Abstract
Zinc is essential for many biological processes, including proper functioning of gametes. We recently reported that zinc levels rise by over 50% during oocyte maturation and that attenuation of zinc availability during this period could be achieved using the membrane-permeable heavy metal chelator N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN). This zinc insufficiency resulted in formation of large polar bodies, failure to establish metaphase II arrest, and impaired establishment of cortical polarity. As these phenotypes resemble those of MOS null oocytes, we examined the impact of zinc insufficiency on the MOS-MAPK pathway. Reduced levels of both MOS protein and phosphorylation of MAP2K1/2 are observed in zinc-insufficient oocytes; however, these differences appear only after completion of the first meiotic division. In addition, activation of the downstream effector of the MOS pathway, MAPK3/1, is not affected by zinc insufficiency, and reduced MOS levels are observed only with the presence of TPEN after the first polar body extrusion. These data are inconsistent with the hypothesis that reduced MOS mediates the observed phenotype. Finally, MOS overexpression does not rescue the phenotype of zinc-insufficient oocytes, confirming that the observed disruption of asymmetric division and spindle abnormalities cannot be attributed to impaired MOS signaling. Zinc-insufficient oocytes do not increase maturation promoting factor (MPF) activity following the first meiotic division, and increasing MPF activity through expression of nondegradable cyclin B1 partially rescues the ability of zinc-insufficient oocytes to enter metaphase II. Although we have shown that zinc has a novel role in the meiotic cell cycle, it is not mediated through the MOS-MAPK pathway.
Keywords: gamete biology, in vitro maturation, meiosis, metal biology, Mos, oocyte development, oocyte, zinc homeostasis
Transition from the first meiotic division to meiosis II arrest in mouse oocytes requires zinc; although the phenotype of zinc-insufficient oocytes resembles that of MOS-null oocytes, this defect cannot be attributed to an impaired MOS pathway signaling.
INTRODUCTION
Zinc is an integral component of hundreds of enzymes, transcription factors, and other molecules involved in a variety of biological functions, and the homeostasis of this trace element is tightly regulated [1]. Absorption, cellular uptake, and proper subcellular distribution of zinc are stringently controlled by numerous zinc transporters and binding proteins [2]. Recent studies have also suggested that zinc may act as an intracellular second messenger capable of transducing extracellular signals to cellular signaling events [3]. Zinc is emerging as a particularly important factor in gamete biology. It is needed for spermatogenesis and can modulate sperm capacitation [4–7], and zinc deficiency has been linked to impairment of sexual development in both males and females, as well as in adverse pregnancy outcomes [1, 8, 9]. While the importance of zinc in the physiology of male germ cells has been established, and the effects of excess or insufficient zinc have been studied in Xenopus laevis oocytes [10–14], few studies to date have looked in detail at the role of zinc in the mammalian oocyte. Therefore, we sought to characterize the role of zinc in mammalian oocyte biology. Recently, we reported that the total zinc content of maturing oocytes undergoes large fluxes, increasing by over 50% during meiotic resumption and decreasing by the two-cell embryo stage. Furthermore, perturbation of intracellular zinc availability interferes with meiotic progression and asymmetric division [15]. This report further characterized the molecular mechanism of this meiotic disruption.
Oocytes in the mammalian ovary arrest at prophase of meiosis I (MI) during prenatal development and do not reinitiate meiosis until cued to do so by the LH surge preceding ovulation [16]. Reinitiation of meiosis, or meiotic maturation, also occurs spontaneously when fully grown oocytes are released from the follicle environment [17]. During oocyte maturation, the membrane of the germ cell nucleus (or germinal vesicle [GV]) breaks down (GVBD), and the first meiotic spindle is formed. Following the first meiotic division in which homologous chromosomes are segregated and cytoplasm is divided asymmetrically to produce the first polar body, the second meiotic spindle forms without an interceding round of DNA replication. These mature eggs arrest for a second time at metaphase of the second meiotic division (MII) until fertilization reinitiates the cell cycle and prompts the separation of sister chromatids and extrusion of the second polar body.
Meiotic divisions are highly asymmetric, ensuring that maternal resources within the ooplasm are conserved for embryonic development while allowing the reduction division that ultimately produces a haploid gamete. We have recently shown that under conditions of zinc insufficiency, this asymmetry is impaired and zinc-insufficient oocytes produce large polar bodies [15]. Spindle migration to the oocyte cortex, a process dependent on actin microfilaments, is required for asymmetric division [18–20]. Localization of the meiotic spindle to the oocyte cortex is accompanied by local changes in cellular architecture and polarization of the oocyte. These changes, collectively referred to as cortical reorganization, result in a polarized oocyte with a cortical granule-free domain (CGFD), a cap of thickened cortical actin, and a domain free of microvilli in the region adjacent to the meiotic spindle [21–23]. In order to ascertain the mechanism by which zinc insufficiency disrupts asymmetric division, we began to investigate pathways known to play a role in establishment of this asymmetry, including the MOS-MAPK pathway.
During oocyte maturation, the product of the c-Mos proto-oncogene (MOS) is translated and accumulates after GVBD [24]. MOS is a mitogen-activated kinase kinase kinase, and its accumulation leads to phosphorylation of mitogen-activated protein kinase kinase 1 and 2 (MAP2K1/2 [previously known as MEK1/2]) followed by phosphorylation of mitogen-activated protein kinase 3 and 1 (MAPK3/1 [previously known as Erk1/2 or p42/p44MAPK]) [25]. Cortical reorganization is a MOS-dependent process [26], and in oocytes of the Mos null mouse model, spindle migration is impaired, cortical reorganization is disrupted, and oocytes produce large polar bodies during the first meiotic division [19, 26–28]. MOS null oocytes fail to activate MAPK3/1, which is thought to be the cause of the major features of the MOS null phenotype [26–29]. In addition to producing large polar bodies, these eggs frequently fail to maintain arrest at MII and display a variety of spindle defects ranging from interphase-like configurations to entry into a third metaphase and formation of monopolar half-spindles [27–29]. Similarities between the phenotypes of zinc-insufficient oocytes and MOS null eggs led us to hypothesize that the MOS-MAPK pathway may be impaired by zinc insufficiency.
Considering the importance of zinc in so many biological systems, as well as emerging evidence supporting a role for zinc as a second messenger involved in cell signaling, we sought to further investigate the role of zinc in mammalian oocyte biology. In a companion paper [15], we have shown that zinc is acquired by the maturing murine oocyte to a level that exceeds the zinc quota of most cells [30, 31]. When we blocked zinc accumulation, the oocyte did not undergo asymmetric cytokinesis, nor did it complete normal meiosis. In this study, we investigate the potential role of zinc in MOS-MAPK signaling during meiotic maturation to understand better how zinc controls meiotic maturation of the mammalian oocyte.
MATERIALS AND METHODS
Reagents and Antibodies
Culture medium, fetal bovine serum (FBS), AlexaFluor-633 and -488 phalloidin conjugates (product codes A22284 and A12379, respectively), AlexaFluor-488-conjugated goat anti-mouse immunoglobulin G (IgG) (product code A11001), and horseradish peroxidase (HRP)-conjugated anti-mouse IgG (code 62–6520) were purchased from Invitrogen (Carlsbad, CA). Anti-phospho-MAP2K1/2 (code 9121) and anti-phospho-MAPK3/1 (code 9106) antibodies were purchased from Cell Signaling (Beverly, MA). Anti-actin (code A2066), anti-α-tubulin (code T9026), biotin-conjugated Lens culinaris agglutinin (LCA) (code L4143), and Texas Red-conjugated avidin (code A2348) were purchased from Sigma-Aldrich (St. Louis, MO). Fluorescein isothiocyanate -streptavidin and Vectashield mounting medium with 4′,6′-diamidino-2-phenylindole (DAPI) were from Vector Laboratories (Burlingame, CA). Anti-MOS antibody (code sc-1093R) was from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-cyclinB1 (code ab72) was purchased from Abcam (Cambridge, MA). HRP-conjugated anti-rabbit IgG (code NA934) and ECL-Plus and ECL-Advanced detection reagents were purchased from Amersham Biosciences (Piscataway, NJ). Other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted.
