Abstract
Endothelial targeting of antioxidant enzymes attenuates acute vascular oxidative stress in animal studies. Superoxide dismutase (SOD) and catalase conjugated with antibodies to Platelet-Endothelial Cell Adhesion Molecule-1 (anti-PECAM/SOD and anti-PECAM/catalase) bind to endothelium, accumulate in the pulmonary vasculature, and detoxify reactive oxygen species. In order to define the role of conjugate size in the efficacy and specificity of endothelial targeting, we synthesized anti-PECAM/enzyme conjugates of controlled size (40 nm–10,000 nm). Binding of anti-PECAM/enzymes to endothelial cells increased with conjugate size from 300 nm to 2 μm (from 2.5 to 8.5% of bound fraction), and was specific, as conjugates did not bind to PECAM-negative cells. Pulmonary uptake of anti-PECAM/enzyme conjugates injected intravenously in mice also increased from 4.5 to 16% of Injected Dose for particles from 200 to 800 nm. However, control conjugates larger than 300 nm showed elevated non-specific pulmonary uptake, indicating that the targeting specificity of anti-PECAM/enzyme conjugates in vivo has a bell-shaped curve with a maximum close to 300-nm diameter. These results show that: i) the size of an antibody/enzyme conjugate modulates efficacy and specificity of targeting, and ii) a size optimum should be defined in vivo to account for parameters that are difficult to model in cell culture.
Keywords: Vascular immunotargeting, endothelium, antioxidant delivery, lungs
1. Introduction
Targeted delivery of therapeutics to endothelial cells holds promise to improve treatment of disease conditions involving these vascular lining cells, including ischemia-reperfusion, inflammation, tumor growth, thrombosis and oxidative stress [1]. This can be achieved by conjugating drugs to antibodies that target specific endothelial surface molecules [2–7]. For example, conjugation of drugs and drug carriers with antibodies to Platelet-Endothelial Cell Adhesion Molecule-1 (anti-PECAM) provides: i) specific binding of conjugates to endothelial cells; ii) intracellular delivery of drugs; ii) preferential accumulation in highly vascularized organs after intravenous injection (especially in the lungs, which is the main target for anti-PECAM conjugates); and, iv) enhanced local therapeutic effects of drugs including genetic materials, as well as anti-thrombotic, anti-inflammatory and anti-oxidant enzymes [8–10].
In particular, endothelial targeting of the antioxidant enzymes superoxide dismutase (SOD) and catalase is being developed as a novel approach for effective containment of acute vascular oxidative stress [5, 9, 11–13]. Anti-PECAM/SOD and anti-PECAM/catalase conjugates detoxify corresponding reactive oxygen species in endothelial cells [14, 15]. After intravenous injection in animals, SOD and catalase conjugated with antibodies to PECAM-1 and other endothelial targets accumulate in the pulmonary vasculature and protect against acute pulmonary oxidative stress, whereas native enzymes, or enzymes conjugated with control IgG, exert neither endothelial targeting nor protection [10, 16, 17]. This approach is especially attractive for potential use in alleviation of lung ischemia/reperfusion injury, such as that associated with transplantation injury [10, 12, 13, 18]. Therefore, understanding of mechanisms and further optimization of endothelial targeting of anti-PECAM/SOD and anti-PECAM/catalase represent important goals.
One particularly intriguing, but still insufficiently understood, aspect is the role of the size of the conjugates in endothelial targeting. Most targeting studies have not measured the size of protein conjugates and fusion constructs, which may vary from nanometers (small fusion proteins) to microns (supramolecular conjugates). One reason for this lack of information is that the synthesis of protein conjugates with controlled and stable size is very challenging. The few studies that have quantitatively measured size by dynamic light scattering (DLS) employed conjugates within a narrow range of 250–350 nm in diameter [10, 14]. Therefore, no systematic studies on the role of size of antibody/enzyme conjugates in endothelial targeting are available. To fill this gap in our knowledge, we designed a series of protein conjugates consisting of antioxidant enzymes coupled to either anti-PECAM or control IgG that ranged in size from ~50 nm to microns and then studied the endothelial targeting of these conjugates using isotope-labeled SOD or catalase in cell cultures and lab animals.
