Abstract
We evaluated replication-defective poxvirus vectors (modified vaccinia Ankara [MVA] and fowlpox [FPV]) in a homologous and heterologous vector prime-boost vaccination regimen containing matching HIV inserts (MVA-HIV and FPV-HIV) given at months 0, 1, 3, 5 and 7 in 150 healthy HIV-negative vaccinia-naïve participants. FPV-HIV alone was poorly immunogenic, while the high dose (109 pfu/2ml) of MVA-HIV alone elicited maximal responses after two injections: CD4+ and CD8+ T-cell responses in 26/55 (47.3%) and 5/60 (8.3%) of participants, respectively and IFN-γ ELISpot responses in 28/62 (45.2%). The infrequent CD8+ T-cell responses following MVA-HIV priming were boosted only by the heterologous (FPV-HIV) construct in 14/27 [51.9%] of participants post-4th vaccination. Alternatively, HIV envelope-specific binding antibodies were demonstrated in approximately two-thirds of recipients of the homologous boosting regimen, but in less than 20% of subjects after the heterologous vector boost. Thus, a heterologous poxvirus vector prime-boost regimen can induce an HIV-specific CD8+ T-cell and CD4+ T-cell responses, which may be an important feature of an optimal regimen for preventive HIV vaccination.
Key words/terms: Preventive HIV vaccine, MVA and Fowlpox vectors, Phase I clinical trial
Introduction
It is expected that a safe and effective vaccine will be required to bring the HIV pandemic under control [1–3]. Considerable evidence indicates the importance of anti-HIV cellular immune responses in the control of acute and chronic HIV-1 infection [4–7] and a possible protective role in HIV acquisition [8, 9]. Thus, a vaccine regimen capable of inducing broadly reactive HIV-specific CD4+ and CD8+ T-cell responses may be required.
A variety of live vector-HIV vaccine constructs have been shown to induce HIV-specific CD8+ and/or CD4+ T-cell responses in clinical trials [10], and analogous constructs are capable of protecting against disease progression in the SIV-rhesus macaque model [11, 12]. To date, most vaccination regimens have employed prime-boost strategies to optimize cellular and/or humoral immune responses. Because anti-vector immunity can limit responses to boosting of viral-vectored vaccines, alternative strategies have been used, including boosts with heterologous vectored constructs, or a combination of vaccine modalities including plasmid DNA or recombinant proteins [13–15].
We studied two pairs of matching poxvirus-vectored HIV vaccine candidates with identical HIV inserts, one Modified vaccinia Ankara (MVA)-based and the other fowlpox virus (FPV)-based. Because of vector limitations, the full complement of HIV inserts were split into two MVA and two FPV constructs; the structural genes env and gag were included in one vector pair, while the regulatory genes tat, rev, nef, and reverse transcriptase (RT) were included in the second vector pair. This study examines the safety and immunogenicity of homologous prime-boost and heterologous prime-boost MVA-HIV and FPV-HIV immunization strategies in healthy human volunteers (HVTN 055).
Materials and Methods
Vaccines
The 2 vector pairs were produced under cGMP conditions by Therion Biologics Corporation, Inc. (Cambridge, MA) in primary chick embryo fibroblasts using an attenuated MVA vector (kindly provided by Dr. Anton Mayr), and an FPV vector derived from the USDA-licensed poultry vaccine, POXVAC-TC (Schering-Plough, Union, NJ) [16]. HIV inserts were derived from a subtype B primary isolate strain C58A1 obtained from an HIV-1-infected infant [17]. The tat, rev, nef and RT genes were modified to be rendered non-functional and the nef gene was modified to abrogate MHC class I downregulation [18–21]. Non-recombinant wild-type MVA and FPV at the same dosage as the poxviral-HIV recombinants were used as control vaccines.
Study design
The study was a randomized, controlled, double-blind, 2-part phase I trial in 150 HIV-uninfected, vaccinia-naïve subjects at NIAID-supported HIV Vaccine Trials Network (HVTN) study sites in the US and Brazil (Table 1). The duration of participant follow-up was 13 months, which included at least 6 months after the final vaccination. Eligible subjects were healthy volunteers, 18–40 years of age, who reported no history of smallpox vaccination and had no evidence of a vaccinial scar on physical examination. Subjects who had previously received HIV vaccines, or who have exhibited hypersensitivity to egg products were excluded. Because myopericarditis of unknown mechanism has been observed following administration of replication-competent vaccinia virus for vaccination against smallpox [22, 23], subjects who had conditions that might complicate the safety assessment were excluded. Such conditions included known cardiac disease, hypertension requiring medication, an abnormal electrocardiogram (ECG) at screening, elevated serum cardiac troponin I or creatine kinase (CK), or two or more cardiac risk factors. Also, Brazilian subjects seropositive for Trypanosoma cruzi were excluded.
Table 1.
Study schema
| Part A | ||||||
|---|---|---|---|---|---|---|
| Group [a] | # recipients Vaccine / Control | Immunization Schedule in Months (Day) | ||||
| 0(0) | 1(28) | 3(84) | 5(140) | 7(196) | ||
| 1 [F/F] | 10 / 2 | F9b | F9 | F9 | F9 | F9 |
| 2 [low dose M/F] | 10 / 2 | M7c | M7 | F9 | F9 | F9 |
| 3 [mid dose M/F] | 10 / 2 | M8d | M8 | F9 | F9 | F9 |
| 4 [M/F] | 10 / 2 | M9e | M9 | F9 | F9 | F9 |
| 5 [M/M] | 10 / 2 | M9 | M9 | M9 | M9 | M9 |
| Total | 50/10 | |||||
| Part B | ||||||
| 6 [F/F] | 25 / 5 | F9 | F9 | F9 | F9 | F9 |
| 7 [M/F] | 25 / 5 | M9 | M9 | F9 | F9 | F9 |
| 8 [M/M] | 25 / 5 | M9 | M9 | M9 | M9 | M9 |
| Total | 75 / 15 | |||||
| Total (Parts A & B) | 125/25 | |||||
| TOTAL | ||||||
| (Vaccine & Control) | 150 | |||||
Composition of groups referred to in text as FPV-HIV alone [F/F], MVA-HIV + FPV-HIV [M/F], and MVA alone [M/M]
F9 indicates FPV-HIV total dose 109 pfu/2 mL
M7 indicates MVA-HIV total dose 107 pfu/2 mL
M8 indicates MVA-HIV total dose 108 pfu/2 mL
M9 indicates MVA-HIV total dose 109 pfu/2 mL
The study had two parts, Part A examined 1-log10 dose escalations of MVA-HIV, from 1 × 107 particle forming units (pfu) to 1 × 109 pfu, while Part B examined the 1× 109 pfu dose of MVA-HIV in an expanded number of participants. FPV-HIV was used at a constant dose of 1 × 109 pfu, which had been previously demonstrated to be safe [24]. Study preparations were given in 1 ml volumes into the left (5 × 108 pfu of vectors containing HIV env/gag) and right (5 × 108 pfu of vectors containing HIV tat/rev/nef-RT) deltoid muscle at months 0, 1, 3, 5 and 7. The heterologous vector groups received MVA-HIV at months 0 and 1, followed by FPV-HIV at months 3, 5 and 7. Blood was drawn for peripheral blood mononuclear cells (PBMC) and sera cryopreservation at baseline, 2 weeks after each vaccination and at the final visit (13 months). For this report, immunogenicity data from participants in Parts A and B who received the same regimen were combined. Standard HVTN protocol operational policies and procedures were followed, including HIV-acquisition risk reduction counseling at each visit, HIV testing at 3–4 month intervals and strict adherence to Good Clinical Practice (GCP) and International Conference on Harmonization (ICH) guidelines. The clinical protocol and informed consent documents were approved by the Institutional Review Board and Institutional Biosafety Committee of each participating institution, as well as the Brazilian national regulatory authorities.
