Abstract
There is an urgent need to develop methods that lower costs of using recombinant human bone morphogenetic proteins (BMPs) to promote bone induction. In this study, we demonstrate the osteogenic effect of a low-molecular weight compound, SVAK-12, that potentiated the effects of BMP-2 in inducing transdifferentiation of C2C12 myoblasts into the osteoblastic phenotype. Here, we report a specific compound, SVAK-12, which was selected based on in silico screenings of small-molecule databases using the homology modeled interaction motif of Smurf1-WW2 domain. The enhancement of BMP-2 activity by SVAK-12 was characterized by evaluating a BMP-specific reporter activity and by monitoring the BMP-2-induced expression of mRNA for osteocalcin and alkaline phosphatase (ALP), which are widely accepted marker genes of osteoblast differentiation. Finally, we confirmed these results by also measuring the enhancement of BMP-2-induced activity of ALP. Smurf1 is an E3 ligase that targets osteogenic Smads for ubiquitin-mediated proteasomal degradation. Smurf1 is an interesting potential target to enhance bone formation based on the positive effects on bone of proteins that block Smurf1-binding to Smad targets or in Smurf1−/− knockout mice. Since Smads bind Smurf1 via its WW2 domain, we performed in silico screening to identify compounds that might interact with the Smurf1-WW2 domain. We recently reported the activity of a compound, SVAK-3. However, SVAK-3, while exhibiting BMP-potentiating activity, was not stable and thus warranted a new search for a more stable and efficacious compound among a selected group of candidates. In addition to being more stable, SVAK-12 exhibited a dose-dependent activity in inducing osteoblastic differentiation of myoblastic C2C12 cells even when multiple markers of the osteoblastic phenotype were parallelly monitored.
Keywords: BMP-2, Smad, Smurf1, Virtual screening, Potentiating
Introduction
The process of cell differentiation is regulated by several cytokines. Among the osteogenic proteins that have been reported, the bone morphogenetic protein (BMP) family members are the most notable cytokines for their unique ability to promote differentiation of mesenchymal stem cells and pre-osteoblastic cells into osteoblasts in vitro and also to induce ectopic bone formation in vivo [1]. Recombinant human BMP-2 was approved by the FDA in 2002 and is being used clinically as a bone graft substitute to achieve spinal fusion and fracture repair. However, the requirement for a 15,000-fold higher concentration in humans (1.5 mg/ml) than in cell culture (100 ng/ml) creates a huge translational barrier for routine clinical use due to high cost [2–6]. In this study, we describe a compound that exhibits a strong potentiating activity on BMP-2.
Smurf1 disruption is a potential therapeutic strategy to increase BMP responsiveness. Interest in Smurf1 as a target is supported by the observation that Smurf1−/− mice exhibit an age-dependent increase in bone mass [7]. Smurf1 transgenic mice showed decreased bone volume and a reduced bone formation rate [8]. LIM Mineralization Protein-1 (LMP-1) can enhance BMP-2 activity by increasing cellular responsiveness to BMP-2 [9, 10]. LMP-1 exerts at least a part of this effect by interacting with the E3 ligase, Smurf1, preventing binding and degradation of Smads 1, 5, and 8 [9]. We modeled the binding domains of Smurf1 and its Smad target peptides in a recent report [11–13]. Our overall goal is to translate our knowledge of the modeled Smurf1-WW2 domain structure into therapeutic applications for clinical bone induction. Unlike recombinantly prepared proteins or synthetic peptides, the use of drug-like, low-molecular weight compounds has several advantages, including blocking all the activity mediated by the Smurf1-WW2 domain, better cell permeability, and better in vivo stability and bioavailability. In this report, compounds selected by virtual screening are evaluated for their osteogenic activity for their potential use as drugs that promote BMP-2 activity.
In this study, we focus on identifying a low-molecular weight compound as a reagent that potentiates BMP-2 activity from a set of lead compounds that were previously selected based on computational homology modeling [11, 12], docking, and in silico screening of commercially available small molecular compounds. The selected compounds were predicted to possess features similar to the PPXY motif of Smads that interact with Smurf1, as determined by Ludi docking scores. The BMP-potentiating activities of the compounds were evaluated by monitoring several markers of the osteoblastic phenotype corresponding to various time points during phenotype differentiation of C2C12 cells toward terminally differentiated osteoblasts. We used this phenotypically stable cultured cell line as an in vitro model system to study inducible effects, in which the direct change is differentiation. The extremely low cost of SVAK-12 and its high stability and availability make it appealing for use in bone regenerative medicine. Our findings serve as a basis to formulate new therapeutic approaches, in which the use of BMP is more affordable.
Materials and methods
Cell culture
Mouse C2C12 cells and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from ATCC (Manassas, VA). The non-heat inactivated fetal bovine serum (FBS) was purchased from HyClone Laboratories, Inc. (Logan, UT). The C2C12 cells at passages 5–10 were subcultured in T-75 cm2 flasks in DMEM supplemented with 10% FBS at 37°C in 5% CO2 with humidification. When the flasks reached 80% confluence, the cells were trypsinized and seeded in triplicate at 200,000 cells/well in a 6-well plate for quantitative real-time RT-PCR and alkaline phosphatase (ALP) assays or at 50,000 cells/well in a 12-well plate for the dual-luciferase reporter assay.
In silico screening of small molecular databases
We used the chemical database (chemicals available for purchase, CAP) (Accelrys, Inc.) for compound screenings. We accessed 70,000 molecules in the database supplied by Accelrys Inc. The WW2-domain structure of Smurf1 served as a model for receptor-based in silico screenings. The screening was performed using the program LUDI, de novo receptor docking procedure. LUDI, which can be considered as a hybrid of a complete pharmacophore and 3D modeling tool, is a method for the de novo design and positioning of ligands for proteins [12, 14]. The LUDI score is a sum of five contributions: ideal hydrogen bonds, perturbed ionic interactions (interaction of donor/acceptor in the receptor, e.g., COO–, or NH3+), lipophilic interactions, the freezing of internal degrees of freedom of the ligand, and the loss of translational and rotational entropy of the ligand. In receptor mode, LUDI searches a fragment library for complementary small molecules that best bind the defined interaction sites. During the “search and fit” the LUDI program also determines the energy estimates, or scores, for each conformation of the selected fragments in the library.
