examined results for several other biomarkers that had not previously been evaluated, most notably adiponectin, an abundant adipocyte hormone that has been associated with lower risk of both diabetes84 and coronary heart disease.85 In pooled analyses, adiponectin levels were significantly increased by alcohol intake. Taken together, these findings extend previous evidence supporting an apparent causal role for alcohol consumption in preventing coronary heart disease through favourable effects on levels of high density lipoprotein cholesterol, fibrinogen, and adiponectin and limited adverse effects on triglycerides at levels of alcohol consumption that are considered “not risky.”
Potential biological mechanisms and clinical context
Our results thus implicate reverse cholesterol transport, haemostasis, and insulin sensitivity in the pathway by which alcohol consumption might prevent cardiovascular disease. The mechanisms by which alcohol influences high density lipoprotein cholesterol, fibrinogen, and adiponectin are not fully understood. In the case of high density lipoprotein cholesterol, various mechanisms have been proposed, including an increased transport rate of lipoproteins36 and increased lipoprotein lipase activity.58 86 The effect on fibrinogen is also not well understood, although alcohol seems to influence the conformation and stability of fibrinogen molecules.87 For adiponectin, one study showed that alcohol consumption increases expression of the ADIPOQ gene in adipose tissue, but little else is known about this effect.51
Our findings need to be put into a clinical context. The significant changes in levels of high density lipoprotein cholesterol, fibrinogen, and adiponectin after alcohol consumption were well within a pharmacologically relevant magnitude. In our systematic review, we determined that alcohol consumption increased high density lipoprotein cholesterol levels by about 0.1 mmol/L overall and in a dose-response manner (0.072 mmol/L for 1-2 drinks a day, 0.10 mmol/L for 2-4 drinks a day, and 0.14 mmol/L for ≥4 drinks a day). This degree of increase is greater than any currently available single pharmacological therapy, including fibrates (approved by the Food and Drug Administration for people with low levels of high density lipoprotein cholesterol). For example, a systematic review of fibrates on high density lipoprotein cholesterol levels showed an overall increase of 2.6 mg/dL88 compared with our findings of alcohol increasing high density lipoprotein cholesterol levels by 3.5-4 mg/dL. Similarly, alcohol consumption decreased fibrinogen levels by 0.20 g/L. Given that an increase of 1 g/L has been associated with a nearly threefold increase in risk of coronary heart disease in pooled cohort studies,89 this magnitude of decrease in fibrinogen could account for a substantial decrease in heart disease among drinkers.90 The clinical implications of alcohol’s effect on adiponectin is less certain since this biomarker is less commonly examined in the clinical setting. An increase of about 0.6 mg/L represents approximately 1 standard deviation in adiponectin levels in the collected trials, or similar to the effect of thiazolinediones on this insulin sensitising adipokine.91
Limitations of the study
Our review has some limitations and caveats. We did not formally search the grey literature, but we are confident that our search of the peer reviewed literature captured all relevant articles. The studies that we pooled did lack uniformity. Duration and dosing of the alcohol interventions were, however, different, as were the characteristics of the participants. Therefore it is possible that potential confounders such as smoking, physical inactivity, body weight, and diet could have affected our findings.92 Also, none of the studies blinded participants to alcohol consumption. However, owing to the taste and physiological effects of alcohol, it may not be possible to blind participants to this intervention. We chose to evaluate stable circulating cellular and molecular biomarkers associated with cardiovascular disease, in particular atherothrombotic and coronary heart disease. More variable measures, such as blood pressure, can be influenced by alcohol in complex, biphasic directions after ingestion and hence are less amenable to being summarised. Our selection of biomarkers for study was guided by links to cardiovascular pathophysiology. Other biomarkers may be of relevance to alcohol’s effects on other health conditions—for example, cancer.12 Lastly, although we found that alcohol consumption has favourable effects on some of the biomarkers associated with coronary heart disease, this remains indirect evidence for the mechanisms by which alcohol may cause cardioprotection.
