Abstract
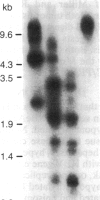
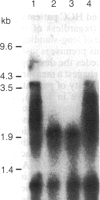
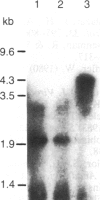
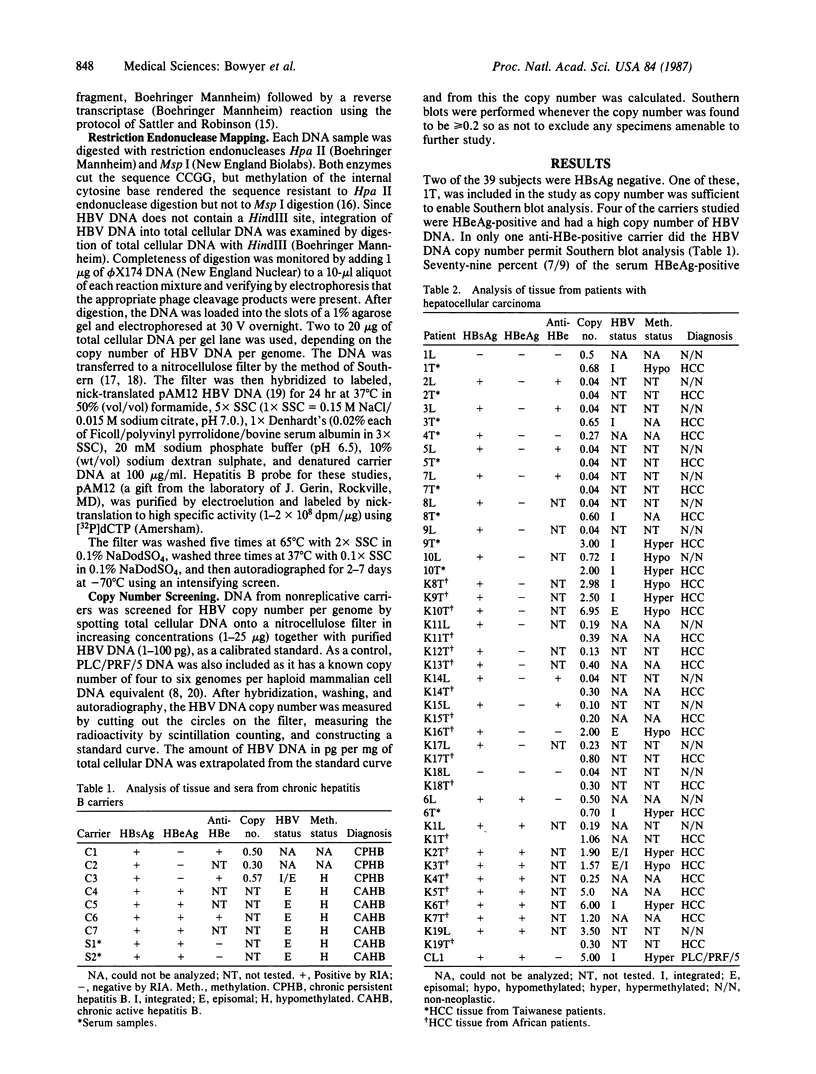
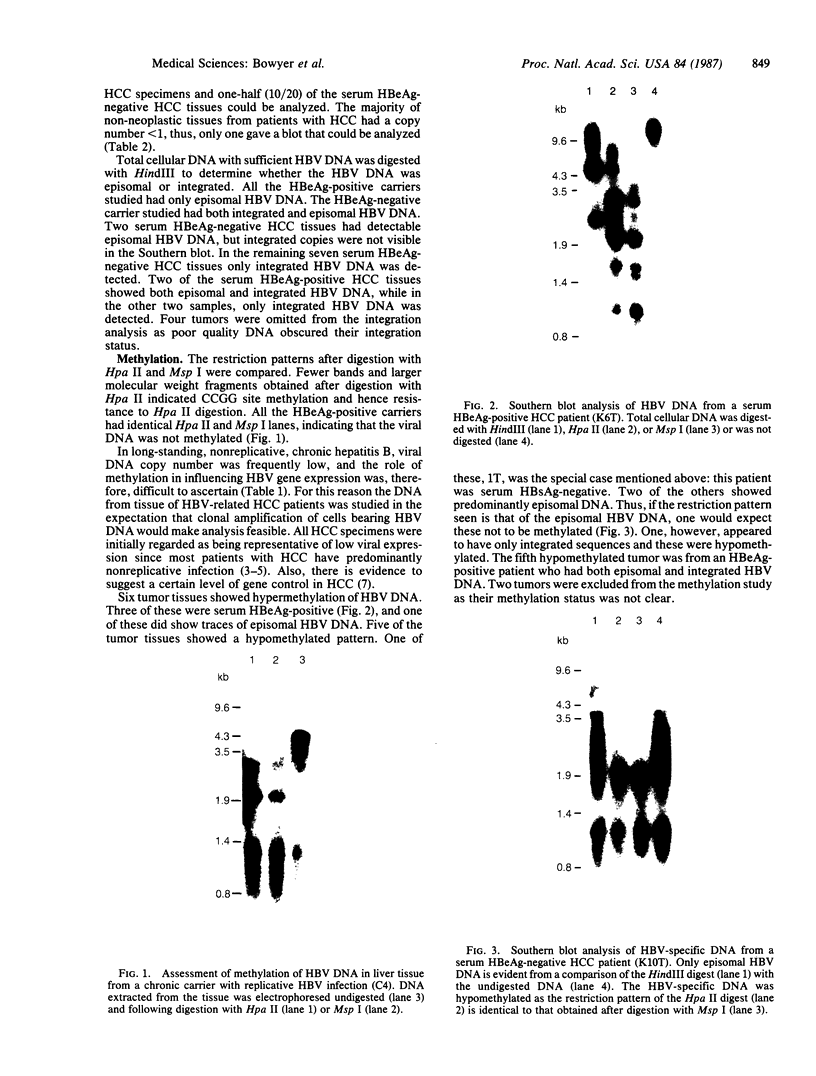
We examined the methylation status of CCGG sites in hepatitis B virus (HBV) DNA to determine whether methylation could be responsible for the selective expression of the HBV surface gene in chronic hepatitis B infection and hepatocellular carcinoma. Infected liver tissue from patients with low levels of viral replication was analyzed for HBV DNA copy number per haploid cell genome. Total cellular DNA, with sufficient HBV DNA, was digested with the restriction endonucleases Msp I and Hpa II, to determine whether the HBV DNA was methylated, or HindIII, to determine whether the HBV DNA was integrated or episomal. The cleavage fragments were analyzed by Southern blotting and hybridization to 32P-labeled HBV DNA. In replicative chronic hepatitis B, hypomethylation of the HBV genome correlated with HBV expression in both virions and infected tissue. In carriers with nonreplicative infection, it was difficult to ascertain the role of methylation as copy number was low. HBV DNA copy number was also low in 17 out of 29 of the tumor tissues tested and as many as 14 out of 16 of the adjacent non-neoplastic tissues tested. Integrated sequences were hypermethylated in the PLC/PRF/5 cell line and in six of the tumor tissues suggesting that methylation plays a role in HBV gene repression. However, since DNA from five other tumors was hypomethylated, the belief that methylation per se is an absolute determinant of HBV core gene repression does not hold for human hepatocellular carcinoma tissue. Additional factors, such as gene rearrangements, therefore, must influence HBV expression in hepatocellular carcinoma.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonino F., Hoyer B., Nelson J., Engle R., Verme G., Gerin J. Hepatitis B virus DNA in the sera of HBsAg carriers: a marker of active hepatitis B virus replication in the liver. Hepatology. 1981 Sep-Oct;1(5):386–391. doi: 10.1002/hep.1840010503. [DOI] [PubMed] [Google Scholar]
- Brechot C., Hadchouel M., Scotto J., Degos F., Charnay P., Trepo C., Tiollais P. Detection of hepatitis B virus DNA in liver and serum: a direct appraisal of the chronic carrier state. Lancet. 1981 Oct 10;2(8250):765–768. doi: 10.1016/s0140-6736(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Dejean A., Carloni G., Bréchot C., Tiollais P., Wain-Hobson S. Organization and expression of hepatitis B sequences cloned from hepatocellular carcinoma tissue DNA. J Cell Biochem. 1982;20(3):293–301. doi: 10.1002/jcb.240200309. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C. Specifically unmethylated cytidylic-guanylate sites in Herpesvirus saimiri DNA in tumor cells. J Virol. 