SUMMARY
As Giardia lamblia is unable to synthesize cholesterol de novo, this steroid might be obtained from the host’s intestinal milieu by endocytosis of lipoproteins. In this work, we identified a putative Giardia lamblia low-density lipoprotein receptor-related proteins (GlLRP), a type-I membrane protein, which shares the substrate-N-terminal binding domain and a FXNPXY-type endocytic motif with human LRPs. Expression of tagged-GlLRP showed that it was localized predominantly in the endoplasmic reticulum, lysosomal-like peripheral vacuoles, and plasma membrane. However, the FXNPXY-deleted GlLRP was retained at the plasma membrane suggesting that it is abnormally transported and processed. The low density lipoprotein and chylomicrons interacted with GlLRP, with this interaction being necessary for lipoprotein internalization and cell proliferation. Finally, we show that GlLRP binds directly to the medium subunit of Giardia adaptor protein 2, indicating that receptor-mediated internalization occurs through an adaptin mechanism.
Keywords: Protozoa, LDL, LRP, receptor-mediated endocytosis, chylomicrons
INTRODUCTION
The low-density lipoprotein receptor-related protein (LRP) is a large, highly conserved receptor that binds numerous types of ligands, raises several different signal transduction pathways, and is implicated in a variety of diseases including Alzheimer's disease, cancer, and HIV (reviewed in (Herz & Strickland, 2001). LRP is a member of the low-density lipoprotein receptor (LDLR) family, which also includes the apoE receptor 2 (ApoER2), the very low density lipoprotein receptor (VLDLR), multiple epidermal growth factor (EGF) repeat-containing protein (MEGF7), megalin, LDL-related protein-1 (LRP1), and LDL-related protein-1b (LRP1b) (Rebeck et al., 2006, Krieger & Herz, 1994, Willnow et al., 1999). The most characteristic structural components of these receptors are five common structural units: (1) cysteine-rich ligand-binding repeats, which form ligand-binding domains, (2) epidermal growth factor (EGF) receptor–like cysteine-rich repeats, (3) YWTD domains, (4) a single membrane-spanning segment, and (5) a cytoplasmic tail that contains between one and three FXNPXY-like motifs (Fig. 1) (Li et al., 2001, Jeon et al., 2001, Takeda et al., 2003). Through its binding domains, LRP interacts and mediates the internalization of a diverse group of molecules, ranging from lipoproteins (including apolipoprotein E, chylomicrons, very-low-density lipoprotein, and lipoprotein lipase), proteinases, and proteinase inhibitor complexes to unrelated molecules (such as lactoferrin and thrombospondin) (reviewed in Herz & Strickland, 2001). Because LRP is a protein which travels along the secretory route, it displays reticular endoplasmic reticulum (ER) and perinuclear Golgi-like staining, in addition to being present at the plasma membrane and within early endosomes (Waldron et al., 2008). During its journey to the cell surface, LRP undergoes sequential cleavage events due to different proteinases (reviewed in Rebeck et al., 2006). LRP is synthesized as a 600 kDa transmembrane glycoprotein, which later becomes cleaved by furin in the trans-Golgi network, thus producing a 515 kDa α-subunit and an 85 kDa β-subunit (Herz et al., 1990). These two subunits remain non-covalently associated during transport to the cell surface as well as during internalization and recycling from early endosomes to the plasma membrane (Herz et al., 1990, Ward et al., 1989). The cytoplasmic tail contains FXNPXY-like motifs that serve as docking sites for the endocytosis machinery and for cytoplasmic adaptors. However, the mechanisms involved in the endocytosis of the members of the LDL receptor family are not completely understood. Moreover, the initial endocytosis rates of individual LDLR family members are significantly different, suggesting that the endocytic functions among these members are distinct.
Figure 1. Giardia LRP as an LDL receptor family member.
(A) LDLR and LRP1 represent two of the receptor family members in mammalian cells. LRP minireceptor (mLRP) is used in mammalian cells to examine the ligand-binding properties of LRP (Obermoeller-McCormick et al., 2001) and the structure shows a strong similarity with GL113565 putative Giardia LRP (GlLRP in red). Each member contains a single transmembrane domain, at least one ligand-binding domain, EGF repeat, YWTD β-propeller, and cytoplasmic FXNPXY-like motif. YWTD β-propeller-like domain in GlLRP is depicted in grey. (B) Structures of four putative calcium-binding pockets from GL113565. Homology-modeled structures are compared to model structures (partial reproductions of human LRP pdb 1N7D). Labels for each structure indicate the amino acid ranges modeled. The top models correspond to the R1’ and R2’ ligand-binding domain calcium pockets while the lower models correspond to the A’ and B’ EGF-precursor-homology domain calcium pockets.
Although host cell cholesterol has been implicated in the survival and replication of many pathogens, the lipid uptake in protozoa parasites has not been widely characterized. The divergent eukaryote Giardia lamblia is unable to synthesize cholesterol de novo, although this steroid might be obtained by trophozoites from the intestinal milieu (Adam, 2001, Lujan et al., 1996b). It has been shown that a portion of the lipid uptake may be associated with endocytosis of lipoproteins by the trophozoite in vitro (Lujan et al., 1996b, Rivero et al., 2010). For more than a decade, different groups have searched for the LDLR in Giardia without success. However, it was suggested that a protein capable of binding LDL may exist in this parasite (Rivero et al., 2010, Lujan et al., 1996b). It was also proposed that cholesterol is captured from LDL by the Ck-like receptor, thus regulating encystation (Kaul & Kaur, 2001). Nevertheless, no classical lipoprotein receptor has been identified until now. Searching the Giardia genome database (GiardiaDB) for genes that codified for homologous LDLR yielded negative results. However, when looking specifically for the characteristic structural motifs of this family receptor, we found a type I membrane protein that contains two cysteine-rich ligand binding repeats and two EGF-precursor domains, is homologous to LRP1 and LRP1b, and has one FXNPXY-like internalization signal (FNSPTY) within its cytoplasmic tail. In this study, we examined whether a putative Giardia LRP (GlLRP) was responsible for the uptake of cholesterol via its interaction with lipoproteins. Our data indicate that evolutionarily conserved motifs in GlLRP mediate direct interactions between GlLRP/lipoproteins and the GlLRP/μ2-adaptin subunit of the clathrin-adaptor protein 2 (AP2) in this parasite.
RESULTS
The GL113565 Giardia protein possesses structurally conserved LDLR domains
Members of the LDLR family are composed of modular domains which include cysteine-rich complement-type repeats, EGF repeats, YWTD β-propeller domains, a transmembrane domain, and a cytoplasmic domain. The extracellular complement-type repeats are found in clusters and are implicated not only in ligand binding but also in calcium binding. In a GiardiaDB survey, we identified two proteins containing calcium-binding EGF-like domains (GL113565 and GL94510), similar to the ones found in the LRP of different cells. These Giardia proteins were predicted type I membrane proteins homologous to human LRP1 and LRP1b, and possessed a signal peptide, a 20 aa transmembrane domain, and a cytoplasmic tail. Interestingly, GL113565 but not GL94510 contained an FXNPXY-like motif (FNSPTY) within its cytoplasmic tail, consistent with the presence of one or more copies of the FXNPXY-like motif in the cytoplasmic domain of the LDLR family members (Fig. 1A). Similar to human LDLR and LRPs, the extracellular domain of GL113565 was composed of epidermal growth factor (EGF) precursor homology domains and several ligand-binding domains (LBD) (Figs. S1 and 1B). Models of GL113565 extracellular domains were obtained by homology modeling using Modeller (Sali et al., 1995), based on the crystal structure of the human LDLR (LDLR) extracellular domain (PDB code 1N7D) at 3.7 Å (Rudenko et al., 2002). The model that was generated was structurally similar to the template and contained two well conserved ligand binding domains, R1’ (residues 134 to 151) and R2’ (178 to 200), and two EGF precursor homology domains, A’ (residues 223 to 246) and B’ (426 to 455) (Fig. 1B). Despite the close resemblance between the extracellular domain structures of GL113565 and LDLR, there was little overall sequence similarity suggesting that GL113565 might possess additional non-conserved ligand-binding domains. The region of GL113565 which lies between the EGF precursor homology domain and the transmembrane domain was enriched in beta-sheets according to secondary structure prediction (beta-propeller-like domain) (Fig. S2). However, we were unable to produce a satisfactory model of this region when using the LDLR beta-propeller domain as a template. Because the Giardia protein GL113565 fulfills all the structural requirements for an LRP, we further tested whether this protein also functioned as an LRP. For ease of understanding, GL113565 will now be referred to Giardia lamblia LRP (GlLRP).