Conclusions and policy implications
This systematic review provides a thorough examination of the literature on the effect of alcohol consumption on biomarkers associated with cardiovascular disease, and produces compelling, indirect evidence in support of a causal protective effect of alcohol. Our companion systematic review assessing alcohol associations with clinically relevant cardiovascular end points offers parallel evidence of the protective effect of alcohol consumption.93 These combined reviews provide a foundation of knowledge on which clinical and public health messaging can be discussed.
What is already known on this topic
Observational studies suggest that moderate alcohol intake is associated with lower risk of various cardiovascular events, particularly coronary heart disease
Interventional studies showed that alcohol favourably influences various biomarkers associated with risk of coronary heart disease
What this study adds
Moderate alcohol consumption had favourable effects on levels of high density lipoprotein cholesterol, apolipoprotein A1, adiponectin, and fibrinogen
These results strengthen the case for a causal link between alcohol intake and reduced risk of coronary heart disease
Contributors: All authors conceived the study and developed the protocol. SB and PR carried out the search, abstracted the data for the analysis, and did the statistical analysis. SB, PR, and WG wrote the first draft of the manuscript. All authors critically reviewed the manuscript for important intellectual content and approved the final version of the manuscript. WG will act as guarantor for the paper.
Funding: This work was supported by a contracted operating grant from Program of Research Integrating Substance Use Information into Mainstream Healthcare (PRISM) funded by the Robert Wood Johnson Foundation project No 58529, with cofunding by the Substance Abuse and Mental Health Services and the Administration Center for Substance Abuse Treatment. SB is supported by a postdoctoral fellowship award from the Alberta Heritage Foundation for Medical Research. PR is supported by a Frederick Banting and Charles Best Canada graduate scholarship from the Canadian Institutes of Health Research. WG is supported by a Canada research chair in health services research and by a senior health scholar award from the Alberta Heritage Foundation for Medical Research. Other than funding, research in this manuscript was done independent of funding agencies. None of the funding agencies played an active role in the preparation, review, or editing of this manuscript.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: All authors had support from a contracted operating grant from Program of Research Integrating Substance Use Information into Mainstream Healthcare (PRISM) funded by the Robert Wood Johnson Foundation project No 58529, with cofunding by the Substance Abuse and Mental Health Services and the Administration Center for Substance Abuse Treatment for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: Statistical code and datasets available from the corresponding author at wghali@ucalgary.ca.
Cite this as: BMJ 2011;342:d636
Web Extra. Extra material supplied by the author
Appendices
Flowchart for meta-analysis
References
- 1.Alcoholic Beverages. Dietary guidelines for Americans 2005. US Government Printing Office, 2005.
- 2.Turner C. How much alcohol is in a “standard drink”? An analysis of 125 studies. Br J Addict 1990;85:1171. [DOI] [PubMed] [Google Scholar]
- 3.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ 1999;319:1523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature 2002;420:868-74. [DOI] [PubMed] [Google Scholar]
- 5.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351:2599-610. [DOI] [PubMed] [Google Scholar]
- 6.Kannel WB. Overview of hemostatic factors involved in atherosclerotic cardiovascular disease. Lipids 2005;40:1215-20. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Wolf PA, Castelli WP, D’Agostino RB. Fibrinogen and risk of cardiovascular disease. JAMA 1987;258:1183-6. [PubMed] [Google Scholar]
- 8.Berg AH, Scherer PE. Adipose tissue, inflammation and cardiovascular disease. Circ Res 2005;96:939-49. [DOI] [PubMed] [Google Scholar]
- 9.Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of cardiovascular disease: a systematic review. J Gen Intern Med 2009;24:S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with cardiovascular and cerebrovascular disease: a systematic review and meta-analysis. J Gen Intern Med 2009;24:S28. [Google Scholar]
- 11.Egger M, Smith GD. Principles of and procedures for systematic reviews. In: Egger M, Smith GD, Altman DG, eds. Systematic reviews in health care. BMJPublishing Group, 2001:23-42.
- 12.Health risks and benefits of alcohol consumption. Alcohol Res Health 2000;24:5-11. [PMC free article] [PubMed] [Google Scholar]
- 13.Juni P, Altman DG, Egger M. Assessing the quality of randomised controlled trials. In: Egger M, Smith GD, Altman DG, eds. Systematic reviews in health care: meta-analysis in context. 2nd ed. BMJ Books, 2007.
