Abstract
Innervation of the cervix is important for normal timing of birth because transection of the pelvic nerve forestalls birth and causes dystocia. To discover whether transection of the parasympathetic innervation of the cervix affects cervical ripening in the process of parturition was the objective of the present study. Rats on Day 16 of pregnancy had the pelvic nerve (PnX) or the vagus nerve (VnX) or both pathways (PnX+VnX) transected, sham-operated (Sham) or nonpregnant rats served as controls. Sections of fixed peripartum cervix were stained for collagen or processed by immunohistochemistry to identify macrophages and nerve fibers. All Sham controls delivered by the morning of Day 22 postbreeding, while births were delayed in more than 75% of neurectomized rats by more than 12 h. Dystocia was evident in more than 25% of the PnX and PnX+VnX rats. Moreover, on prepartum Day 21, serum progesterone was increased severalfold in neurectomized versus Sham rats. Assessments of cell nuclei counts indicated that the cervix of neurectomized rats and Sham controls had become equally hypertrophied compared to the unripe cervix in nonpregnant rats. Collagen content and structure were reduced in the cervix of all pregnant rats, whether neurectomized or Shams, versus that in nonpregnant rats. Stereological analysis of cervix sections found reduced numbers of resident macrophages in prepartum PnX and PnX+VnX rats on Day 21 postbreeding, as well as in VnX rats on Day 22 postbreeding compared to that in Sham controls. Finally, nerve transections blocked the prepartum increase in innervation that occurred in Sham rats on Day 21 postbreeding. These findings indicate that parasympathetic innervation of the cervix mediates local inflammatory processes, withdrawal of progesterone in circulation, and the normal timing of birth. Therefore, pelvic and vagal nerves regulate macrophage immigration and nerve fiber density but may not be involved in final remodeling of the extracellular matrix in the prepartum cervix. These findings support the contention that immigration of immune cells and enhanced innervation are involved in processes that remodel the cervix and time parturition.
Keywords: cervical remodeling, cervix, collagen, fiber density, macrophage, parturition, progesterone
Pelvic or vagal neurectomy of pregnant rats forestalls parturition and prevent cervical ripening.
INTRODUCTION
The cervix is a well-innervated component of the female reproductive tract. In contrast to the uterus, which is relatively denervated with pregnancy [1–3], the density of nerve fibers in the cervix is increased manifold by the day before birth in rodents [4, 5] and in women is sustained during the peripartum period [6, 7]. The cervix receives sympathetic innervation from the hypogastric nerve [8, 9], as well as parasympathetic projections through the pelvic and vagus nerves [10–13]. Hypogastric and pelvic nerve pathways to the cervix connect with sensory and autonomic areas in the thoracolumbar spinal cord, which have ascending connections with integrative centers in the brainstem and hypothalamus [14, 15]. These integrative centers receive input from and project to the vagus nerve [16, 17]. Vagal connections with the cervix are reported in nonpregnant rodents [18, 19] and women [18, 20], but their importance for parturition is not known. The possibility that innervation may convey signals that affect cervical function comes from the finding that activity by nerves that project to the cervix is increased with pregnancy in rats [21].
Understanding the contribution of innervation to the process of parturition comes from evidence that indicates that transection of the hypogastric nerve, i.e., sympathetic innervation, does not affect cervical ripening or birth in rats [4, 5]. By contrast, it has been known for a long time that input from the pelvic nerve is involved in the process of parturition at term. In rats, transection of the sensory branch of the pelvic nerve was found to result in interference with birth [11, 22–28], delayed timing and labor, and, commonly, dystocia. Moreover, pelvic neurectomy has been reported to acutely decrease [13] or increase [25, 26] serum progesterone in pregnant rats, depending on whether neurectomy was performed early or late in pregnancy, respectively. Although little is known about the birth process in women with damage to innervation of the cervix or major spinal cord damage, complete transection extending to as high as the sixth cervical vertebra does not block the perception of labor pains [29]; these patients often deliver by cesarean section. Thus, evidence suggests that innervation may be important for the mechanism that remodels the cervix and the process of birth at term. In the process of remodeling of the prepartum cervix, proinflammatory processes are associated with degradation of the extracellular matrix [30, 31]. Neurogenic inflammation has been proposed to contribute to immigration of immune cells and importation of leukocyte collagenases that degrade collagen and disrupt the structure of the cervix [4, 32–36]. The lack of histological analysis of the cervix after neurectomy led to the major objective of the present study, which tested the hypothesis that nerve transections disrupt ripening of the cervix and processes that time parturition. Present findings indicate that transections of the pelvic and vagus nerves forestall birth through a mechanism that differentially regulates macrophage immigration.
MATERIALS AND METHODS
Animals
Adult Long Evans rats were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Rats were individually housed in a temperature- and humidity-controlled vivarium room with lights-on at 0700 h for 12 h. Rats were provided food and water ad libitum. Animal care followed guidelines set by the National Institutes of Health and, experimental procedures were approved by the local institutional animal care and use committee.
Surgery and Nerve Transections
Several days after rats were acclimatized to the vivarium, surgery was performed on Day 15 postbreeding, under isoflurane-oxygen gas anesthesia. Rats were randomly assigned to transection of the pelvic nerve (PnX; n = 22) or the vagus nerve (VnX; n = 16) or both nerves (PnX+VnX; n = 12). Under aseptic conditions, the bilateral pelvic neurectomy procedure followed those previously described in the rat [11, 37]. Briefly, a lower abdominal midline incision was made; bowels and uterine horns were packed rostrally with a saline-moistened gauze; and after blunt dissection, the bifurcation of the common iliac vein was identified. The pelvic floor area that contained the communicating blood vessels from the internal iliac across the rectum to the cervix was cleared of adipose tissue. Methylene blue dye was applied with a syringe to visualize myelinated nerves [38]. The pelvic nerve was identified as crossing the surface of the common iliac artery before branching into smaller fibers amid the complex of blood vessels over the rectum that project into the cervix. The main trunk and nerve fiber projections across the rectum to the cervix were cut, and a segment was removed with microforceps. Methylene blue was again applied to confirm the absence of nerves. The bladder and ureter complex was reflected over the midline, across the previous field of surgery, and the same procedures were repeated on the opposite side.