GlLRP is highly processed
We generated three constructs expressing GlLRP (LRP-HA, HA-LRP, and LRP-FXNPXY-HA) (Fig. 2A). To facilitate immunodetection, an HA epitope was included at the C-terminus of GlLRP generating the version LRP-HA. To obtain the N-terminus-tagged GlLRP, the HA epitope was inserted immediately after GlLRP’s signal peptide, producing the HA-LRP fusion protein. The LRP-FXNPXY-HA truncated version of GlLRP was generated by deletion of the FXNPXY-like motif and addition of the HA epitope at the C-terminus. Giardia trophozoites with stable expression of these receptors were initially identified via immunofluorescence with anti-HA monoclonal antibody (mAb), further purified by subcloning, and confirmed by immunoblotting. Thus, we used selected lrp-ha, ha-lrp, and lrp-FXNPXY-ha strains expressing the LRP-HA, HA-LRP, and LRP-FXNPXY-HA proteins, respectively, to investigate GlLRP. Consequently, the HA-fusion proteins allowed us to independently observe the carboxyl and the amino termini of GlLRP (LRP-HA and HA-LRP, respectively) by immunoblot analysis of stably transfected cells. The molecular weight of the most significant bands detected were approximately 116 kDa, 64 kDa, and 52 kDa (Fig. 2B, -inh). The ~116 kDa band observed for both LRP-HA and HA-LRP might represent the endoplasmic reticulum precursor while the other bands might represent the mature subunits of GlLRP after protease processing as was shown for the LRP family (Duckert et al., 2004). To assess whether a furin-like protease is involved in the maturation of GlLRP, the transgenic cells were grown in medium containing the furin inhibitor PMSF (Ashworth et al., 1999a, Ashworth et al., 1999b) before analysis by immunoblotting. The results showed that the proteolytic process of GlLRP was completely prevented by 170 μg/ml of PMSF, indicated by the presence of only the 116 kDa form in both cells (Fig. 2B, PMSF). Interestingly, addition of the cysteine protease inhibitor E64 significantly reduced LRP processing, although the main bands were still present after treatment (Fig. 2B, E64). The ~64 kDa band, expected to contain the transmembrane domain and cytoplasmic tail, was observed for LRP-HA while the ~52 kDa band, possibly representing the extracellular subunit of GlLRP, was observed only for HA-LRP (Fig. 2A-B). Addition of the serine protease inhibitors TLCK and TPCK did not have any effect on GlLRP processing (not shown). These results suggest that GlLRP goes through a combination of furin-like and cysteine protease proteolytic events. Moreover, the cleavage performed by a furin-like protease seemed to be necessary for the further action of cysteine proteases likely in the PVs. When LRP-FXNPXY-HA was analyzed by immunoblotting before and after the treatment with the proteases inhibitors, only the 116 kDa form was observed. It is thus possible that the deletion of the FXNPXY-like motif in LRP-FXNPXY-HA impaired the proteolytic maturation of GlLRP and the degradation following internalization, as was previously suggested for LRP1 (Reekmans et al., 2010).
Figure 2. Processing of GlLRP in stably transfected trophozoites.
(A) Schematic representation of HA-tagged LRPs is depicted in comparison to the full-length endogenous GlLRP. The putative RPRNT site of furin cleavage (residues 491 to 495) is represented by an arrow. The presumed subunits (52 and 64 kDa) generated upon furin cleavage are indicated. The HA epitope is shown in black. (B) LRP-HA, HA-LRP, and LRP-FXNPXY-HA are detected by immunoblotting using anti-HA mAb in 103 lrp-ha, ha-lrp, and lrp-FXNPXY-ha trophozoites, respectively. Note the significant processing of LRP-HA and HA-LRP in comparison to LRP-FXNPXY-HA (-inh). Addition of the furin-like inhibitor PMSF completely prevented the processing of LRP-HA and HA-LRP (PMSF). Treatment with the cysteine protease inhibitor E64 reduced LRP-HA and HA-LRP cleavage although the main bands of 52 and 64 kDa are still observed (E64). No inhibitory effect is observed for LRP-FXNPXY-HA after treatment (PMSF and E64). Relative molecular weights of protein standards (kDa) are indicated on the left.
Deletion of the FXNPXY-like motif impaired ER localizationof GlLRP
Previous studies have shown that LRP1 and LRP1b are mainly retained at the ER, a required step in assuring the correct folding and maturation of these receptors (Reekmans et al., 2010). To investigate whether this was also the case for GlLRP, we performed colocalization studies and compared the subcellular localization of LRP-HA, HA-LRP, and LRP-FXNPXY-HA with the distribution of the ER protein BiP by direct immunofluorescence labeling and confocal laser scanning microscopy (Fig. 3). Both LRP-HA and HA-LRP were found to display a reticular pattern, colocalizing with BiP. However, when LRP-FXNPXY-HA was analyzed, this variant receptor was distributed in the cytoplasm and did not colocalize with BiP (Fig. 3). This suggests that the FXNPXY-like motif is essential for early steps in the GlLRP biosynthesis.
Figure 3. ER retention of GlLRP is avoided by deletion of the FXNPXY-like motif.
Direct IFA and confocal microscopy shows that both LRP-HA and HA-LRP (green) strongly colocalize (merge) with the ER protein BiP (red). LRP-FXNPXY-HA (green) is not present in the ER, showing mostly a cytoplasmic distribution. Inserts show the differential interference contrast (DIC) microscopy. All images were acquired and processed identically. Bars, 10 μm.
GlLRP localizes on the cell surface
After passage through the secretory pathway, the LRPs remain on the cell surface where they bind different substrates via their extracellular portion. To explore cell surface localization of GlLRP, lrp-ha, ha-lrp, and lrp-FXNPXY-ha cells were tested by IFA on fixed, non-permeabilized cells, with HA-LRP being the only GlLRP version detected (not shown). Coimmunostaining of fixed, non-permeabilized ha-lrp cells with anti-HA mAb and anti-surface protein 9B10 mAb, showed colocalization of both proteins at the plasma membrane (Fig. 4). However, IFA of lrp-ha and lrp-FXNPXY-ha cells selectively permeabilized with saponin showed colocalization of LRP-HA and FXNPXY-HA with VSP9B10 (Fig. 4). These experiments also confirmed the predicted topology of the receptor with the N-terminus exposed to the extracellular space and the C-terminus located inside the cell.
Figure 4. Surface localization of GlLRP.
Direct IFA and fluorescence microscopy show that LRP-HA, HA-LRP, and LRP-FXNPXY-HA (green) colocalize with the variant-specific surface protein VSP9B10 (red) at the plasma membrane. lrp-ha, and lrp-FXNPXY-ha trophozoites were treated with 0.05% saponin after fixation to selectively permeabilize the plasma membrane. ha-lrp cells were fixed and stained without permeabilization (*). Differential interference contrast (DIC) microscopy is shown on the right. All images were acquired and processed identically. Bars, 10 μm.
Because total internal reflection fluorescence microscopy (TIRFM) is suitable for imaging transmembrane receptors located at the plasma membrane, we used this technique to corroborate cell surface localization of GlLRP (Fig. 5A) (Axelrod, 1989). For this, Giardia cells expressing HA-tagged LRPs were fixed, incubated with saponin (except for ha-lrp), labeled with FITC-anti-HA mAb, and, after a second fixation and attachment, imaged using TIRFM optics. In agreement with previous studies examining LRP1 (Herz et al., 1988, Zemskov et al., 2007), we observed that the signal was restricted to fluorescent structures close to the plasma membrane of LRP-HA, HA-LRP, and LRP-FXNPXY-HA in lrp-ha, ha-lrp, and lrp-FXNPXY-ha trophozoites, respectively (Fig. 5B). Because the region visualized using TIRFM is at least a few hundred nanometers wide, the cytoplasmic zone immediately beneath the plasma membrane is necessarily visualized in addition to the plasma membrane. To analyze whether the signal obtained allowed the selective visualization of GlLRP in surface regions, the anti-VSP9B10 mAb and the PV-marker LysoSensor (Touz et al., 2002b) were used for positive and negative controls, respectively. We observed that this methodology was able to detect the surface signal from VSP9B10 but not from the PV-fluorescent marker LysoSensor in saponin-permeabilized cells (Fig. 5B).
Figure 5. Plasma membrane localization of GlLRP by TIRFM.
(A) Schematic illustration of a Giardia trophozoite attached to the glass slide by its dorsal side during TIRFM. When the incidence angle of laser excitation entirely reflects the illuminating beam back, a specific fluorescent excitation (evanescent wave) is induced in a very thin optical section from the glass surface (≤100 nm in depth). This evanescent wave is an electromagnetic field which decays exponentially; thus, only the fluorophores nearest the glass surface (e.g. plasma membrane proteins) are selectively excited ( ) (Adapted from Axelrod, 2003). (B) TIRFM show LRP-HA, HA-LRP, LRP-FXNPXY-HA, and VSP9B10 at the plasma membrane. The PV marker LysoSensor (LS) does not present fluorescence at the surface. The trophozoites were treated with 0.05% saponin after fixation to selectively permeabilize the plasma membrane except for ha-lrp cells that were fixed and stained without permeabilization (*). Phase contrast images are depicted on the left panels (PC). All images were acquired and processed identically. Bars, 10 μm.