Animals
CD1 mice were maintained in accordance with the policies of Northwestern University's Animal Care and Use Committee and National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Mice were housed and bred in a controlled barrier facility within Northwestern University's Center for Comparative Medicine (Chicago, IL) and were provided with water and Teklad Global (Madison, WI) irradiated 2919 chow, which does not contain soybean or alfalfa meal and therefore contains minimal phytoestrogens, ad libitum. Temperature, humidity, and photoperiod (14L:10D) were kept constant.
Oocyte Collection and In Vitro Maturation
Immature (17- to 21-day-old) female CD1 mice were given intraperitoneal injections of 5 IU equine chorionic gonadotropin (Calbiochem, La Jolla, CA) in 100 μl of sterile phosphate-buffered saline (PBS). Then, 44 to 48 h later, mice were anesthetized with isoflurane and euthanized by cervical dislocation. Ovaries were dissected into Leibovitz L-15 medium containing 1% FBS, and large antral follicles were punctured using 28-gauge needles to release cumulus oocyte complexes (COCs). COCs were then either collected rapidly to minimize spontaneous maturation or kept in dissection medium containing 0.2 mM 3-isobutyl-1-methylxanthine (IBMX) for up to 2 h to prevent germinal vesicle breakdown. COCs were washed through several drops of culture medium consisting of minimum essential medium (MEM)-alpha supplemented with 10% FBS, 100 μM l-glutamine, 1.5 IU/ml human chorionic gonadotropin, and 5 ng/ml epidermal growth factor and placed in culture medium with or without 10 μM N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) (1 mM stock solution in water was added to medium at a 1:100 dilution; affinity constants were 1016, 1015, and 1010 M−1 for Zn2+, Fe2+, and Mn2+, respectively [32]). Medium containing alpha-MEM was preequilibrated for several h at 37°C in 5% CO2; and incubations were performed in drops of medium covered with embryo quality mineral oil. In some experiments, COCs were transferred from control to TPEN-containing medium (or vice versa) at various time points of culture. COCs were washed through at least 3 drops of the terminal medium before transfer to culture drops.
Analysis of First Polar Body Timing
After 6.25 h of culture in the presence or absence of TPEN, oocytes were manually stripped of their surrounding cumulus cells by repeated pipetting using a capillary with a narrow bore. Groups of 10–15 oocytes were placed in individual drops of the same culture medium (with or without TPEN) under oil and imaged and scored for the presence of a first polar body every 15 min using an inverted Leica DM IRB model microscope with transmitted light.
SDS-PAGE and Western Blotting
After culture for 4 to 18 h (depending on the experiment), oocytes were collected and prepared for Western blotting. COCs were briefly incubated in 30 μg/ml hyaluronidase and pipetted several times to remove cumulus cells. Denuded oocytes were imaged and transferred in a minimal amount of medium to microcentrifuge tubes and immediately lysed in 10 μl of 1× SDS-PAGE sample buffer [33]. Samples were quickly frozen and stored at −80°C. Fifteen to 50 oocytes were collected for each sample, depending on the experiment and antibodies used. Samples were heated to 95°C for 5 min and separated by SDS-PAGE using an Invitrogen NuPAGE system. Bis-Tris gels (10%) were run with 3-(N-morpholino)propanesulfonic acid-SDS running buffer and transferred to Immobilon-P polyvinylidene fluoride membranes (Millipore, Billerica, MA) at 25 V for 2 h at room temperature. Blots were blocked in 5% non-fat dry milk in TBS-T (20mM Tris, pH7.4, 137mM NaCl, 0.1% Tween20 [v/v]). Primary antibodies were diluted 1:1000 in 3% BSA-TBS-T (anti-MOS and anti-cyclinB1 were used at 1:500 dilution). HRP-conjugated secondary antibodies were diluted 1:2000 in 1% non-fat dry milk-TBS-T. Detection was performed using ECL-Plus reagent (Amersham). Biomax MR films (Kodak, Rochester, NY) were exposed and developed, or an Alpha-Innotech (San Learndo, CA) MultiImage II system was used. Densitometry analysis was performed using ImageJ version 1.40g software (http://rsb.info.nih.gov/ij/) and/or Alpha-Innotech FluoroChemHD2 software. All protein levels were normalized to actin, and comparisons of normalized values for 2–5 independent experiments were performed using a two-tailed Student t-test.
Immunofluorescence and Scanning Laser Confocal Microscopy
Oocytes were denuded of cumulus cells and fixed in 4% paraformaldehyde for 30 min at 37°C. Prior to fixation, the oocytes to be used for cortical granule (CG) staining with LCA were incubated briefly in acidic Tyrode solution (Millipore) to remove their zonae pellucidae. Oocytes were blocked and permeabilized in wash solution containing 0.2% azide, 2% normal goat serum, 1% BSA, 0.1 M glycine, 0.2% non-fat dry milk, and 0.1% Triton X-100 in PBS at 37°C for at least 15 min. Oocytes were incubated with primary antibodies (1:100 dilution) or LCA (10 μg/ml) for 1 h at room temperature, followed by three washes in wash solution. They were then incubated for 1 h in secondary antibodies (5 μg/ml) and phalloidin (2 U/ml) or avidin (5 μg/ml) conjugates, washed three additional times, and mounted in Vectashield with DAPI with or without 2 μg/ml propidium iodide. Microscopy was performed using a Leica SP5 inverted laser-scanning confocal microscope with a 40× or 63× oil immersion objective (Leica Microsystems, Heidelberg, Germany). Images were processed using LAS AF software (Leica Microsystems).
RNA Microinjection
The expression vector pRN3MOS was kindly provided by M.H. Verlhac [34]; the vector pRN3-CCNB1(Δ90)-EGFP was kindly provided by Karen Schindler [35]. Microinjection of MOS cRNA into GV stage oocytes was performed essentially as previously described [34]. Briefly, an mMESSAGE mMACHINE T3 kit (Ambion, Austin, TX) was used to in vitro-transcribe capped MOS RNA from linearized plasmid. RNA was purified with RNeasy columns (Qiagen, Valencia, CA) and eluted in Tris-EDTA buffer at a final concentration of 0.5 μg/μl, and aliquots were stored at −80°C. COCs were collected as described above in medium containing 0.2 mM IBMX, and oocytes with intact GVs were manually stripped of cumulus cells prior to injection. In vitro-synthesized RNA (3–10 pl) was injected into the oocyte cytoplasm, using an Eppendorf FemtoJet pressure microinjector with Femtotip injection capillaries. Oocytes were kept in L-15 medium supplemented with 1% FBS and 0.2 mM IBMX during injection. They were then cultured in alpha-MEM containing IBMX and 10% FBS for 6 h to allow overexpression of MOS protein. Ten to 15 oocytes were collected for Western blotting to confirm MOS overexpression, while remaining oocytes were transferred to in vitro maturation medium (IVM) with or without 10 μM TPEN for 16 h, as described above. Resumption of meiotic maturation was facilitated by removal of IBMX from the culture medium. Oocytes were processed for Western blotting or immunofluorescence as described above.
CCNB1(Δ90)-EGFP cRNA was produced and injected as it was for MOS, except that oocytes were in vitro-matured in the presence of 10 μM TPEN for 10 to 12.5 h prior to injection so that injections occurred around the time of the MI–MII transition. Injections were performed in L-15 medium containing 3 mg/ml BSA and 10 μM TPEN. Following injections, cells were cultured in TPEN containing IVM medium for an additional 3.5 to 6 h (16 h total) before being fixed and stained for spindles and actin as described above.