Safety assessment
Participants were instructed in standardized self-reporting of body temperature and the grading of injection site reactions and systemic symptoms and collected this information for 3 days following each injection on study-specific diary cards. They also returned to the clinic 2 weeks after each vaccination for clinical evaluation and laboratory testing of hematologic, renal and hepatic analytes [25]. Adverse events including reactogenicity events were graded using a standardized DAIDS toxicity table [26]. All subjects were followed for interval cardiac symptoms at each visit, serum creatine kinase (CK) and troponin I two weeks after each vaccination and ECGs two weeks after the first and final vaccinations. Additional cardiac work-ups were performed as indicated for subjects having signs or symptoms suggestive of cardiac involvement.
Immunogenicity assessment
Intracellular cytokine staining assay
PBMC were isolated from sodium heparin-anticoagulated blood and cryopreserved within 8 hours of venipuncture [27]. HIV-specific T-cells were detected by intracellular cytokine staining (ICS) as previously described [28, 29]. Global potential T-cell epitope (G-PTE) [30] pools for HIV Env, Gag, Pol and Nef were used at a final concentration of 1 μg/ml. Each pool included 160 peptides, and a total of 9 pools were used (3 for Env and Pol, 2 for Gag and 1 for Nef). HIV Rev and Tat peptide pools were not available for testing. Either an 8-color (for post-3rd and 4th vaccination samples [28]) or a 10-color (for post-2nd vaccination and final study visit samples [29]) ICS assay was used. For response rates and magnitude of cells producing IFN-γ and/or IL-2, combined data from the two assays was reported. However, TNF-α expression has not been cross-validated between the two assays; therefore, only data from the 8-color assay are presented for the polyfunctionality assessment. Criteria for an evaluable response and positivity were determined as previously described [28] based upon background-adjusted measurements and adequate number of T-cells examined. Criteria for evaluability were applied separately for CD4+ and CD8+ T-cells, so the total number of specimens for each analysis may differ.
Ex-vivo Interferon-γ Elispot assay
Assessments were performed using cryopreserved PBMCs (as described above) obtained from study participants 2 weeks after the 2nd and 4th vaccinations in a standardized, validated bulk IFN-γ ELISpot assay as described elsewhere [31]. The same G-PTE peptide pools that were used in the ICS assay were employed for in-vitro stimulations. Positivity criteria for a T-cell response was determined by the data-free resampling method, testing for a > 2-fold increase in the mean of the experimental wells over the mean of the control wells, adjusting for multiple peptide pools [32]. In addition to statistical significance, the mean background-subtracted response for the peptide pool had to be > 50 SFC/106 PBMC for the peptide pool to be considered positive.
Anti-gp120 & p24 ELISA
Anti-Gag and anti-Env binding antibody responses were determined by HVTN-validated non-commercial ELISAs [33]. Sera were tested in plates coated with purified p24 (Protein Sciences, Inc.) and gp120 (Protein Sciences, Inc.). Positivity was scored using duplicate measurements for antigen-containing minus non-antigen-containing wells that had an optical density (OD) ≥ 0.2.
HIV-1 neutralization assay
Neutralizing antibodies against HIV-1 were measured as a function of reductions in Tat-regulated luciferase reporter gene expression after a single round of infection in TZM-bl cells. Sera were tested for the ability to neutralize the homologous C58A1 vaccine strain and strain MN that is highly sensitive to neutralization [34, 35]. The assay was considered positive if the titer was ≥ 25.
Statistical analyses
Analyses were performed by treatment group regardless of the number of study injections received. Differences in reaction symptom severity were tested with Kruskal Wallis tests. Since there were no clinically significant differences in the incidence or severity of local and systemic reactions in recipients of the two poxviral-HIV constructs versus empty vector controls, controls were combined with their respective study groups for reactogenicity analysis. Immunogenicity response rates were presented with 95% confidence intervals [36]. Differences in immunogenicity response rates between treatment groups and gender were tested with Fisher exact tests. Because of overlap of peptides between peptide pools for the same HIV protein, for HIV proteins requiring multiple in-vitro peptide pools, the magnitude of a T-cell response was defined as that of the pool with the maximum response. Accordingly, the overall magnitude of HIV-specific response was calculated by summing across the maximum pools for each protein. Differences in response magnitudes between treatment groups were tested with Wilcoxon rank sum tests. To control for correlation within an individual, generalized estimating equation (GEE) methods [37] were used to assess the effect of boosting within a treatment group on response rates. Differences in response to Gag and Env proteins within a treatment group were assessed with McNemar tests. P-values were considered statistically significant for p < 0.05 and were unadjusted for multiple comparisons.
Results
Study subjects
One hundred-fifty participants, 136 in the US and 14 in Brazil, were enrolled over a period of approximately 21 months; 60 in Part A and 90 in Part B (Table 2). Overall, 51% of the subjects were male, 37% were non-white or of Latino ethnicity and the median age was 24 years. One hundred forty-five (97%), 138 (92%), 130 (87%), and 106 (71%) subjects received their second, third, fourth and fifth vaccinations, respectively. Twenty-one subjects missed the last vaccination because Therion Biologics, Inc. discontinued business operations. Twenty-three additional subjects had vaccinations discontinued due to intercurrent adverse events (n=7; one participant had severe myalgias and arthalgias consistent with vaccine reactogenicity, however, the AEs in the others were not felt to be related to vaccination), missed vaccination window (n=9), subject refusal (n=3), pregnancy (n=2), subject relocation (n=2). One of the participants who refused further vaccinations did so due to moderate myalgias and headache judged to be probably related to the 2nd vaccination of the high dose of MVA-HIV. All participants who missed vaccinations were followed for safety assessments for the duration of the study unless they had relocated.
Table 2.