- 14.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
- 15.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith GD, Altman DG, eds. Systematic reviews in health care: meta-analysis in context. 2nd ed. BMJ Books, 2007.
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [PubMed] [Google Scholar]
- 20.Baer DJ, Judd JT, Clevidence BA, Muesing RA, Campbell WS, Brown ED, et al. Moderate alcohol consumption lowers risk factors for cardiovascular disease in postmenopausal women fed a controlled diet. Am J Clin Nutr 2002;75:593-9. [DOI] [PubMed] [Google Scholar]
- 21.Bantle AE, Thomas W, Bantle JP. Metabolic effects of alcohol in the form of wine in persons with type 2 diabetes mellitus. Metabolism 2008;57:241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belfrage P, Berg B, Cronholm T, Elmqvist D, Hagerstrand I, Johansson B, et al. Prolonged administration of ethanol to young, healthy volunteers: effects on biochemical, morphological and neurophysiological parameters. Acta Med Scand 1973;552:1-44. [PubMed] [Google Scholar]
- 23.Belfrage P, Berg B, Hagerstrand I, Nilsson-Ehle P, Tornqvist H, Wiebe T. Alterations of lipid metabolism in healthy volunteers during long-term ethanol intake. Eur J Clin Invest 1977;7:127-31. [DOI] [PubMed] [Google Scholar]
- 24.Bertiere MC, Betoulle D, Apfelbaum M, Girard-Globa A. Time-course, magnitude and nature of the changes induced in HDL by moderate alcohol intake in young non-drinking males. Atherosclerosis 1986;61:7-14. [DOI] [PubMed] [Google Scholar]
- 25.Beulens JW, van Beers RM, Stolk RP, Schaafsma G, Hendriks HF. The effect of moderate alcohol consumption on fat distribution and adipocytokines. Obesity 2006;14:60-6. [DOI] [PubMed] [Google Scholar]
- 26.Beulens JW, van Loon LJ, Kok FJ, Pelsers M, Bobbert T, Spranger J, et al. The effect of moderate alcohol consumption on adiponectin oligomers and muscle oxidative capacity: a human intervention study. Diabetologia 2007;50:1388-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beulens JW, van den BR, Kok FJ, Helander A, Vermunt SH, Hendriks HF. Moderate alcohol consumption and lipoprotein-associated phospholipase A2 activity. Nutr Metab Cardiovasc Dis 2008;18:539-44. [DOI] [PubMed] [Google Scholar]
- 28.Beulens JW, de Zoete EC, Kok FJ, Schaafsma G, Hendriks HF. Effect of moderate alcohol consumption on adipokines and insulin sensitivity in lean and overweight men: a diet intervention study. Eur J Clin Nutr 2008;62:1098-105. [DOI] [PubMed] [Google Scholar]
- 29.Burr ML, Fehily AM, Butland BK, Bolton CH, Eastham RD. Alcohol and high-density-lipoprotein cholesterol: a randomized controlled trial. Br J Nutr 1986;56:81-6. [DOI] [PubMed] [Google Scholar]
- 30.Cartron E, Fouret G, Carbonneau MA, Lauret C, Michel F, Monnier L, et al. Red-wine beneficial long-term effect on lipids but not on antioxidant characteristics in plasma in a study comparing three types of wine—description of two O-methylated derivatives of gallic acid in humans. Free Radic Res 2003;37:1021-35. [DOI] [PubMed] [Google Scholar]
- 31.Clevidence BA, Reichman ME, Judd JT, Muesing RA, Schatzkin A, Schaefer EJ, et al. Effects of alcohol consumption on lipoproteins of premenopausal women. A controlled diet study. Arterioscler Thromb Vasc Biol 1995;15:179-84. [DOI] [PubMed] [Google Scholar]
- 32.Contaldo F, D’Arrigo E, Carandente V, Cortese C, Coltorti A, Mancini M, et al. Short-term effects of moderate alcohol consumption on lipid metabolism and energy balance in normal men. Metabolism 1989;38:166-71. [DOI] [PubMed] [Google Scholar]