Sperm Chromatin Microinjection
Preparation of heat-inactivated sperm heads and microinjection of sperm chromatin into the oocyte cortex were performed essentially as previously described [21] with slight modification. Prior to injection, oocytes were matured in vitro in the presence or absence of TPEN, as detailed above, and injections were performed at room temperature in drops of L-15 medium containing 0.05% polyvinyl alcohol (with or without 10 μM TPEN) under mineral oil. Microinjection capillaries designed for mouse intracytoplasmic sperm injection (6-μm-inner diameter, 25° angle, nonpolished end) were purchased from Eppendorf and were back-filled with Fluorinert FC-77 (Sigma-Aldrich) rather than mercury. Following injection, oocytes were cultured for 2–4 h in IVM medium with or without TPEN and then fixed and processed for CG staining as described above.
Histone H1 and MBP kinase assay
Histone H1 and myelin basic protein (MBP) kinase activities were assayed in single oocytes as described previously [36]. Dried gels were exposed to Kodak Biomax MR films for 6 to 18 h, using Kodak intensifying screens, and films were developed, scanned, and analyzed using ImageJ software.
RESULTS
Zinc Insufficiency Disrupts Meiotic Maturation and Asymmetric Division
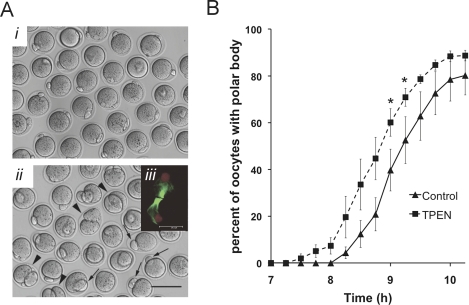
We first sought to further characterize meiotic maturation of zinc-insufficient oocytes. We have recently shown that attenuation of intracellular zinc signaling during IVM of mouse oocytes disrupts asymmetric division and leads to a telophase I-like meiotic arrest [15]. COCs matured in vitro in the presence of the membrane-permeable heavy metal chelator, TPEN, at 10 μM produce polar bodies on average 50% larger in diameter than controls, with some oocytes dividing symmetrically (Fig. 1A [15]). Moreover, instead of establishing a bipolar MII spindle, all TPEN-treated oocytes display a telophase arrest-like microtubule conformation with a chromatin mass rather than discrete chromosomes (Fig. 1A, iii). In initial experiments, it was noted that oocytes collected after 8 to 10 h of IVM were more likely to have already extruded a polar body when they were cultured in the presence of TPEN. More detailed analysis demonstrated that the kinetics of first-polar body extrusion are indeed more rapid when COCs are cultured in the presence of TPEN (Fig. 1B). After 9 h of IVM, 60% ± 13% of zinc-insufficient oocytes had produced a polar body, compared to 40% ± 20% of control oocytes (P < 0.05 by Student t-test). An average difference of 29 min was observed between the point at which 50% of TPEN oocytes had produced a polar body versus that of controls. The percentage of oocytes that eventually produced polar bodies was not different between TPEN-treated and control groups; over 96% had polar bodies by 15 h IVM. The rate of GVBD also did not differ between groups, with the majority of oocytes undergoing GVBD within 60 min of removal from IBMX (data not shown).
FIG. 1.
The first meiotic division in zinc-insufficient oocytes is accelerated and produces a large first polar body. A) Denuded oocytes are shown from COCs matured in vitro in control medium (i) or medium containing 10 μM TPEN (ii). Bar = 100 μm. A, iii) inset shows a Z stack projection of confocal images of the spindle from an oocyte matured in the presence of TPEN; microtubules are shown in green and chromatin in red; bar = 20μm. Arrows (ii) mark oocytes with large polar bodies; arrowheads show examples of symmetric division. B) Timing of first polar body extrusion for oocytes IVM in control (solid line) or TPEN-containing medium (dashed line) based on the percentage of oocytes displaying a polar body. Data are average percentages over five separate experiments ± SEM; a total of 207 control and 240 TPEN-treated oocytes were scored. Asterisks indicate statistical significance according to Student t-test (P < 0.05).
Zinc-Insufficient Oocytes Have Reduced Levels of MOS and Phosphorylated MAP2K1/2 but Not Phosphorylated MAPK3/1
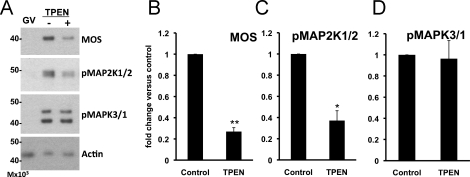
Oocytes lacking MOS protein exhibit large polar bodies [27], similar to those observed in zinc-insufficient oocytes. We therefore hypothesized that zinc insufficiency disrupts MOS pathway signaling, leading to the observed phenotype. To test this hypothesis, we measured levels of MOS protein and the phosphorylation status of the downstream components, MAP2K1/2 and MAPK3/1, by immunoblotting to determined whether the MOS-MAPK pathway is disrupted in zinc-insufficient oocytes. Oocytes matured in the presence of TPEN for 16–18 h contained lower levels of both MOS protein and phosphorylated MAP2K1/2 than control IVM MII eggs (P < 0.05) (Fig. 2, A–C). Despite the decrease in the two upstream signaling molecules, levels of phospho-MAPK3/1 were not significantly different between the groups (Fig. 2D).
FIG. 2.
Oocytes matured in the presence of TPEN have reduced levels of MOS and phospho-MAP2K1/2, while phospho-MAPK3/1 levels remain unchanged. A) Western blot analysis of freshly isolated intact (GV) oocytes and oocytes in vitro matured in the absence (−) or presence (+) of 10 μM TPEN for 16 to 18 h. B–D) Graphs present densitometric analyses of at least three experiments normalized to actin, with 25 to 40 oocytes per well ± SEM. Single asterisks indicate statistical significance according to Student t-test (P < 0.05; double asterisks indicate P < 0.005).
Cortical Reorganization Is Perturbed in Zinc- Insufficient Oocytes
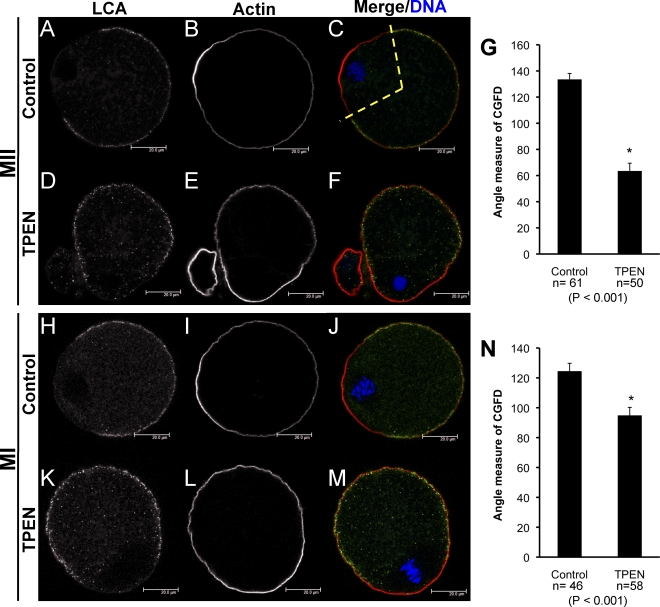
Normally, translocation of the meiotic spindle to the cortex during oocyte maturation results in reorganization of the overlying region of the cortex, including formation of a CGFD, thickening of cortical actin to form an actin cap, and loss of microvilli in the overlying domain [22, 23]. MOS null eggs also fail to undergo cortical reorganization, both in the region surrounding the MII spindle and in the cortical regions surrounding exogenously introduced chromatin [19, 26]. We therefore investigated whether cortical reorganization is also disrupted in zinc-insufficient oocytes. Eggs were fixed after 15–16 h of IVM, stained for CGs and actin, and imaged using confocal microscopy in order to assay formation of the CGFD and cortical actin cap; representative images from control and TPEN-treated groups are shown in Figure 3. Formation of a large CGFD in the region overlying the MII spindle is observed in control eggs (Fig. 3, A and C); however, zinc-insufficient oocytes display incomplete clearing of CGs in the cortical region overlying oocyte chromatin, even though this chromatin is often properly localized to the cortical region (Fig. 3, D and F). In addition, restriction of CGs to the cortical region is slightly reduced in zinc-insufficient oocytes. Measurements of the extent of the CGFD also show that this domain is significantly smaller in oocytes matured in the presence of TPEN (Fig. 3G; P < .001). While zinc insufficiency disrupts CGFD formation, the cortical actin cap is observed in both TPEN-treated oocytes and control eggs (Fig. 3, B and E).