Participant demographics and number of study vaccinations receiveda
| Characteristic | FP/FPb | 107 MVA/FP | 108 MVA/FP | 109 MVA/FPb | 109 MVA/MVAb | Total |
|---|---|---|---|---|---|---|
| N=42 | N=12 | N=12 | N=42 | N=42 | N=150 | |
| Country | ||||||
| US | 37 (88.1%) | 12 (100.0%) | 12 (100.0%) | 37 (88.1%) | 38 (90.5%) | 136 (90.7%) |
| Brazil | 5 (11.9%) | 0 (0.0%) | 0 (0.0%) | 5 (11.9%) | 4 (9.5%) | 14 (9.3%) |
| Gender | ||||||
| Male | 23 (54.8%) | 9 (75.0%) | 7 (58.3%) | 17 (40.5%) | 20 (47.6%) | 76 (50.7%) |
| Female | 19 (45.2%) | 3 (25.0%) | 5 (41.7%) | 25 (59.5%) | 22 (52.4%) | 74 (49.3%) |
| Race | ||||||
| White - non-Hispanic | 22 (52.4%) | 12 (100.0%) | 10 (83.3%) | 25 (59.5%) | 25 (59.5%) | 94 (62.7%) |
| African American - non-Hispanic | 1 (2.4%) | 0 (0.0%) | 0 (0.0%) | 5 (11.9%) | 3 (7.1%) | 9 (6.0%) |
| Hispanic | 15 (35.7%) | 0 (0.0%) | 1 (8.3%) | 10 (23.8%) | 13 (31.0%) | 39 (26.0%) |
| Asian | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Multiracial | 4 (9.5%) | 0 (0.0%) | 0 (0.0%) | 1 (2.4%) | 1 (2.4%) | 6 (4.0%) |
| Other | 0 (0.0%) | 0 (0.0%) | 1 (8.3%) | 1 (2.4%) | 0 (0.0%) | 2 (1.3%) |
| Age | ||||||
| 18 – 20 years | 14 (33.3%) | 2 (16.7%) | 2 (16.7%) | 5 (11.9%) | 7 (16.7%) | 30 (20.0%) |
| 21 – 30 years | 24 (57.1%) | 10 (83.3%) | 8 (66.7%) | 29 (69.0%) | 30 (71.4%) | 101 (67.3%) |
| 31 – 40 years | 4 (9.5%) | 0 (0.0%) | 2 (16.7%) | 8 (19.0%) | 5 (11.9%) | 19 (12.7%) |
| Median | 24 | 24 | 24 | 24 | 24 | 24 |
| Range | 18, 36 | 19, 30 | 19, 37 | 18, 40 | 19, 35 | 18, 40 |
| # of subjects (% of group) who received the indicated set of study injections | ||||||
| 1st | 42 (100%) | 12 (100%) | 12 (100%) | 42 (100%) | 42 (100%) | 150 (100%) |
| 2nd | 41 (97.6%) | 11 (91.7%) | 11 (91.7%) | 42 (100%) | 40 (95.2%) | 145 (96.7%) |
| 3rd | 39 (92.9%) | 11 (91.7%) | 11 (91.7%) | 39 (92.9%) | 38 (90.5%) | 138 (92.0%) |
| 4th | 36 (85.7%) | 11 (91.7%) | 10 (83.3%) | 38 (90.5%) | 35 (83.3%) | 130 (86.7%) |
| 5th | 28 (66.7%) | 10 (83.3%) | 10 (83.3%) | 32 (76.2%) | 26 (61.9%) | 106 (70.7%) |
Groups include both vaccine and control recipients. If a study injection was missed, all future injections were discontinued, but participant data were included in the analysis per ITT
Includes participants from study Parts A & B who received the same regimen and dose of vaccine.
Safety
The study preparations were generally well-tolerated; no adverse events (AEs) met the criteria for filing an FDA Safety Report and no participant acquired HIV infection. One or more AEs were reported by 91% of study participants; however 97% of these were graded as mild or moderate. AEs were infrequently considered to be definitely/probably related (6%) or possibly related (4%) to the study preparations. The MVA-HIV dose escalation in Part A proceeded without complication (data not shown) and therefore the 1 × 109 pfu dose was employed in Part B. No significant abnormalities of laboratory parameters were detected in any group.
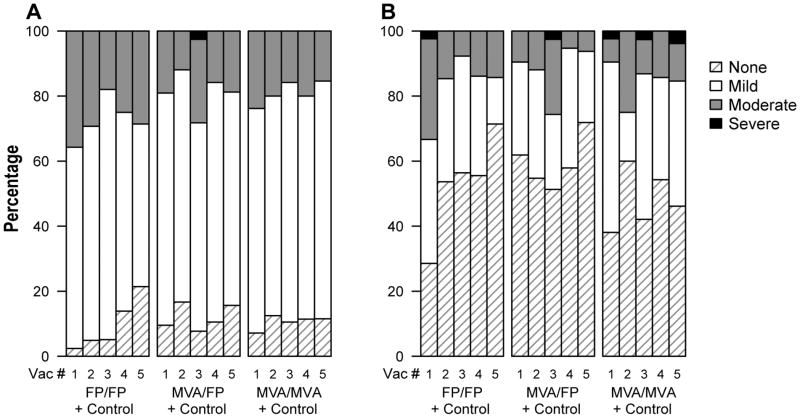
Overall, the reactogenicity profile did not differ appreciably among study groups (Figure 1). Pain and/or tenderness at the injection site was common, but for most the maximal intensity was mild (Figure 1A); only 1 vaccinee reported severe pain and tenderness, after the first FPV-HIV boost in the M/F group. Systemic symptoms were less common and most frequently graded as mild (Figure 1B). Six participants (one of whom in the mid-dose MVA group and not included in Figure 1B) reported one or more severe systemic symptoms, typically consisting of myalgia, malaise, headache, fatigue and/or arthralgia that began within 12–18 hours of vaccination, usually accompanied by other less severe local and/or systemic symptoms. In addition, one of these participants also had severe local pain and tenderness at the injection site as a part of the constellation of symptoms. Of the 6 participants with severe reactions, three experienced them after an FPV-HIV injection and three had them after an MVA-HIV injection. Febrile reactions were rare, only one participant had a temperature of > 39.4 degrees centigrade. Local and systemic reactions typically resolved without treatment within 48 hours and reactions rarely increased after subsequent injections with the same vector.
Figure 1. Maximum severity of local pain or tenderness (panel A) and of systemic symptoms (panel B) following each of the 5 study injections.
Onset of a reaction was within the first 3 days following a study injection. Reactions were followed to resolution to determine the maximum severity. Systemic reactions included malaise/fatigue, headache, chills, myalgia, arthralgia, nausea and vomiting. Groups include both vaccinees and controls who received empty pox vectors. Data are included from all participants in Part B of the study, plus those from Part A who received the same regimen and dosage as the Part B groups.
Although several participants had additional cardiac evaluations for chest symptoms, minor ECG changes, or elevated CKs, there was no evidence that these events were related to vaccination or that any participant developed subclinical or overt myopericarditis. All participants with an elevated CK reported recent strenuous exercise and the abnormalities resolved promptly.
Immunogenicity
Due to the large number of participants who did not receive their 5th (final) vaccination, this report focuses on immune responses after the first four vaccinations and at the end of the study. Immunogenicity data from all participants in Part A and B of the study who received the same regimens and dosages were combined for this analysis. Overall, the FPV-HIV alone regimen [F/F] was poorly immunogenic and except for HIV-specific CD4+ T-cell and anti-p24 antibody responses after the 3rd vaccination (Tables 3 & 4), the response rates were not appreciably greater than the false positivity rate of the assays (< 3% response in control recipients). In comparison, the M/M and M/F regimens were significantly more immunogenic and thus were examined more extensively. Of note, although the group size was small in the dose escalation phase of the study, the lower dose MVA-HIV priming appeared to have induced cellular immune responses in a similar proportion of study subjects (Table 3) as those who received the 109 pfu dose of MVA-HIV.
Table 3.