- 33.Couzigou P, Fleury B, Crockett R, Rautou JJ, Blanchard P, Lemoine F, et al. High density lipoprotein cholesterol and apoprotein A1 in healthy volunteers during long-term moderate alcohol intake. Ann Nutr Metab 1984;28:377-84. [DOI] [PubMed] [Google Scholar]
- 34.Crouse JR, Grundy SM. Effects of alcohol on plasma lipoproteins and cholesterol and triglyceride metabolism in man. J Lipid Res 1984;25:486-96. [PubMed] [Google Scholar]
- 35.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA 2002;287:2559-62. [DOI] [PubMed] [Google Scholar]
- 36.De Oliveira E, Silva ER, Foster D, McGee HM, Seidman CE, Smith JD, et al. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation 2000;102:2347-52. [DOI] [PubMed] [Google Scholar]
- 37.Djurovic S, Berge KE, Birkenes B, Braaten O, Retterstol L. The effect of red wine on plasma leptin levels and vasoactive factors from adipose tissue: a randomized crossover trial. Alcohol Alcohol 2007;42:525-8. [DOI] [PubMed] [Google Scholar]
- 38.Estruch R, Sacanella E, Badia E, Antunez E, Nicolas JM, Fernandez-Sola J, et al. Different effects of red wine and gin consumption on inflammatory biomarkers of atherosclerosis: a prospective randomized crossover trial. Effects of wine on inflammatory markers. Atherosclerosis 2004;175:117-23. [DOI] [PubMed] [Google Scholar]
- 39.Fraser GE, Anderson JT, Foster N, Goldberg R, Jacobs D, Blackburn H. The effect of alcohol on serum high density lipoprotein (HDL). A controlled experiment. Atherosclerosis 1983;46:275-86. [DOI] [PubMed] [Google Scholar]
- 40.Frimpong NA, Lapp JA. Effects of moderate alcohol intake in fixed or variable amounts on concentration of serum lipids and liver enzymes in healthy young men. Am J Clin Nutr 1989;50:987-91. [DOI] [PubMed] [Google Scholar]
- 41.Glueck CJ, Hogg E, Allen C, Gartside PS. Effects of alcohol ingestion on lipids and lipoproteins in normal men: isocaloric metabolic studies. Am J Clin Nutr 1980;33:2287-93. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg DM, Garovic-Kocic V, Diamandis EP, Pace-Asciak CR. Wine: does the colour count? Clin Chim Acta 1996;246:183-93. [DOI] [PubMed] [Google Scholar]
- 43.Gottrand F, Beghin L, Duhal N, Lacroix B, Bonte JP, Fruchart JC, et al. Moderate red wine consumption in healthy volunteers reduced plasma clearance of apolipoprotein AII. Eur J Clin Invest 1999;29:387-94. [DOI] [PubMed] [Google Scholar]
- 44.Hagiage M, Marti C, Rigaud D, Senault C, Fumeron F, Apfelbaum M, et al. Effect of a moderate alcohol intake on the lipoproteins of normotriglyceridemic obese subjects compared with normoponderal controls. Metabolism 1992;41:856-61. [DOI] [PubMed] [Google Scholar]
- 45.Hansen AS, Marckmann P, Dragsted LO, Finne NI, Nielsen SE, Gronbaek M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur J Clin Nutr 2005;59:449-55. [DOI] [PubMed] [Google Scholar]
- 46.Hartung GH, Foreyt JP, Mitchell RE, Mitchell JG, Reeves RS, Gotto AM Jr. Effect of alcohol intake on high-density lipoprotein cholesterol levels in runners and inactive men. JAMA 1983;249:747-50. [PubMed] [Google Scholar]
- 47.Hartung GH, Reeves RS, Foreyt JP, Patsch W, Gotto AM Jr. Effect of alcohol intake and exercise on plasma high-density lipoprotein cholesterol subfractions and apolipoprotein A-I in women. Am J Cardiol 1986;58:148-51. [DOI] [PubMed] [Google Scholar]