Each molecule in our databases with a molecular weight between 200 and 700 was docked into the targeted binding site in the WW2 domain of Smurf1. Based on the binding models of these compounds predicted by LUDI, the top 10% of the high-scoring compounds from the CAP database were extracted. This output was further refined by excluding redundant analogs of the same scaffold. Of the best-scored 300 compounds selected, samples of 54 compounds were obtained from different vendors.
RNA extraction and reverse transcription
The C2C12 cells were plated at a density of 200,000 cells/well in 6-well plates and grown overnight in DMEM containing 10% FBS. On day 2, the culture medium was replaced with DMEM containing 2% FBS and the cells were treated with various concentrations of selected compound (diluted from 10 mg/ml stock solutions prepared in DMSO) for 24 h. In control cultures, a DMSO solvent concentration of 0.01% (v/v) was applied. On day 3, medium was replaced with fresh DMEM containing 2% FBS and the cells were treated with BMP-2 for 24 h.
Total RNA was harvested using the RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). The harvested RNA was digested with RNase-free DNase I (Qiagen, Valencia, CA) to remove DNA contamination. The concentration of the isolated RNA was determined by measuring the absorbance at 260 nm wavelength with a spectrophotometer (Model DU 640, Beckman Coulter, Inc. Brea, CA). The ratio of A260/A280 was between 1.6 and 1.8. Reverse transcription was carried out to synthesize cDNA in a 100 μl volume with 2 μg of total RNA, 10× RT buffer, 5.5 mM MgCl2, 2 mM dNTP mixture, 0.125 μM oligo d(T), 0.125 μM random primer, 40 U of RNase inhibitor, and 125 U of MultiScribe (Applied Biosystems, Foster City, CA) for 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was performed to determine the mRNA expression level of ALP and osteocalcin. The sequences of the primers were as follows: ALP (forward, 5′-TCA GGG CAA TGA GGT CAC ATC-3′; reverse, 5′-CAC AAT GCC CAC GGA CTT C-3′), osteocalcin (forward, 5′-CGG CCC TGA GTC TGA CAA AG-3′; reverse, 5′-CTC GTC ACA AGC AGG GTC AA-3′). Twenty-five microliters of reaction volume included 5 μl of cDNA, 0.5 μl of 10 μM of each primer and 12.5 μl of 2× SYBR green master mix (Applied Biosystems). Real-time PCR was performed with the following three-step protocol: step 1, 50°C for 2 min; step 2, 95°C for 10 min; step 3, 40 cycles of 95°C for 15 s and 62°C for 1 min using the 7500 real-time PCR System (Applied Biosystems, Foster City, CA). To confirm the amplification specificity, the PCR products were subjected to a dissociation curve analysis. The threshold cycles (Ct) of each reaction were normalized to those obtained for 18S mRNA using the −ΔΔCt method (Applied Biosystems). All PCR reactions were performed in triplicate.
Alkaline phosphatase assay
The C2C12 cells were plated at 200,000 cells/well in 6-well plates and grown overnight in DMEM containing 10% FBS. On day 2, the culture medium was replaced with DMEM containing 2% FBS and the cells were treated with various concentrations of compound for 24 h. On day 3, the medium was replaced with fresh DMEM containing 2% FBS and the cells were treated with 50 ng/ml of BMP-2 for 72 h. The cells were washed with phosphate-buffered saline (PBS) and lysed by addition of lysis buffer (10 mM Tris–HCl pH 8.0, 1 mM MgCl2, and 0.5% Triton X-100). The cell lysates were centrifuged for 5 min at 13,000×g. The supernatant was removed and the aliquots were assayed for ALP activity and protein amount. The ALP activity was measured in triplicate using an ALP assay kit (Sigma-Aldrich, St. Louis, MO) in microtiter plates. The protein amount was determined with Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA) using bovine serum albumin (BSA) as a standard. The ALP activity (nmoles of p-nitrophenol per ml) was normalized to the protein amount (nmoles of p-nitrophenol per μg).
Dual-luciferase reporter assay
The BMP-specific Smad1-driven 9 × GCCG (a consensus binding sequence for Smad1) reporter plasmid was kindly provided by Dr. Miyazono (The Institute of Japanese Foundation for Cancer Research, Tokyo). The C2C12 cells were trypsinized and seeded in triplicate wells at 50,000 cells/well in 12-well plates on day 1. On day 2, the cells were cotransfected with the 9 × GCCG-reporter construct and the renilla-luciferase control vector using SuperFect (Qiagen, Valencia, CA) for 24 h. A total of 1 μg of plasmids was used for co-transfection in each well and the concentration of renilla-luciferase vector was 1/15 of the 9 × GCCG-reporter plasmid. On day 3, medium was replaced with DMEM containing 2% FBS and the cells were treated with various concentrations of compound. On day 4, the cells were treated with BMP-2. On day 5, the luciferase activities were measured in 20 μl of cell-lysate using the dual-luciferase assay system (Promega, Madison, WI) with a luminometer (LumiCount; Packard Bioscience, Meriden, CT) following the manufacturer’s instructions. The luciferase activity was expressed as relative units of luciferase (RUL; a ratio of firefly luciferase to renilla-luciferase activity).