HIV-specific T-cell response rates.
| Assay | PBMC Timepointa | Controls | FP Aloneb (95 CI)c | MVA 107 pfu/2mL+ FP (95 CI) | MVA 108 pfu/2mL+ FP (95 CI) | MVA 109 pfu/2mL + FPb (95 CI) | MVA 109 pfu/2mL Aloneb (95 CI) | p-valued |
|---|---|---|---|---|---|---|---|---|
| T-cell Responses: | ||||||||
| ICSe CD4+ T-cells | ||||||||
| Post-2nd | 0/14 = 0.0f (0.0, 21.5) | ND | ND | ND | 14/27 = 51.9 (34.0, 69.3) | 12/28 = 42.9 (26.5, 60.9) | 0.59 | |
| Post-3rd | 0/22 = 0.0 (0.0, 14.9) | 5/28 = 17.9 (7.9, 35.6) | 5/9 = 55.6 (26.7, 81.1) | 7/9 = 77.8 (45.3, 93.7) | 15/27 = 55.6 (37.3, 72.4) | 12/29 = 41.4 (25.5, 59.3) | 0.42 | |
| Post-4th | 0/22 = 0.0 (0.0, 14.9) | 2/30 = 6.7 (1.8, 21.3) | 6/9 = 66.7 (35.4, 87.9) | 5/9 = 55.6 (26.7, 81.1) | 15/27 = 55.6 (37.3, 72.4) | 11/28 = 39.3 (23.6, 57.6) | 0.28 | |
| 13 months | 2/23 = 8.7 (2.4, 26.8) | 1/28 = 3.6 (0.6, 17.7) | 5/8 = 62.5 (30.6, 86.3) | 4/9 = 44.4 (18.9, 73.3) | 7/24 = 29.2 (14.9, 49.2) | 4/28 = 14.3 (5.7, 31.5) | 0.31 | |
| ICSe CD8+ T-cells | ||||||||
| Post-2nd | 0/14 = 0.0 (0.0, 21.5) | ND | ND | ND | 5/30 = 16.7 (7.3, 33.6) | 0/30 = 0.0 (0.0, 11.4) | 0.052 | |
| Post-3rd | 1/22 = 4.5 (0.8, 21.8) | 1/28 = 3.6 (0.6, 17.7) | 2/8 = 25.0 (7.1, 59.1) | 3/9 = 33.3 (12.1, 64.6) | 8/27 = 29.6 (15.9, 48.5) | 4/29 = 13.8 (5.5, 30.6) | 0.20 | |
| Post-4th | 0/22 = 0.0 (0.0, 14.9) | 1/31 = 3.2 (0.6, 16.2) | 4/9 = 44.4 (18.9, 73.3) | 3/9 = 33.3 (12.1, 64.6) | 14/27 = 51.9 (34.0, 69.3) | 6/28 = 21.4 (10.2, 39.5) | 0.026 | |
| 13 months | 1/24 = 4.2 (0.7, 20.2) | 2/31 = 6.5 (1.8, 20.7) | 3/8 = 37.5 (13.7, 69.4) | 3/9 = 33.3 (12.1, 64.6) | 11/26 = 42.3 (25.5, 61.1) | 4/28 = 14.3 (5.7, 31.5) | 0.033 | |
| IFN-γ ELISpot | ||||||||
| Post-2nd | 0/16 = 0.0 (0.0, 19.4) | ND | ND | 2/10 = 20.0 (5.7, 51.0) | 12/29 = 41.4 (25.5, 59.3) | 16/33 = 48.5 (32.5, 64.8) | 0.62 | |
| Post-4th | 0/22 = 0.0 (0.0, 14.9) | 2/31 = 6.5 (1.8, 20.7) | 6/9 = 66.7 (35.4, 87.9)( | 8/9 = 88.9 (56.5, 98.0) | 17/26 = 65.4 (46.2, 80.6) | 13/28 = 46.4 (29.5, 64.2) | 0.18 |
Note. ND = assay not performed for the group
Denotes timepoint of 2 weeks after the indicated vaccination.
Includes participants from study Parts A & B who received the same regimen and dose of vaccine.
95% confidence intervals are calculated using the score test method [36].
P-values are from Fisher’s exact tests.
ICS response as indicated by secretion of IL-2 and/or IFN-γ to Env, Gag, Pol and Nef peptide pools.
Percent positive
Table 4.
HIV-binding antibody response rates.
| Assay | PBMC timepointa | Controls | FP Aloneb (95 CI)c | MVA 107 pfu/2mL+ FP (95 CI) | MVA 108 pfu/2mL + FP (95 CI) | MVA 109 pfu/2mL + FPb (95 CI) | MVA 109 pfu/2mL Aloneb (95 CI) | p-valued |
|---|---|---|---|---|---|---|---|---|
| Binding antibody: | ||||||||
| p24 | Baseline | 1/25 = 4.0e (0.7, 19.5) | 1/35 = 2.9 (0.5, 14.5) | 1/10 = 10.0 (0.0, 40.4) | 0/10 = 0.0 (0.0, 27.8) | 3/35 = 8.6 (3.0, 22.4) | 1/35 = 2.9 (0.5, 14.5) | |
| Post-3rd | 1/23 = 4.3 (0.8, 21.0) | 4/28 = 14.3 (5.7, 31.5) | 1/9 = 11.1 (2.0, 43.5) | 1/9 = 11.1 (2.0, 43.5) | 11/30 = 36.7 (21.9, 54.5) | 6/30 = 20.0 (9.5, 37.3) | 0.25 | |
| Post-4th | ND | 0/30 = 0.0 (0.0, 11.4) | 0/9 = 0.0 (0.0, 29.9) | 0/9 = 0.0 (0.0, 29.9) | 11/29 = 37.9 (22.7, 56.0) | 6/29 = 20.7 (9.8, 38.4) | 0.25 | |
| 13 months | ND | 0/31 = 0.0 (0.0,11.0) | 0/8 = 0.0 (0.0, 32.4) | 0/9 = 0.0 (0.0, 29.9) | 1/27 = 3.7 (0.7, 18.3) | 1/29 = 3.4 (0.6, 17.2) | 1.00 | |
| gp120 | Baseline | 0/25 = 0.0 (0.0, 13.3) | 0/35 = 0.0 (0.0, 9.9) | 0/10 = 0.0 (0.0, 27.8) | 0/10 = 0.0 (0.0, 27.8) | 0/35 = 0.0 (0.0, 9.9) | 0/35 = 0.0 (0.0, 9.9) | |
| Post-3rd | 0/23 = 0.0 (0.0, 14.3) | 0/28 = 0.0 (0.0, 12.1) | 1/9 = 11.1 (2.0, 43.5) | 2/9 = 22.2 (6.3, 54.7) | 5/30 = 16.7 (7.3, 33.6) | 20/30 = 66.7 (48.8, 80.8) | < 0.001 | |
| Post-4th | ND | 0/30 = 0.0 (0.0, 11.4) | 2/9 = 22.2 (6.3, 54.7) | 1/9 = 11.1 (2.0, 43.5) | 3/29 = 10.3 (3.6, 26.4) | 20/29 = 69.0 (50.8, 82.7) | < 0.001 | |
| 13 months | ND | 0/31 = 0.0 (0.0, 11.0) | 0/8 = 0.0 (0.0, 32.4) | 0/9 = 0.0 (0.0, 29.9) | 0/28 = 0.0 (0.0, 12.1) | 0/29 = 0.0 (0.0, 11.7) | 1.00 |
Note. ND = assay not performed for the group
Denotes time-point of 2 weeks after the indicated vaccination.
Includes participants from both study Parts A & B who received the same regimen and dose of vaccine.
95% confidence intervals are calculated using the score test method [36].
P-values are from Fisher’s exact tests.