1. Materials and Methods
2.1.Materials and cells
Cytochrome c, xanthine oxidase, xanthine (3,7-dihydro-purine-2,6-dione), dimethylformamide (DMFA) and fetal bovine serum were purchased from Sigma (St. Louis, MO). Cu, Zn-superoxide dismutase (SOD) and catalase from bovine liver and streptavidin are from Calbiochem (San Diego, CA). Radioisotope-containing sodium iodide (Na125I) was obtained from PerkinElmer (Wellesley, MA). Succinimidyl-6-[biotinamido]hexanoate (NHS-LC-biotin), 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (SMCC), N-succinimidyl-S-acetylthioacetate (SATA) , HABA (4′-hydroxyazobenzene-2-carboxylic acid) and Iodogen were from Pierce Biotechnology (Rockford, IL). Anti-PECAM monoclonal antibodies used were: (1) mAb 62, a monoclonal mouse IgG2α antibody directed against human Platelet-Endothelial Cell Adhesion Molecule-1 (PECAM) [9, 19]; (2) mAb 390, a rat monoclonal antibody reacting with murine PECAM [20], and (3) MEC-13.3, a rat IgG2α monoclonal antibody against murine PECAM from BD Biosciences (San Jose, CA). All cell culture medium components were from Life Technologies (Gaithersburg, MD) unless otherwise noted. Rat IgG was from Jackson Immuno-Research Laboratory, Inc. (West Grove, PA). Human umbilical vein endothelial cells (HUVEC) were purchased from Clonetics (San Diego, CA).
2.2. Biotinylation of proteins and biotin measurement
Proteins were biotinylated with NHS-LC-biotin freshly dissolved in DMFA to a concentration of 0.1 M. SOD was biotinylated at 15:1 NHS-LC-biotin:protein molar excess. IgG were biotinylated at different ratio to modulate conjugates size. After 2 h of incubation on ice, free biotin was removed by dialysis against phosphate-buffered saline (PBS). Protein-bound fraction of biotin in b-SOD and b-IgG was measured by HABA assay as we described earlier [21].
2.3. Measurements of SOD activity
Activity of SOD was measured using ferricytochrome c assay [22]. Xanthine/xanthine oxidase system was used as a source of superoxide anion and cytochrome c as the indicating scavenger of the radical competing with SOD. Working solution (0.6 ml) contained 50 mM phosphate buffer (pH 7.8), 0.1 EDTA, 50 μM xanthine, 20 μM cytochrome c and 10 μl of SOD-containing sample. Reaction was initiated by the addition of 10 μl of 0.2 U/ml xanthine oxidase and the absorbance was monitored at 550 nm using Cary 50 spectrophotometer (Varian, Palo Alto, CA). One unit of SOD is defined as the amount of enzyme, which inhibits the rate of cytochrome c reduction by 50% at pH 7.8 and 25ºC. Each measurement was performed at least three times and results were expressed as mean±SD.
2.4. SOD and catalase radiolabeling
SOD and catalase were radiolabeled with Na125I using Iodogen as recommended by the manufacturer. Iodogen was first dissolved in chloroform and a thin film was casted in glass tube by drying under nitrogen flow. After the iodination reaction was performed (15 min at 25ºC), an excess of free label was removed by gel-filtration chromatography using Bio-Spin 6 Chromatography columns (Bio-Rad Labs, Hercules, CA), while iodinated enzymes were transferred to PBS.
2.5.Preparation of immunoconjugates
Two modes of enzyme conjugation to antibody were used, using streptavidin-biotin system and chemical conjugation using SATA and SMCC cross-linkers. Biotinylated SOD was conjugated with biotinylated IgG (anti-PECAM or non-immune IgG) via streptavidin as described elsewhere [9, 21, 23]. The molar ratio of enzyme to antibody was kept 1:1. In second technique, hetero-bifunctional cross-linker SMCC was used to introduce stable maleimide reactive group into enzyme molecule. The reaction was performed at 20–100 fold molar excess of SMCC at room temperature during 1 h. In parallel sulfhydryl groups were introduced in the molecule of antibody or control IgG through primary amine using SATA at 20-fold molar excess of SATA at room temperature for 30 min. Sulfhydryl groups were de-protected using hydroxylamine and antibody was conjugated with SA at 2:1 IgG to SA molar ratio. At each step unreacted components were removed using Spin Protein Columns (G-25 Sephadex, Roche Applied Science, Indianapolis, IN). The effective diameter of the obtained conjugates was measured by Dynamic light scattering apparatus 90Plus Particle Sizer (Brookhaven Instruments Corp., NY). Radiolabeled anti-PECAM conjugates were prepared by introducing from 5 to 10 mol% of 125I-enzyme to unlabeled enzyme prior to its conjugation.
2.6. Cell culture
HUVEC were maintained in M199 medium (GIBCO, Grand Island, NY) with 15% fetal bovine serum supplemented with 100 μg/ml heparin (Sigma), 2 mM L-glutamine (GIBCO), 15 μg/ml endothelial cell growth supplement (Upstate, Lake Placid, NY), 100 U/ml penicillin, and 100 μg/ml streptomycin (GIBCO). Cells were grown to confluency for higher PECAM expression level.
2.7. Binding of anti-PECAM conjugates to endothelial cells
HUVEC were plated in 24 well culture dishes and grown to confluent culture. Radiolabeled conjugates were added to cells at sequential dilutions. Cells were incubated at 37ºC for 1 h, unbound label were washed out and cells were lysed with lysis buffer (1% Triton X-100, 1.0 M NaOH). Bound radioactivity was measured using a Wallac 1470 Wizard™ gamma counter (Gaithersburg, MD). Bound material was expressed as ng of enzyme per well. Each point was performed in quadruplicates and results were expressed as Mean±SEM.