FIG. 3.
CG distribution is disrupted in zinc-insufficient oocytes. Confocal images of LCA-labeled CGs are shown in panels A, D, H, and K; CG staining appears in green in the merged images (C, F, J, M). Oocytes were also stained for actin (B, E, I, L; red in merged images), and DAPI-stained chromatin is shown in blue (C, F, J, M). Proper CG redistribution and actin cap formation in a control IVM MII egg are shown in panels A–C. Reduced formation of the CGFD is observed in oocytes matured in the presence of TPEN for 15 h (D and F); actin cap formation is shown in panels E and F. Cortical reorganization in MI oocytes matured for 7.5 h in control (H–J) or TPEN-containing medium (K–M) are shown. Representative images of at least 20 oocytes analyzed per group are shown. Measurement of the angle occupied by the CGFD for control and TPEN-treated oocytes after 15–16 h and after 7.5 h of IVM are shown in panels G and N, respectively; the region measured is demonstrated by the dotted yellow line in panel C. Error bars show SEM; asterisk indicates statistical significance according to Student t-test (P values and number of oocytes measured are indicated below the graphs).
Since the failed asymmetry of the first meiotic division of zinc-insufficient oocytes indicates that oocyte polarity may be disrupted at MI, we went on to look at CGFD and actin cap formation after 7.5 h of IVM, a time at which the MI spindle has formed and migrated to the cortex in most oocytes. As in the case described above, actin cap formation occurred in MI oocytes incubated in both control and TPEN-containing medium for the full 7.5 h (Fig. 3, I and L). CGFD formation in zinc-insufficient MI oocytes was also disrupted but to a lesser degree than that after 15–16 h of culture. Again, less complete clearing of CGs in the cortical region overlying the MI spindle was observed, the region occupied by the CGFD was smaller, and an increased number of CGs failed to localize to the oocyte cortex in TPEN-treated oocytes versus that in controls (Fig. 3, H–N). Spindle localization to the oocyte cortex was observed in a similar proportion of control and TPEN-treated oocytes by 7.5 h of IVM, indicating that spindle migration does occur in zinc-insufficient oocytes.
Zinc Insufficiency Affects Late-Stage Meiotic Maturation
In order to establish a timeline showing when zinc action is important during meiotic maturation, oocytes were collected at various time points during IVM with and without TPEN treatment; and components of the MOS pathway were analyzed by immunoblotting. While slight decreases in the levels of MOS and phospho-MAP2K1/2 were observed after 10 to 12 h of culture under zinc-insufficient conditions, differences were not statistically significant until 16 h of culture (Fig. 4, A and B). Again, phospho-MAPK3/1 levels in zinc-insufficient oocytes did not vary significantly from control levels (Fig. 4C). Of note, no significant differences in levels of MOS-MAPK pathway components were observed until after first polar body extrusion had occurred (at approximately 9 h of culture).
FIG. 4.
The critical window for zinc action in maintaining MOS levels is during the later portion of meiotic maturation. Western blot analysis shows changes in relative levels of MOS, phoshpo-MAP2K1/2, and phospho-MAPK3/1 in oocytes in vitro matured in the presence of TPEN versus levels in control oocytes at time points from 4 to 16 h, as indicated. Graphs present densitometric analysis to show protein levels relative to that of controls for each time point ± SEM, with normalization to actin (A–C). D–I) Western blot analysis of oocytes matured for 18 h with 10 μM TPEN present for a portion of the culture period, as designated graphically in D–F. Designation C14 indicates that oocytes were cultured in control medium for the first 14 h of culture, then transferred to TPEN-containing medium for the remaining 4 h of the 18-h culture period; T4 indicates culture in the presence of TPEN for the first 4 h followed by 14 h in control medium; and so on. Protein levels were normalized to that of actin, and error bars show SEM. Graphs present combined data from two to five experiments for each condition with 20 to 50 oocytes per well; asterisks indicate statistical significance according to Student t-test (P < .05).
To further delineate the window of time during meiotic maturation during which zinc bioavailability is critically important, TPEN was added to IVM cultures during different time periods at the beginning or end of an 18-h culture period. These extended IVM cultures were used to ensure that robust differences in MOS levels could be detected. Immunoblot analysis shows that addition of TPEN only during later events of meiotic maturation resulted in significant decreases in MOS and phospho-MAP2K1/2 levels (Fig. 4, D–I). TPEN treatment at only the beginning of the 18-h culture period, for as long as 12 h, did not result in reduced MOS or phospho-MAP2K1/2 levels. Even a treatment of up to 14 h in TPEN-containing medium followed by 4 h in control medium resulted in only a slight decrease in MOS protein levels. However, the presence of TPEN at the end of the culture period, even when added at 10 to 12 h of culture (i.e., after the first meiotic division, during the last 6 to 8 h of culture), resulted in MOS levels significantly lower than those of oocytes matured in control medium. As in other experiments, phospho-MAPK3/1 levels were not reduced, despite reduction in components of the upstream pathway. Curiously, in some cases, levels of MAPK3/1 phosphorylation were higher than those of controls, even when MOS and MAP2K1/2 levels were significantly reduced (Fig. 4, G–I).
MOS Overexpression Fails to Rescue Zinc-Insufficient Oocytes
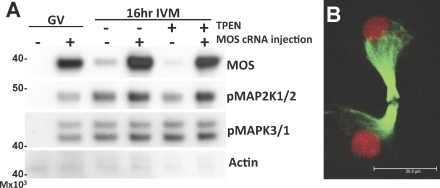
Because results of the above experiments indicate that the decrease in MOS occurs too late to have a role in establishing the phenotype of zinc-insufficient oocytes, we tested the effects of MOS overexpression in zinc-insufficient oocytes to verify that restoration of MOS levels is not sufficient to rescue the observed phenotype. Oocytes were injected with in vitro-transcribed MOS RNA and incubated for 6 h in the presence of IBMX to maintain GV arrest and to allow protein overexpression, followed by IVM under zinc-sufficient or -insufficient conditions. While injected oocytes showed robust expression of MOS, along with activation of downstream signaling components, MAP2K1/2 and MAPK3/1 (Fig. 5A), the telophase arrest-like spindles that are a hallmark of the zinc-insufficient phenotype were still observed (Fig. 5B). Of 33 MOS-overexpressing oocytes matured in the presence of TPEN, none formed MII spindles. In addition, neither polar body size nor CGFD formation was rescued in MOS-overexpressing oocytes cultured in TPEN (see Supplemental Figure S1 [available online at www.biolreprod.org]). Some MOS-injected oocytes underwent GVBD while still in medium containing IBMX, as has been previously reported with oocytes cultured in the presence of dibutyryl cAMP [34].
FIG. 5.