Percent positive
Intracellular cytokine staining assay: CD4+ and CD8+ T-cell responses
Effect of boosting
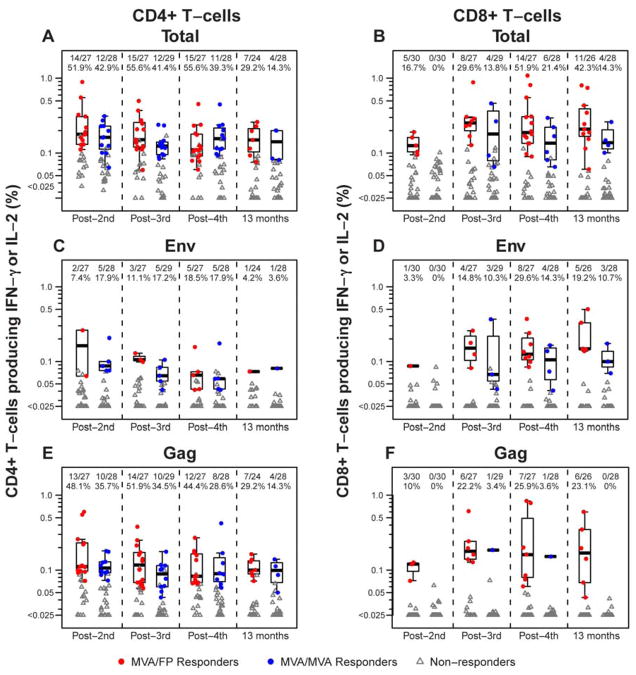
Participants in the M/F and M/M groups received the same study preparations at the first 2 vaccination timepoints and consequently HIV-specific CD4+ T-cell responses were similar (Table 3); overall, assays were positive in 26 of 55 subjects (47.3%, combining the groups; confidence intervals overlap in the range of 7.3–11.4%) and for responders the median magnitude was between 0.1–0.2 % of CD4+ T-cells producing IFN-γ and/or IL-2 (Figure 2A). The 3rd and 4th vaccinations did not significantly increase the frequency of CD4+ T-cell responses in either group, nor were there significant differences between them. For both groups, responses were diminished at the final study visit compared to the post-4th vaccination timepoint (29.2% [p = 0.03] and 14.3% [p = 0.02] in the M/F and M/M groups, respectively).
Figure 2. Ex-vivo HIV-specific CD4+ and CD8+ T-cells induced by heterologous vector (M/F) and homologous vector (M/M) regimens.
The percentage of CD4+ (panels A, C and E) and CD8+ (panels B, D and F) T-cells producing γ-interferon (IFN- γ) and/or interleukin-2 (IL-2) in response to Env, Gag, Pol and/or Nef peptide pools (panels A and B), Env peptide pools alone (panels C and D) or Gag peptide pools alone (panels E and F) 2 weeks after the indicated immunization and at the final study visit as measured by intracellular staining assay. Responders are shown in colored circles (red = M/F; blue = M/M; groups include all participants in Part B of the study, plus those from Part A who received the same regimen and dosage as the Part B groups) and non-responders in gray triangles. The boxplots show the distribution of responses in positive responders only. The box indicates the median and interquartile range (IQR); whiskers extend to the furthest point within 1.5 times the IQR from the upper or lower quartile. Numbers at the top of each panel show the number of responders / number with assay result and the percent with positive response. Pol and Nef are not shown due to infrequent responses.
Unlike CD4+ T-cell responses, HIV-specific CD8+ T-cell responses in the M/F group increased with subsequent vaccinations (16.7% post 2nd vs 51.9% post 4th vaccinations; p=0.03; Table 3). Significantly more M/F participants had CD8+ responses as compared to the M/M group after the 4th vaccination (51.9% vs. 21.4%, respectively; p=0.026; Table 3). This difference was found to be persistent at the final visit (42.3% vs. 14.3%, respectively; p=0.033). For the M/F group the CD8+ T-cell magnitudes for responders tended to be slightly higher than the CD4+ magnitudes, with medians ranging from 0.19–0.25% of CD8+ T-cells producing IFN-γ and/or IL-2 (Figure 2B).
Concurrent HIV-specific CD4+ and CD8+ T-cell responses in an individual vaccine recipient were seen more frequently among the M/F group. Such responses were detected in 7 of 16 (43.8%), 8 of 21 (38.1%) and 5 of 13 (38.5%) of vaccinees in this group after the 3rd and 4th vaccinations and at the final study visit, respectively, whereas they were present in only 1 of 15 (6.7%), 3 of 14 (21.4%) and 0 of 8 subjects in the M/M groups at the corresponding time points. However, only the difference post 3rd vaccination was statistically significant (p=0.04). No differences in CD4+ or CD8+ response rates or magnitudes were observed between men and women for either group or combining across groups post 2nd vaccination (data not shown).
Responses to individual gene products
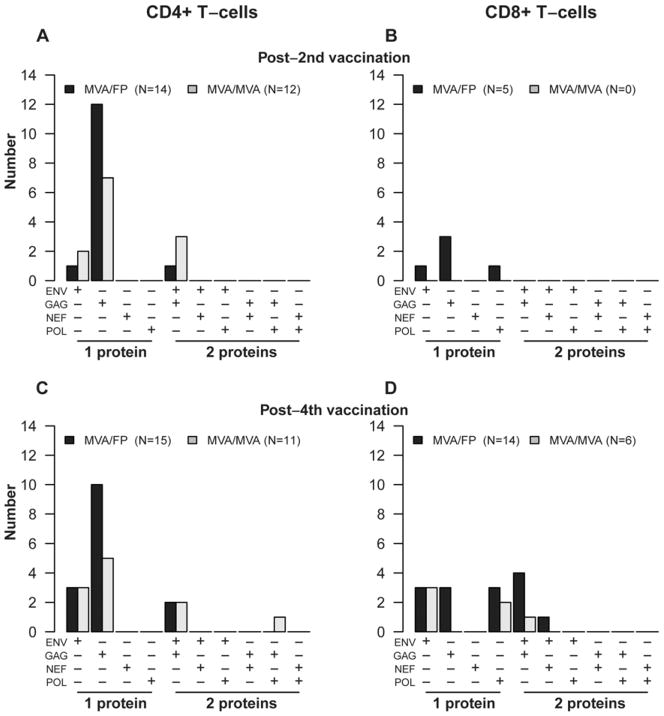
HIV-specific CD4+ and CD8+ T-cell responses in all groups were targeted primarily at Gag and Env peptide pools (Figure 2C–F), but rarely to Pol and Nef pools. CD4+ T-cell responses were more frequently directed at Gag than Env peptides at all timepoints in both groups (Figure 2C and E). These differences were statistically significant for the M/F group after the 2nd (p=0.003) and 3rd (p= 0.003) vaccinations as well as at the final study visit (p= 0.03). In contrast, CD8+ T-cell responses were more evenly distributed to Gag and Env among the M/F group, but directed almost entirely at Env among the M/M group (Figure 2D and F). The median magnitude of CD4+ T-cell response to Gag and Env were relatively modest at 0.06 – 0.16% of CD4+ T-cells producing IFN-γ and/or IL-2 in both groups, as was that for CD8+ T-cell responses, which was in the range of 0.07 –0.18% of CD8+ T-cells producing IFN-γ and/or IL-2. In addition, CD4+ and CD8+ T-cell responses tended to be directed toward a single HIV gene product in both groups (Figure 3).
Figure 3. HIV protein-specific CD4+ and CD8+ T-cell responses induced by heterologous vector (M/F) and homologous vector (M/M) regimens.
Number of study participants with HIV gene-specific responses among CD4+ T-cells at 2 weeks post 2nd vaccination (panel A); CD8+ T-cells at 2 weeks post 2nd vaccination (panel B), CD4+ T-cells at 2 weeks post 4th vaccination (panel C), and CD8+ T-cells at 2 weeks post 4th vaccination (panel D).as measured by intracellular staining assay. Groups include all participants in Part B of the study, plus those from Part A who received the same regimen and dosage as the Part B groups.
No participants had positive responses to >2 proteins at these timepoints.