) (Adapted from Axelrod, 2003). (B) TIRFM show LRP-HA, HA-LRP, LRP-FXNPXY-HA, and VSP9B10 at the plasma membrane. The PV marker LysoSensor (LS) does not present fluorescence at the surface. The trophozoites were treated with 0.05% saponin after fixation to selectively permeabilize the plasma membrane except for ha-lrp cells that were fixed and stained without permeabilization (*). Phase contrast images are depicted on the left panels (PC). All images were acquired and processed identically. Bars, 10 μm.
GlLRP is associated with lipoproteins
Mammalian LRP is larger than but structurally similar to other members of the LDLR protein family. Whereas LDLR acts in lipoprotein metabolism binding LDL via the single protein apolipoprotein B-100 (apoB-100), the LRP appears to be important for the clearance of apo-B48–containing lipoproteins such as chylomicrons. Recently, we showed that LDL is internalized and delivered to the PVs by adaptin-mediated endocytosis in Giardia, a process which requires specific binding of LDL to a receptor (Rivero et al., 2010). To analyze whether GlLRP functions as an LDL-receptor in this parasite, we performed an assay of fluorescent Bodipy-LDL uptake in lrp-ha, followed by immunofluorescence and confocal microscopy analyzing different optical section planes from the surface (Fig. 6, a-e). The results showed that Bodipy-LDL partially colocalized with LRP-HA in a cluster-like pattern on transgenic trophozoites.
Figure 6. LDL and Giardia LRP colocalize in patches.
Direct IFA and confocal laser scanning microscopy of lrp-ha cells using anti-HA mAb (LRP-HA in red) and Bodipy-LDL (green) show partial colocalization (merge in yellow) at different optical section plains from the surface. From the dorsal to the ventral plane: (a) 0.0 μm, (b) 1.2 μm, (c) 2.4 μm, (d) 3.2 μm, and (e) 5.2 μm. Differential interference contrast (DIC) microscopy images of each section are depicted on the left. All images were acquired and processed identically. Bars, 10 μm.
To determine the in vivo interaction between LRP and LDL, lrp-ha, ha-lrp, and lrp-FXNPXY-ha trophozoites were grown in lipoprotein-deficient medium followed by incubation in labeling medium containing LDL for up to 2 h. After removing the unbound LDL by washing, LDL was immunoprecipitated with an antibody against ApoB and the immunoprecipitate was subsequently separated by SDS–PAGE and detected by immunoblot using a directly labeled anti-HA mAb (revealing the HA-tagged LRPs). The IPP assays showed that the tagged-LRPs were able to bind LDL because they were all coimmunoprecipitated with the anti-ApoB mAb, suggesting a functional interaction (Fig. 7A). Apo-B48, a truncated apoB-containing the amino-terminal portion of apo-B100, is required for the assembly of chylomicrons in the intestine. Our recent studies showed that LDL and chylomicrons, but not ApoA-containing high density lipoprotein (HDL), impaired LDL endocytosis (Rivero et al., 2010). To determine whether GlLRP might also be a receptor for chylomicrons, we repeated the immunoprecipitation utilizing the anti-ApoB mAb (that recognizes both ApoB100 and ApoB48 apoproteins) now challenging the transgenic and wild-type trophozoites with purified chylomicrons. We observed that the tagged-LRPs co-precipitated with ApoB suggesting that GlLRP is involved in the uptake of cholesterol from chylomicrons in this parasite (Fig. 7B). IPP control utilizing LRP-HA strain extracts in the absence of added LDL or chylomicrons showed no detection of the HA-tagged LRP (Fig. 7A and B, control). There was also no indication of tagged LRP when wild-type cells or transgenic cells were immunoprecipitated with anti-ApoB or a non-related anti-V5 mAb, respectively (not shown). When the trophozoites were grown in medium containing the protease inhibitor PMSF before lipoprotein challenge, only the 116 kDa band was observed after ApoB immunoprecipitation confirming that the LRP precursor was able to bind LDL and chylomicrons (Fig. 7C). These results represent the first demonstration of the interaction between lipoproteins and a membrane receptor in this parasite.
Figure 7. Giardia LRP binds lipoproteins.
Immunoblotting after IPP utilizing anti-ApoB mAb. (A) LRP-HA coimmunoprecipitates with ApoB in lrp-ha, ha-lrp, and lrp-FXNPXY-ha trophozoites after 2 h of LDL uptake. The control cells without LDL do not show nonspecific binding under the same IPP conditions. (B) LRP-HA coprecipitates with ApoB after 2 h of incubation with chylomicrons (2 h). There is no label of LRP-HA when the transgenic cells were treated in the absence of chylomicrons (control). (C) Addition of the protease inhibitor PMSF shows the coimmunoprecipitation of the LDL-ApoB and the putative precursor of GlLRP. (D) Coimmunoprecipitation of chylomicrons-ApoB and GlLRP precursor after addition of PMSF. Controls included the omission of lipoprotein. The immunoblot experiments were performed utilizing alkaline phosphatase-labeled anti-HA mAb. Relative molecular weights of protein standards are indicated on the left in kDa.
GlLRP down- and up-regulation controls LDL internalization
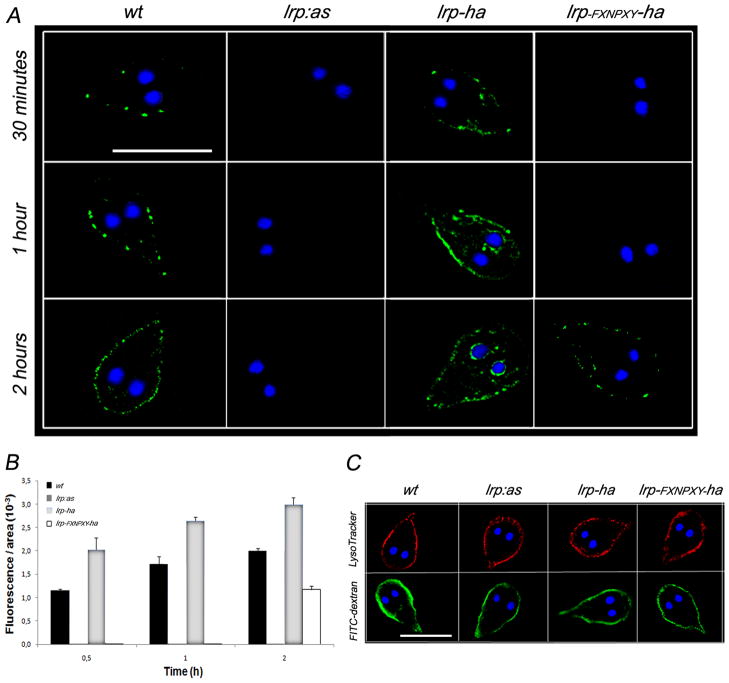
We sought to establish a functional connection between GlLRP and LDL endocytosis by reducing GlLRP expression via an antisense RNA approach (Fig. S3A). After transfection and stable selection of trophozoites deficient in GlLRP (lrp:as), the depletion of GlLRP expression by antisense production was determined by semi-quantitative RT-PCR. Quantification of the corresponding mRNA showed that the amount of Giardia lrp decreased by 83 ± 5% (n=5) in trophozoites expressing the antisense. This depletion, however, had no effect on the steady-state levels of the constitutively expressed glutamate dehydrogenase enzyme mRNA (gdh) (Fig. S3B). To study whether LDL was indeed internalized by GlLRP binding, we compared the endocytic uptake and PV delivery of Bodipy-LDL in wild-type, lrp:as (GlLRP depleted), lrp-ha (GlLRP overexpressed), and lrp-FXNPXY-ha trophozoites. A significant reduction of LDL internalization was observed when comparing wild-type (wt) and lrp:as trophozoites from up to 2 h (Fig. 8A). Conversely, an increase in the LDL internalization over time was detected for lrp-ha cells compared with wild-type (Fig. 8A). When lrp-FXNPXY-ha trophozoites were tested, the internalization of Bodipy-LDL was highly delayed showing LDL label in the PVs later than 2 hours (Fig. 8A). It is possible that this effect has been a result of the combination of LDL binding to LRP-FXNPXY-HA, which may be unable to internalize LDL, and to the native LRP. Follow up of LDL internalization in wild-type, lrp:as, lrp-ha, and lrp-FXNPXY-ha trophozoites for 24 h did not significantly change the observed pattern. Quantitative data from three different uptake experiments are shown in Fig. 8B. Finally, the controls utilizing the fluid-phase marker FITC-dextran or the PV-marker LysoTracker (Touz et al., 2003, Rivero et al., 2010) did not display differences in the fluid-phase uptake between these cells (Fig. 8C). Overall, the uptake results clearly support our hypothesis that GlLRP is involved in LDL endocytosis in this parasite.