Overexpression of MOS in zinc-insufficient oocytes does not rescue transition to meiosis II. A) Western blot analysis shows overexpression of MOS protein and phosphorylation of MAP2K1/2 and MAPK3/1 in oocytes injected with MOS cRNA (+) or buffer only (−). Actin is shown as a loading control. Oocytes with intact GV were cultured in IBMX-containing medium for 6 h to allow protein expression, followed by 16 h of culture in control or TPEN-containing medium. B) Three-dimensional projection of a confocal Z stacks through the oocyte spindle structure in MOS-overexpressing oocytes cultured in TPEN-containing medium for 16 h shows a telophase arrest-like spindle with decondensing chromatin. A total of 33 MOS-overexpressing oocytes were analyzed; a representative image is shown. Microtubules appear in green, chromatin in red. Bar = 20 μm.
Exogenous Chromatin Induces Actin Cap but Not CGFD Formation in Zinc-Insufficient Oocytes
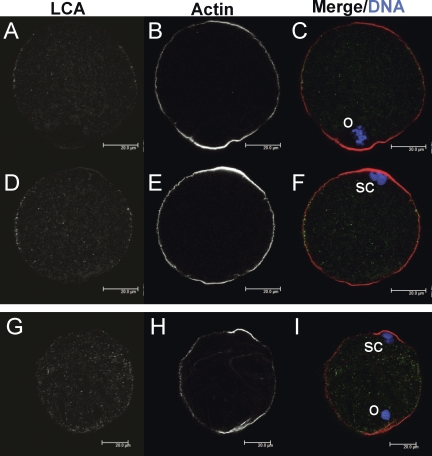
As the above results indicate that MOS-MAPK pathway signaling is not involved in the zinc-insufficient phenotype, the cause of the observed disruption of cortical reorganization remains an open question. In order to investigate whether the altered state of endogenous chromatin in zinc-insufficient oocytes is responsible for this failure, exogenous chromatin was injected into the oocyte cortex to determine whether cortical reorganization could be induced. Sperm heads were used as a convenient source of chromatin and were heat-inactivated to prevent oocyte activation. Oocytes underwent IVM in the presence or absence of TPEN for 12–14 h prior to injection and were cultured for an additional 2–4 h after injection to allow sperm chromatin-induced cortical reorganization. Oocytes were fixed, processed, and imaged as described above to observe cortical reorganization. Control eggs show actin cap and CGFD formation both in the region surrounding the endogenous MII spindle (Fig. 6, A-C) and in the region proximal to injected sperm chromatin (Fig. 6, D–F). While TPEN-treated oocytes show actin cap formation in cortical domains adjacent to both endogenous and sperm chromatin (Fig. 6, H and I), CGFD formation occurs to a lesser extent than in controls (Fig. 6, G and I). Measurement of the CGFD induced by exogenous chromatin shows that the domain is significantly larger in controls (96° ± 7°; n = 23) than in TPEN-treated oocytes (9° ± 4°; n = 21; P < 0.001).
FIG. 6.
Exogenous chromatin induces actin cap formation but not CG redistribution in zinc-insufficient oocytes. Confocal images from two different planes of the same control IVM MII egg are shown in panels A–F. A–C) Images are in the plane of the egg's MII spindle (o), while images in panels D–F are in the plane of the exogenously introduced heat-inactivated sperm chromatin (sc). The egg shown was injected with three sperm nuclei. G–I) Images from a single plane of a TPEN IVM oocyte injected with sperm chromatin, with both the oocyte's endogenous chromatin (o) and microinjected sperm chromatin (sc) in the same plane. LCA-labeled CGs are shown in panels A, D, and G and appear in green in merged images (C, F, I). Oocytes were also stained for actin (B, E, H; red in merged images), and DAPI-stained chromatin is shown in blue (C, F, I). At least 20 control and TPEN-treated IVM oocytes injected with sperm chromatin were analyzed over three separate experiments; representative images are shown. Bars = 20 μm.
Zinc-Insufficient Oocytes Fail to Increase MPF Activity after the First Meiotic Division
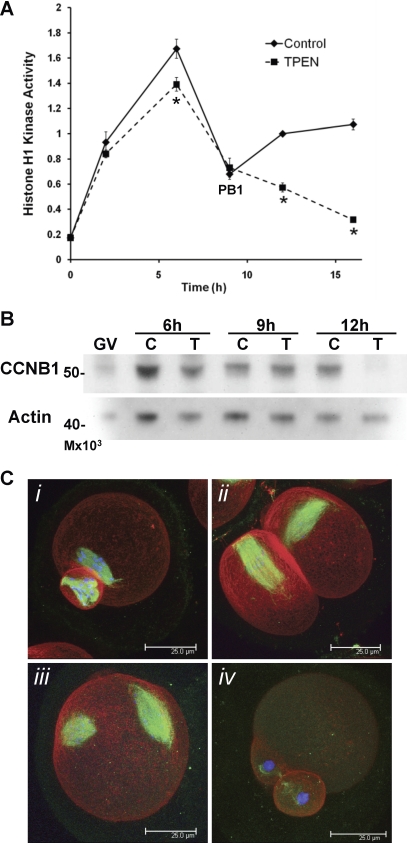
To further investigate causes of the apparent meiotic arrest and failure to establish an MII spindle in zinc-insufficient oocytes, the activity of MPF, a complex of CDK1 and cyclin B1 (CCNB1), was measured in single oocytes by its ability to act as a histone H1 kinase. Histone H1 kinase activity, as assayed by histone H1 phosphorylation, increased after GVBD (2 h IVM) in both control and zinc-insufficient oocytes and continued to rise in MI oocytes (6 h IVM) (Fig. 7A). All oocytes assayed at 9 h IVM had already produced a polar body, and as expected, MPF activity decreased at the time of the first polar body extrusion. However, while MPF activity increased as control oocytes transitioned into MII (12 h IVM), it continued to decrease in zinc-insufficient oocytes and remained low even after 16 h IVM (Fig. 7A). Myelin basic protein (MBP) kinase activity, reflecting activity of MAPK, was not different between control and zinc-insufficient oocytes through the MI–MII transition and was slightly increased in TPEN-treated oocytes after 16 h in culture (Supplemental Fig. S2). This is consistent with the observed phosphorylation of MAPK3/1, which was also similar between TPEN-treated and control oocytes (Fig. 2 and 4).
FIG. 7.
Zinc-insufficient oocytes fail to increase MPF activity following the first meiotic division. A) Kinetics of histone H1 kinase activity over the time course of IVM are shown. Values are in arbitrary units and represent densitometric analysis normalized to control oocytes matured for 12h ± SEM. Four to eleven oocytes were assayed for each data point. Asterisks indicate statistical significance according to Student t-test (P < 0.01). B) Western blot analysis for CCNB1 in oocytes matured in control (C) or TPEN (T)-containing medium for 6, 9, or 12 h. The experiment was repeated three times; a representative blot is shown. C) Three-dimensional projected confocal Z stacks of eggs stained for tubulin (green), actin (red), and DNA (blue) are shown. Following injection with CCNB1(Δ90)-EGFP cRNA during IVM in TPEN-containing medium, 26% of the injected oocytes had MII spindle-like structures (C, i and ii), while 32% did not produce polar bodies and had two spindle-like structures (C, iii), and 38% remained in MI (not shown). Uninjected oocytes matured in the presence of TPEN displayed telophase I-arrested spindles (C, iv). A total of 156 injected oocytes were analyzed. Bar = 25 μm.
MPF activity is controlled at the level of CCNB1 accumulation and degradation during both meiosis and mitosis [37]. Western blot analysis of CCNB1 over the time course of IVM under zinc-sufficient and -insufficient conditions showed a dramatic reduction in CCNB1 levels after 12 h (Fig. 7B). MPF activity is additionally controlled by both activating and inhibitory phosphorylation of CDK1 [38, 39]. Elevation of the inhibitory phosphorylation of CDK1 at tyrosine 15 was also observed by Western blotting in some but not all experiments after 12 h IVM in the presence of TPEN (data not shown), indicating that MPF activity may be controlled at multiple levels in the case of zinc-insufficiency.