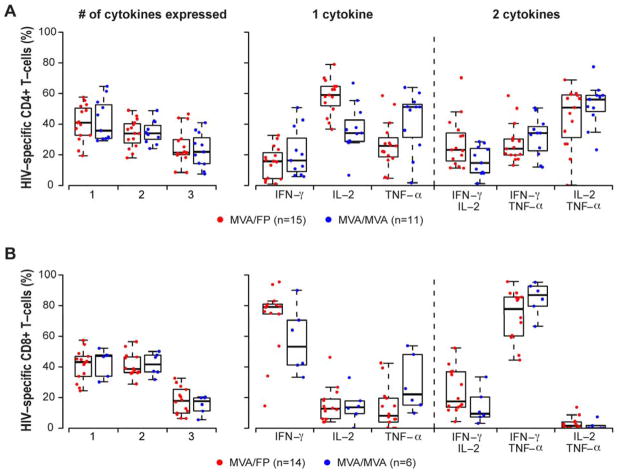
Polyfunctional responses after the 4th vaccination
A similar proportion of post-4th vaccination PBMCs from responders in the M/F and M/M groups were shown to be secreting one, two or three cytokines when exposed to HIV peptides in the ICS assay (Figure 4). For CD4+ T-cell responders, the median percentages of cells secreting one or two cytokines were 34–41% while the medians were 21–22% for cells secreting all three. Single responses more frequently consisted of IL-2 among the M/F group (p <0.001) and trended towards TNF-α in the M/M group (p = 0.06), whereas dual responses most often consisted of IL-2 + TNF-α in both groups. Alternatively, for CD8+ T-cells, median percentages were 39–47% of cells secreting one or two cytokines while medians were 18% for cells secreting all three; single cytokine responses were most frequently IFN-γ in both groups while dual responses tended to consist of the combination of IFN-γ and TNF-α.
Figure 4. HIV-specific CD4+ and CD8+ T-cells producing multiple cytokines induced by heterologous vector (M/F) and homologous vector (M/M) regimens at 2 weeks post 4th vaccination.
For each panel, the left graph shows the percentage of the HIV-specific CD4+ (panel A) or CD8+ (panel B) T-cells (red = MVA/FP; blue = MVA/MVA; groups include all participants in Part B of the study, plus those from Part A who received the same regimen and dosage as the Part B groups) that are producing one, two or three cytokines for positive responders detected by intracellular staining assay. The right graph depicts the γ-interferon (IFN-γ), interleukin-2 (IL-2) or tumor necrosis factor-α (TNF-α) in those cells producing one cytokine (1st panel on right), and the percentage of cells co-producing cytokine pairs in those producing two cytokines (2nd panel on the right). The boxplots indicate the median and IQR; whiskers extend to the furthest point within 1.5 times the IQR from the upper or lower quartile.
Interferon-γ ELISpot responses
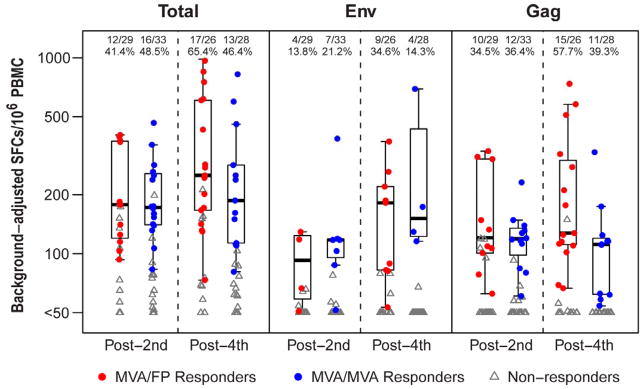
IFN-γ ELISpot responses were detected in PBMCs from 45% of subjects from the M/F and M/M groups combined after the 2nd MVA-HIV vaccination and were boosted to 65% of subjects in the M/F group after the 4th vaccination (Figure 5). Median spot forming cells (SFC)/106 PBMCs were in the 170–250 range in both groups at both timepoints. As in the ICS assay, IFN-γ ELISpot responses primarily targeted Gag and Env peptide pools, and most frequently targeted Gag peptides after the heterologous vector boost.
Figure 5. HIV-specific Interferon-γ ELISpot assay response rates and magnitude of background-adjusted spot forming cells (SFCs) per 106 PBMC induced by heterologous vector (M/F) and homologous vector (M/M) regimens.
Total is the response to Env, Gag, Pol and/or Nef peptide pools. Responders are shown in colored circles (red = M/F; blue = M/M; groups include all participants in Part B of the study, plus those from Part A who received the same regimen and dosage as the Part B groups) and non-responders in gray triangles. The boxplots show the distribution of responses in positive responders only. The box indicates the median and interquartile range (IQR); whiskers extend to the furthest point within 1.5 times the IQR from the upper or lower quartile. Numbers at the top of each panel show the number of responders / number with assay result and the percent with positive response. Pol and Nef are not shown due to infrequent responses.
Binding and neutralizing antibody responses
Anti-Gag binding antibody responses were detected in the sera from 11 of 30 (36.7%) and 6 of 30 (20%) of participants 2 weeks after the third immunization in the M/F and M/M groups, respectively. However, anti-gp120 responses were detected in 66.7% (20/30) of sera from the M/M group at that timepoint, which was significantly greater than the corresponding anti-gp120 responses among the M/F group (5/30, 16.7%; p<0.001; Table 4). In all cases, the antibodies were short-lived and rarely detectable at the final study visit. Neutralizing antibody responses were detected in only 6 study participants distributed among the study groups (one received FPV-HIV alone, one received MVA-HIV alone and 4 received the heterologous boost) and were of low titer (range 26–101).
Discussion
This report indicates that the MVA-HIV and FPV-HIV candidate vaccines were well tolerated when administered in both homologous and heterologous vector regimens, and heterologous boosting was superior at inducing a balanced HIV-specific CD4+ and CD8+ T-cell response. FPV-HIV was poorly immunogenic when given alone, but significantly boosted CD8+ T-cell responses in participants primed with two doses of MVA-HIV. Overall, the heterologous regimen induced HIV-specific CD4+ and CD8+ T-cell responses in approximately half of the participants after the second boost, and concurrent CD4+ and CD8+ T-cell responses detected in 38% of responders. CD4+ T-cell responses tended to be directed at Gag, while CD8+ T-cell responses were more evenly directed at Gag and Env, but both tended to target a single HIV protein at a time. Although the bulk IFN-γ ELISpot assays do not distinguish CD4+ from CD8+ T-cell phenotypes, it confirmed the ICS findings that the heterologous vector regimen induced better HIV-specific cellular responses than the homologous regimens. In contrast to studies of another poxvirus vector, NYVAC-HIV-C [38], we observed no gender differences in T-cell response rate or magnitude. It is unclear why the non-structural HIV proteins were so poorly immunogenic in our study, however, we were not able to perform a thorough assessment because only Pol and Nef peptide pools were available for use in the in-vitro assays. In addition, only responses to the HIV reverse transcriptase (RT) portion of Pol could be expected as only that part of Pol was contained in the vaccine constructs.
While the main focus of this study was to assess the tolerability of escalating doses of MVA-HIV and the cellular immunogenicity of these poxvirus-vectored HIV vaccine regimens, we also conducted a limited assessment of HIV-specific humoral immunogenicity. Interestingly, we saw that gp120 binding antibody responses were better in the group that received the homologous vector boosting regimen. However, binding assays were not performed after the priming vaccinations alone, so it is difficult to make firm conclusions about the contribution of the later boosts, aside from the fact that while the FPV-HIV preparation was able to boost CD8+ T-cell responses directed at Env peptide pools, it was not capable of doing so for gp120 binding antibodies.