Figure 8. The endocytosis of LDL depends of the level of GlLRP expression.
(A) Immunofluoresce microscopy shows that Bodipy-LDL is endocytosed to the PVs in wild-type trophozoites (wt) but not in lrp:as trophozoites (up to 2 h). lrp-ha cells overexpressing LRP-HA show an increase of LDL internalization over time. lrp-FXNPXY-ha trophozoites show a remarkable delay in LDL endocytosis and PV delivery. The most representative effect is shown for each type of cell. Bar, 10 μm. Nuclear DNA was labeled with DAPI (blue). DAPI: 4',6-diamidino-2-phenylindole. (B) Histograms of fluorescent images (fluorescence/area × 103) show the relative amount of Bodipy-LDL internalized by parasites in control (wt) and transgenic cells (lrp-ha, lrp- FXNPXY-ha, and lrp:as). All images were equally processed; the threshold value was determined and is exclusive in each image. The results are presented here as the average (± S.D.) of ten determinations. (C) Controls adding LysoTracker Red or FITC-dextran for 30 min shows no alteration of fluid-phase endocytosis in all cell types. Bar, 10 μm.
Reduction of GlLRP expression impairs Giardia growth
Some studies have suggested that the acquisition of cholesterol via LDL and chylomicrons is necessary to support Giardia growth (Rivero et al., 2010, Lujan et al., 1996b). Thus, it is possible that GlLRP is also required for trophozoite replication. To analyze Giardia growth in the absence of GlLRP, time-course curves were performed in wt and lrp:as cells without the addition of puromycin. Similar to the effect of LDL depletion (Rivero et al., 2010), a significant effect of lrp:as cells on the growth curve was observed at 24 h post-culturing with the maximum effect being achieved at 48 h (Fig. 9). At this time-point no cell deterioration was observed. Trophozoites transfected with an empty vector or an unrelated antisense RNA were unable to produce growth inhibition demonstrating that the effect observed might be specifically attributed to GlLRP depletion (Fig. 9).
Figure 9. GlLRP depleted cells show a decrease in growth.

Cell growth curves of wt and lrp:as trophozoites cultured in presence of LDL for 6, 12, 24, 36, and 48 h. A decrease of cell proliferation capability for lrp:as trophozoites is evident. Control cells containing the empty vector or gsp:as cells do not show any defect in cell growth comparing with wt. Data represent 2 ± s.d. for n=6 of three independent experiments.
GlLRP might be endocytosed by Giardia adaptor protein 2
The results presented strongly indicate that the binding and endocytosis of LDL depend on GlLRP. In addition, we have previously shown that LDL endocytosis was blocked by downregulation of the medium subunit (μ2) of the heterotetrameric clathrin-adaptor protein AP2 (Rivero et al., 2010). Taken together, these results suggest that AP2 might be involved in the binding and internalization of GlLRP in this parasite. To investigate whether GlLRP and μ2 directly interact, we used a yeast two-hybrid assay, in which full-length Giardia LRP or LRP-FXNPXY was expressed in frame with the activation domain encoded by pGADT7 (AD), whileμ2 was expressed in frame with the DNA binding domain encoded by pGBKT7 (BD). The expression of approximately equal levels of protein from all constructs was confirmed by immunoblots of yeast protein extracts following transformation (data not shown). The interaction of GlLRP with μ2 was observed by the growth of yeast cells on medium lacking leucine, tryptophan, and histidine (TDO), which selects for colonies expressing interacting proteins (Fig. 10A). By using a high-stringency medium that also lacked adenine (QDO), we demonstrated that this interaction was both strong and direct (Fig. 10A). Importantly, the LRP-FXNPXY-AD construct, which lacks the FXNPXY-like motif of GlLRP, exhibited no growth in combination with μ2 (Fig. 10A, TDO-QDO). To confirm the μ2-GlLRP interaction, we performed immunoprecipitation assays using lrp-ha and lrp-FXNPXY-ha trophozoites and a mAb that specifically recognizesμ2. As shown in figure 10B, LRP-HA coimmunoprecipitated with μ2 (LRP-HA, line 2) while LRP-FXNPXY-HA was unable to precipitate in concert with μ2 (LRP-FXNPXY-HA, line 2), reinforcing the idea that the endocytic motif is crucial for GlLRP-μ2 interaction.
Figure 10. GlLRP interacts with AP2 via its cytoplasmic endocytic motif.
(A) The yeast two- hybrid assay demonstrates that Giardia LRP but not LRP-FXNPXY (LRP lacking the endocytic motif) specifically interacts with μ2. β2 and the empty AD vector were used as positive and negative controls, respectively. (B) Immunoblotting using HRP-conjugated anti-HA mAb shows LRP-HA and LRP-FXNPXY-HA input before IPP (lines 1). After IPP assays using anti-μ2 mAb, LRP-HA but not LRP-FXNPXY-HA coprecipitates with μ2 (lines 2). Control using non-transfected cells in IPP shows no specific interaction (lines 3). MW standards are shown on the left of the panel in kDa.
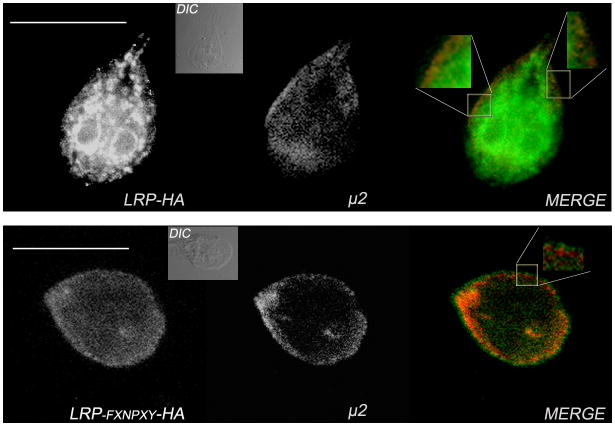
The colocalization of GlLRP and μ2 was analyzed by direct immunofluorescence and confocal microscopy utilizing the anti-μ2 mAb in lrp-ha trophozoites. Previously, we showed that μ2 localized mainly in the PVs, with some cytoplasmic and plasma membrane localization also observed in growing trophozoites (Rivero et al., 2010). Although most of the LRP-HA labeling was noted in association with the ER, partial colocalization with μ2 was detected in the PVs (Fig. 11A). An analysis of 100 cells confirmed that the colocalization was similar in all cells. Interestingly, in many of these cells, a nuclear localization of LRP-HA was detected, thus implying the participation of this receptor in cell signaling, as has been extensively described for the LRP family (see discussion). Additional studies detecting LRP-FXNPXY-HA and μ2 in lrp-FXNPXY-ha trophozoites demonstrated that LRP-FXNPXY-HA localized predominantly to the plasma membrane and did not colocalize with μ2 in the PVs (Fig. 11B). In contrast with the results obtained from the expression of LRP-HA, LRP-FXNPXY-HA was neither retained in the ER nor detected in the PVs or nuclei, suggesting an important role of the FXNPXY-like motif in GlLRP transport and subcellular localization.
Figure 11. The subcellular colocalization between GlLRP and μ2 depends on the FXNPXY-like motif.
(A) Direct IFA and confocal microscopy illustrating that LRP-HA and μ2 partially colocalize in the PVs (colocalization in yellow, magnifications) in lrp-ha trophozoites. (B) LRP- FXNPXY-HA is found predominantly at the plasma membrane and does not colocalize with μ2, neither by confocal microscopy examination nor by colocalization analysis (insert). Differential interference contrast microscopy (DIC) is depicted in the inserts. Bars, 10 μm.
DISCUSSION
The present study establishes that, in the absence of the LDLR, a protein homologous to LDLR-related protein 1 participates in the endocytosis of ApoB-lipoproteins, a process that also depends upon the clathrin-adaptor protein 2 (AP2) in Giardia. GlLRP shares structural elements with all members of the LDLR family, with the extracellular segments, which are responsible for the binding and release of their lipoprotein ligands, containing ligand-binding domains and epidermal growth factor (EGF)-like cysteine rich-repeats. The YWTD motif, which is proposed to form a β-propeller domain, is not conserved in the GlLRP sequence, thus suggesting that the lipoprotein release at low pH and the recycling of the receptor to the cell surface might be somewhat different in this parasite (Rudenko et al., 2002). GlLRP also contains a single membrane-spanning region and a cytoplasmic tail containing an FXNPXY-like motif, which serves to regulate the endocytic and signaling functions of these receptors (Goretzki & Mueller, 1998, Trommsdorff et al., 1998, Misra et al., 1999, Trommsdorff et al., 1999). Given the lack of an LDLR-homologous protein, GlLRP seems to be a reliable candidate for lipoprotein binding and cholesterol uptake by Giardia trophozoites.