Expression of Nondegradable CCNB1 Partially Rescues Zinc-Insufficient Oocytes
In order to test whether decreased CCNB1 levels were indeed responsible for the low MPF activity and failure of zinc-insufficient oocytes to progress to MII, oocytes cultured in the presence of TPEN during IVM were injected with cRNA for a truncated form of CCNB1, Δ90, which lacks an N-terminal anaphase-promoting complex/cyclosome (APC/C) interaction domain and does not become ubiquitinylated upon APC/C activation [40] fused with EGFP. Oocytes were maintained in TPEN-containing medium during the entire culture period and were injected at between 10 and 12.5 h of culture to avoid early arrest due to high MPF levels preventing the first meiotic division, as much as was possible. After 16 h total culture, the CCNB1(Δ90)-EGFP expression led to a partial rescue of the usual TPEN phenotype, with only 3% of injected eggs displaying telophase I arrest compared with 97% of uninjected oocytes treated with TPEN in parallel (Fig. 7C, iv). Twenty-six percent of injected oocytes divided to produce first polar bodies and had MII spindle-like structures ranging from partially organized metaphase plates to spindle-like microtubules with chromosomes dispersed along their length (Fig. 7C, i and ii). Eggs that had divided symmetrically usually had spindle-like structures in both daughter cells. Remaining oocytes had not produced a polar body and were either arrested in MI (38%) or had two spindle-like structures (32%) (Fig. 7C, iii), most likely due to increased MPF activity after karyokinesis but before completion of cytokinesis. A total of 156 injected oocytes from three separate experiments were analyzed.
DISCUSSION
Zinc Is Essential for Oocyte Maturation
We demonstrate here that zinc bioavailability is required during the transition from completion of the first meiotic division to the establishment of the meiosis II spindle. Oocytes matured in vitro under zinc-insufficient conditions undergo an accelerated first meiotic division, produce large first polar bodies, arrest with a telophase I-like spindle configuration without discrete chromosomes, and fail to form a meiosis II spindle (Fig. 1 [15]). By using a model zinc insufficiency during IVM, we can now begin to decipher the underlying mechanism of zinc action in oocyte biology.
Zinc is Required for Complete Cortical Reorganization
During both meiosis and mitosis, asymmetric division requires cortical polarization [41, 42]. Our data provide evidence that zinc is required for complete cortical reorganization to take place (Fig. 3). These results also introduce a disconnect between formation of the actin cap, which develops appropriately in zinc-insufficient oocytes, and redistribution of CGs, which is disrupted in zinc-insufficient oocytes with smaller and less complete CGFDs (Fig. 3). In addition, because exogenously introduced chromatin also fails to induce full CGFD formation (Fig. 6), this disruption is likely due to effects of zinc insufficiency on cellular machinery controlling polarization and not secondary to the disrupted chromatin conformation observed in zinc-insufficient oocytes. Polarization prior to asymmetric division essentially implicates cell fate decisions before cell division actually occurs. Thus, successful polarization is required to produce cells capable of accomplishing different purposes. In fact, when asymmetric division fails during female meiosis, the fate of the daughter cell destined to become the oocyte or polar body can be disrupted. When oocytes that divide symmetrically at the first meiotic division during IVM under zinc-insufficient conditions are rescued with exogenous zinc within 12 h of culture, a portion of these oocytes will produce two metaphase II-arrested spindles, one in each cell [15]. Similarly, expression of a dominant-negative form of ARF1 in mouse oocytes, which also leads to symmetric division during meiosis I, causes generation of two MII oocytes, both of which can be activated subsequently either parthenogenetically or through sperm injection [43]. Through its role in establishing polarity and proper asymmetric division, zinc also has an essential function in determining oocyte versus polar body cell fate.
Zinc Action Is Not Mediated Through MOS-MAPK Signaling
While the importance of zinc in the establishment of oocyte polarity and asymmetric division is apparent, the mechanism by which zinc insufficiency disrupts this process is less clear. The MOS-MAPK pathway has been shown to be required for both proper asymmetric division and establishment of cortical polarity [19, 26, 27]; therefore, potential disruption of MOS pathway signaling was a logical target for investigation. Although features of the phenotype of zinc-insufficient oocytes bear striking resemblance to that of MOS null oocytes, our data indicate that disturbance of MOS pathway signaling is not the primary cause of this phenotype.
We observed a decrease in the levels of MOS protein and MAP2K1/2 phosphorylation in oocytes in vitro matured under zinc-insufficient conditions (Fig. 2); however, this decrease was gradual and only became statistically significant after 16 h of IVM in the presence of TPEN, well after extrusion of the first polar body at approximately 9 h (Fig. 4). Since disrupted asymmetric division and establishment of the telophase arrest-like spindle occur before the reduction in MOS and MAP2K1/2 activation, it is unlikely that this reduction could be the cause of the observed zinc insufficiency phenotype. In addition, phosphorylation of the pathway's downstream effector, MAPK3/1, was not reduced significantly at any time point tested (Fig. 4), and MAPK activity assayed in single oocytes was not reduced by zinc insufficiency (Supplemental Fig. S2). The phenotype of MOS null eggs has been attributed to the absence of MAPK3/1 activation, as inhibition of MAP2K1/2, the only known MAPK3/1 kinases, also causes production of large polar bodies [44, 45], and cortical reorganization is thought to depend on activation of MYLK by MAPK3/1 [26]. Both the late timing of reduction in MOS and the lack of change in the pathway's main effector in zinc-insufficient oocytes led us to conclude that reduced MOS is not responsible for the observed phenotype.
In contrast to the reduced MOS levels observed after 16 h of IVM, zinc insufficiency during the early part of culture resulted in increased MOS protein levels relative to those of controls (Fig. 4). This effect is likely due to the apparent acceleration of meiotic progression in zinc-insufficient oocytes. While early formation of the first polar body was observed under zinc-insufficient conditions (Fig. 1B), this feature was not observed in MOS null oocytes, which have been reported to undergo meiosis I with normal kinetics [28, 29], reinforcing our conclusion that zinc insufficiency is operating through a MOS-independent pathway. This accelerated meiosis I division could potentially contribute to the large polar body phenotype observed in zinc-insufficient oocytes, given that cytokinesis occurring prior to spindle migration to the cortex could lead to production of large polar bodies [46]. Additionally, this feature may indicate involvement of zinc in spindle assembly checkpoint (SAC) machinery, as Tsurumi et al. [47] have demonstrated that expression of dominant-negative mutants of several SAC components led to acceleration of meiosis I. However, as the difference in meiosis I timing observed between zinc-sufficient and -insufficient oocytes is small (approximately 29 min, compared to differences of 3 h or more with mutant SAC components [47]), and because localization of the MI spindle to the cortex was observed in most zinc-insufficient oocytes, there could be other explanations for defective asymmetric division in zinc-insufficient oocytes.
Microinjection of MOS cRNA into GV-arrested oocytes resulted in overexpression of MOS protein and phosphorylation of MAP2K1/2 and MAPK3/1, which was maintained after 16 h of culture under zinc-insufficient conditions (Fig. 5A). However, activation of the MOS-MAPK pathway failed to rescue the telophase arrest-like spindle phenotype characteristic of zinc-insufficient oocytes (Fig. 5B), and neither polar body size nor CGFD formation was rescued by MOS overexpression (Supplemental Fig. S1). These results, consistent with the timing data presented above, strongly implicate a MOS-independent mechanism under the control of zinc as a critical component during oocyte maturation.