- 48.Hartung GH, Foreyt JP, Reeves RS, Krock LP, Patsch W, Patsch JR, et al. Effect of alcohol dose on plasma lipoprotein subfractions and lipolytic enzyme activity in active and inactive men. Metabolism 1990;39:81-6. [DOI] [PubMed] [Google Scholar]
- 49.Imhof A, Plamper I, Maier S, Trischler G, Koenig W. Effect of drinking on adiponectin in healthy men and women: a randomized intervention study of water, ethanol, red wine, and beer with or without alcohol. Diabetes Care 2009;32:1101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen T, Retterstol LJ, Sandset PM, Godal HC, Skjonsberg OH. A daily glass of red wine induces a prolonged reduction in plasma viscosity: a randomized controlled trial. Blood Coagul Fibrinolysis 2006;17:471-6. [DOI] [PubMed] [Google Scholar]
- 51.Joosten MM, Beulens JW, Kersten S, Hendriks HF. Moderate alcohol consumption increases insulin sensitivity and ADIPOQ expression in postmenopausal women: a randomised, crossover trial. Diabetologia 2008;51:1375-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karlsen A, Retterstol L, Laake P, Kjolsrud-Bohn S, Sandvik L, Blomhoff R. Effects of a daily intake of one glass of red wine on biomarkers of antioxidant status, oxidative stress and inflammation in healthy adults. ESPEN 2007;2:e127-33. [Google Scholar]
- 53.Malmendier CL, Delcroix C. Effect of alcohol intake on high and low density lipoprotein metabolism in healthy volunteers. Clin Chim Acta 1985;152:281-8. [DOI] [PubMed] [Google Scholar]
- 54.McConnell MV, Vavouranakis I, Wu LL, Vaughan DE, Ridker PM. Effects of a single, daily alcoholic beverage on lipid and hemostatic markers of cardiovascular risk. Am J Cardiol 1997;80:1226-8. [DOI] [PubMed] [Google Scholar]
- 55.Mezzano D, Leighton F, Martinez C, Marshall G, Cuevas A, Castillo O, et al. Complementary effects of Mediterranean diet and moderate red wine intake on haemostatic cardiovascular risk factors. Eur J Clin Nutr 2001;55:444-51. [DOI] [PubMed] [Google Scholar]
- 56.Mezzano D, Leighton F, Strobel P, Martinez C, Marshall G, Cuevas A, et al. Mediterranean diet, but not red wine, is associated with beneficial changes in primary haemostasis. Eur J Clin Nutr 2003;57:439-46. [DOI] [PubMed] [Google Scholar]
- 57.Naissides M, Mamo JC, James AP, Pal S. The effect of chronic consumption of red wine on cardiovascular disease risk factors in postmenopausal women. Atherosclerosis 2006;185:438-45. [DOI] [PubMed] [Google Scholar]
- 58.Nishiwaki M, Ishikawa T, Ito T, Shige H, Tomiyasu K, Nakajima K, et al. Effects of alcohol on lipoprotein lipase, hepatic lipase, cholesteryl ester transfer protein, and lecithin:cholesterol acyltransferase in high-density lipoprotein cholesterol elevation. Atherosclerosis 1994;111:99-109. [DOI] [PubMed] [Google Scholar]
- 59.Pace-Asciak CR, Rounova O, Hahn SE, Diamandis EP, Goldberg DM. Wines and grape juices as modulators of platelet aggregation in healthy human subjects. Clin Chim Acta 1996;246:163-82. [DOI] [PubMed] [Google Scholar]
- 60.Pikaar NA, Wedel M, van der Beek EJ, van Dokkum W, Kempen HJ, Kluft C, et al. Effects of moderate alcohol consumption on platelet aggregation, fibrinolysis, and blood lipids. Metabolism 1987;36:538-43. [DOI] [PubMed] [Google Scholar]
- 61.Retterstol L, Berge KE, Braaten O, Eikvar L, Pedersen TR, Sandvik L. A daily glass of red wine: does it affect markers of inflammation? Alcohol Alcohol 2005;40:102-5. [DOI] [PubMed] [Google Scholar]
- 62.Romeo J, Warnberg J, Nova E, Diaz LE, Gonzalez-Gross M, Marcos A. Changes in the immune system after moderate beer consumption. Ann Nutr Metab 2007;51:359-66. [DOI] [PubMed] [Google Scholar]