As in the case of other LRP receptors, we found that GlLRP was highly proteolyzed. It is possible that GlLRP might be cleaved at a site that matches the consensus sequence recognized by furin, a processing proteinase that frequently serves to activate or modify cell surface receptors and secreted proteins upon exit from the secretory pathway (Steiner, 1998). Notably, furin also cleaves members of the Notch family of cell surface signaling proteins, which, as in LRP, contain multiple EGF repeats in their extracellular domains (Logeat et al., 1998). Although there is no experimental evidence, the furin precursor putative serine protease (GL2897) has been found in the Giardia genome and its proteolytic activity has been proposed (Touz et al., 2002a). In this sense, immunoblotting of different versions of tagged-LRP showed that the ER precursor form was partially cleaved mainly into 52 kDa and 64 kDa forms, likelyby a furin-like protease. We observed additional shedding of GlLRP and experimentally confirmed that the action of a cysteine protease was involved. Remarkably, deletion of the FXNPXY-like motif impaired GlLRP proteolysis, suggesting a key role of this motif in the process of maturation and degradation. When we analyzed the subcellular localization of full-length tagged-LRPs, the receptor was observed to be somehow retained at the ER and localized in the PVs and plasma membrane. Conversely, experiments utilizing lrp-FXNPXY-ha trophozoites revealed that the majority of the LRP-FXNPXY-HA pool was not retained at the ER before being delivered to the plasma membrane. We also observed that LRP-FXNPXY-HA showed a punctuate cytoplasmic staining, and it is possible that a portion of the immature ‘non-cleavable’ GlLRP pool did not accumulate in the ER but was rather degraded by the proteasome complex, as was recently described for LRP1 (Reekmans et al., 2010). Although LRP-FXNPXY-HA possesses several predicted ubiquitination sites (Tung & Ho, 2008), and the proteosome has been described in Giardia (Gallego et al., 2007), further studies will be necessary to show whether this mechanism of degradation applies for LRP-FXNPXY-HA. On the other hand, the LRP-FXNPXY-HA that reached the cell surface was unable to be internalized because no distinguishable PV-localization pattern was found. These results argue in favor of a mechanism where GlLRP is shortly retained at the ER for maturation and processing before being delivered to the plasma membrane. Subsequently, GlLRP is internalized via the FXNPXY-like motif to the PVs where it might be degraded or recycled back to the plasma membrane (see below).
Giardia trophozoites do not have the capacity of de novo cholesterol synthesis. Instead, they appear to satisfy their lipid requirements by obtaining cholesterol and phosphatidylcholine from the external environment in the duodenum and jejunum in vivo, and from the serum component of the modified TYI-S-33 medium in vitro (Keister, 1983). In axenic cultures, the cholesterol and phospholipids are supplied by lipoproteins, beta-cyclodextrins, and bile salts, with the transfer of lipids to the parasite surface being facilitated by bile salts (Lujan et al., 1996b). In a recent report, we showed that Giardia trophozoites are able to acquire LDL from the culture medium, with this lipoprotein being critical for parasite growth and survival (Rivero et al., 2010). Here, we showed that in vitro growth of parasites was significantly inhibited by the reduction of GlLRP expression, providing additional evidence of the importance of cholesterol acquisition from serum lipoproteins. We recently showed that the endocytosis of LDL depended on AP2, suggesting that LDL is internalized via a receptor-mediated process (Rivero et al., 2010). In the current study, we found that GlLRP plays a role in the binding and endocytosis of apoB–containing lipoproteins, probably as a mechanism for cholesterol acquisition. Subcellular localization and protein-protein interaction experiments demonstrate that LDL and GlLRP interact via ApoB, with GlLRP participating in the binding and uptake of LDL by trophozoites when the expression of the receptor was down- or up-regulated. Interestingly, we observed that GlLRP behaved like an ApoB-receptor in terms of its interactions not only with LDL, but also with chylomicrons. This observation strongly suggests that GlLRP plays a fundamental role in the uptake of chylomicrons from the host intestine and is able to recognize ApoB100 apoprotein from LDL in axenic cultures as a way of adapting to a new environment. Our findings are supported by several studies, which show the importance of LRP in the uptake of apo-B48-containing lipoproteins (Veniant et al., 1998, Yu et al., 2001) as well as in the clearance of lipoproteins in animals that are deficient in the LDL receptor (Willnow et al., 1995, Willnow & Herz, 1995, Veniant et al., 1998).
One common characteristic of LDLR family members is that they have at least one copy of the FXNPXY-like sequence in their cytoplasmic tail, which serves either as the signal for endocytosis or as a binding element for cellular adaptor proteins involved in signal transduction (Harris-White & Frautschy, 2005). Despite the fact that no LRP-like adaptor proteins that contain a PTB domain, such as ARH or Dab, have been found in the Giardia genome, we demonstrated here that GlLRP binds to the medium subunit (μ2) of AP2 and that this interaction depends on the FXNPXY-like motif. This finding is in agreement with others that show a failure in GlLRP trafficking to the PVs when it lacks the FXNPXY-like motif. Additionally, it has been shown by surface plasmon resonance and photoaffinity labeling that the FXNPXY-like motif binds to μ2 purified from bovine- brain-coated vesicles (Boll et al., 2002). Interestingly, in spite of the strong interaction found between ARH and LRP1 in an in vitro binding assay, the subcellular localization of LRP1 was not affected in the liver of ARH-deficient mice, whereas LDLR was found to be redistributed from intracellular localizations to the cell membrane (Jones et al., 2003). This effect underscores the importance of the availability of intracellular adaptor proteins in the determination of the specific cellular function of lipoprotein receptors.
Given the similarities between Giardia LRP and other members of the LRP family, it is also possible that this protein binds several other substrates essential for growth, such as growth factors (Lujan et al., 1994) or proteases. Therefore, it is possible that GlLRP is multifunctional and is involved in both receptor-mediated endocytosis (which leads to the degradation of the endocytosed material) and in cell signaling. In mammalian cells, proteolytic cleavage of LRP, either within the membrane by gamma-secretase activity or close to the cytoplasmic leaflet, results in the release of this domain into the cytoplasm, where it modulates signaling or is further translocated to other subcellular compartments (Liu et al., 2007). Our preliminary results suggest that the degradation of GlLRP might in part be due to the action of a gamma-secretase-like complex (Rivero, unpublished results). Further investigations are required to reveal if GlLRP provides crosstalk with the signaling pathways to regulate cell growth and differentiation.
In summary, results from the current investigation reveal that a receptor containing the characteristics of an LDLR family member is present in Giardia trophozoites. This receptor (GlLRP) is implicated in the binding and internalization of lipoproteins showing once more the capacity of this parasite to acquire essential components from different environments. It is, however, important to assess the binding of other ligands as well. An important subject for future studies will be to analyze whether GlLRP binding and processing are involved in transcription regulation or activation of components of the signaling pathways during Giardia growth and differentiation.
EXPERIMENTAL PROCEDURES
Antibodies and other reagents
Anti-HA, HRP-labeled anti-HA, alkaline phosphatase(AP)-labeled anti-HA, and FITC-labeled anti-HA mAbs were purchased from Sigma (St. Louis, MO). 9C9 mAb was employed to detect the ER-BiP protein (Lujan et al., 1996a). 9B10 mAb was used for the detection of VSP9B10 (Nash et al., 2001). Anti-μ2 mAb 2F5 was used for the μ2 subunit of AP2 (Rivero et al., 2010). Alexa Fluor 555 was used for the primary antibody label (Zenon Tricolor Mouse IgG1 Labeling Kit, Molecular Probes, Invitrogen, Carlsbad, CA). Bodipy-labeled LDL was purchased from Molecular Probes-Invitrogen. Human chylomicrons were acquired from Athens Research & Technology. The L-trans-epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and phenylmethylsulfonyl fluoride (PMSF) were purchased from Sigma (St. Louis, MO). Saponin and Triton X-100 were also purchased from Sigma (St. Louis, MO).. LysoSensorTM Green DND-189 and LysoTrackerTM Red DND-99 used to label PVs were acquired from Molecular Probes-Invitrogen (Carlsbad, CA). The 20 kDa FITC-dextran was purchased from Sigma (St. Louis, MO).
Sequence Alignment
MUSCLE, ClustalW2 and manual curation were iteratively used to produce the pairwise alignment between Giardia LRP and human LDLR and LRP1 sequences. Jalview was used to manually curate and produce publication images.
Structure modelling
Modeller 9v7 was used to model putative GlLRP calcium-binding pockets using pdb 1N7D as a template (Sali et al., 1995). Modelling was performed using symmetry restraints for identical residues between the template and model sequences which interact with calcium atoms. One hundred models were built for each pocket, and the model with the best Modeller objective function values and DOPE scores was depicted (Shen & Sali, 2006).