Zinc Is Required for Entry into Meiosis II
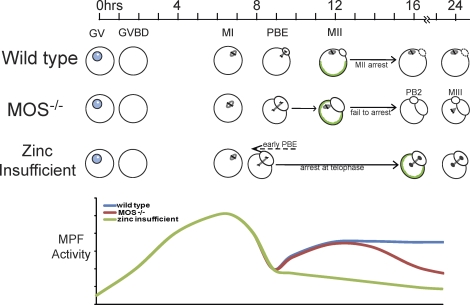
While the results presented herein essentially rule out MOS-MAPK as being the primary pathway disrupted by zinc-insufficiency during oocyte maturation, zinc obviously plays an important role in some cellular mechanism(s) critical for proper meiotic maturation. Components of the zinc-insufficient phenotype, expanded upon by these studies, give us additional clues as to where zinc could be important. In addition to the failed asymmetric division initially observed, we have shown that establishment of cortical polarization is disrupted in zinc-insufficient oocytes, and, importantly, the telophase arrest-like spindle phenotype has called our attention to the meiosis I–meiosis II transition. The dynamics of MPF activity are critical for progression through meiosis. The activity of MPF, a complex of CDK1 and CCNB1, rises as meiotic maturation begins, allowing formation of the MI spindle. MPF activity declines briefly via limited degradation of CCNB1 to allow anaphase I and the first meiotic division to progress and then increases again and is maintained at high levels to allow establishment of the MII spindle and arrest at this point, until fertilization or activation [48]. We show here that in zinc-insufficient oocytes, MPF activity does not increase following the first meiotic division and remains low (Fig. 7A); this appears to be mediated through failure to reaccumulate CCNB1 following the first meiotic division (Fig. 7B) and is further supported by the partial rescue of the zinc insufficiency phenotype by expression of a nondegradable CCNB1 (Fig. 7C). This differs from the case of MOS-null oocytes, in which MPF activity increases after meiosis I but is not maintained, leading to a premature second meiotic division [28]. Zinc-insufficient oocytes fail to enter MII, while MOS null oocytes enter MII but fail to arrest. In this sense, it appears that zinc-insufficiency interferes with the ability to establish cytostatic factor (CSF) activity, rather than the failure to maintain CSF that has been shown in MOS null oocytes [28, 48]. Our model for the relationship between MOS null and zinc-insufficient oocytes and the reflected dynamics of MPF activity is shown in Figure 8. Although the absence of MOS protein and zinc insufficiency led to visibly similar phenotypes, different mechanisms are at play.
FIG. 8.
Proposed model of the relationship between zinc-insufficient and MOS null oocytes. Timeline shows number of h after initiation of IVM. GVBD and formation of the MI spindle proceed normally in both MOS null and zinc-insufficient oocytes. The MI spindle in MOS null oocytes does not migrate to the oocyte cortex, and the first meiotic division produces a large polar body [19]. Spindle migration appears to occur normally in zinc-insufficient oocytes; however, the first meiotic division is slightly accelerated and produces a large polar body. MOS null oocytes fail to arrest in MII, and a majority expel a second polar body and transition into a third M phase state with monopolar spindles [28]. Zinc-insufficient oocytes do not form an MII spindle and instead arrest with telophase-like spindles. CG reorganization (green outlines within cells) is disrupted in both MOS null eggs and zinc-insufficient oocytes. A schematic of MPF activity is shown below. MPF activity increases after GVBD in wild-type, MOS null, and zinc-insufficient oocytes and then declines just before polar body extrusion (PBE), allowing anaphase to take place. In wild type and MOS null oocytes, MPF activity rises once again as the MII spindle is established and is maintained at high levels in MII arrested eggs, while in MOS null eggs, MPF activity once again drops as oocytes expel a second polar body [28]. After the decline with the first meiotic division, MPF activity in zinc-insufficient oocytes remains low, and these cells fail to establish an MII spindle. Although MOS absence and zinc insufficiency produce visibly similar phenotypes, different mechanisms are involved.
With a focus on the meiosis I–meiosis II transition and establishment of CSF in mind, the recently characterized F-box protein 43 (FBXO43; previously known as Emi2) emerges as a promising candidate zinc-regulated factor that controls meiotic progression. FBXO43 is an inhibitor of the APC/C ubiquitin ligase responsible for targeting CCNB1 for degradation [49, 50]. FBXO43 is a key component of CSF, and the mechanism leading to FBXO43 degradation cued by the calcium oscillations at fertilization has been worked out over the last few years [51]. Identification of a zinc-regulated molecular pathway that controls the transition of oocytes out of meiosis I and into metaphase II arrest is a fascinating new use of an inorganic signaling pathway in a biological system.
We have demonstrated that zinc is critical for progression of oocytes through the meiosis I–meiosis II transition and that, although zinc-insufficient oocytes resemble MOS null oocytes, impaired MOS-MAPK pathway signaling is not the cause of this phenotype. While additional pathways remain to be explored, this work illustrates the importance of a previously under-recognized role for zinc in oocyte biology.
Supplementary Material
Acknowledgments
We thank Marie-Helene Verlhac for the generous gift of the MOS RNA expression vector and Karen Schindler for providing the pRN3-Δ90CCNB1-EGFP construct. We also thank Francesca Duncan for helpful advice and critical reading of the manuscript and gratefully acknowledge Jennifer Jozefik, Sarah Kiesewetter, and Dragan Mackovic for providing animal care and technical assistance.
Footnotes
Supported by National Institutes of Health/National Institute of Child Health and Human Development (NIH/NICHD) Hormonal Signals that Regulate Ovarian Development Grant P01 HD-021921 and the W.M. Keck Foundation Medical Research Award; M.L.B. and A.M.K. were fellows of the Reproductive Biology Training Grant NIH/NICHD T32 HD-07068.