Vagus nerve transections required an upper abdominal incision to the intercostal margin to expose the liver. The hepatogastric ligament was cut, and a moist gauze was inserted rostrally to retract the liver and expose the subdiaphragmatic portion of the esophagus. Methylene blue was topically applied with a syringe to visualize the bifurcated vagus nerve along the dorsal surface of the esophagus; and, after both pathways were cut and a segment removed with microforceps, methylene blue was again applied to confirm transection. The double neurectomy procedure combined both approaches as detailed above. Following surgery, the abdomen was closed with a running suture stitch, and the skin was secured with surgical staples. Two groups of sham-operated rats (Shams) were subjected to the same anesthesia and surgical procedures with the upper or lower abdomen opened, organs manipulated as in the dissection, and then closed (n = 16), or in 5 rats, a complete procedure with applications of methylene blue dye to visualize pelvic or vagus nerves (n = 3 or 2, respectively) but no transection.
Postsurgery, abdominal pressure was applied by gentle rubbing to all rats twice each day to evacuate the bladder. Rats were asphyxiated with Co2 within 3 h of lights-on or within 3 h of birth. A blood sample was immediately obtained by cardiac puncture from rats in each group to assess serum progesterone and corticosterone (0.0002–0.01 ml of serum, as used in kits from Cayman Chemical Company, Ann Arbor, MI). Coefficients of variability were <10%, and assay sensitivities were <0.008 ng/ml. The cervix was excised, post-fixed, embedded in paraffin, and sectioned longitudinally at 10 μm, and sections were mounted on positively charged glass slides and stained as detailed below.
The day and time of birth were recorded as the appearance of the first pup or the persistence of a substantial bloody vaginal discharge, indicating prolonged and overt stressful delivery. Rats with incomplete delivery and one rat that had not delivered by the morning of day 24 were euthanized for animal welfare concerns. Postsurgery, more than 90% of the PnX rats expressed a substantial volume of urine after twice daily gentle rubbing pressure and had distended bladders postmortem, as previously reported [11, 28]. After the vagotomy procedure, 82% of rats had a distended stomach, an outcome comparable to that in a previous report [39]. The combined procedure to transect the pelvic and vagus nerves resulted in 76% of rats with both a distended bladder and stomach. Overall, 19 postneurectomy rats that did not have a distended bladder and/or distended stomach were excluded from the study.
Processing and Analysis of Cervix
Procedures used to stain cervical sections with picrosirius red for analysis of collagen content and structure, as well as to enumerate cell nuclei, macrophages, and nerve fibers, using immunohistochemistry and image analyses have been previously detailed [4, 5, 40]. Briefly, the optical density (OD) of polarized light from birefringence of type I collagen [13] from picrosirius red-stained sections of cervix was normalized to the number of cell nuclei/area for each rat, to account for the hypertrophy of tissue in pregnant rat cervix (less that of the extracellular matrix per field of view). An automated image analysis protocol was used to count ED-1 antibody-labeled macrophages. Briefly, a photomicrograph of the field of view in the microscope was captured and, using Image Pro Plus 6 software, criteria were set based upon color and measurements for a specific immunohistochemical stain. These settings were in the eye dropper mode (degree of sensitivity, color index, minimum number of pixels) and count/size mode (object pixel connections, smoothing, fill holes, and area range). In a survey of sections, macrophage numbers were confirmed by direct manual counts, and a random sample of data was independently evaluated by multiple coauthors blinded to the experimental design. To estimate the area of the cervix with nerve fibers, a stereologic approach was taken to assess the area of tissue that contained processes labeled with peripherin, an antibody to neurofilament IV, as previously described [4, 41]. Three cervix sections per rat and eight nonoverlapping 10 × 10 grid placements per section, i.e., 152 100 μm2, were analyzed. As before, all data were normalized to cell nuclei number/area for each individual to account for hypertrophy and hyperplasia of the reproductive tract tissue. Based upon postmortem evaluation of cervix morphology, nonpregnant rats were in the metestrus or diestrus phase of the ovarian cycle, which is characterized by low serum progesterone concentration.
Statistics
Data were normally distributed, and individual comparisons were made by ANOVA or Student t-test. Following ANOVA, Tukeys test was used for individual comparisons. In a few instances Levenes test for homogeneity of variance was significant, and data were analyzed using the Kruskal-Wallis test. Data are the means ± SEM. A P value of < 0.05 was considered significant.
RESULTS
Effects of Neurectomy on Timing of Birth and Hormones in Circulation
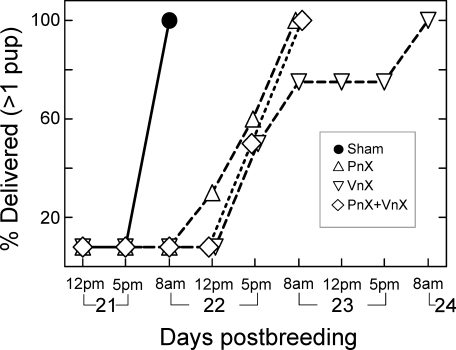
All Sham-operated pregnant rats, including those treated with methylene blue, delivered by the morning of Day 22 postbreeding. Pups were estimated to have been born between 0700 and 1000 h, within 3 h of lights-on, comparable to that in previous reports [42–45]. By contrast, births were delayed in 91% of PnX rats, 73% of VnX rats, and 86% of PnX+VnX rats. In neurectomized rats with delayed birth (Fig. 1), at least one pup had delivered by 1700 h of Day 22 postbreeding in 60% of PnX rats (n = 6 of 10), 50% of VnX rats (n = 4 of 8), and 50% of PnX+VnX rats (n = 3 of 6). By the morning of Day 23, all PnX and all PnX+VnX rats had given birth, while 2 rats in the VnX group remained pregnant until the morning of Day 24 postbreeding. On average, at least one pup had been delivered by PnX, VnX, and PnX+VnX rats on Day 23 ± 0.04, Day 23 ± 0.2, and Day 23 ± 0.1 postbreeding, respectively. Thus, births were delayed in about half of the neurectomized rats by an estimated minimum of 10 h, beginning on the evening of Day 22, and by 24 h or more in the remaining rats by the morning of Day 23 postbreeding or beyond. In addition, more than 25% of rats in the PnX and PnX+VnX groups experienced dystocia; pups were present in the birth canal or were retained in utero. All pups from VnX rats delivered without apparent complications. Of the 67 postpartum rats that met criteria for successful nerve transection, i.e., had distended bladders and or stomachs, 5 neurectomized rats delivered at normal term (1 PnX, 1 PnX+VnX, and 3 VnX rats). Data from these rats were considered postpartum outliers, although their removal from the data set did not affect the statistical analyses.