Giardia cell lines and vectors
Trophozoites of the isolate WB, clone 1267 (Nash et al., 1988) were cultured in TYI-S-33 medium supplemented with 10% adult bovine serum and 0.5 mg/ml bovine bile, as previously described (Keister, 1983). These trophozoites were used as hosts for the expression of transgenic genes and as non-transfected controls. The GlLRP open reading frame was amplified from genomic DNA using the f1 (CATTCCATGGATGCAGTGATCTACGGATTGCTTGTC) and r1 (CATTGATATCATATGTGGGGCTATTGAAGTCTTCAT) primers and cloned into the plasmid pTubHAc-pac (Touz et al., 2003) to generate the pLRP-HA vector. LRP cDNA lacking the endocytic motif was amplified using the f1 and r1’ (CATTGATATCGAAGTCTTCATTGCTTC) primers and cloned into the pTubHAc-pac vector to generate the pLRP-FXNPXY-HA expression plasmid. To generate the N-terminus HA-tagged GlLRP, a lrp ORF lacking the signal peptide was amplified by using the f2 (CAATACGCGTACCTACATACTGCTATCTCTGTTGTTGA) and r2 (CAATGCGGCCGCCTAATATGTGGGGCTATTGAAGTCTTC) primers and cloned into the pTubH7Pac-HA vector (Kulakova et al., 2006). All vectors contained a puromycin cassette under the control of the endogenous non-regulated gdh promoter for cell selection. Stable trophozoite transfection was performed as previously described (Touz et al., 2005, Touz et al., 2004, Elmendorf et al., 2001, Singer et al., 1998, Yee & Nash, 1995). Drug-resistant trophozoites were usually apparent by 7–10 days post-transfection.
Inhibitor assays
The activities of furin and cysteine proteases were monitored using the specific inhibitors PMSF and E64, respectively. Briefly, 104 lrp-ha, ha-lrp, and lrp-FXNPXY-ha trophozoites were grown in complete medium containing 170 μg/ml of PMSF, 10 E64 μg/ml, or DMSO (for control) for 24 h (Touz et al., 2002b). The trophozoites were then chilled, collected by centrifugation and analyzed by immunoblotting.
Immunoblot analysis
Immunoblot assays were performed as previously reported (Touz et al., 2005). Briefly, 10 μg of total protein was incubated with sample buffer, boiled for 10 min, and separated in 10% Bis-Tris gels. Samples were transferred to nitrocellulose membranes, blocked with 5% skim milk and 0.1% Tween-20 in TBS, and then incubated with primary antibody diluted in the same buffer. After washing and incubation with an enzyme-conjugated secondary antibody, proteins were visualized with the SuperSignal West Pico Chemiluminescent Substrate (Pierce) and autoradiography, or by using BCIP/NBT substrate (BioRad). Controls included the omission of the primary antibody, the use of an unrelated antibody, or assays using non-transfected cells.
Immunofluorescence assay
Trophozoites were washed with PBSm (1% growth medium in PBS, pH 7.4) and allowed to attach themselves to slides at 37°C. After fixation with 4% formaldehyde, the cells were washed and blocked with PBS containing 10% normal goat serum and 0.1% Triton X-100. The cells were then incubated with specific Abs in PBS containing 3% normal goat serum and 0.1% Triton-X100, followed by incubation with FITC or Texas Red-conjugated goat anti-mouse secondary antibody. For cell surface localization of LRP-HA and LRP-FXNPXY–HA, the transgenic cells were blocked and the plasma membrane selectively permeabilized with 0.05% saponin (Wassler et al., 1987, Delcamp et al., 1998, Newman et al., 2009). For surface staining of HA-LRP, detergent treatment was omitted. For direct double staining, the anti-HA mAb (Sigma, St. Louis, MO) was labeled with Zenon Alexa Fluor 488 and was used to detect HA-tagged LRP (final dilution of anti-HA 1:500), while 9C9, 2F5, and 9B10 mAbs were labeled with Zenon Alexa Fluor 555 (1:200 final dilution), following the manufacturer suggested protocol (Zenon Tricolor Mouse IgG1 Labeling Kit, Molecular Probes, Invitrogen Corporation, Carlsbad, CA). Controls included the omission of the primary antibody and the staining of wild-type cells. Finally, preparations were washed and mounted in Vectashield mounting medium. Fluorescence staining was visualized with a motorized FV1000 Olympus confocal microscope (Olympus UK Ltd, UK), using 63× or 100× oil immersion objectives (NA 1.32, zoom X). The fluorochromes were excited using an argon laser at 488 nm and a krypton laser at 568 nm. DAPI was excited with ultraviolet light using a 364 nm Argon laser. Detector slits were configured to minimize crosstalk between the channels. Differential interference contrast images were collected simultaneously with the fluorescence images, by the use of a transmitted light detector. Images were processed using FV10-ASW 1.4 Viewer and Adobe Photoshop 8.0 (Adobe Systems) software. Colocalization and deconvolution were performed using MetaMorph software (Molecular Devices, Silicon Valley, CA). Fluorescent images were observed with an inverted microscope (Carl Zeiss Axiovert 35M) equipped with epifluorescence and differential interference contrast (DIC) optics using a 100× oil immersion objectives (Carl Zeiss) and were captured under regular fluorescence microscopy with a silicon-intensified target camera (SIT-C2400; Hamamatsu Phototonics, Bridgewater, NJ). The images were digitized directly into a Metamorph/Metafluor Image Processor (Universal Imaging Corporation, West Chester, PA).
Total internal reflection fluorescence microscopy (TIRFM)
ha-lrp trophozoites were grown in complete medium, collected, fixed using 1% glutaraldehyde, stained with FITC-labeled anti-HA mAb (diluted 1:200) followed by a second fixation with 1% glutaraldehyde/0.1 M glycine and attached to polylysine-treated cover slips. Alternatively, the lrp-ha and lrp-FXNPXY-ha trophozoites were permeabilized with 0.05% saponin in PBS after the first glutaraldehyde fixation and then processed as described. For positive and negative controls, anti-VSP9B10 mAb and the PV-marker LysoSensor were used, respectively, in cells fixed and treated with saponin before TIRFM. Trophozoites were observed using a 60x 1.45 numerical aperture objective equipped for through-the-objective TIRF illumination using a 488 nm argon laser on a Nikon TE2000-U microscope with filter cubes optimized for fluorescein/GFP (Chroma Technology, Rockingham, VT). Images were captured with a cooled CCD ORCA II-ER (HAMAMATSU Photonics K.K., Shizuoka, Japan) camera and MetaMorph software (Molecular Devices, Silicon Valley, CA).
Bodipy-LDL
For colocalization of LDL/GlLRP, lrp-ha trophozoites were cultured for two days in a medium deficient in lipoprotein (Sigma), followed by a medium exchange for labeling buffer (50 mM glucose, 10 mM cysteine, 2 mM ascorbic acid in PBS, pH 7.1) containing 7.5 μg Bodipy-labeled LDL. After 30 min at 37°C, the trophozoites were fixed, labeled with Alexa Fluor 555-anti-HA mAb, and visualized by confocal microscopy. Twenty confocal sections of 0.4 μm were taken parallel to the coverslip (z sections).
Immunoprecipitation assay (IPP)
Wild-type, lrp-ha, ha-lrp, and lrp-FXNPXY-ha trophozoites previously challenged with 10 μg/ml of Bodipy-LDL (Invitrogen) or 100 μg/ml of chylomicrons (Athens Research & Technology) were harvested and resuspended in 1 ml of cold lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 1% Triton X-100) for 1 h at 4°C. The lysate was centrifuged at 10,000 x g for 10 min at 4°C, and the supernatant mixed with anti-ApoB mAb and incubated overnight at 4°C. Protein A-agarose beads (50 μl; Qiagen, Valencia, CA) were added to each sample and incubated for 4 h at 4°C. Beads were pelleted at 700 x g and washed four times with washing buffer (50 mM NaH2PO4, pH 8.0; 300 mM NaCl; 0.1% Triton X-100). Beads were resuspended in sample buffer and boiled for 10 min before immunoblot analysis using AP-labeled anti-HA mAb. Controls included testing of transgenic cell without addition of LDL or chylomicrons. Also, wild-type cells and a non-related mAb were used as controls of this assay. The IPP assay was repeated by growing the cells in growth medium containing 170 μg/ml of PMSF before lipoprotein challenge and processed as described above. For the IPP shown in figure 10B, no inhibitors were used. In this assay the 2F5 mAb was utilized to immunoprecipitate μ2.