A number of other studies have been conducted with poxvirus-vectored vaccine regimens in the HIV, malaria and tuberculosis fields, including the heterologous MVA and FPV vector combination [39–47] and the same poxviral-vectored products in HIV-infected adolescents [16]. However, the majority of these studies employed bulk IFN-γ ELISpot as the primary cellular immunogenicity readout and ICS and/or CD4+/CD8+ T-cell depleted INF-γ ELISpot assays were performed only for small subgroups. In addition, it is difficult to compare our results to those from HIV immunotherapy studies as their subjects had already been primed through natural infection. This existing literature does suggest that MVA-vectored constructs induce primarily a CD4+ T-cell response, but the optimal number of priming vaccinations and the precise contribution of a heterologous vector boost has not been clearly delineated. It is unknown why MVA vector regimens tend to induce stronger HIV-specific CD4+ T-cell responses to HIV inserts while replication-defective adenovirus vectors induce stronger CD8+ T-cell responses, but the heterologous pox-vector boost does appear to provide additional antigenic exposure to boost the CD8+ T-cell response. Of note, Precopio et al. demonstrated vigorous anti-vector CD8+ T-cell responses by ICS after three, but not two, intramuscular injections of the same MVA (Therion Biologics), given on the same 0, 1 and 3 month schedule [48]. This suggests that in our study, strong vector-specific cellular immune responses induced by homologous vector boosting may have out-competed the insert-specific response and suppressed the boosting effect of additional vaccinations. It would be of interest to determine if removal of immunodominant epitopes in MVA can lead to improvement of insert-specific cellular responses.
The lack of correlates of protection from HIV infection and/or disease continues to complicate development of an efficacious HIV-1 vaccine. Nevertheless, clues may be inferred from challenge studies of analogous vaccines in the simian immunodeficiency virus (SIV) model and the results of efficacy trials in humans. A study of Therion’s MVA and FPV vectors with similarly engineered SIV inserts showed an increase in the magnitude of SIV-specific IFN-γ ELISpot responses after FPV-SIV boosting of macaques that received a heterologous regimen as compared to a homologous MVA-SIV regimen [49]. Particularly instructive are the findings of the phase IIB study of the adenovirus-serotype 5 (Ad5)-HIV-gag/pol/nef candidate vaccine developed by Merck conducted in the Americas and the Phase III study of a combination regimen of canarypox-HIV-gag/protease/env vaccine (vCP1521) boosted by recombinant HIV glycoprotein 120 subunit vaccine (AIDSVAX B/E) in Thailand (Protocol RV144). The Ad5-HIV vaccine induced relatively strong HIV-specific CD8+ T-cell responses in most study participants but modest HIV-specific CD4+ T-cell and antibody responses, and failed to protect participants from infection or reduce setpoint HIV RNA levels [29, 50]. Alternatively, the canarypox-HIV plus rgp120 regimen induced modest HIV-specific CD8+ and CD4+ T-cell responses, but better T-cell help as indicated by lymphoproliferation, and binding antibody responses (all directed primarily against env) and had an efficacy of 31% against HIV infection [13]. Although far from definitive, the results from the study in Thailand suggest that a balanced immune response including HIV-specific CD4+ T-cell and antibody responses and a more modest CD8+ T-cell response may be important characteristics of a successful vaccination strategy. In our study, CD4+ T-cells secreting IL-2 alone were seen more frequently in the M/F group, possibly indicating a T helper response similar to that seen in RV 144 [51, 52].
In conclusion, while it is unknown if the immune responses induced by the heterologous pox-vector regimen described here may recapitulate some of the important protective aspects of the canarypox-HIV plus rgp120 regimen, this regimen can augment both CD4+ and CD8+ T-cell immune responses that may be important components of an effective preventive HIV vaccine and should be considered for additional larger scale testing.
Acknowledgments
We thank the study participants for their time and effort, the teams of dedicated individuals at the study sites as well as members of the HVTN Laboratory Program, SCHARP, and Core staff who contributed to the study implementation and analysis. In addition, the following individuals deserve personal recognition:
UR: Nega Goji, Catherine Bunce, Carrie Dykes, Mhorag Hay
FHCRC: Chris Galloway, David Berger, Sara Mostad
UAB: Scott Parker
SLU: Geoff Gorse, Robert Belshe, Bernard Chaitman, Heidi Israel, Gwen Pendleton
Sao Paulo: Sirlene Caminada, Jose Valdez Ramalho Madruga, Gabriela Calazans, Esper Kallas
Rio: Mauro Schechter, Regina Ferro do Lago, Praca Onze Study Team
Laboratory Program: Kent Weinhold, John Hural, Don Carter
SCHARP: Gina Escamilla, Liza Noonan, Sara Jasinski
DAIDS: Alan Fix, Jorge Flores, Chris Butler
Therion: Mary Lou Horzempa, Linda Gritz, Alicia Gomez Yafal, Patricia Greenhalgh, Kelledy Manson
Also, technical assistance in the preparation of the manuscript was provided by Patricia Bedford and Rachel Tompa.
2.) Financial support: Division of AIDS, National Institutes of Allergy and Infectious Diseases, National Institutes of Health (grants 1U01 AI046747, 1U01 AI068614, 1U01 AI046703, 1U01 AI068635, 1U01 AI046725, 1U01 AI068618, 1U01 AI46725, 1U01 AI AI047980, 1U01 AI069511, 1U01 AI047996; 1U01 AI069452, 1U01 AI048021, 1U01 AI046747, 1U01 AI 068614, 1U01 AI069414, 1U01 AI069420, AI026507). This publication was also made possible by Grant Number UL1 RR 024160 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Footnotes
Potential conflicts of interest statement: none reported
Previous presentation
Presented in part at “AIDS Vaccine 2007”, Seattle, WA, 20-23 August 2007.
Trial registration. Clinicaltrials.gov identifier NCT00083603.