FIG. 1.
Percent of rats that delivered at least one pup relative to the day postbreeding in Sham-operated (Sham, n = 13) or nerve transected groups (PnX, pelvic; VnX, vagus; PnX+VnX, pelvic and vagus). By 1700 h (indicated as 5pm in figure) of Day 22 postbreeding, 2 of 9 PnX rats, 3 of 6 VnX rats, and 3 of 5 PnX+VnX rats had delivered a pup. Symbols for groups on Days 22 and 23 are offset to visualize data.
Progesterone in circulation was elevated in neurectomized rats with delayed birth compared to that in Sham-operated controls. Serum progesterone concentration in Sham-operated rats on Day 21 of pregnancy was 20 ± 2 ng/ml (n = 3) on the day before birth, and was 21 ± 2 ng/ml (n = 6) postpartum on Day 22 postbreeding, i.e., the day of birth. In neurectomized rats on Day 21 of pregnancy, serum progesterone was 74 ± 12 ng/ml (n = 8, an average of 62 ng/ml for 3 Day 21 PnX rats, 54 ng/ml for 2 Day 21 VnX rats, and 126 ng/ml for 2 Day 21 PnX+VnX rats), significantly greater than that in Day 21 prepartum Shams (P < 0.05, Student t-test). By the next day (day 22 postbreeding with delayed birth), serum concentrations were reduced in neurectomized rats to 20 ± 2 ng/ml (n = 10) and to 26 ± 5 ng/ml on Days 22.5–24 (postpartum, n = 11), equivalent to that in Sham controls.
In addition, serum corticosterone concentrations were increased in neurectomized rats compared to that in Sham controls. On Day 21 of pregnancy, serum corticosterone in 2 Sham-operated rats was 104 ± 3 ng/ml compared to 392 ± 49 ng/ml in neurectomized rats (averages of 369 ng/ml for 3 Day 21 PnX rats, 448 ng/ml for 2 Day 21 VnX rats, and 378 ng/ml for 3 Day 21 PnX+VnX rats), significantly greater than that in Day 21 prepartum Shams (P < 0.05, Student t-test). On Day 22 postbreeding, corticosterone concentrations in prepartum neurectomized rats with delayed birth remained elevated, i.e., 271 ± 33 ng/ml (n = 8), compared to that in prepartum Shams on Day 21 (above) or in postpartum Day 22 Shams (70 ± 35 ng/ml, n = 6). By contrast, serum corticosterone in postpartum neurectomized rats with delayed birth was 105 ± 33 ng/ml (≥Day 22.5, n = 13) comparable to concentrations found in prepartum and postpartum groups of Sham rats (P > 0.05).
Characteristics of Remodeling Associated with Birth
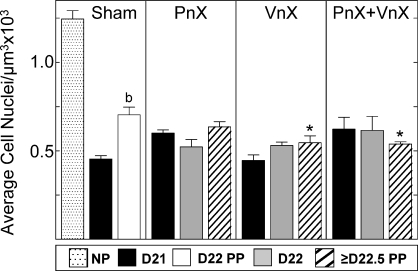
Growth in the cervix with pregnancy was evident in all groups. In the cervix from Sham-operated rats on Day 21 of postbreeding, the luminal epithelium increased in thickness, while in stroma, more space surrounded fewer cell nuclei in each microscopic field of view compared to that in the cervix from nonpregnant rats. Numbers of cell nuclei per area of cervix decreased by Day 21 postbreeding in all groups of pregnant and postpartum rats, whether Sham or neurectomized, relative to that in nonpregnant Shams (Fig. 2). Thus, neurectomy did not block the growth of the cervix with pregnancy. On Day 22 postbreeding, 24 h later and approximately 2–4 h after birth, the density of cell nuclei in the cervix from postpartum Sham rats increased compared to that in Day 21 prepartum Sham group. Postpartum VnX and PnX+VnX, but not PnX, rats had a reduced cell nuclei density compared to that in postpartum cervix from Sham controls.
FIG. 2.
Data are mean densities of cell nuclei (±SEM) (3 sections/rat) in the cervix of Sham-operated rats (Sham, n = 14; 4–6/group), PnX pregnant rats (n = 22; 4–11/group), VnX rats (n = 16; 4–8/group), or PnX+VnX rats (n = 12; 3–5/group) with respect to day (D) postbreeding. All prepartum [71] or postpartum (PP) groups were significantly reduced compared to the nonpregnant (NP) group (n = 3) (ANOVA, F = 19.30, df = 11). b, P < 0.05 versus Day 21 Sham (ANOVA, F = 110.4, df = 2). *, P < 0.05 versus Day 22 PP Sham (ANOVA, F = 5.004, df = 3).