Statistics and calculations
Results are presented as the mean of three determinations (n) with error bars representing the standard error of the mean (SEM). Experimental results that are visually represented are from consistent experiments where one representative experimental result is shown. Statistical significance (P < 0.05) was calculated using a one-way analysis of variance (ANOVA) with Bonferroni post-hoc test (equal variances assumed) or Dunnett’s T3 post-hoc test (equal variances not assumed) using Statistical Products for Social Sciences Version 16.0 (SPSS 16.0) for Windows (SPSS, Chicago, IL) to compare various treatments in multi-group analysis. Statistical probability of P < 0.05 was considered significant and is denoted as (*) in the figures.
Determination of EC50
The EC50 values were calculated by determining the concentration by which 50% of maximum activity was reached using the sigmoidal fit equation. The 50% effective concentrations were determined with the standard curve analysis of SigmaPlot 8.02. The nonlinear regression equation is y = min + (max − min)/(1 + (x/EC50)Hill Slope) where y is the observed responses; x is the dose concentration; max and min are approximated by the program automatically during the calculation. Values were not extrapolated beyond the tested range of concentrations.
Results
Modeling and assignment of template structure to a Smurf1-interacting peptide and its target Smurf1-WW2 domain
Smurf1 interaction with Smads is based on the presence of unique residues in WW2-domain of Smurf1 (Fig. 1) [11–13]. These observations prompted us to embark on a drug design project based on the interacting domain of Smurf1.
Fig. 1.
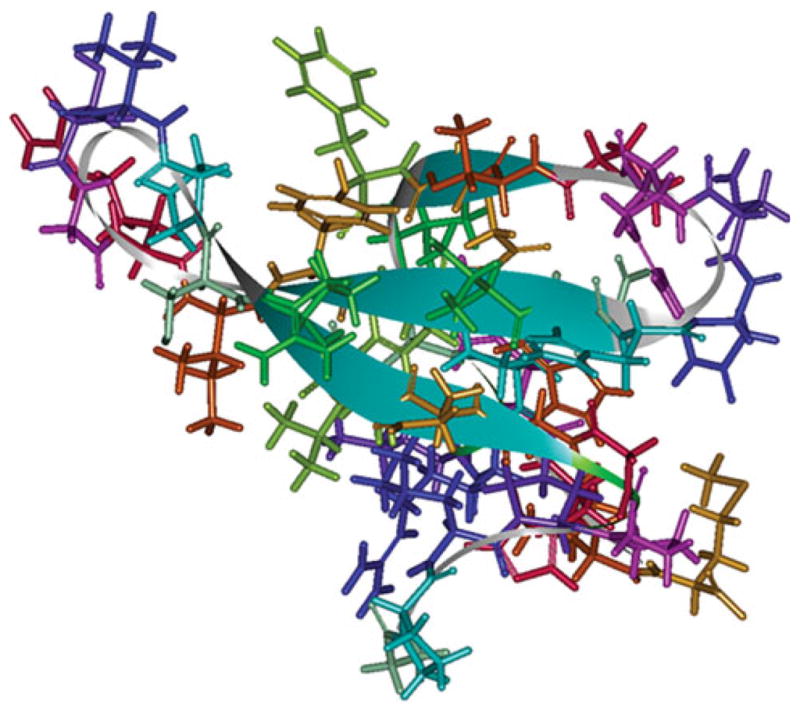
Optimized structure of the WW2 domain of Smurf1. The anti-parallel β-sheet structures are shown in ribbon form
Since X-ray crystallographic or NMR-based structural data are lacking for the Smurf1-WW2 domain but homologous structures are available in the protein data bank, we used the homologous structures to discern a template model structure for the Smurf1-WW2 domain. The WW domains are mined from the SWISS-PROT and SP-TrEMBL protein databases. We applied the “FindPattern” function in the Genetics Computer Group (GCG) software to search in databases for related “chordate” sequences for the Smurf1-WW2 domain. Modeling of a template structure for interacting domains is a critical pre-requisite for the subsequent identification of interacting residues. We accomplished this task for the Smad motif that interacts with Smurf1 [11, 12]. We determined the model structure for the WW2-domain of Smurf1 and a Smurf1-interacting peptide motif as an example of Smurf1 target peptide (Fig. 2). To build template models, we used MODELLER as well as SWISS_MODEL [15, 16] protein structure homology modeling servers (accessible via the ExPasy server at http://swissmodel.expasy.org). We also used the HOMOLOGY module of the INSIGHT-II software of Accelrys Inc. to model the target peptide structure. The HOMOLOGY module of INSIGHT-II helps in extracting the equivalent coordinates of the similar structure to build a model structure for the WW2-domain. Once a backbone and side chains of the target peptide were built, we optimized the structure using the energy minimization protocol of the DISCOVER module of INSIGHT-II. In order to model the conformation of the target peptide, we used the related peptide fragment from the rat Enac Bp2 peptide complex (1I5H, PDB code). This complex has a WW domain bound to a peptide fragment that is similar to the N-terminal 10 residues of a 10-residue model peptide that interacts with Smurf1, with three prolyl residues complexed with a homologous WW domain [11–13]. The resultant model of the 10 residue structure was considered as an example of Smurf1 target proteins. We compared these template models to obtain an acceptable model based on evaluation by the WHAT-CHECK and PRO-CHECK programs to correct the errors. These methods yielded optimum structure with stereochemically and energetically favored configuration.
Fig. 2.