2.8. Pulmonary uptake and blood level of anti-PECAM conjugates in vivo
Animal experiments were performed according to the protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. Ten micrograms of radiolabeled anti-PECAM/enzyme or IgG/enzyme were injected IV in C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) via the tail vein. After 1 h, blood samples were withdrawn and animals were sacrificed. Internal organs were harvested, rinsed with saline, blotted dry, and weighed. Tissue radioactivity in organs and 100-μl samples of blood was determined in a gamma counter. The level of 125I in the organs was used to calculate parameters enzyme uptake (see [19] for detailed explanations) expressed as mean±SD. Total dose circulating in blood was calculated considering that mouse blood volume equals normally to 7.2% of animal weight. Immunospecificity index (ISI), parameter of targeting specificity, was calculated as %ID/g of targeted carrier (anti-PECAM/enzyme) normalized per blood level and divided by same parameter of control non-targeted counterpart (IgG/enzyme).
2.9. Statistical analysis
Statistical difference among groups was determined using one-way analysis of variance (ANOVA). When statistically significant differences were found (p<0.05) individual comparisons were made using the Student t-test upon normality confirmation and Holm-Sidak method (SigmaPlot 11.0).
2. Results
3.1 Synthesis of antibody-enzyme conjugates of controlled size
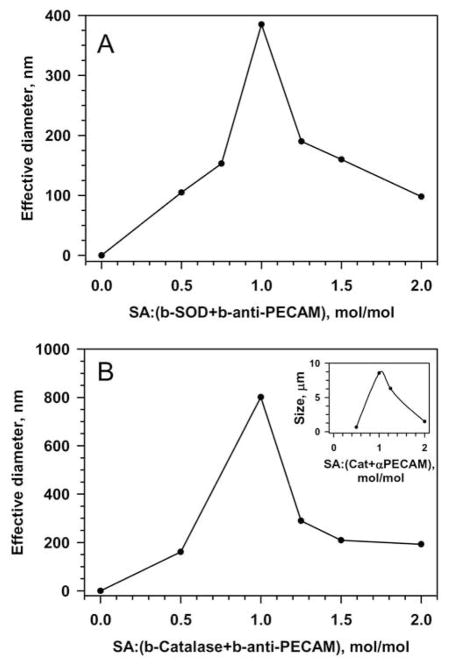
We conjugated SOD and catalase to anti-PECAM using two methods and found that in both approaches the size of the resultant anti-PECAM/enzyme conjugates could be controlled by modulating the reaction conditions. In the first approach, we used a modular streptavidin-biotin cross-linking technique: the enzymes and immunoglobulins were biotinylated (4–7 biotin residues per protein molecule) and cross-linked by streptavidin (SA) [14]. DLS showed that this allowed us to modulate the size of conjugates from ~50 nm to several microns by changing the ratio between SA and biotinylated proteins (Fig. 1).
Figure 1.
Modulation of streptavidin cross-linker input controls size of conjugates formed from biotinylated anti-PECAM and biotinylated enzymes. A. Anti-PECAM/SOD conjugates. B. Anti-PECAM/catalase conjugates. Inset, using highly biotinylated catalase forms larger particles. Size was measured using Dynamic Light Scattering (DLS). Representative curves are shown.
Using proteins with variable degree of biotinylation allows for further “tuning” of the conjugate size. For example, using anti-PECAM with ~4 vs ~6 biotin residues per molecule provided SOD conjugates with maximal size approximately 200 vs 400 nm (data not shown). Similarly, variations of streptavidin input and degree of biotinylation of catalase provided anti-PECAM/catalase conjugates of diverse size ranging from ~30 nm to few microns (Fig. 1B). Similarly to other protein macrocomplexes such as formation of multimolecular immune complexes, a molar excess of either component (biotinylated proteins or streptavidin) serves as an inhibitor of the polymerization reaction and yields smaller conjugates comparing with conjugates formed at optimal molar ratio that in most situations tends to be close to equimolar ratio (i.e. the equimolar ratio between biotin-binding sites of streptavidin and number of biotin residues of biotinylated proteins).