- 63.Romeo J, Warnberg J, Diaz LE, Gonzalez-Gross M, Marcos A. Effects of moderate beer consumption on first-line immunity of healthy adults. J Physiol Biochem 2007;63:153-9. [DOI] [PubMed] [Google Scholar]
- 64.Roth MJ, Baer DJ, Albert PS, Castonguay TW, Dorgan JF, Dawsey SM, et al. Relationship between serum leptin levels and alcohol consumption in a controlled feeding and alcohol ingestion study. J Natl Cancer Inst 2003;95:1722-5. [DOI] [PubMed] [Google Scholar]
- 65.Schneider J, Liesenfeld A, Mordasini R, Schubotz R, Zofel P, Kubel F, et al. Lipoprotein fractions, lipoprotein lipase and hepatic triglyceride lipase during short-term and long-term uptake of ethanol in healthy subjects. Atherosclerosis 1985;57:281-91. [DOI] [PubMed] [Google Scholar]
- 66.Senault C, Betoulle D, Luc G, Hauw P, Rigaud D, Fumeron F. Beneficial effects of a moderate consumption of red wine on cellular cholesterol efflux in young men. Nutr Metab Cardiovasc Dis 2000;10:63-9. [PubMed] [Google Scholar]
- 67.Sharpe PC, McGrath LT, McClean E, Young IS, Archbold GP. Effect of red wine consumption on lipoprotein (a) and other risk factors for atherosclerosis. QJM 1995;88:101-8. [PubMed] [Google Scholar]
- 68.Sierksma A, van der Gaag MS, Kluft C, Hendriks HF. Effect of moderate alcohol consumption on fibrinogen levels in healthy volunteers is discordant with effects on C-reactive protein. Ann NY Acad Sci 2001;936:630-3. [DOI] [PubMed] [Google Scholar]
- 69.Sierksma A, van der Gaag MS, van Tol A, James RW, Hendriks HF. Kinetics of HDL cholesterol and paraoxonase activity in moderate alcohol consumers. Alcohol Clin Exp Res 2002;26:1430-5. [DOI] [PubMed] [Google Scholar]
- 70.Sierksma A, van der Gaag MS, Kluft C, Hendriks HF. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur J Clin Nutr 2002;56:1130-6. [DOI] [PubMed] [Google Scholar]
- 71.Sierksma A, Vermunt SHF, Lankhuizen IM, van der Gaag MS, Scheek LM, Grobbee DE, et al. Effect of moderate alcohol consumption on parameters of reverse cholesterol transport in postmenopausal women. Alcohol Clin Exp Res 2004;28:662-6. [DOI] [PubMed] [Google Scholar]
- 72.Sierksma A, Patel H, Ouchi N, Kihara S, Funahashi T, Heine RJ, et al. Effect of moderate alcohol consumption on adiponectin, tumor necrosis factor-alpha, and insulin sensitivity. Diabetes Care 2004;27:184-9. [DOI] [PubMed] [Google Scholar]
- 73.Suzukawa M, Ishikawa T, Yoshida H, Hosoai K, Nishio E, Yamashita T, et al. Effects of alcohol consumption on antioxidant content and susceptibility of low-density lipoprotein to oxidative modification. J Am Coll Nutr 1994;13:237-42. [DOI] [PubMed] [Google Scholar]
- 74.Thornton J, Symes C, Heaton K. Moderate alcohol intake reduces bile cholesterol saturation and raises HDL cholesterol. Lancet 1983;2:819-22. [DOI] [PubMed] [Google Scholar]
- 75.Tsang C, Higgins S, Duthie GG, Duthie SJ, Howie M, Mullen W, et al. The influence of moderate red wine consumption on antioxidant status and indices of oxidative stress associated with CHD in healthy volunteers. Br J Nutr 2005;93:233-40. [DOI] [PubMed] [Google Scholar]
- 76.Valimaki M, Taskinen MR, Ylikahri R, Roine R, Kuusi T, Nikkila EA. Comparison of the effects of two different doses of alcohol on serum lipoproteins, HDL-subfractions and apolipoproteins A-I and A-II: a controlled study. Eur J Clin Invest 1988;18:472-80. [DOI] [PubMed] [Google Scholar]