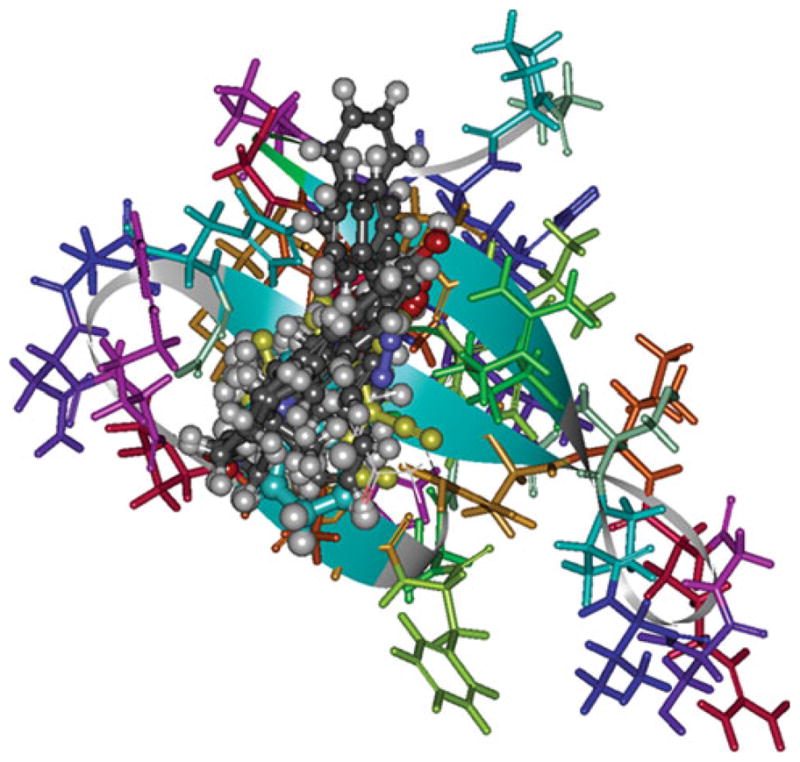
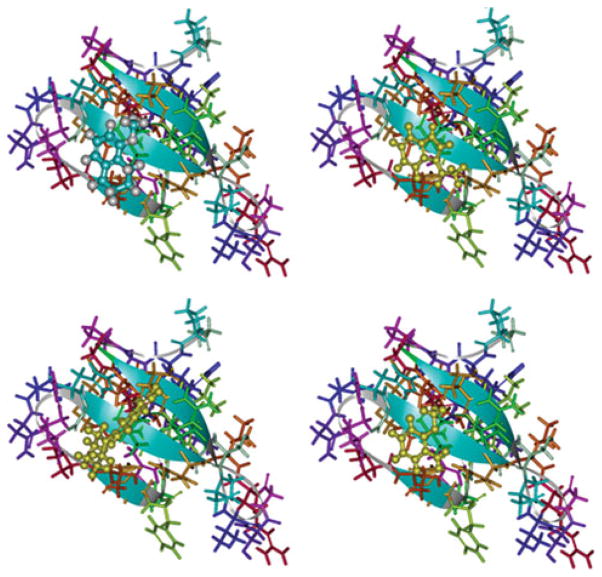
Surface region of the Smurf1-WW2 domain is shown, where the CAP small molecules are targeted. A group of consensus high-scoring molecules are shown interacting with Smurf1-WW2 domain residues. The WW2 domain atoms are shown in stick form, while the compounds are shown in ball and stick form
Virtual screening and selection of compounds
The LUDI de novo design method of Accelrys Inc. was used to identify the compounds that potentially block the Smurf1-WW2 domain. The important binding region within the WW2 domain was identified based on various studies on WW domains in the literature. The region around the residues, Tyr18, Val20, His22, Arg25, Thr27, and Phe29, was defined as the binding site with an appropriate radius of 9 Å for LUDI docking covering the entire binding interface (Fig. 2). The LUDI search against the region gave about 400 molecules with a Ludi score >300. In Fig. 2, we show a cluster of 10 top scoring molecules that occupy the binding region. The Ludi scoring approach was used to select 300 top scoring molecules. From these, we obtained 54 compounds from commercial sources for biochemical evaluation in living cells. Binding poses of four anonymous compounds are shown in Fig. 3.
Fig. 3.
Binding poses are shown for four selected high scoring low-molecular weight molecules that were in the pool of 300 consensus high-scoring molecules used for virtual testing. The WW2 domain atoms are shown in stick form, while the compounds are shown in ball and stick form
Optimization of a cell-based method to monitor BMP-2-induced responses
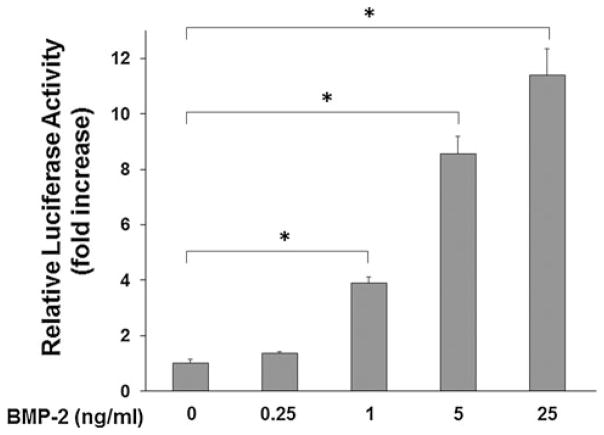
The mouse-derived C2C12 myoblasts served as an experimentally tractable model system for investigating the molecular basis of transdifferentiation toward the osteoblastic phenotype. We recently developed a sensitive and reliable cell-based assay utilizing a Smad1-specific luciferase reporter plasmid containing a multimerized—GCCG—motif (9 copies) to monitor transcriptional activity driven by activated Smad1 in C2C12 cells [17]. The BMP-specific Smad1-driven 9 × GCCG-reporter construct has been widely used to assay BMP activity in many cell types at a typical concentration of 50–100 ng/ml of BMP-2. To select a sub-optimal dose of BMP-2 for studying the potentiating effect of selected compounds, we performed the reporter assay with lower BMP-2 concentrations ranging from 0.25 to 25 ng/ml. The concentration range of BMP-2 required for activating the reporter assay was established (Fig. 4). The results from this experiment allowed us to select a sub-optimal dose (1.0 μg/ml) of BMP-2 to assess the potentiating effects of compounds in subsequent experiments.