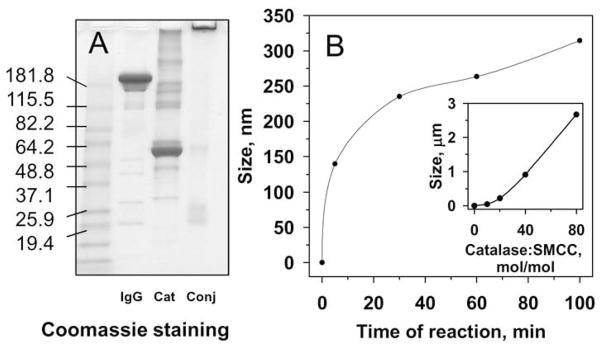
Conjugation using the bi-functional cross-linker SATA/SMCC at an optimal molar ratio of modified proteins resulted in highly effective conjugation (Fig.2A), which eliminated the need for separation of impurities. Similarly to streptavidin-biotin conjugation, this method allowed control of size of protein conjugates (Fig.2B). DLS showed that conjugates of desired size could be produced by varying either the time of reaction (Fig.2B) or the extent of protein modification (Fig.2B, inset). For example, catalase conjugation using SATA/SMCC cross-linker at a controlled extent of protein modification yielded a series of anti-PECAM/catalase conjugates for in vivo studies with effective mean diameters of 40, 80 and 300 nm and 2.0 μm (Fig.1B).
Figure 2.
Preparation of conjugates using SATA/SMCC linker. A. Non-reducing SDS-gel electrophoresis of initial components IgG and catalase (Cat) and IgG/catalase conjugate preparation (conj). Coomassie staining of 4–15% gradient gel. B. Effects of reaction time on conjugate size. Catalase was modified at ratio catalase:SMCC 1:20 and IgG at IgG:SATA ratio 1:20. Conjugation reaction was performed for indicated time before size measurements. Inset: Effects of extent of catalase modification on conjugate size. Catalase was modified as indicated, while IgG:SATA molar ratio was fixed at 1:20. Proteins were mixed and conjugation reaction was performed for 60 min at room temperature before size measurements.
3.2.Characterization of anti-PECAM conjugates in vitro and binding to endothelial cells
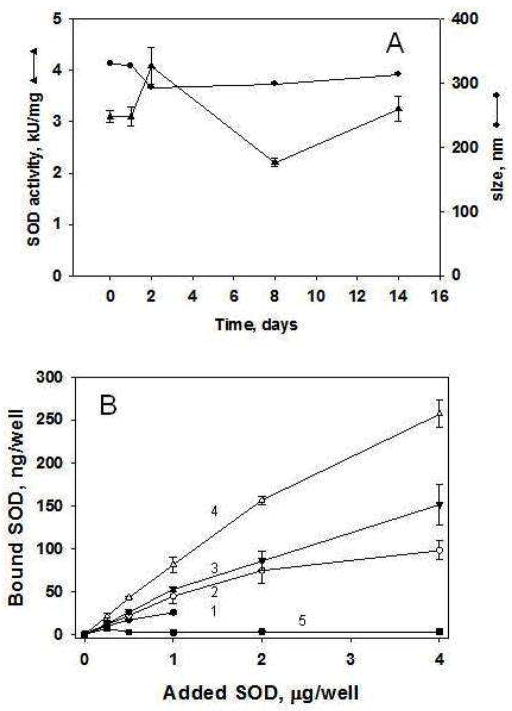
Both conjugation methods yielded SOD and catalase conjugates retaining approximately 75%–80% of enzymatic activity, measured by assays of cytochrome c oxidation and H2O2 decay, respectively (not shown). Conjugates stored either at −20ºC in 50% glycerol or at −80°C in 7% sucrose retained their catalytic activity and size for a prolonged time. For example, Figure 3A shows the stability of an anti-PECAM/SOD conjugate with an initial size ~300 nm during two weeks of storage at −80°C in 7% sucrose. Storage of sub-micron conjugates at these conditions for at least up to two months did not change their properties (data not shown).
Figure 3.
Stability of the anti-PECAM/enzyme conjugates and binding to endothelial cells. A, Conjugate size (●) and enzymatic activity (▲) after storage in 50% glycerol at −20ºC for at least 2 weeks. B. Binding of conjugates to endothelial cells. Cells grown to confluence were incubated with anti-PECAM/125I-SOD conjugates of indicated size for 1 h at 37ºC, unbound material was washed with PBS and cells-bound conjugate was measured in a gamma-counter. Comparison of 300 nm (1, ●), 400 nm (2, ○), 700 nm (3, ▼) and 2.0 μm (4, △) conjugates binding to HUVEC vs PECAM-negative REN cells (5, ■). Mean±SD of four repeats are shown.
In initial studies of the effects of the conjugate size on endothelial targeting, we tested the binding of anti-PECAM/125I-SOD conjugates to endothelial cells in culture. The mass of the conjugate bound to target cells achieved the highest level of 250 ng/well at a concentration of 4.0 μg/ml of SOD for conjugates with an average diameter 2.0 μm (Fig. 3B). Further, the amount of SOD delivered to cells was directly proportional to the conjugate size: conjugates with mean diameter of 400 nm vs 2 microns achieved 4.4% vs 8.9% of added SOD as calculated from the linear segment of the binding curves at 1 μg/ml added SOD (Fig. 3B). Binding of the conjugates was specific, as they did not bind to PECAM-negative REN cells (Fig. 3B, inset), used as negative control, as in previous studies of PECAM-directed endothelial targeting [21].