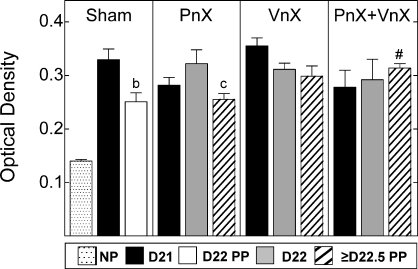
With reduced cell nuclei density (perceived as a consequence of increased cell size and extracellular area, rather than increased vascularity) the content and organization of collagen in the cervix declined with pregnancy. In nonpregnant Shams, collagen fibers in the cervix were darkly stained with picrosirius red, and fibrils were tightly packed in a striated arrangement. In sections of cervix from all pregnant rats, the intensity and area of birefringence was reduced, and collagen fibrils appeared loosely packed amid lightly stained pockets. The OD of polarized light, inversely related to collagen content and structure, increased in sections of cervix from all pregnant rats by Day 21, relative to that in nonpregnant rats, irrespective of neurectomy (Fig. 3). Transection of the pelvic nerve did not prevent the postpartum reduction in OD in the cervix (≥Day 22.5), i.e., increase in collagen content and structure that occurred in postpartum Sham and PnX rats. However, OD of postpartum cervix sections from the PnX+VnX group was significantly increased compared to that of postpartum PnX rats, an indication that vagotomy blocks restoration of collagen in the extracellular matrix. Among the peripartum VnX and PnX+VnX groups, the OD of cervix sections was unchanged and comparable to that in prepartum Day 21 Sham rats. Thus, vagus nerve transection blocked the postpartum increase in collagen content and reorganization of structure found in postpartum Sham and PnX groups.
FIG. 3.
Data shown are the mean ODs ± SEM of picrosirius red-stained sections (3 sections/rat; n = 3–10 rats/group) normalized to cell nuclei density to correct for hypertrophy of cervix with pregnancy. Photomicrographs of birefringent polarized light were converted to grayscale images. Regions of reduced collagen content and scattered fibrils were inversely related to OD as previously described [5, 40] (details of NIH Image J analyses are in Materials and Methods). Compared to OD of nonpregnant controls, OD was significantly increased (less collagen content and structure) in cervix of prepartum [71] and postpartum (PP) groups (P < 0.05; ANOVA, F = 5.984, df = 11). Rat numbers/group are the same as those specified in Figure 2 legend. b, P < 0.05 versus Day (D) 21 Sham (P < 0.05, t-test; t = 3.039, df = 6); c, P < 0.05 versus Day 22 PnX rats (delayed birth) (ANOVA, F = 4.094, df = 2); #, P < 0.05 versus ≥ Day 22.5 PP PnX rats (ANOVA, F = 4.411, df = 2).
Based upon bright-field analysis of cell morphology and stereological analysis of sections, macrophages were more prevalent in the cervix of pregnant Sham rats than in nonpregnant controls. ED-1 antibody-labeled macrophages were dark brown and often surrounded violet-colored hematoxylin-counterstained cell nuclei. By postbreeding Day 21, the presence of macrophages increased in the cervix of Sham-operated rats, in particular throughout the subepithelial stroma and smooth muscle regions near blood vessels (Fig. 4, left panel photomicrographs). This increase in resident macrophages was blocked by pelvic neurectomy, i.e., in PnX and PnX+VnX groups but not in VnX rats on Day 21 postbreeding (Fig. 4 right panels, P < 0.05; ANOVA, F = 3.82, df = 11). In neurectomized rats that had not given birth by the morning of Day 22 postbreeding, the numbers of macrophages present in PnX and PnX+VnX rats were increased compared to that in nonpregnant rats. Rather, macrophage numbers declined in the prepartum cervix of Day 22 VnX rats compared to that in the Day 21 VnX group. In postpartum VnX rats with delayed birth, the census of macrophages remained reduced, significantly lower than that in postpartum Shams. Thus, a reduced presence of macrophages was found in prepartum neurectomized rats, either on Day 21 or 22 postbreeding in association with delayed birth.
PLATE I. Figures 4 and 5.
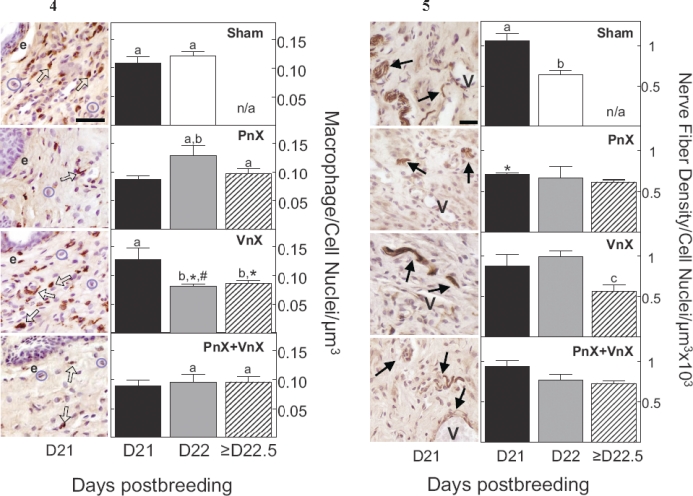
Fig 4. Left panels) Macrophages stained with ED-1 antibody (specific dark brown-stained cells indicated by open arrows) and cell nuclei counterstained with hematoxylin (indicated by circles of pale violet stain) in a section of cervix on Day 21 of pregnancy from corresponding Sham and neurectomized groups. e, endothelium. Bar = 50 μm. Right panels) Data are mean numbers of macrophages in the cervix (±SEM) (3 sections/rat; numbers of rats/group are specified in Fig. 2 legend) normalized to cell nuclei number/μm3 to account for variability among individuals or field of view and hypertrophy with pregnancy. Day (D) 21 and 22 groups are prepartum, while Day 22 Shams (white bar, top panel) and Day ≥22.5 groups (cross-hatched bar) are postpartum at term or with delayed birth, respectively. The density of macrophages in the nonpregnant Sham group was 0.048 ± 0.011 (n = 3, data not shown). n/a, not applicable, since all Sham controls delivered by Day 22 postbreeding. a, P < 0.05 versus nonpregnant Sham (ANOVA, F = 3.823, df = 11); b, P < 0.05 versus prepartum Day 21, same group (ANOVA; PnX, F = 3.6, df = 2; VnX, F = 5.6, df = 2); *, P < 0.05 versus postpartum Day 22 Shams (ANOVA, F = 2.7, df = 3); #, P < 0.05 versus Day 22 PnX rats with delayed birth (Student t-test, t = 2.6, df = 6).