Fig. 4.
Determination of BMP-2 activity in a cell-based reporter assay. A dose-dependent induction of luciferase activity by BMP-2 is shown in C2C12 cells transfected with the BMP-specific and Smad1-driven 9 × GCCG-reporter plasmid for 24 h. Luciferase activities were determined in triplicate. Asterisks denote statistical significance, determined as described in “Materials and methods,” between the indicated treatments (P < 0.05)
SVAK-12 potentiates BMP-2-induced Smad1-driven luciferase reporter activity
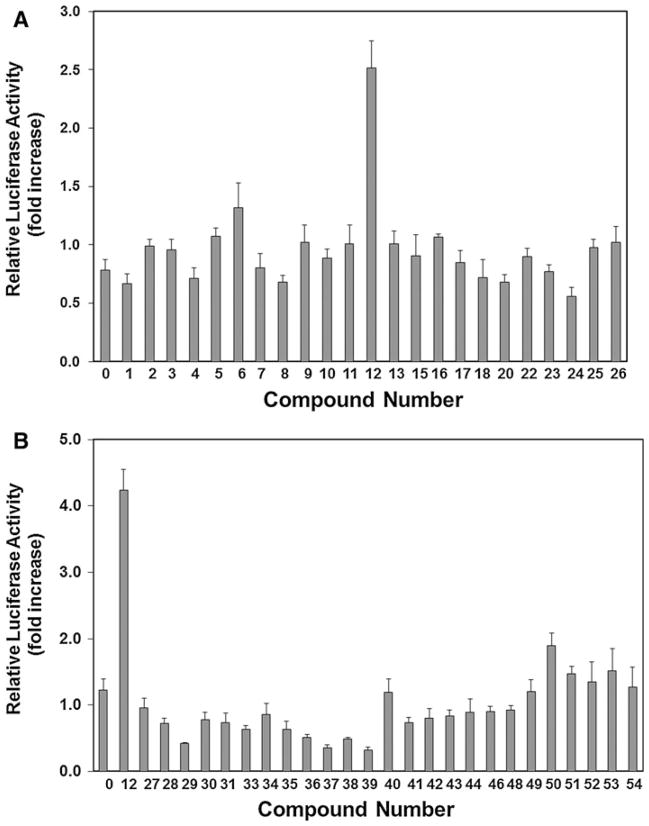
In an effort to identify a novel small drug-like molecule that could disrupt Smurf1–Smad1/5 interaction we used the computer simulation techniques of molecular docking for virtual screening. Compounds were ranked according to their relative binding energy, favorable shape complementarity, and potential to form hydrogen bonds within the modeled Smurf1-WW2 domain hydrophobic pocket [11–13]. For each docked compound, the best-scoring complexes were ranked, clustered, and re-ranked using calculations which included more accurate estimates of de-solvation. Finally, a diversity selection, followed by visual inspection using 3D stereo-graphics of putative compounds, were employed as filters for discovery of potential “lead” compounds. A representative sample of the most favorable compounds (54 compounds) was tested experimentally for their ability to potentiate BMP-2 activity in our luciferase reporter assay using C2C12 cells. Out of these candidate lead compounds, we selected SVAK-12 for the current studies as a promising synthetic compound (Fig. 5a, b). We focused on additional characterization of SVAK-12 activity because it showed relatively higher activity and its parent compound and the related chemical derivatives were pharmacologically more defined. The solvent dimethylsulfoxide (DMSO) controls showed only basal activity similar to no treatment controls. The DMSO solvent concentration of 0.01% (v/v) was not toxic to cells as determined by cell number, total protein amount and cell phenotype consistent with the literature (data not shown). At concentrations higher than 1.0 μg/ml, SVAK-12 caused lifting of cells from plates leading to decreased number of cells at the end of the experiment.
Fig. 5.
Determination of the efficacy of various selected compounds to enhance BMP-induced luciferase activity. Relative activities of a representative set of compounds in the luciferase reporter assay are shown. Compounds were tested at a concentration of 1.0 μg/ml, while BMP-2 was used at 1.0 ng/ml. The 1.0 μg/ml compound concentration for screening was selected empirically. Data points were determined in triplicate. In “no compound” controls, cells were treated with DMSO (0.01%) alone
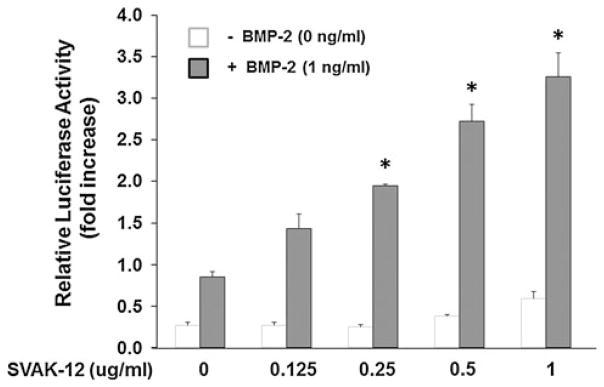
Next, we determined the effectiveness of compound SVAK-12 on potentiation of BMP-2 activity over the concentration range from 0.125 to 1.0 μg/ml while keeping the BMP-2 concentration at 1 ng/ml in the luciferase reporter assay (Fig. 6). SVAK-12 caused a dose-dependent enhancement of the luciferase activity with an optimum enhancement of 3.8-fold (P < 0.05) observed at a concentration of 1.0 μg/ml when compared to BMP-2 alone. The concentration required for half-maximal activation, EC50 value, of 2.6 μM was calculated from the Hill plot. The EC50 value was generated from fitted curves by solving for the x-intercept at the 50% activity level of the Y-intercept as described in “Materials and methods.” The non-sigmoidal nature of the dose–response curves of the compound is due to cell lifting induced at concentrations at or just above the maximal effective dose. After assay optimization, which included determining optimal BMP-2 concentration, plating density of cells, and compound incubation time, the promoter assay was shown to display the dynamic range and reproducibility required for a screening assay.