3.3. Effects of conjugate size on endothelial targeting in vivo
To study endothelial targeting in animals, we traced isotope-labeled enzyme conjugates after IV injection in mice. In accordance with the literature [5, 24], non-conjugated SOD and catalase injected in control mice were rapidly eliminated from the circulation by kidney and liver, respectively, and showed no appreciable uptake (<5% of injected dose per gram, ID/g) in the vascularized organs including lungs (not shown).
On the contrary, anti-PECAM/enzyme conjugates accumulated in the lungs after IV injection in wild-type C57BL/6J mice, but not in PECAM-1 knock-out (PECAM KO) mice (Fig. 4). In correlation with cell culture data, amount of pulmonary uptake increased with size of both anti-PECAM/catalase (Fig.4, A–C) and anti-PECAM/SOD (Fig.4, D–F). For example, 80 and 300 nm anti-PECAM/catalase conjugates showed marked accumulation in the lungs (32±1.6 vs. 84±14 %ID/g; Fig.4, B and C, gray bars), whereas 40 nm conjugate showed relatively limited uptake, although clearly exceeding that observed in PECAM KO mice (17.3±1.1 vs 4.1±0.1 %ID/g, p<0.001) (Fig. 4, A). The fact that the conjugates did not accumulate in the lungs of PECAM KO mice confirmed that pulmonary uptake reflects specific endothelial targeting provided by conjugate binding to PECAM-1 (Fig.4, A–C, black bars).
Figure 4.
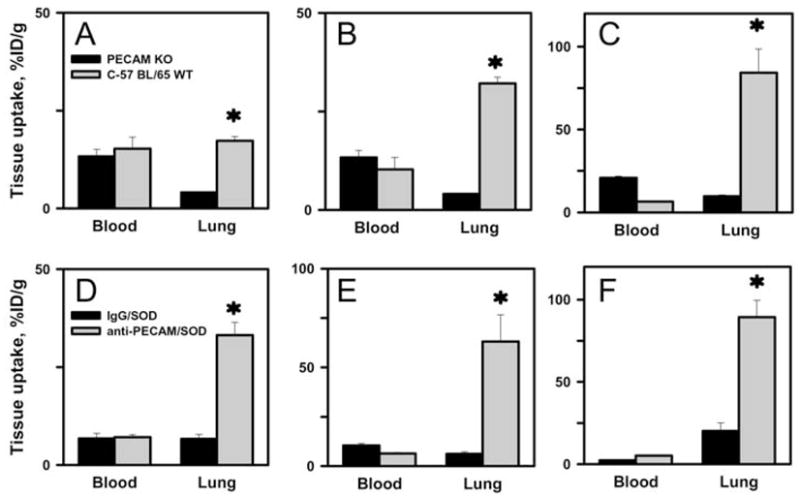
Blood and lung level of antibody-enzyme conjugates of various sizes in vivo. A–C. Blood level and pulmonary uptake of anti-PECAM/catalase conjugates in PECAM KO mice (PECAM KO, black bars) vs wild type mice (WT, gray bars). D–F. Blood level and pulmonary uptake of control IgG/SOD (black bars) vs anti-PECAM/SOD (gray bars) conjugates. 125-I-labeled conjugates were injected IV, tissues were harvested in 1 h. A, 40 nm; B, 80 nm; C, 300 nm. D, 200 nm; E, 300 nm; F, 800 nm. * p<0.001 targeting vs. non-targeting conditions by t-test.
Figure 4D–F, showing data tracing anti-PECAM/SOD vs control IgG/SOD conjugates with mean diameters of 200, 300 and 800 nm confirmed specific PECAM-directed targeting to the endothelial cells in the pulmonary vasculature. As in the case of anti-PECAM/catalase, anti-PECAM/SOD conjugates accumulated in the mouse lungs markedly more effectively than control IgG/SOD counterparts (Fig.4, D–F). The extent of pulmonary uptake also was proportional to the size of the SOD conjugates: as size increased from 200 to 300 and 800 nm pulmonary uptake of anti-PECAM/SOD conjugates was augmented from 33±3.2 to 63±14 and 89±12 %ID/g, respectively (p<0.001).