REFERENCES
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev 1993; 73: 79 118 [DOI] [PubMed] [Google Scholar]
- Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci 2004; 61: 49 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Sakata-Sogawa K, Hasegawa A, Suzuki T, Kabu K, Sato E, Kurosaki T, Yamashita S, Tokunaga M, Nishida K, Hirano T. Zinc is a novel intracellular second messenger. J Cell Biol 2007; 177: 637 645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JC, Nolan JP, Hammerstedt RH, Bavister BD. Role of zinc during hamster sperm capacitation. Biol Reprod 1994; 51: 1238 1247 [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Lamothe G, Villemure M. Control of superoxide and nitric oxide formation during human sperm capacitation. Free Radic Biol Med 2009; 46: 1420 1427 [DOI] [PubMed] [Google Scholar]
- Hamdi SA, Nassif OI, Ardawi MS. Effect of marginal or severe dietary zinc deficiency on testicular development and functions of the rat. Arch Androl 1997; 38: 243 253 [DOI] [PubMed] [Google Scholar]
- Lewis B, Aitken RJ. A redox-regulated tyrosine phosphorylation cascade in rat spermatozoa. J Androl 2001; 22: 611 622 [PubMed] [Google Scholar]
- Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update 2007; 13: 163 174 [DOI] [PubMed] [Google Scholar]
- Jameson S. Zinc status in pregnancy: the effect of zinc therapy on perinatal mortality, prematurity, and placental ablation. Ann N Y Acad Sci 1993; 678: 178 192 [DOI] [PubMed] [Google Scholar]
- Bruinsma JJ, Jirakulaporn T, Muslin AJ, Kornfeld K. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev Cell 2002; 2: 567 578 [DOI] [PubMed] [Google Scholar]
- Falchuk KH, Montorzi M, Vallee BL. Zinc uptake and distribution in Xenopus laevis oocytes and embryos. Biochemistry 1995; 34: 16524 16531 [DOI] [PubMed] [Google Scholar]
- Nomizu T, Falchuk KH, Vallee BL. Zinc, iron, and copper contents of Xenopus laevis oocytes and embryos. Mol Reprod Dev 1993; 36: 419 423 [DOI] [PubMed] [Google Scholar]
- Sun L, Chai Y, Hannigan R, Bhogaraju VK, Machaca K. Zinc regulates the ability of Cdc25C to activate MPF/cdk1. J Cell Physiol 2007; 213: 98 104 [DOI] [PubMed] [Google Scholar]
- Wallace RA, Misulovin Z. The role of zinc and follicle cells in insulin-initiated meiotic maturation of Xenopus laevis oocytes. Science 1980; 210: 928 930 [DOI] [PubMed] [Google Scholar]
- Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 2010; 6: 674 681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon D, Tsafriri A, Lindner HR, Cordova T, Harell A. Serum gonadotrophin levels in pro-oestrous rats in relation to the resumption of meiosis by the oocytes. J Reprod Fertil 1972; 31: 51 58 [DOI] [PubMed] [Google Scholar]
- Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 1965; 208: 349 351 [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Josefowicz WJ, Letourneau GE. Meiotic maturation of mouse oocytes in vitro: inhibition of maturation at specific stages of nuclear progression. J Cell Sci 1976; 22: 531 545 [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Lefebvre C, Guillaud P, Rassinier P, Maro B. Asymmetric division in mouse oocytes: with or without Mos. Curr Biol 2000; 10: 1303 1306 [DOI] [PubMed] [Google Scholar]
- Leader B, Lim H, Carabatsos MJ, Harrington A, Ecsedy J, Pellman D, Maas R, Leder P. Formin-2, polyploidy, hypofertility and positioning of the meiotic spindle in mouse oocytes. Nat Cell Biol 2002; 4: 921 928 [DOI] [PubMed] [Google Scholar]
- Deng M, Kishikawa H, Yanagimachi R, Kopf GS, Schultz RM, Williams CJ. Chromatin-mediated cortical granule redistribution is responsible for the formation of the cortical granule-free domain in mouse eggs. Dev Biol 2003; 257: 166 176 [DOI] [PubMed] [Google Scholar]
- Longo FJ, Chen DY. Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev Biol 1985; 107: 382 394 [DOI] [PubMed] [Google Scholar]
- Nicosia SV, Wolf DP, Inoue M. Cortical granule distribution and cell surface characteristics in mouse eggs. Dev Biol 1977; 57: 56 74 [DOI] [PubMed] [Google Scholar]
- Paules RS, Buccione R, Moschel RC, Vande Woude GF, Eppig JJ. Mouse Mos protooncogene product is present and functions during oogenesis. Proc Natl Acad Sci U S A 1989; 86: 5395 5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, de Pennart H, Maro B, Cobb MH, Clarke HJ. MAP kinase becomes stably activated at metaphase and is associated with microtubule-organizing centers during meiotic maturation of mouse oocytes. Dev Biol 1993; 158: 330 340 [DOI] [PubMed] [Google Scholar]
- Deng M, Williams CJ, Schultz RM. Role of MAP kinase and myosin light chain kinase in chromosome-induced development of mouse egg polarity. Dev Biol 2005; 278: 358 366 [DOI] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude GF. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proc Natl Acad Sci U S A 1996; 93: 7032 7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Weber M, Geraud G, Colledge WH, Evans MJ, Maro B. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development 1996; 122: 815 822 [DOI] [PubMed] [Google Scholar]
- Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E. Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod 1996; 55: 1315 1324 [DOI] [PubMed] [Google Scholar]
- Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2001; 292: 2488 2492 [DOI] [PubMed] [Google Scholar]
- Suhy DA, Simon KD, Linzer DI, O'Halloran TV. Metallothionein is part of a zinc-scavenging mechanism for cell survival under conditions of extreme zinc deprivation. J Biol Chem 1999; 274: 9183 9192 [DOI] [PubMed] [Google Scholar]
- Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem 1985; 260: 2719 2727 [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680 685 [DOI] [PubMed] [Google Scholar]
- Verlhac MH, Lefebvre C, Kubiak JZ, Umbhauer M, Rassinier P, Colledge W, Maro B. Mos activates MAP kinase in mouse oocytes through two opposite pathways. Embo J 2000; 19: 6065 6074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler K, Schultz RM. CDC14B acts through FZR1 (CDH1) to prevent meiotic maturation of mouse oocytes. Biol Reprod 2009; 80: 795 803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Hayashi H, Schultz RM. Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development 2000; 127: 4147 4156 [DOI] [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 1989; 339: 280 286 [DOI] [PubMed] [Google Scholar]
- Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 1992; 257: 1955 1957 [DOI] [PubMed] [Google Scholar]
- Kaldis P, Russo AA, Chou HS, Pavletich NP, Solomon MJ. Human and yeast cdk-activating kinases (CAKs) display distinct substrate specificities. Mol Biol Cell 1998; 9: 2545 2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick S, Nixon VL, Chang HY, Herbert M, Levasseur M, Jones KT. Maintenance of sister chromatid attachment in mouse eggs through maturation-promoting factor activity. Dev Biol 2004; 275: 68 81 [DOI] [PubMed] [Google Scholar]
- Cowan CR, Hyman AA. Asymmetric cell division in C. elegans: cortical polarity and spindle positioning. Annu Rev Cell Dev Biol 2004; 20: 427 453 [DOI] [PubMed] [Google Scholar]
- Deng M, Suraneni P, Schultz RM, Li R. The Ran GTPase mediates chromatin signaling to control cortical polarity during polar body extrusion in mouse oocytes. Dev Cell 2007; 12: 301 308 [DOI] [PubMed] [Google Scholar]
- Wang S, Hu J, Guo X, Liu JX, Gao S. ADP-ribosylation factor 1 regulates asymmetric cell division in female meiosis in the mouse. Biol Reprod 2009; 80: 555 562 [DOI] [PubMed] [Google Scholar]
- Tong C, Fan HY, Chen DY, Song XF, Schatten H, Sun QY. Effects of MEK inhibitor U0126 on meiotic progression in mouse oocytes: microtuble organization, asymmetric division and metaphase II arrest. Cell Res 2003; 13: 375 383 [DOI] [PubMed] [Google Scholar]
- Yu LZ, Xiong B, Gao WX, Wang CM, Zhong ZS, Huo LJ, Wang Q, Hou Y, Liu K, Liu XJ, Schatten H, Chen DY, et al. MEK1/2 regulates microtubule organization, spindle pole tethering and asymmetric division during mouse oocyte meiotic maturation. Cell Cycle 2007; 6: 330 338 [DOI] [PubMed] [Google Scholar]
- Metchat A, Akerfelt M, Bierkamp C, Delsinne V, Sistonen L, Alexandre H, Christians ES. Mammalian heat shock factor 1 is essential for oocyte meiosis and directly regulates Hsp90alpha expression. J Biol Chem 2009; 284: 9521 9528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurumi C, Hoffmann S, Geley S, Graeser R, Polanski Z. The spindle assembly checkpoint is not essential for CSF arrest of mouse oocytes. J Cell Biol 2004; 167: 1037 1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick S, Jones KT. How eggs arrest at metaphase II: MPF stabilisation plus APC/C inhibition equals cytostatic factor. Cell Div 2007; 2: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgwick S, Hansen DV, Levasseur M, Jackson PK, Jones KT. Mouse Emi2 is required to enter meiosis II by reestablishing cyclin B1 during interkinesis. J Cell Biol 2006; 174: 791 801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S, Yoshida N, Amanai M, Ohgishi M, Fukui T, Fujimoto S, Nakano Y, Kajikawa E, Perry AC. Mammalian Emi2 mediates cytostatic arrest and transduces the signal for meiotic exit via Cdc20. Embo J 2006; 25: 834 845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Kornbluth S. Across the meiotic divide: CSF activity in the post-Emi2/XErp1 era. Journal of Cell Science 2008; 121: 3509 3514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.