Fig. 6.
SVAK-12 potentiates BMP-2 responsiveness in the Smad1-driven reporter assay. A 3.8-fold enhancement of luciferase activity was observed at a SVAK-12 concentration of 1.0 μg/ml when compared to BMP-2 alone (1 ng/ml). Data points were determined in triplicate. Statistical significance from the BMP-2 control group was (P < 0.05) obtained as detailed in “Materials and methods” and is denoted by asterisks
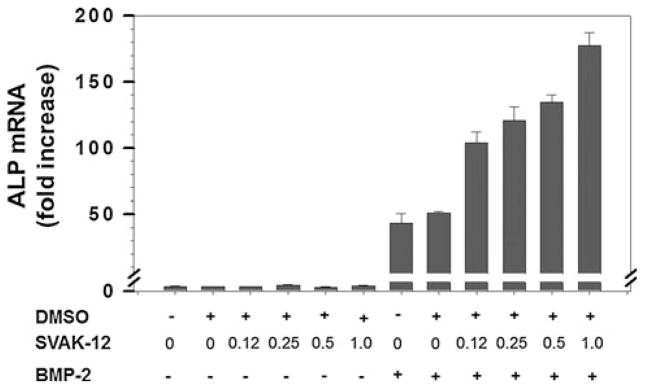
BMP-2-induced alkaline phosphatase and osteocalcin mRNA levels are enhanced by the compound SVAK-12
We tested whether the compound, SVAK-12, that enhanced BMP-induced reporter activity would also exhibit potentiating activity on BMP-2-induced marker gene expression. We determined the effectiveness of compound SVAK-12 over the concentration range from 0.125 to 1.0 μg/ml while keeping the BMP-2 concentration constant at 20 ng/ml by determining ALP mRNA levels (Fig. 7). SVAK-12 caused a dose-dependent increase in the BMP-induced ALP mRNA level with the maximal 3.5-fold increase (P < 0.05) compared to BMP-2 alone observed at a compound with a maximal concentration of 1.0 μg/ml. BMP-2 alone induced a 43- or 51-fold increase in ALP mRNA in the absence or the presence of DMSO (0.01%), respectively, when compared to the “no treatment” control (Fig. 7).
Fig. 7.
SVAK-12 enhances the BMP-induced increase of the ALP mRNA level in C2C12 cells. The compound dose-dependently enhanced the BMP-2 induced ALP mRNA level. The peak 3.5-fold enhancement of ALP mRNA level was observed at a SVAK-12 concentration of 1.0 μg/ml. Quantitation of the mRNA was based on triplicate determinations. Asterisks denote significant difference from BMP alone in the presence of an equal amount of DMSO (0.01%) as in compound treatments. Statistical significance (P < 0.05) among different groups was obtained as detailed in the “Materials and methods” section
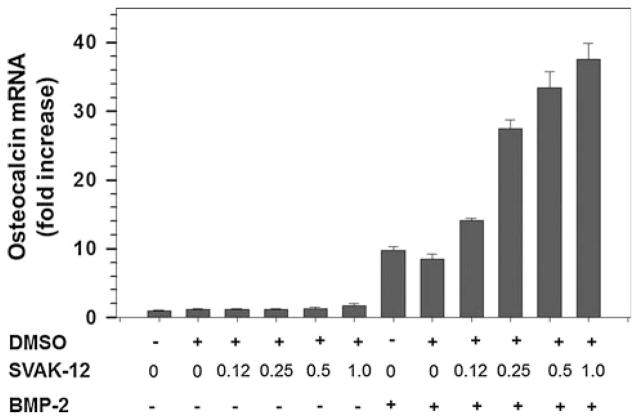
We also tested whether the compound, SVAK-12, that enhanced BMP-induced reporter activity and ALP mRNA expression would exhibit potentiating activity by increasing BMP-2-induced osteocalcin gene expression. Such an observation would strengthen its biological role in promoting the osteoblastic phenotype. We determined the effectiveness of compound SVAK-12 over the concentration range from 0.125 to 1.0 μg/ml while keeping the BMP-2 concentration constant at 20 ng/ml, as shown in Fig. 8. SVAK-12 caused a dose-dependent increase in the BMP-induced osteocalcin mRNA level with a maximal 4.4-fold increase (P < 0.05) compared to BMP-2 alone observed at a compound concentration of 1.0 μg/ml. BMP-2 alone induced a 9- or 7-fold increase in osteocalcin mRNA in the absence or the presence of DMSO (0.01%), respectively, when compared to “no treatment” control (Fig. 8). An EC50 value in the range of 1.5–5.3 μM was estimated for the expression of both ALP and osteocalcin mRNAs.
Fig. 8.