These data show that the efficacy of the pulmonary delivery of anti-PECAM/enzyme conjugates, expressed as percent of total lung uptake, was proportional to their size (Fig.5, panel A: 300 nm vs. 40 nm, P<0.001; 300 nm vs. 80 nm, P<0.01; 80 nm vs. 40 nm, P<0.05; panel C: 800 nm vs. 200 nm, P<0.001; 800 nm vs. 300 nm, P<0.01; 300 nm vs. 200 nm, P<0.05; by Holm-Sidak method). In order to rigorously analyze the specificity of the endothelial targeting manifested by the pulmonary accumulation of conjugates, we have calculated the ratio of uptake of targeted vs. non-targeted formulation normalized by their respective blood levels, i.e., the immunospecificity index (ISI, the most accurate measure of targeting specificity) [5]. Pulmonary ISI of anti-PECAM/enzyme conjugates increased with size increase to 300 nm (Fig. 5, panels B&D). However, further larger conjugate size (i.e., 800 nm) diminished the pulmonary ISI of anti-PECAM/SOD (Fig.5D), because control IgG/SOD of this size showed enhanced pulmonary uptake. Therefore, anti-PECAM-enzyme conjugates with size close to 300 nm diameter showed maximally specific endothelial targeting in the vasculature vs. small conjugates (200 nm) and large (800 nm) conjugates. It is not known yet how sharp the optimal size range is. Based on in vivo studies with polymer particles [25, 26] it may extend within few hundred nanometers. In this case, it is plausible that conjugates with average diameter close to 400–500 nm may exert superior specificity; yet this does not seem likely due to dramatic reduction of specificity of 800 nm vs 300 nm conjugates.
Figure 5.
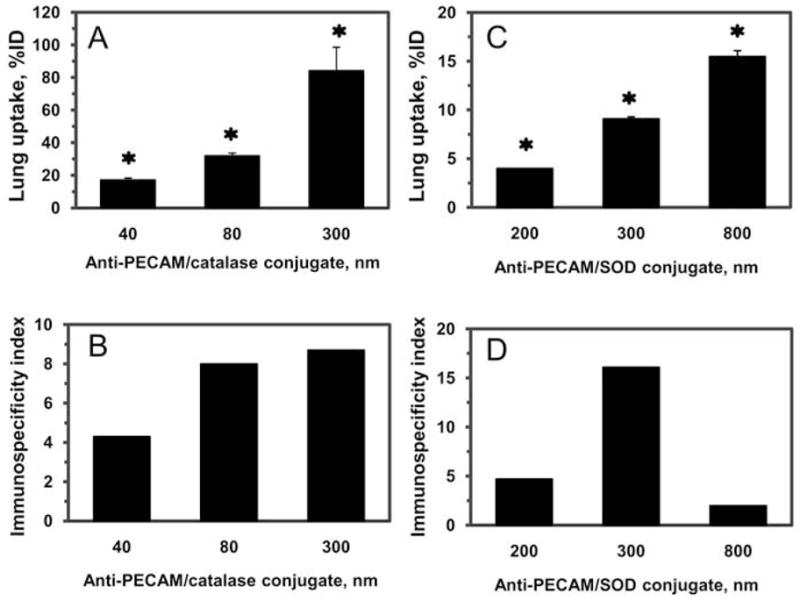
Lung uptake (A and C) and immunospecificity index (ISI; B and D) of anti-PECAM/catalase (A and B) and anti-PECAM/SOD (C and D) conjugates of various size. Radiolabeled conjugates were injected i.v. and tissue was harvested 1 h post-injection. The immunospecificity index was calculated as ratio of targeted vs non-immune counterparts. Tissue uptake is shown as mean±SEM (n=3–5). * p<0.001 targeted conjugate vs. non-immune counterparts by t-test.
4. Discussion
Carrier geometry plays a key role in drug delivery [27]. For example, the size and shape of polymer carriers markedly influences their longevity in the circulation, their uptake by phagocytes and clearing tissues, their internalization by target cells and degradation rate, as well as drug loading and release [26, 28, 29]. In the context of pulmonary endothelial targeting, recent studies showed that the geometry (i.e., size and shape) of polymer carriers carrying anti-PECAM and other affinity moieties modulate the efficacy and specificity of pulmonary accumulation of circulating carriers [25].
However, the role of size of protein conjugates has been much less thoroughly addressed; in part, because it is more difficult to devise protein conjugates with controlled size than it is to manufacture controlled-sized polymer carriers [27]. Nevertheless, protein conjugates and fusion constructs represent potentially useful classes of targeted therapeutics and many experimental agents in this class have shown promising therapeutic and prophylactic effects in animal studies.
This study describes the synthesis of differently sized antibody/enzyme conjugates (represented by SOD and catalase conjugated with IgG and PECAM antibodies) and testing their ability to target endothelial cells. Conjugation of drug cargoes to antibodies and antibody fragments via streptavidin is widely employed in animal studies [21]. This modular approach provides supramolecular conjugates with unique targeting features including enhanced uptake by target cells [3, 9]. From the translational perspective, however, it is important to note that an alternative conjugation technique (streptavidin is not used in patients in the USA) can also be adapted to produce stable antibody-enzyme conjugates of desirable size within a wide range of conjugate diameters (Fig. 2).
Targeting efficacy was directly proportional to the conjugate size in both cell culture and in vivo (Fig. 3 and 4). This can be explained by the increasing: i) drug load; and ii) number of antigen-binding sites of the conjugates due to enhanced number of antibody molecules per conjugate particle, which resulted in higher affinity of binding to target. However, the elevated pulmonary uptake of large IgG/enzyme conjugates revealed that the specificity of in vivo targeting diminishes when the conjugate size begins to exceed 300 nm in average diameter (Fig. 5).