Fig 5. Left panels) Cervix from rats on Day (D) 21 of postbreeding. Nerve fibers are stained dark brown with peripherin antibody (arrows) and counterstained with hematoxylin (nuclei are pale violet). Open spaces are blood vessels (V), some of which contain erythrocytes. Group designations and number of rats/group are the same as in Figure 4. Bar = 25 μm. Right panels) Data are mean nerve fiber densities in cervix of Sham and neurectomized groups (±SEM) (3 sections/rat, n = 3–10 rats/group) normalized to cell nuclei number/μm3 × 103 to adjust for variability among individuals and hypertrophy with pregnancy. The area of cervix with nerve fibers in the nonpregnant Sham group was 0.49 ± 0.03 (n = 3, data not shown). a, P < 0.05 versus nonpregnant Sham (Kruskal-Wallis); b, P < 0.05 versus prepartum Day 21 Sham group (ANOVA, F = 20.98, df = 2); c, P < 0.05 versus Day 22 VnX group with delayed birth (ANOVA, F = 5.412, df = 2); *, P < 0.05 versus prepartum Day 21 Sham (ANOVA, F = 3.306, df = 6).
In all rats, peripherin-stained nerve fibers were evident in sections of cervix. Fibers were dark brown with punctate stain, as if in cross-section or thin section, and of various lengths, with beaded varicosities (Fig. 5, left panel photomicrographs). Fibers were occasionally bundled and of varied in length. Nerve fibers were often located around blood vessels and were prominent throughout the stroma and perimetrium of the cervix. As with macrophages, more nerve fibers/area were present in the prepartum cervix of Sham rats than in nonpregnant rats, the density of nerve fibers/area of cervix increased in Sham controls on Day 21 postbreeding (Fig. 5, right panels). This increase was not found in Day 21 neurectomized rats. Rather, nerve fiber density was reduced in PnX rats versus Sham rats on Day 21 postbreeding (P < 0.05; ANOVA, F = 3.306, df = 6). Moreover on Day 22 postbreeding, all neurectomized rats with delayed birth had the same low density of nerve fibers in the cervix as in nonpregnant controls. Postpartum, nerve fiber density in the cervix of Day 22 Shams at term and ≥Day 22.5 VnX rats with delayed birth declined from the peak in prepartum D21 Sham and D22 VnX groups. Overall, the area of cervix with nerve fibers in all postpartum groups was the same as that in nonpregnant controls. Thus, transection of parasympathetic pathways blocks changes in nerve fiber density in the peripartum cervix.
DISCUSSION
The present findings indicate that the pelvic and vagus nerves are involved in timing of birth and the process of cervical remodeling in rats at term. Transection of the two parasympathetic pathways that innervate the cervix delayed births. Although growth and remodeling of the extracellular matrix with pregnancy did not appear to be affected by nerve transections, each neurectomy, or their combination, prolonged serum progesterone production and reduced the number of macrophages in the prepartum cervix. The novel contribution of the present study was to provide evidence of a neural mechanism near term that is involved in the recruitment of macrophages in the cervix and that affects the decline in progesterone in circulation.
The present findings in Sham controls replicate our previous reports in both rats [5] and mice [32, 40] for prepartum remodeling of cervix, in terms of hypertrophy, degradation of extracellular matrix, macrophage recruitment, and increased presence of nerve fibers. The function served by the pelvic nerve for some of these processes associated with parturition may differ from that mediated by the vagus nerve in innervation of the cervix. Although nerve transections blocked the increase in area of cervix with nerve fibers that occurred in Sham controls, recruitment of macrophages on Day 21 of pregnancy was blocked in PnX and PnX+VnX rats but not VnX rats. These results suggest that the pelvic nerve regulates immigration of macrophages in the prepartum cervix. By contrast, increased presence of macrophages in the prepartum cervix of PnX rats with delayed birth on Day 22 of pregnancy may reflect signals conveyed by the vagus nerve or the decline of progesterone in circulation. Progesterone withdrawal is unlikely to be the sole consideration for immigration of macrophages, because fewer macrophages were present with reduced serum progesterone in the cervix from VnX rats on Day 22 postbreeding. Moreover, the distinct roles of the pelvic and vagus nerves in recruitment of macrophages on Day 21 and Day 22 of pregnancy, respectively, is supported by findings in PnX+VnX rats in which the census of resident macrophages in the prepartum cervix was unchanged (data for postbreeding Day 21 = 22), even though serum progesterone had declined. Differences in macrophage immigration among groups suggest that one nerve tract does not necessarily compensate for loss of the other pathway. Thus, each parasympathetic projection could serve a distinct and specific function related to recruitment or possibly activation of proinflammatory processes in the prepartum cervix.
With the present analyses of collagen stain, innervation does not appear to regulate growth or degradation of the extracellular matrix of the cervix as pregnancy nears term. With pregnancy, increased size of the cervix, indicated by reduced cell nuclei density, as well as reduced collagen content and structure, indicated by increased OD of birefringence from picrosirius red-stained sections, was the same in neurectomized rats as in Sham controls. Hypertrophy and reduced collagen matrix in the prepartum cervix in neurectomized rats that do not deliver on time is consistent with evidence that cervical remodeling occurs in PGF2α receptor-deficient mice, even though birth is delayed [40]. The finding that extracellular matrix degradation was not obstructed by neurectomy is also consistent with recent reports by Mackay et al. [28] and Shi et al. [35] who used light-induced autofluorescence to quantify collagen cross-linkage and evaluate cervical resistance to stretch. These results in rats subjected to bilateral pelvic neurectomy, as well as new findings for effects of vagotomy, support the conclusion that loss of parasympathetic innervation does not reverse remodeling of the extracellular matrix in the cervix, as pregnancy progresses to term. The sustained reduction of collagen content and structure in the cervix of peripartum neurectomized rats may reflect not only reduced numbers but potentially the activity of resident macrophages. It also remains to be determined if another immune cell, such as neutrophils with capabilities for collagenolytic activity, is recruited into the cervix. Contributions of the hormone relaxin are another critical component for softening and remodeling of the cervix [46] and, along with sustained levels of progesterone, may contribute to maintaining a remodeled extracellular matrix in neurectomized rats late in pregnancy. Thus, evidence collectively supports the conclusions that assessments by OD or luminescence reflect the dramatic remodeling of the cervix with pregnancy but not necessarily the transition to effacement, where cervical structure disappears into the lower uterine segment that connects the uterus to vagina before birth.