SVAK-12 enhances the BMP-induced osteocalcin mRNA level in C2C12 cells. The peak 4.4-fold enhancement of the osteocalcin mRNA level induced by BMP-2 was observed at a SVAK-12 compound concentration of 1.0 μg/ml. Quantitation of mRNA was based on triplicate determinations. Asterisks denote significant difference from BMP alone in the presence of equal amount of DMSO (0.01%) as in compound treatments. Statistical significance (P < 0.05) among different groups was obtained as detailed in “Materials and methods” section
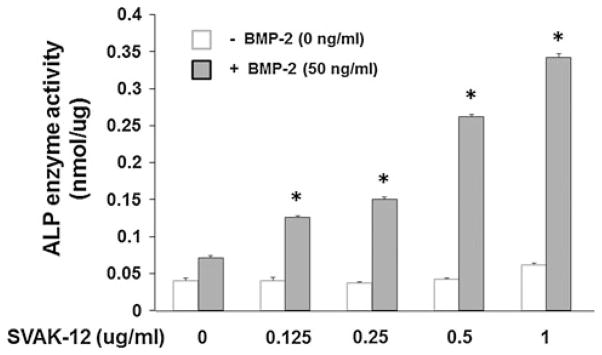
BMP-2-induced ALP enzyme activity is enhanced by the compound SVAK-12
We used SVAK-12 in the concentration range of 0.125–1.0 μg/ml while keeping the BMP-2 concentration constant at 50 ng/ml to evaluate its enhancement of the BMP-induced ALP activity. The ALP enzyme activity assay demonstrated that the compound dose-dependently enhanced the BMP-2-induced ALP activity. The peak activity of the maximal 4.8-fold increase (P < 0.001) was observed at a compound concentration of 1.0 μg/ml when compared to BMP-2 alone, further confirming the earlier observations (Fig. 9). An EC50 value of 3.7 μM was estimated for the activity of ALP. These results support our hypothesis that SVAK-12 is potentially a low-cost substitute for a recombinantly prepared protein in enhancing BMP-induced osteoblastic responses in cells.
Fig. 9.
Enhancement of BMP-2-induced ALP enzyme activity by SVAK-12. The compound dose-dependently enhanced the ALP activity induced by 50 ng/ml of BMP-2. The peak 4.8-fold enhancement of BMP-2-induced ALP activity was observed at a SVAK-12 compound concentration of 1.0 μg/ml. Data points were determined in triplicate. Asterisks denote significant difference from BMP alone control in the presence of equal amount of DMSO (0.01%) as in compound treatments. Statistical significance (P < 0.001) among different treatment groups was obtained as detailed in “Materials and methods”
Discussion
Increasing cellular responsiveness to BMP would be advantageous since bone formation in humans is requiring such high dose of rhBMPs. LMP-1 is an example of a naturally occurring protein that increases cellular response to BMPs. In this study, we sought to find a synthetic compound that blocks Smurf1 activity to enhance BMP responsiveness. We identified a unique compound, SVAK-12, that promotes the BMP-induced expression of phenotypic markers characteristic of a differentiated osteoblast. The reporter assays using BMP-specific Smad1-driven 9 × GCCG-reporter plasmid, real-time RT-PCR for ALP and osteocalcin mRNA expression, and ALP enzyme activity assay all showed that the compound potentiated BMP-2 activity.
Small synthetic chemical compounds (<700 Da), can be more easily manufactured than recombinant proteins and are more amenable to production and scale-up for low-cost use. Small-molecule compounds with potential BMP-potentiating activity will provide a pharmacological approach to help further extend the therapeutic use of BMPs. Any identification of a low-cost compound that potentiates a BMP-induced cellular response will broaden the use of BMP-2 for efficient bone regeneration application. Therefore, we extended the use of our cell-based assay using the Smad1-specific luciferase reporter to test potentially active candidates among the synthetic compounds that were selected as “leads” after extensive chemo-informatic analyses of the Smurf1-WW2 domain and its interacting site in target proteins. We selected SVAK-12 as one such active “lead” candidates.
To test the validity of the cell-based reporter assay further, we examined the potentiating activity of SVAK-12 on BMP-2-induced cellular responses. Real-time RT-PCR for ALP and osteocalcin mRNA expression and the ALP enzyme assay in C2C12 cells also demonstrated a dose-dependent activity of SVAK-12 in potentiating BMP-2-induced signaling. From these results, we conclude that the activity of SVAK-12 is not dependent on any particular assay as similar BMP-potentiation effects were observed both in transfected and non-transfected cells.
We applied lower concentrations of BMP-2 (0.125–5 ng/ml) than are typically used (50–100 ng/ml) so as to improve the sensitivity of our reporter assay in detecting enhancement of BMP-2 activity. We showed that the specificity of the assay was well maintained despite monitoring multiple cellular outputs at various time points. The compound SVAK-12 elevated the BMP-2-induced response significantly even at a dose of 0.1 μg/ml. The effective dose of compound remained consistent (0.25 μg/ml) among multiple assays. This suggested that the biological markers in C2C12 cells that we chose to investigate are tightly controlled by the same BMP-signaling pathway. All these studies suggest that the compound, SVAK-12 or a potentially more efficacious derivative, may be useful in potentiating the BMP-2 responsiveness of cells. The combined use of such a compound with BMP-2 may reduce the high doses of BMP-2 protein currently required for its effective clinical use. The abundance and low cost of SVAK-12 makes it a highly desirable drug candidate. Thus, SVAK-12 may be a useful pharmacological tool that induces BMP-mediated osteoblastic progression.
In summary, we established the 9 × GCCG reporter transfected C2C12 cells as a sensitive cell-based sensor of BMP-2 osteogenic effects. This system allowed us to monitor successfully the complex process of BMP-induced transdifferentiation of C2C12 cells toward the osteoblastic phenotype by determining various cellular markers at different time points in living cells. Also the identification and activity determination of SVAK-12 through novel chemo-informatics approach, virtual screenings and cell-based biochemical evaluations lay promising ground for further applications in bone biology. Future experiments will evaluate enhancement of BMP-2-induced bone formation by SVAK-12 or its more efficacious derivatives in animal models.
Our results provide support for the idea that low-molecular weight compounds can inhibit protein–protein interactions that normally occur over relatively large surfaces. Also, our results raise the possibility of developing drugs that target Smurf1 via a novel mode of action and may inspire future rational development of compounds with the ability to modulate WW domain-dependent functions in other proteins. The rational development of compound SVAK-12 proves that small-molecule compounds can provide all the necessary requirements to block Smurf1 function. Currently, the compound selected in this study forms the basis of a medicinal chemistry effort to produce new chemical entities with optimized drug-like properties such as potency and bioavailability. Such compounds could have future applications in the treatment of disease by specifically modulating the WW domain-dependent interactions of a target protein. However, a major challenge is to further develop such compounds into high affinity, specific, and active drugs at low concentrations.