GlLRP downregulation
1-2 2 4 4 of the gllrp ORF was amplified using the f3 (CATTGATATCATGCATGCAGTGATCTACGGATTGCTTGTC) and r3 (CATTCCATGGTTAGTGGTGGGAGGAATAGAGCA) primers, restricted with EcoRV and NcoI enzymes and ligated to pTubHAc-pac vector in the opposite direction, resulting in the antisense vector that was used for inhibition of GlLRP expression. The pLRP-AS vector was used to stably transfect the Giardia clone WB1267, producing lrp:as cells deficient in GlLRP. Antisense production as well as gllrp depletion was confirmed by RT-PCR before performing the uptake experiments.
Semi-quantitative RT-PCR
The total RNA from wild-type and lrp:as cells was isolated using Trizol reagent (Invitrogen), and a second purification was performed using the SV Total RNA Isolation System (Promega). Semi-quantitative (sq) RT-PCR was performed as previously described (Rivero et al., 2010). For detection of endogenous gllrp mRNA, the f4 (CGTGCGCATCACCTTTTACGATAGTAT) and r1 primers corresponding to the 3’ 2451-3213 nt were used. To detect the gllrp antisense, the r3 primer was added during the reverse transcription step, followed by the addition of the f3 primer for PCR amplification. These assays were performed four times in duplicates.
Uptake experiments
The Bodipy-LDL uptake assays were performed as reported (Rivero et al., 2010). Briefly, wild-type, lrp:as, ha-lrp, and lrp-FXNPXY-ha trophozoites were cultured for two days in medium deficient in lipoprotein and the uptake was performed by exchanging the medium for labeling buffer containing 7.5 μg Bodipy-labeled LDL. After different time periods at 37°C, the trophozoites were washed and visualized by fluorescence microscopy. All images were acquired and processed identically. Fluorescence images were measured by Metamorph software version 7.0. All the images were equally processed; the threshold value was determined and exclusive in each image. Controls for this experiment included the addition of 2 mg/ml FITC-dextran (Sigma) or 10 μM of LysoTracker Red DND-99 (Molecular Probes-Invitrogen) to the tested trophozoites for 30 min.
Growth curve
Tubes containing 7 ml of growth medium were inoculated with 104 trophozoites from wild-type (wt) or GlLRP antisense transgenic (lrp:as) trophozoites logarithmic phase cultures. Every 6 h, the medium was decanted and the tubes were chilled on ice for 20 min to detach adherent living trophozoites. The number of viable cells was determined by counting on a hemocytometer. Cells expressing the empty antisense vector or a non-related GSP:antisense vector (Touz et al., 2002a) were used as control.
Yeast two-hybrid assay
The MATCHMAKER Two-Hybrid System was used following the manufacturer’s recommended protocol (Clontech, Palo Alto, CA). The two-hybrid pGADT7-Rec(LEU2) vector (GAL4 transcription activation domain; AD) containing the sequences for gllrp or lrp-FXNPXY (lacking the FXNPXY-like motif sequence (3’-AATAGCCCCACATAT) were used as bait, while μ2 gene was inserted into the pGBKT7(TRP1) vector (GAL4 DNA binding domain; BD), yielding the pLRP-AD, pLRP-FXNPXY-AD, and pμ2-BD vectors, respectively. The AH109 transformants were cultured at 30°C for 4–5 days on plates with minimal medium lacking leucine and tryptophan (-L/-T) to test for positive transformation, or in the absence of leucine, tryptophan, and histidine (TDO, triple dropout medium) to study specific protein interactions as previously described (Touz et al., 2004). Controls included the pβ2-AD/pμ2-BD interaction (positive control) and the empty pGADT7/pμ2-BD vector (negative control).
Statistics
Descriptive statistics included the calculation of the means and standard deviations of the control and experimental groups. A comparison of the means was performed using the Independent-Samples Student’s t-test from the SPSS Statistic program. A p≤0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr Mariano Bisbal and Ignacio Jausoro for TIRF microscopy assistance. We also thank members of our laboratory for helpful discussions. The project was supported by Grant Number R01TW00724 from the Fogarty International Center. The content is solely the responsibility of the authors and does not necessary represent the official views of the Fogarty International Center or the National Institutes of Health. This research was also supported in part by the Academy of Science for the Developing World (TWAS), Agencia Nacional para la Promoción de la Ciencia y Tecnología (FONCyT) PICT2004-PICT2007, and the National Council for Sciences and Technology (CONICET) PIP2005-6563.
References
- Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev. 2001;14:447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth JL, Kelly V, Rock MJ, Shuttleworth CA, Kielty CM. Regulation of fibrillin carboxy-terminal furin processing by N-glycosylation, and association of amino-and carboxy-terminal sequences. J Cell Sci. 1999a;112(Pt 22):4163–4171. doi: 10.1242/jcs.112.22.4163. [DOI] [PubMed] [Google Scholar]
- Ashworth JL, Murphy G, Rock MJ, Sherratt MJ, Shapiro SD, Shuttleworth CA, Kielty CM. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J. 1999b;340(Pt 1):171–181. [PMC free article] [PubMed] [Google Scholar]
- Axelrod D. Total internal reflection fluorescence microscopy. Methods Cell Biol. 1989;30:245–270. doi: 10.1016/s0091-679x(08)60982-6. [DOI] [PubMed] [Google Scholar]
- Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 2003;361:1–33. doi: 10.1016/s0076-6879(03)61003-7. [DOI] [PubMed] [Google Scholar]
- Boll W, Rapoport I, Brunner C, Modis Y, Prehn S, Kirchhausen T. The mu2 subunit of the clathrin adaptor AP-2 binds to FDNPVY and YppO sorting signals at distinct sites. Traffic. 2002;3:590–600. doi: 10.1034/j.1600-0854.2002.30808.x. [DOI] [PubMed] [Google Scholar]
- Delcamp TJ, Dales C, Ralenkotter L, Cole PS, Hadley RW. Intramitochondrial [Ca2+] and membrane potential in ventricular myocytes exposed to anoxia-reoxygenation. Am J Physiol. 1998;275:H484–494. doi: 10.1152/ajpheart.1998.275.2.H484. [DOI] [PubMed] [Google Scholar]
- Duckert P, Brunak S, Blom N. Prediction of proprotein convertase cleavage sites. Protein Eng Des Sel. 2004;17:107–112. doi: 10.1093/protein/gzh013. [DOI] [PubMed] [Google Scholar]
- Elmendorf HG, Singer SM, Pierce J, Cowan J, Nash TE. Initiator and upstream elements in the alpha2-tubulin promoter of Giardia lamblia. Mol Biochem Parasitol. 2001;113:157–169. doi: 10.1016/s0166-6851(01)00211-0. [DOI] [PubMed] [Google Scholar]
- Gallego E, Alvarado M, Wasserman M. Identification and expression of the protein ubiquitination system in Giardia intestinalis. Parasitol Res. 2007;101:1–7. doi: 10.1007/s00436-007-0458-2. [DOI] [PubMed] [Google Scholar]
- Goretzki L, Mueller BM. Low-density-lipoprotein-receptor-related protein (LRP) interacts with a GTP-binding protein. Biochem J. 1998;336(Pt 2):381–386. doi: 10.1042/bj3360381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-White ME, Frautschy SA. Low density lipoprotein receptor-related proteins (LRPs), Alzheimer's and cognition. Curr Drug Targets CNS Neurol Disord. 2005;4:469–480. doi: 10.2174/156800705774322102. [DOI] [PubMed] [Google Scholar]
- Herz J, Hamann U, Rogne S, Myklebost O, Gausepohl H, Stanley KK. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. Embo J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Kowal RC, Goldstein JL, Brown MS. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. Embo J. 1990;9:1769–1776. doi: 10.1002/j.1460-2075.1990.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon H, Meng W, Takagi J, Eck MJ, Springer TA, Blacklow SC. Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol. 2001;8:499–504. doi: 10.1038/88556. [DOI] [PubMed] [Google Scholar]
- Jones C, Hammer RE, Li WP, Cohen JC, Hobbs HH, Herz J. Normal sorting but defective endocytosis of the low density lipoprotein receptor in mice with autosomal recessive hypercholesterolemia. J Biol Chem. 2003;278:29024–29030. doi: 10.1074/jbc.M304855200. [DOI] [PubMed] [Google Scholar]
- Kaul D, Kaur M. Receptor-Ck regulates membrane-bound 125 kDa protein having affinity for genomic sterol regulatory sequence. Mol Cell Biochem. 2001;216:141–143. doi: 10.1023/a:1011044503773. [DOI] [PubMed] [Google Scholar]
- Keister DB. Axenic culture of Giardia lamblia in TYI–S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- Kulakova L, Singer SM, Conrad J, Nash TE. Epigenetic mechanisms are involved in the control of Giardia lamblia antigenic variation. Mol Microbiol. 2006;61:1533–1542. doi: 10.1111/j.1365-2958.2006.05345.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Cam J, Bu G. Low-density lipoprotein receptor family: endocytosis and signal transduction. Mol Neurobiol. 2001;23:53–67. doi: 10.1385/MN:23:1:53. [DOI] [PubMed] [Google Scholar]