- 77.Valimaki M, Laitinen K, Ylikahri R, Ehnholm C, Jauhiainen M, Bard JM, et al. The effect of moderate alcohol intake on serum apolipoprotein A-I-containing lipoproteins and lipoprotein (a). Metabolism 1991;40:1168-72. [DOI] [PubMed] [Google Scholar]
- 78.Van der Gaag MS, van Tol A, Scheek LM, James RW, Urgert R, Schaafsma G, et al. Daily moderate alcohol consumption increases serum paraoxonase activity; a diet-controlled, randomised intervention study in middle-aged men. Atherosclerosis 1999;147:405-10. [DOI] [PubMed] [Google Scholar]
- 79.Van der Gaag MS, van Tol A, Vermunt SH, Scheek LM, Schaafsma G, Hendriks HF. Alcohol consumption stimulates early steps in reverse cholesterol transport. J Lipid Res 2001;42:2077-83. [PubMed] [Google Scholar]
- 80.Van Golde PM, Hart HC, Kraaijenhagen RJ, Bouma BN, van de Wiel A. Regular alcohol intake and fibrinolysis. Neth J Med 2002;60:285-8. [PubMed] [Google Scholar]
- 81.Vazquez-Agell M, Sacanella E, Tobias E, Monagas M, Antunez E, Zamora-Ros R, et al. Inflammatory markers of atherosclerosis are decreased after moderate consumption of cava (sparkling wine) in men with low cardiovascular risk. J Nutr 2007;137:2279-84. [DOI] [PubMed] [Google Scholar]
- 82.Watzl B, Bub A, Pretzer G, Roser S, Barth SW, Rechkemmer G. Daily moderate amounts of red wine or alcohol have no effect on the immune system of healthy men. Eur J Clin Nutr 2004;58:40-5. [DOI] [PubMed] [Google Scholar]
- 83.Mukamal KJ, Jadhav PP, D’Agostino RB, Massaro JM, Mittleman MA, Lipinska I, et al. Alcohol consumption and hemostatic factors: analysis of the Framingham Offspring Cohort. Circulation 2001;104:1367-73. [DOI] [PubMed] [Google Scholar]
- 84.Heidemann C, Sun Q, van Damm RM, Meigs JB, Zhang C, Tworogger SS, et al. Total and high molecular weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med 2008;149:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004;291:1730-7. [DOI] [PubMed] [Google Scholar]
- 86.Taskinen MR, Nikkila EA, Valimaki M, Sane T, Kuusi T, Kesaniemi YA, et al. Alcohol-induced changes in serum lipoproteins and in their metabolism. Am Health J 1987;113:458-64. [DOI] [PubMed] [Google Scholar]
- 87.Gorinstein S, Caspi A, Zemser M, Libman I, Goshev I, Trakhtenberg S. Plasma circulating fibrinogen stability and moderate beer consumption. J Nutr Biochem 2003;14:710-6. [DOI] [PubMed] [Google Scholar]
- 88.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta regression analysis. BMJ 2009;338:b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, Wilson AC, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 2005;294:2848. [DOI] [PubMed] [Google Scholar]
- 90.Kaptoge S, White IR, Thompson SG, Wood AM, Lewington S, Lowe GD, et al. Associations of plasma fibrinogen levels with established cardiovascular disease risk factors, inflammatory markers, and other characteristics: individual participant meta-analysis of 154,211 adults in 31 prospective studies: the fibrinogen studies collaboration. Am J Epidemiol 2007;166:867-79. [DOI] [PubMed] [Google Scholar]
- 91.Riera-Guardia N, Rothenbacher D. The effect of thiazolidinediones on adiponectin serum level: a meta-analysis. Diabetes Obes Metab 2008;10:367-75. [DOI] [PubMed] [Google Scholar]
- 92.Naimi TS, Brown DW, Brewer RS, Giles WH, Mensah G, Serdula MK, et al. Cardiovascular risk factors and confounders among nondrinking and moderate-drinking US adults. Am J Prev Med 2005;28:369-73. [DOI] [PubMed] [Google Scholar]
- 93.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendices
Flowchart for meta-analysis