Acknowledgments
We thank Boojala VB Reddy for assistance in homology modeling. We also thank Vandana Voleti for assistance in computational analyses.
Abbreviations
- BMP
Bone morphogenetic protein
- Smurf1
Smad ubiquitin regulatory factor-1
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- ALP
Alkaline phosphatase
- DMSO
Dimethylsulfoxide
- RUL
Relative units of luciferase
- FBS
Fetal bovine serum
- CAP database
Chemicals available for purchase database
Footnotes
Disclosures All the biochemical studies in this work were performed at the Atlanta Veterans Affairs Medical Center and partly supported by NIH grant # R01 AR53093 (Boden) and a VA Merit award to Dr. Titus. In the past and not related to this work, Dr. Boden has received compensation as a consultant for Medtronic Sofamor Danek and for intellectual property. Emory University and some of the authors have/may receive royalties in the future related to LMP-1. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Contributor Information
Satoshi Kato, Atlanta VA Medical Center and Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA 30329, USA. Department of Orthopaedic Surgery, Kanazawa University School of Medicine, Kanazawa 920-8641, Japan.
Sreedhara Sangadala, Email: ssangad@emory.edu, Atlanta VA Medical Center and Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA 30329, USA. VAMC-Research Service, 1670 Clairmont Rd., Decatur, GA 30033, USA.
Katsuro Tomita, Department of Orthopaedic Surgery, Kanazawa University School of Medicine, Kanazawa 920-8641, Japan.
Louisa Titus, Atlanta VA Medical Center and Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA 30329, USA.
Scott D. Boden, Atlanta VA Medical Center and Department of Orthopaedics, Emory University School of Medicine, Atlanta, GA 30329, USA
References
- 1.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 2.Polly DW, Jr, Ackerman SJ, Shaffrey CI, Ogilvie JW, Wang JC, Stralka SW, Mafilios MS, Heim SE, Sandhu HS. A cost analysis of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion. Orthopedics. 2003;26:1027–1037. doi: 10.3928/0147-7447-20031001-12. [DOI] [PubMed] [Google Scholar]
- 3.Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine. 2002;27:2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, Schimandle JH, Hutton WC. The use of an osteoinductive growth factor for lumbar spinal fusion. Part I: Biology of spinal fusion. Spine. 1995;20:2633–2644. doi: 10.1097/00007632-199512150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kusumoto K, Bessho K, Fujimura K, Akioka J, Okubo Y, Wang Y, Iizuka T, Ogawa Y. Osteoinduction by recombinant human bone morphogenetic protein-2 in muscles of non-human primates. J Int Med Res. 2002;30:251–259. doi: 10.1177/147323000203000305. [DOI] [PubMed] [Google Scholar]
- 6.Ackerman SJ, Mafilios MS, Polly DW., Jr Economic evaluation of bone morphogenetic protein versus autogenous iliac crest bone graft in single-level anterior lumbar fusion: an evidence-based modeling approach. Spine. 2002;27:S94–S99. doi: 10.1097/00007632-200208151-00017. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita M, Ying S, Zhang G, Li C, Cheng SY, Deng C, Zhang Y. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell. 2005;121:101–113. doi: 10.1016/j.cell.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao M, Qiao M, Harris SE, Oyajobi BO, Mundy GR, Chen D. Smurf1 inhibits osteoblast differentiation and bone formation in vitro and in vivo. J Biol Chem. 2004;279:12854–12859. doi: 10.1074/jbc.M313294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangadala S, Boden SD, Viggeswarapu M, Liu Y, Titus L. LIM mineralization protein-1 potentiates bone morphogenetic protein responsiveness via a novel interaction with Smurf1 resulting in decreased ubiquitination of Smads. J Biol Chem. 2006;281:17212–17219. doi: 10.1074/jbc.M511013200. [DOI] [PubMed] [Google Scholar]
- 10.Sangadala S, Okada M, Liu Y, Viggeswarapu M, Titus L, Boden SD. Engineering, cloning and functional characterization of recombinant LIM mineralization protein-1 containing an N-terminal HIV-derived membrane transduction domain. Protein Expr Purif. 2009;65:165–173. doi: 10.1016/j.pep.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Sangadala S, Boden SD, Metpally RP, Reddy BV. Modeling and analysis of molecular interaction between Smurf1-WW2 domain and various isoforms of LIM mineralization protein. Proteins. 2007;68:690–701. doi: 10.1002/prot.21429. [DOI] [PubMed] [Google Scholar]
- 12.Bohm HJ. The computer program LUDI: a new method for the de novo design of enzyme inhibitors. J Comput Aided Mol Des. 1992;6:61–78. doi: 10.1007/BF00124387. [DOI] [PubMed] [Google Scholar]
- 13.Sangadala S, Metpally RP, Reddy BV. Molecular interaction between Smurf1 WW2 domain and PPXY motifs of Smad1, Smad5, and Smad6—modeling and analysis. J Biomol Struct Dyn. 2007;25:11–23. [PubMed] [Google Scholar]
- 14.Bohm HJ. On the use of LUDI to search the fine chemicals directory for ligands of proteins of known three-dimensional structure. J Comput Aided Mol Des. 1994;8:623–632. doi: 10.1007/BF00123669. [DOI] [PubMed] [Google Scholar]
- 15.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 16.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada M, Sangadala S, Liu Y, Yoshida M, Reddy BV, Titus L, Boden SD. Development and optimization of a cell-based assay for the selection of synthetic compounds that potentiate bone morphogenetic protein-2 activity. Cell Biochem Funct. 2009;27:526–534. doi: 10.1002/cbf.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]