A prepartum fall in serum progesterone concentrations is considered a critical component in the mechanism of parturition in rodents [47]. The decline in progesterone in circulation before birth was common to all groups in the study but was postponed, and, as previously found, births were delayed by pelvic neurectomy [11, 25, 26]. A neurectomy-induced delay in the fall in systemic progesterone contrasts with reports that earlier in pregnancy, transection of the pelvic nerve, as well as the vagus nerve, was associated with reduced serum progesterone [13, 48], the significance of which is uncertain because preterm birth did not occur. Nonetheless, the finding that innervation of the cervix may influence ovarian function during pregnancy could reflect efferent control by a local and more central parasympathetic pathway [49, 50]. After pelvic nerve transections, there is a loss of afferent connections to local interneurons in the thoracolumbar spinal cord [14], as well as to ascending sensory pathways in the dorsal columns and autonomic intermediolateral columns of the spinal cord that project to brainstem regions, i.e., solitary nucleus and dorsal motor nucleus. These regions receive input from the vagus nerve [17, 51–53]. Integration of signals in the brainstem could then influence the ovaries through either a descending spinal cord projection or more directly through an extraspinal vagal pathway [54, 55]. Such pathways may also affect adrenal corticosterone production which is elevated following neurectomy.
This is the first study of the effect of parasympathetic nerve transections on serum corticosterone in pregnant rodents. In contrast to the lack of effect of vagotomy on serum corticosterone in nonpregnant rats [56, 57], present results indicate that transection of the pelvic and/or vagus nerve increased serum corticosterone more than threefold compared to peripartum Sham controls or in intact pregnant rats [58]. Thus, findings in this study suggest parasympathetic restraint of corticosteroid production during pregnancy may be mediated through spinal cord or vagal afferent pathways that innervate the adrenal gland [59]. The stress of surgery or consequences of neurectomy may also contribute to alterations in serum corticosterone concentrations as pregnancy nears term. However, stress-induced increases in serum corticosterone in rats late in pregnancy do not affect serum progesterone or the timing of parturition [59] and may not impact the fetus due to placental metabolism [60]. In this study, growth and remodeling of the extracellular matrix were equivalent in prepartum neurectomized and Sham rats, despite elevated maternal serum corticosterone in the former but not latter group. While stress is more commonly associated with preterm birth [61], maternal corticosteroids appear to have little benefit with respect to cervical remodeling or the process of parturition in primates or women [62, 63]. Finally, a few neurectomized rats with a distended bladder and/or stomach delivered on time without any apparent affect on parameters associated with remodeling of the cervix. Thus, our findings also suggest that stress-related distension of internal organs or surgery cannot account for the predominant outcomes of the study in which parasympathetic denervation altered immigration of macrophages into the cervix and delayed birth. Overall, these findings suggest that maternal corticosterone is unlikely to contribute to the mechanism that times parturition in the rat.
The contribution of parasympathetic innervation for recruitment of macrophages and timing birth at term adds to multiple trajectories that contribute to the process of parturition. Even though systemic withdrawal of progesterone, cervical ripening, and labor occurred in neurectomized rats, births were well beyond the normal delivery time. The delay in birth in PnX rats in the present study replicates previous findings [11, 22–26, 28]. Delivery eventually occurs in the absence of a critical neural signal for timing birth. The possibility that severed nerves have functionally reconnected within the 7-day postsurgery period is unlikely given the regrowth rate of the peripheral or vagus nerve following damage [64, 65]. The eventual delivery by neurectomized rats raises the possibility that auxiliary factors, which are independent of innervation, may promote luteolysis, as well as inflammatory processes that could promote recruitment or activate resident immune cells, whatever the census. The convergence of multiple pathways in the mechanism that times birth at term has been proposed [30, 31, 66], in part to explain premature cervical ripening and preterm birth. The newly appreciated importance of innervation for timing of birth may also provide insight into reports in women with spinal cord injuries who experience more severe and frequent complications related to birth, as well as a higher cesarean delivery rate compared to a normative demographic of patients [67–69]. Of clinical interest, it remains to be determined if function of the cervix as pregnancy nears term may be impaired in women with undiagnosed parasympathetic nerve damage or aberrant innervation, potentially a consequence of complications from previous birth experiences [70].
In summary, the findings indicate that loss of pelvic and/or vagus innervation delays birth and differentially regulates macrophage recruitment in the cervix of rats. Although nerve transections do not affect growth or degradation of the extracellular matrix of the cervix with pregnancy, parasympathetic denervation temporarily forestalls the systemic decline in circulating progesterone. These results raise the possibility that innervation of the cervix may directly and, through affects on ovarian production of progesterone, indirectly regulate proinflammatory processes that time ripening and birth at term. These findings extend our understanding to include the contribution of neural signaling in the complex of multiple inputs that promotes cervical ripening and uterine contractions in the final common pathway that times parturition at term.
Acknowledgments
We thank Jonathan W. Boyd, M.D. for help in developing surgical techniques and John Chrisler and John Hough for technical support and expertise. We also thank Gwen Foster for tissue section analysis.
Footnotes
Supported in part by National Institutes of Health Grant HD-054931.