1982 Aug;43(2):427–435. doi: 10.1128/jvi.43.2.427-435.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Gray P., Valenzuela P., Rall L. B., Rutter W. J. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature. 1980 Jul 31;286(5772):535–538. doi: 10.1038/286535a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M., Mohandas T., Shapiro L. J. Cell cycle-specific reactivation of an inactive X-chromosome locus by 5-azadeoxycytidine. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1215–1219. doi: 10.1073/pnas.79.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew M. C., Desmyter J., De Groote G., Frösner G., Roggendorf M., Deinhardt F. Hepatocellular cancer in Southern African blacks: HBeAg, anti-HBe, IgM-anti-HBc and other markers of hepatitis B. Prog Med Virol. 1981;27:41–48. [PubMed] [Google Scholar]
- Marion P. L., Salazar F. H., Alexander J. J., Robinson W. S. State of hepatitis B viral DNA in a human hepatoma cell line. J Virol. 1980 Feb;33(2):795–806. doi: 10.1128/jvi.33.2.795-806.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Robinson W. S. Integrated hepatitis B virus DNA sequences specifying the major viral core polypeptide are methylated in PLC/PRF/5 cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2534–2538. doi: 10.1073/pnas.80.9.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sattler F., Robinson W. S. Hepatitis B viral DNA molecules have cohesive ends. J Virol. 1979 Oct;32(1):226–233. doi: 10.1128/jvi.32.1.226-233.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Dusheiko G. M., Bowyer S., Kew M. C. Hepatitis B virus replication in southern Africa blacks with HBsAg-positive hepatocellular carcinoma. Hepatology. 1984 Jul-Aug;4(4):608–610. doi: 10.1002/hep.1840040405. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor E., Gerety R. J., Vogel C. L., Bayley A. C., Anthony P. P., Chan C. H., Barker L. F. Hepatitis B virus infection and primary hepatocellular carcinoma. J Natl Cancer Inst. 1977 May;58(5):1197–1200. doi: 10.1093/jnci/58.5.1197. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Akahane Y., Gotanda T., Mishiro T., Imai M., Miyakawa Y., Mayumi M. Demonstration of hepatitis B e antigen in the core of Dane particles. J Immunol. 1979 Jan;122(1):275–279. [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978 Sep;5(9):3231–3236. doi: 10.1093/nar/5.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G. H., Korba B. E., Lechner J. F., Tokiwa T., Gazdar A. F., Seeley T., Siegel M., Leeman L., Autrup H., Harris C. C. High-frequency transfection and cytopathology of the hepatitis B virus core antigen gene in human cells. Science. 1983 Oct 28;222(4622):385–389. doi: 10.1126/science.6194563. [DOI] [PubMed] [Google Scholar]