References
- 1.Fauci AS, Johnston MI, Dieffenbach CW, Burton DR, Hammer SM, Hoxie JA, et al. HIV vaccine research: the way forward. Science. 2008;321:530–2. doi: 10.1126/science.1161000. [DOI] [PubMed] [Google Scholar]
- 2.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–4. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 3.Letvin NL. Moving forward in HIV vaccine development. Science. 2009;326:1196–8. doi: 10.1126/science.1183278. [DOI] [PubMed] [Google Scholar]
- 4.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–10. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–6. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 7.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 9.Rowland-Jones SL, Nixon DF, Gotch F, McMichael A, Kroll JS, Hallam N, et al. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–1. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Jin X. HIV vaccine could use a little help. Human Vaccines. 2010;6:1–6. doi: 10.4161/hv.6.6.11285. [DOI] [PubMed] [Google Scholar]
- 11.Barouch DH. Challenges in the development of an HIV-1 vaccine. Nature. 2008;455:613–9. doi: 10.1038/nature07352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14:617–21. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 14.Santra S, Liao HX, Zhang R, Muldoon M, Watson S, Fischer W, et al. Mosaic vaccines elicit CD8(+) T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med. 2010;16:324–8. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barouch DH, O’Brien KL, Simmons NL, King SL, Abbink P, Maxfield LF, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010;16:319–23. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenough TC, Cunningham CK, Muresan P, McManus M, Persaud D, Fenton T, Barker P, et al. Safety and immunogenicity of recombinant poxvirus HIV-1 vaccines in young adults on highly active antiretroviral therapy. Vaccine. 2008;26:6883–6893. doi: 10.1016/j.vaccine.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pikora CA, Sullivan JL, Panicali D, Luzuriaga K. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J Exp Med. 1997;185:1153–61. doi: 10.1084/jem.185.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice AP, Carlotti F. Structural analysis of wild-type and mutant human immunodeficiency virus type 1 tat proteins. J Virol. 1990;64:6018–26. doi: 10.1128/jvi.64.12.6018-6026.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malim MH, McCarn DF, Tiley LS, Cullen BR. Mutational definition of the human immunodeficiency virus type 1 rev activation domain. J Virol. 1991;65:4248–4254. doi: 10.1128/jvi.65.8.4248-4254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg ME, Iafrate AJ, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 nef regulate trafficking of class I MHC complexes. EMBO Journal. 1998;17:2777–89. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowe DM, Parmar V, Kemp SD, Larder BA. Mutational analysis of two conserved sequence motifs in HIV-1 reverse transcriptase. FEBS Lett. 1991;282:231–4. doi: 10.1016/0014-5793(91)80484-k. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Cardiac adverse events following smallpox vaccination---United States, 2003. MMWR Morb Mort Wkly Rep. 2003;52:248–50. [PubMed] [Google Scholar]
- 23.Eckart RE, Love SS, Atwood JE, Arness MK, Cassimatis DC, Campbell CL, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44:201–5. doi: 10.1016/j.jacc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, et al. Overall survival analysis of a phase II randomized controlled trial of a poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Bruyn G, Rossini AJ, Chiu Y-L, Holman D, Elizaga ML, Frey SE, et al. Safety profile of recombinant canarypox HIV vaccines. Vaccine. 2004;22:704–13. doi: 10.1016/j.vaccine.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 26.DAIDS Regulatory Compliance Center; [Accessed 11 March 2010]. Division of AIDS table for grading the severity of adult and pediatric adverse events, version 1.0, December 2004; clarification August 2009. Website. http://rcc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GradingTable_Clarification_August2009_Final.doc. [Google Scholar]
- 27.Bull M, Lee D, Stucky JA, Chiu YL, Rubin A, Horton H, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322:57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horton H, Thomas EP, Stucky JA, Frank I, Moodie Z, Huang Y, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T-cells induced by vaccination. J Immunol Methods. 2007;323:39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McElrath MJ, DeRosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li F, Malhotra U, Gilbert PB, Hawkins NR, Duerr AC, McElrath JM, et al. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine. 2006;24:6893–6904. doi: 10.1016/j.vaccine.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Russell ND, Graham BS, Keefer MC, McElrath MJ, Self SG, Weinhold KJ, Montefiori DC, et al. Phase 2 study of an HIV-1 canarypox vaccine (vCP1452) alone and in combination with rgp120: negative results fail to trigger a phase 3 correlates trial. J Acquir Immune Defic Syndr. 2007;44:203–12. doi: 10.1097/01.qai.0000248356.48501.ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moodie Z, Price L, Gouttefangeas C, Mander A, Janetzki S, Löwer M, Welters MJ, et al. Response definition criteria for ELISPOT assays revisited. Cancer Immunol Immunother. 2010;59:1489–1501. doi: 10.1007/s00262-010-0875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goepfert PA, Tomaras G, Horton H, Montefiori D, Ferrari G, Deers M, et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25:510–8. doi: 10.1016/j.vaccine.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 34.Polonis VR, Brown BK, Rosa-Borges A, Zolla-Pazner S, Dimitrov DS, Zhang MY, et al. Recent advances in the characterization of HIV-1 neutralization assays for standard evaluation of the antibody response to infection and vaccination. Virology. 2008;375:315–20. doi: 10.1016/j.virol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Mascola JR, D’Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, et al. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol. 2005;79:10103–7. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. The American Statistician. 1998;52:119–26. [Google Scholar]
- 37.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 38.McCormack S, Stohr W, Barber T, Bart P-A, Harari A, Moog C, et al. EV02: A phase I trial to compare the safety and immunogenicity of HIV DNA-C prime-NYVAC-C boost to NYVAC-C alone. Vaccine. 2008;26:3162–74. doi: 10.1016/j.vaccine.2008.02.072. [DOI] [PubMed] [Google Scholar]
- 39.Dorrell L, Yang H, Ondondo B, Dong T, di Gleria K, Suttill A, Conlon C, et al. Expansion and diversification of virus-specific T cells following immunization of human immunodeficiency virus type 1 (HIV-1)-infected individuals with a recombinant modified vaccinia virus Ankara/HIV-1 Gag vaccine. J Virol. 2006;80:4705–16. doi: 10.1128/JVI.80.10.4705-4716.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harari A, Bart P-A, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205:63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, Nanvubya A, Bashir F, et al. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine. 2008;26:2788–95. doi: 10.1016/j.vaccine.2008.02.071. [DOI] [PubMed] [Google Scholar]
- 42.Ramanathan VD, Kumar M, Mahalingam J, Sathyamoorthy P, Narayanan PR, Solomon S, Panicali D, et al. A phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type-1 subtype C modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS Res Hum Retroviruses. 2009;25:1107–16. doi: 10.1089/aid.2009.0096. [DOI] [PubMed] [Google Scholar]
- 43.Vasan S, Schlesinger SJ, Chen Z, Hurley A, Lombardo A, Than S, Adesanya P, et al. Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B′/C candidate vaccine. PLoS ONE. 2010;5:e8816. doi: 10.1371/journal.pone.0008816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicol MP, Grobler LA. MVA-85A, a novel candidate booster vaccine for the prevention of tuberculosis in children and adults. Curr Opin Mol Ther. 2010;12:24–34. [PubMed] [Google Scholar]
- 45.Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, Hill AVS. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–55. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 46.Bejon P, Mwacharo J, Kai OK, Todryk S, Keating S, Land T, Gilbert SC, et al. Immunogenicity of the candidate malaria vaccines FP9 and modified vaccinia Ankara encoding the pre-erythrocytic antigen ME-TRAP in 1–6 year old children in a malaria endemic area. Vaccine. 2006;24:4709–15. doi: 10.1016/j.vaccine.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Bejon P, Kai OK, Mwacharo J, Keating S, Lang T, Gilbert SC, Peshu N, et al. Alternating vector immunizations encoding pre-erythrocytic malaria antigens enhance memory responses in a malaria endemic area. Eur J Immunol. 2006;36:2264–72. doi: 10.1002/eji.200636187. [DOI] [PubMed] [Google Scholar]
- 48.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ responses. J Exp Med. 2007;204:1405–16. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santra S, Sun Y, Parvani JG, Philippon V, Wyand MS, Manson K, et al. Heterologous prime/boost immunization of rhesus monkeys by using diverse poxvirus vectors. J Virol. 2007;81:8563–70. doi: 10.1128/JVI.00744-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganusov VV, Milutinovic D, DeBoer RJ. IL-2 regulated expansion of CD4+ T-cell populations by affecting cell death: insights from modeling CFSE data. J Immunol. 2007;179:950–7. doi: 10.4049/jimmunol.179.2.950. [DOI] [PubMed] [Google Scholar]
- 52.Deenick EK, Gett AV, Hodgkin PD. Stochastic model of T-cell proliferation: a calculus revealing IL-2 regulation of precursor frequencies, cell cycle time and survival. J Immunol. 2003;170:4963–72. doi: 10.4049/jimmunol.170.10.4963. [DOI] [PubMed] [Google Scholar]