REFERENCES
- Haase EB, Buchman J, Tietz AE, Schramm LP. Pregnancy-induced uterine neuronal degeneration in the rat. Cell Tissue Res 1997; 288: 293 306 [DOI] [PubMed] [Google Scholar]
- Chavez-Genaro R, Lombide P, Anesetti G. A quantitative study of rat uterine sympathetic innervation during pregnancy and postpartum. Reprod Fertil Dev 2006; 18: 525 531 [DOI] [PubMed] [Google Scholar]
- Wikland M, Lindblom B, Dahlstrom A, Haglid KG. Structural and functional evidence for the denervation of human myometrium during pregnancy. Obstet Gynecol 1984; 64: 503 509 [PubMed] [Google Scholar]
- Kirby LS, Kirby MA, Warren JW, Tran LT, Yellon SM. Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig 2005; 12: 578 585 [DOI] [PubMed] [Google Scholar]
- Boyd JW, Lechuga TJ, Ebner CA, Kirby MA, Yellon SM. Cervix remodeling and parturition in the rat: lack of a role for hypogastric innervation. Reproduction 2009; 137: 739 748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryman I, Norstrom A, Dahlstrom A, Lindblom B. Immunohistochemical evidence for preserved innervation of the human cervix during pregnancy. Gynecol Obstet Invest 1987; 24: 73 79 [DOI] [PubMed] [Google Scholar]
- Tingaker BK, Johansson O, Cluff AH, Ekman-Ordeberg G. Unaltered innervation of the human cervix uteri in contrast to the corpus during pregnancy and labor as revealed by PGP 9.5 immunohistochemistry. Eur J Obstet Gynecol Reprod Biol 2005; 125: 66 71 [DOI] [PubMed] [Google Scholar]
- Houdeau E, Rousseau A, Meusnier C, Prud'homme MJ, Rousseau JP. Sympathetic innervation of the upper and lower regions of the uterus and cervix in the rat have different origins and routes. J Comp Neurol 1998; 399: 403 412 [PubMed] [Google Scholar]
- Baljet B, Drukker J. The extrinsic innervation of the pelvic organs in the female rat. Acta Anat (Basel) 1980; 107: 241 267 [DOI] [PubMed] [Google Scholar]
- Papka RE, McNeill DL, Thompson D, Schmidt HH. Nitric oxide nerves in the uterus are parasympathetic, sensory, and contain neuropeptides. Cell Tissue Res 1995; 279: 339 349 [DOI] [PubMed] [Google Scholar]
- Carlson RR, De Feo VJ. Role of the pelvic nerve vs. the abdominal sympathetic nerves in the reproductive function of the female rat. Endocrinology 1965; 77: 1014 1022 [DOI] [PubMed] [Google Scholar]
- Yang M, Zhao X, Miselis RR. The origin of catecholaminergic nerve fibers in the subdiaphragmatic vagus nerve of rat. J Auton Nerv Syst 1999; 76: 108 117 [DOI] [PubMed] [Google Scholar]
- Burden HW, Leonard M, Smith CP, Louis TM, Lawrence IE. The effects of pelvic neurectomy on collagen in the cervix of the pseudopregnant rat. Anat Rec 1984; 210: 575 581 [DOI] [PubMed] [Google Scholar]
- Kirby MA, Groves M, Yellon SM. Retrograde tracing of spinal cord connections to the cervix with pregnancy in mice. Reproduction 2009; 139: 645 653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM, Grisham LA, Rambau GM, Lechuga TJ, Kirby MA. Pregnancy-related changes in connections from the cervix to forebrain and hypothalamus in mice. Reproduction 2010; 140: 155 164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 2003; 464: 36 48 [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Berkley KJ. Spinal and vagal influences on the responses of rat solitary nucleus neurons to stimulation of uterus, cervix and vagina. Brain Res 1995; 702: 251 254 [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Bianca R, Sansone G, Gomez LE, Cueva-Rolon R, Beyer C, Whipple B. Brain-mediated responses to vaginocervical stimulation in spinal cord-transected rats: role of the vagus nerves. Brain Res 1996; 708: 128 134 [DOI] [PubMed] [Google Scholar]
- Collins JJ, Lin CE, Berthoud HR, Papka RE. Vagal afferents from the uterus and cervix provide direct connections to the brainstem. Cell Tissue Res 1999; 295: 43 54 [DOI] [PubMed] [Google Scholar]
- Whipple B, Komisaruk BR. Brain (PET) responses to vaginal-cervical self-stimulation in women with complete spinal cord injury: preliminary findings. J Sex Marital Ther 2002; 28: 79 86 [DOI] [PubMed] [Google Scholar]
- Liu B, Tong C, Eisenach JC. Pregnancy increases excitability of mechanosensitive afferents innervating the uterine cervix. Anesthesiology 2008; 108: 1087 1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden HW, Price GT, Renegar RH, Hodson CA. Effects of peripheral nerve lesions during pregnancy on parturition in rats. Anat Embryol (Berl) 1990; 182: 499 501 [DOI] [PubMed] [Google Scholar]
- Higuchi T, Uchide K, Honda K, Negoro H. Pelvic neurectomy abolishes the fetus-expulsion reflex and induces dystocia in the rat. Exp Neurol 1987; 96: 443 455 [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez M, Cruz Y, Pacheco P, Aguilar-Roblero R, Hudson R. The sensory but not musc-ular pelvic nerve branch is necessary for parturition in the rat. Physiol Behav 1998; 63: 929 932 [DOI] [PubMed] [Google Scholar]
- Louis TM, Lawrence IE, Jr, Becker RF, Burden HW. Prostaglandin F2alpha prostaglandin E2, progesterone, 20alpha- dihydroprogesterone and ovarian 20alpha hydroxysteroid dehydrogenase activity in preparturient pelvic neurectomized rats. Proc Soc Exp Biol Med 1978; 158: 631 636 [DOI] [PubMed] [Google Scholar]
- Renegar RH, Steel M, Burden HW, Hodson CA. Endocrine parameters associated with disruption of parturition after bilateral pelvic neurectomy. Proc Soc Exp Biol Med 1992; 201: 28 33 [DOI] [PubMed] [Google Scholar]
- Spies HG, Forbes VM, Clegg MT. The influence of coitus, sucking, and prolactin injections on pregnancy in pelvic neurectomized rats. Proc Soc Exp Biol Med 1971; 138: 470 474 [Google Scholar]
- Mackay LB, Shi SQ, Garfield RE, Maner WL. The effect of bilateral pelvic neurectomy on uterine and abdominal electrical and pressure activity, as measured by telemetry in conscious, unrestrained pregnant rats. J Perinat Med 2009; 37: 313 319 [DOI] [PubMed] [Google Scholar]