It is not clear why sub-micron sized IgG/enzyme conjugates exert elevated non-specific uptake in the pulmonary vasculature. They are smaller than limiting size of the capillaries (i.e., ~800 nm vs ~5 μm), which argues against mechanical entrapment in the microvasculature. Studies in cell culture show no indication of enhanced non-specific cellular adhesion of larger conjugates (Fig. 3B). Perhaps, large conjugates possessing multiple copies of IgG Fc-fragments more extensively interact with Fc-receptor bearing cells in circulation (white blood cells and platelets), causing their agglomeration and retention in the capillaries. If this is the case, exceeding optimal size may cause adverse effects (see below); hence, understanding of the mechanism of non-specific uptake is worth further inquiry.
Non-specific vascular uptake of sub-optimally large antibody/enzyme conjugates may have important implications. First, the Fc-fragment-mediated mechanism postulated above may predispose to inflammation and thrombosis. Second, conjugates non-specifically retained in the vasculature are likely to reside in the lumen, without entering endothelial cells. Previous studies showed that for intra-endothelial delivery, anti-PECAM conjugates must interact specifically with PECAM-1, causing endocytic signaling mediated by the PECAM-1 cytosolic domain [30]. Most likely, antibody/enzyme conjugates non-specifically retained in a vessel will not induce endocytosis; hence their effects will be limited to the bloodstream. This location may be sufficient and even preferential for some therapeutics (e.g., fibrinolytics), but intracellular delivery is required for action of many other drugs [4].
In this study, we have focused on the ability of size to affect conjugate targeting to endothelium. Depending on the nature of the cargo, another important factor to consider is the ultimate fate of the conjugates, that is whether they stay on the cell surface (which would be critical for an anti-thrombotic agent) or whether they are internalized, and to which compartment (as might be critical for RNA, DNA, or anti-oxidants). In previous studies, we have shown that optimal size range for internalization of protein anti-PECAM conjugates by endothelium is between ~100 and 500 nm, whereas larger conjugates are poorly internalized despite highly effective and specific binding to endothelial cells [31]. Taken together with the data from this paper, our results suggest that a diameter close to 300 nm of anti-PECAM/enzyme conjugates is optimal for the specific intra-endothelial delivery.
Acknowledgments
This work was supported in part by National Institutes of Health Grants RO1 HL073940 and HL087036 and Project 4 of PO1 HL079063 (to VRM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ding BS, Dziubla T, Shuvaev VV, Muro S, Muzykantov VR. Advanced drug delivery systems that target the vascular endothelium. Mol Interv. 2006;6(2):98–112. doi: 10.1124/mi.6.2.7. [DOI] [PubMed] [Google Scholar]
- 2.Massey KA, Schnitzer JE. Caveolae and Cancer. Recent Results Cancer Res. 180:217–231. doi: 10.1007/978-3-540-78281-0_13. [DOI] [PubMed] [Google Scholar]
- 3.Valadon P, Darsow B, Buss TN, Czarny M, Griffin NM, Nguyen HN, Oh P, Borgstrom P, Chrastina A, Schnitzer JE. Designed auto-assembly of nanostreptabodies for rapid tissue-specific targeting in vivo. J Biol Chem. 285(1):713–722. doi: 10.1074/jbc.M109.061838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv. 2005;2(5):909–926. doi: 10.1517/17425247.2.5.909. [DOI] [PubMed] [Google Scholar]
- 5.Muzykantov VR, Atochina EN, Ischiropoulos H, Danilov SM, Fisher AB. Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc Natl Acad Sci U S A. 1996;93(11):5213–5218. doi: 10.1073/pnas.93.11.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasqualini R, McDonald DM, Arap W. Vascular targeting and antigen presentation. Nat Immunol. 2001;2(7):567–568. doi: 10.1038/89704. [DOI] [PubMed] [Google Scholar]
- 7.Robbins GP, Saunders RL, Haun JB, Rawson J, Therien MJ, Hammer DA. Tunable Leuko-polymersomes That Adhere Specifically to Inflammatory Markers. Langmuir. doi: 10.1021/la1017032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding BS, Hong N, Murciano JC, Ganguly K, Gottstein C, Christofidou-Solomidou M, Albelda SM, Fisher AB, Cines DB, Muzykantov VR. Prophylactic thrombolysis by thrombin-activated latent prourokinase targeted to PECAM-1 in the pulmonary vasculature. Blood. 2008;111(4):1999–2006. doi: 10.1182/blood-2007-07-103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, Harshaw DW, Schultz L, Fisher AB, Albelda SM. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci U S A. 1999;96(5):2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuvaev VV, Christofidou-Solomidou M, Bhora F, Laude K, Cai H, Dikalov S, Arguiri E, Solomides CC, Albelda SM, Harrison DG, Muzykantov VR. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther. 2009;331(2):404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med. 2006;5(1):47–78. doi: 10.2165/00151829-200605010-00004. [DOI] [PubMed] [Google Scholar]