- Liu CX, Ranganathan S, Robinson S, Strickland DK. gamma-Secretase-mediated release of the low density lipoprotein receptor-related protein 1B intracellular domain suppresses anchorage-independent growth of neuroglioma cells. J Biol Chem. 2007;282:7504–7511. doi: 10.1074/jbc.M608088200. [DOI] [PubMed] [Google Scholar]
- Bessia C, Brou C, LeBail O, Jarriault S, Seidah NG, Israel A. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc Natl Acad Sci U S A. 1998;95:8108–8112. doi: 10.1073/pnas.95.14.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Conrad JT, Nash TE. Increased expression of the molecular chaperone BiP/GRP78 during the differentiation of a primitive eukaryote. Biol Cell. 1996a;86:11–18. doi: 10.1111/j.1768-322x.1996.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Helman LJ, Nash TE. Insulin-like growth factors stimulate growth and L-cysteine uptake by the intestinal parasite Giardia lamblia. J Biol Chem. 1994;269:13069–13072. [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Nash TE. Lipid requirements and lipid uptake by Giardia lamblia trophozoites in culture. J Eukaryot Microbiol. 1996b;43:237–242. doi: 10.1111/j.1550-7408.1996.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Misra UK, Gawdi G, Gonzalez-Gronow M, Pizzo SV. Coordinate regulation of the alpha(2)-macroglobulin signaling receptor and the low density lipoprotein receptor-related protein/alpha(2)-macroglobulin receptor by insulin. J Biol Chem. 1999;274:25785–25791. doi: 10.1074/jbc.274.36.25785. [DOI] [PubMed] [Google Scholar]
- Nash TE, Aggarwal A, Adam RD, Conrad JT, Merritt JW., Jr Antigenic variation in Giardia lamblia. J Immunol. 1988;141:636–641. [PubMed] [Google Scholar]
- Nash TE, Lujan HT, Mowatt MR, Conrad JT. Variant-specific surface protein switching in Giardia lamblia. Infect Immun. 2001;69:1922–1923. doi: 10.1128/IAI.69.3.1922-1923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman ZL, Leppla SH, Moayeri M. CA-074Me protection against anthrax lethal toxin. Infect Immun. 2009;77:4327–4336. doi: 10.1128/IAI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermoeller-McCormick LM, Li Y, Osaka H, FitzGerald DJ, Schwartz AL, Bu G. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J Cell Sci. 2001;114:899–908. doi: 10.1242/jcs.114.5.899. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, LaDu MJ, Estus S, Bu G, Weeber EJ. The generation and function of soluble apoE receptors in the CNS. Mol Neurodegener. 2006;1:15. doi: 10.1186/1750-1326-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reekmans SM, Pflanzner T, Gordts PL, Isbert S, Zimmermann P, Annaert W, Weggen S, Roebroek AJ, Pietrzik CU. Inactivation of the proximal NPXY motif impairs early steps in LRP1 biosynthesis. Cell Mol Life Sci. 2010;67:135–145. doi: 10.1007/s00018-009-0171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero MR, Vranych CV, Bisbal M, Maletto BA, Ropolo AS, Touz MC. Adaptor Protein 2 Regulates Receptor-Mediated Endocytosis and Cyst Formation in Giardia lamblia. Biochem J. 2010 doi: 10.1042/BJ20100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G, Henry L, Henderson K, Ichtchenko K, Brown MS, Goldstein JL, Deisenhofer J. Structure of the LDL receptor extracellular domain at endosomal pH. Science. 2002;298:2353–2358. doi: 10.1126/science.1078124. [DOI] [PubMed] [Google Scholar]
- Sali A, Potterton L, Yuan F, van Vlijmen H, Karplus M. Evaluation of comparative protein modeling by MODELLER. Proteins. 1995;23:318–326. doi: 10.1002/prot.340230306. [DOI] [PubMed] [Google Scholar]
- Shen MY, Sali A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006;15:2507–2524. doi: 10.1110/ps.062416606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer SM, Yee J, Nash TE. Episomal and integrated maintenance of foreign DNA in Giardia lamblia. Mol Biochem Parasitol. 1998;92:59–69. doi: 10.1016/s0166-6851(97)00225-9. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Takeda T, Yamazaki H, Farquhar MG. Identification of an apical sorting determinant in the cytoplasmic tail of megalin. Am J Physiol Cell Physiol. 2003;284:C1105–1113. doi: 10.1152/ajpcell.00514.2002. [DOI] [PubMed] [Google Scholar]
- Touz MC, Conrad JT, Nash TE. A novel palmitoyl acyl transferase controls surface protein palmitoylation and cytotoxicity in Giardia lamblia. Mol Microbiol. 2005;58:999–1011. doi: 10.1111/j.1365-2958.2005.04891.x. [DOI] [PubMed] [Google Scholar]
- Touz MC, Gottig N, Nash TE, Lujan HD. Identification and characterization of a novel secretory granule calcium-binding protein from the early branching eukaryote Giardia lamblia. J Biol Chem. 2002a;277:50557–50563. doi: 10.1074/jbc.M202558200. [DOI] [PubMed] [Google Scholar]
- Touz MC, Kulakova L, Nash TE. Adaptor protein complex 1 mediates the transport of lysosomal proteins from a Golgi-like organelle to peripheral vacuoles in the primitive eukaryote Giardia lamblia. Mol Biol Cell. 2004;15:3053–3060. doi: 10.1091/mbc.E03-10-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touz MC, Lujan HD, Hayes SF, Nash TE. Sorting of encystation-specific cysteine protease to lysosome-like peripheral vacuoles in Giardia lamblia requires a conserved tyrosine-based motif. J Biol Chem. 2003;278:6420–6426. doi: 10.1074/jbc.M208354200. [DOI] [PubMed] [Google Scholar]
- Touz MC, Nores MJ, Slavin I, Carmona C, Conrad JT, Mowatt MR, Nash TE, Coronel CE, Lujan HD. The activity of a developmentally regulated cysteine proteinase is required for cyst wall formation in the primitive eukaryote Giardia lamblia. J Biol Chem. 2002b;277:8474–8481. doi: 10.1074/jbc.M110250200. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273:33556–33560. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Tung CW, Ho SY. Computational identification of ubiquitylation sites from protein sequences. BMC Bioinformatics. 2008;9:310. doi: 10.1186/1471-2105-9-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniant MM, Zlot CH, Walzem RL, Pierotti V, Driscoll R, Dichek D, Herz J, Young SG. Lipoprotein clearance mechanisms in LDL receptor-deficient "Apo-B48-only" and "Apo-B100-only" mice. J Clin Invest. 1998;102:1559–1568. doi: 10.1172/JCI4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron E, Heilig C, Schweitzer A, Nadella N, Jaeger S, Martin AM, Weggen S, Brix K, Pietrzik CU. LRP1 modulates APP trafficking along early compartments of the secretory pathway. Neurobiol Dis. 2008;31:188–197. doi: 10.1016/j.nbd.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Ward DM, Ajioka R, Kaplan J. Cohort movement of different ligands and receptors in the intracellular endocytic pathway of alveolar macrophages. J Biol Chem. 1989;264:8164–8170. [PubMed] [Google Scholar]
- Wassler M, Jonasson I, Persson R, Fries E. Differential permeabilization of membranes by saponin treatment of isolated rat hepatocytes. Release of secretory proteins. Biochem J. 1987;247:407–415. doi: 10.1042/bj2470407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow TE, Armstrong SA, Hammer RE, Herz J. Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc Natl Acad Sci U S A. 1995;92:4537–4541. doi: 10.1073/pnas.92.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow TE, Herz J. Animal models for disorders of hepatic lipoprotein metabolism. J Mol Med. 1995;73:213–220. doi: 10.1007/BF00189920. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Nykjaer A, Herz J. Lipoprotein receptors: new roles for ancient proteins. Nat Cell Biol. 1999;1:E157–162. doi: 10.1038/14109. [DOI] [PubMed] [Google Scholar]
- Yee J, Nash TE. Transient transfection and expression of firefly luciferase in Giardia lamblia. Proc Natl Acad Sci U S A. 1995;92:5615–5619. doi: 10.1073/pnas.92.12.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KC, Chen W, Cooper AD. LDL receptor-related protein mediates cell-surface clustering and hepatic sequestration of chylomicron remnants in LDLR-deficient mice. J Clin Invest. 2001;107:1387–1394. doi: 10.1172/JCI11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemskov EA, Mikhailenko I, Strickland DK, Belkin AM. Cell-surface transglutaminase undergoes internalization and lysosomal degradation: an essential role for LRP1. J Cell Sci. 2007;120:3188–3199. doi: 10.1242/jcs.010397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.