- 12.Nowak K, Hanusch C, Nicksch K, Metzger RP, Beck G, Gebhard MM, Hohenberger P, Danilov SM. Pre-ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin-converting-enzyme antibodies. Eur J Cardiothorac Surg. 2009 doi: 10.1016/j.ejcts.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Nowak K, Weih S, Metzger R, Albrecht Ii RF, Post S, Hohenberger P, Gebhard MM, Danilov SM. Immunotargeting of catalase to lung endothelium via anti-ACE antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol. 2007 doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 14.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther. 2007;323(2):450–457. doi: 10.1124/jpet.107.127126. [DOI] [PubMed] [Google Scholar]
- 15.Sweitzer TD, Thomas AP, Wiewrodt R, Nakada MT, Branco F, Muzykantov VR. PECAM-directed immunotargeting of catalase: specific, rapid and transient protection against hydrogen peroxide. Free Radic Biol Med. 2003;34(8):1035–1046. doi: 10.1016/s0891-5849(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 16.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, Ng K, Sweitzer T, Arguiri E, Shuvaev V, Solomides CC, Albelda SM, Muzykantov VR. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2003;285(2):L283–292. doi: 10.1152/ajplung.00021.2003. [DOI] [PubMed] [Google Scholar]
- 17.Muzykantov VR, Atochina EN, Kuo A, Barnathan ES, Notarfrancesco K, Shuman H, Dodia C, Fisher AB. Endothelial cells internalize monoclonal antibody to angiotensin-converting enzyme. Am J Physiol. 1996;270(5 Pt 1):L704–713. doi: 10.1152/ajplung.1996.270.5.L704. [DOI] [PubMed] [Google Scholar]
- 18.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, Muro S, Buerk DG, Solomides CC, Albelda SM, Patterson GA, Muzykantov VR. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol. 2003;21(4):392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 19.Scherpereel A, Rome JJ, Wiewrodt R, Watkins SC, Harshaw DW, Alder S, Christofidou-Solomidou M, Haut E, Murciano JC, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J Pharmacol Exp Ther. 2002;300(3):777–786. doi: 10.1124/jpet.300.3.777. [DOI] [PubMed] [Google Scholar]
- 20.Yan HC, Pilewski JM, Zhang Q, DeLisser HM, Romer L, Albelda SM. Localization of multiple functional domains on human PECAM-1 (CD31) by monoclonal antibody epitope mapping. Cell Adhes Commun. 1995;3(1):45–66. doi: 10.3109/15419069509081277. [DOI] [PubMed] [Google Scholar]
- 21.Shuvaev VV, Dziubla T, Wiewrodt R, Muzykantov VR. Streptavidin-biotin crosslinking of therapeutic enzymes with carrier antibodies: nanoconjugates for protection against endothelial oxidative stress. Methods Mol Biol. 2004;283:3–20. doi: 10.1385/1-59259-813-7:003. [DOI] [PubMed] [Google Scholar]
- 22.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 23.Scherpereel A, Wiewrodt R, Christofidou-Solomidou M, Gervais R, Murciano JC, Albelda SM, Muzykantov VR. Cell-selective intracellular delivery of a foreign enzyme to endothelium in vivo using vascular immunotargeting. Faseb J. 2001;15(2):416–426. doi: 10.1096/fj.00-0022com. [DOI] [PubMed] [Google Scholar]
- 24.Bayati A, Kallskog O, Odlind B, Wolgast M. Plasma elimination kinetics and renal handling of copper/zinc superoxide dismutase in the rat. Acta Physiol Scand. 1988;134(1):65–74. doi: 10.1111/j.1748-1716.1988.tb08460.x. [DOI] [PubMed] [Google Scholar]
- 25.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther. 2008;16(8):1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canelas DA, Herlihy KP, DeSimone JM. Top-down particle fabrication: control of size and shape for diagnostic imaging and drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(4):391–404. doi: 10.1002/wnan.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simone EA, Dziubla TD, Muzykantov VR. Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv. 2008;5(12):1283–1300. doi: 10.1517/17425240802567846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garnacho C, Shuvaev V, Thomas A, McKenna L, Sun J, Koval M, Albelda S, Muzykantov V, Muro S. RhoA activation and actin reorganization involved in endothelial CAM-mediated endocytosis of anti-PECAM carriers: critical role for tyrosine 686 in the cytoplasmic tail of PECAM-1. Blood. 2008;111(6):3024–3033. doi: 10.1182/blood-2007-06-098657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood. 2002;99(3):912–922. doi: 10.1182/blood.v99.3.912. [DOI] [PubMed] [Google Scholar]