- Wanner MB, Rageth CJ, Zach GA. Pregnancy and autonomic hyperreflexia in patients with spinal cord lesions. Paraplegia 1987; 25: 482 490 [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 2009; 297: R525 R545 [DOI] [PubMed] [Google Scholar]
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 2007; 25: 69 79 [DOI] [PubMed] [Google Scholar]
- Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod 1999; 61: 879 883 [DOI] [PubMed] [Google Scholar]
- Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Invest 2003; 10: 323 338 [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol 2005; 66: 161 173 [DOI] [PubMed] [Google Scholar]
- Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Changes in cervical resistance and collagen fluorescence during gestation in rats. J Perinat Med 1999; 27: 188 194 [DOI] [PubMed] [Google Scholar]
- Yellon SM, Burns AE, See JL, Lechuga TJ, Kirby MA. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol Reprod 2009; 81: 1 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco P, Martinez-Gomez M, Whipple B, Beyer C, Komisaruk BR. Somato-motor components of the pelvic and pudendal nerves of the female rat. Brain Res 1989; 490: 85 94 [DOI] [PubMed] [Google Scholar]
- Seif C, Martinez Portillo FJ, Osmonov DK, Bohler G, van der Horst C, Leissner J, Hohenfellner R, Juenemann KP, Braun PM. Methylene blue staining for nerve-sparing operative procedures: an animal model. Urology 2004; 63: 1205 1208 [DOI] [PubMed] [Google Scholar]
- Cueva-Rolon R, Sansone G, Bianca R, Gomez LE, Beyer C, Whipple B, Komisaruk BR. Vagotomy blocks responses to vaginocervical stimulation after genitospinal neurectomy in rats. Physiol Behav 1996; 60: 19 24 [DOI] [PubMed] [Google Scholar]
- Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod 2008; 78: 438 444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci 2009; 16: 257 264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosc MJ. Photoperiodic regulation of the time of birth in rats: involvement of circadian endogenous mechanisms. Physiol Behav 1990; 48: 441 446 [DOI] [PubMed] [Google Scholar]
- Lincoln DW, Porter DG. Timing of the photoperiod and the hour of birth in rats. Nature 1976; 260: 780 781 [DOI] [PubMed] [Google Scholar]
- Murakami N, Abe T, Yokoyama M, Katsume A, Kuroda H, Etoh T. Effect of photoperiod, injection of pentobarbitone sodium or lesion of the suprachiasmatic nucleus on pre-partum decrease of blood progesterone concentrations or time of birth in the rat. J Reprod Fertil 1987; 79: 325 333 [DOI] [PubMed] [Google Scholar]
- Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res 2008; 1213: 98 110 [DOI] [PubMed] [Google Scholar]
- Sherwood OD. Relaxin. Knobil E, Neill JD.(Eds.), The Physiology of Reproduction, 2nd ed. New York: Raven Press, 1994: 862 1009 [Google Scholar]
- Kuon RJ, Shi SQ, Maul H, Sohn C, Balducci J, Maner WL, Garfield RE. Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle. AM J Obstet Gynecol 2010; 202: 455.e1 455.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence IE, Jr, Burden HW, Louis TM. Effect of abdominal vagotomy of the pregnant rat on LH and progesterone concentrations and fetal resorption. J Reprod Fertil 1978; 53: 131 136 [DOI] [PubMed] [Google Scholar]
- Klein CM, Burden HW. Anatomical localization of afferent and postganglionic sympathetic neurons innervating the rat ovary. Neurosci Lett 1988; 85: 217 222 [DOI] [PubMed] [Google Scholar]
- Roche PJ, Parkington HC, Gibson WR. Pregnancy and parturition in rats after sympathetic denervation of the ovary, oviduct and utero-tubal junction. J Reprod Fertil 1985; 75: 653 661 [DOI] [PubMed] [Google Scholar]
- Puder BA, Papka RE. Hypothalamic paraventricular axons projecting to the female rat lumbosacral spinal cord contain oxytocin immunoreactivity. J Neurosci Res 2001; 64: 53 60 [DOI] [PubMed] [Google Scholar]
- Puder BA, Papka RE. Activation and circuitry of uterine-cervix-related neurons in the lumbo-sacral dorsal root ganglia and spinal cord at parturition. J Neurosci Res 2005; 82: 875 889 [DOI] [PubMed] [Google Scholar]
- Papka RE, Hafemeister J, Storey-Workley M. P2X receptors in the rat uterine cervix, lumbo-sacral dorsal root ganglia, and spinal cord during pregnancy. Cell Tissue Res 2005; 321: 35 44 [DOI] [PubMed] [Google Scholar]
- Allen LG, Lawrence IE, Jr, Burden HW, Hodson CA. Effects of abdominal vagotomy on serum LH concentrations in female rats. J Reprod Fertil 1985; 74: 87 94 [DOI] [PubMed] [Google Scholar]
- Gerendai I, Toth IE, Boldogkoi Z, Medveczky I, Halasz B. CNS structures presumably involved in vagal control of ovarian function. J Auton Nerv Syst 2000; 80: 40 45 [DOI] [PubMed] [Google Scholar]
- Hansen MK, Nguyen KT, Fleshner M, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol 2000; 278: R331 R336 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fernandez I, Gonzalo-Sanz LM. Vagal influence on the adrenocortical function of the rat. Rev Esp Fisiol 1987; 43: 203 207 [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology 1984; 114: 1635 1644 [DOI] [PubMed] [Google Scholar]
- Coupland RE, Parker TL, Kesse WK, Mohamed AA. The innervation of the adrenal gland. III. Vagal innervation. J Anat 1989; 163: 173 181 [PMC free article] [PubMed] [Google Scholar]
- Staud F, Mazancova K, Miksik I, Pavek P, Fendrich Z, Pacha J. Corticosterone transfer and metabolism in the dually perfused rat placenta: effect of 11beta-hydroxysteroid dehydrogenase type 2. Placenta 2006; 27: 171 180 [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J 2001; 5: 119 125 [DOI] [PubMed] [Google Scholar]
- Novy MJ, Walsh SW. Dexamethasone and estradiol treatment in pregnant rhesus macaques: effects on gestational length, maternal plasma hormones, and fetal growth. Am J Obstet Gynecol 1983; 145: 920 931 [DOI] [PubMed] [Google Scholar]
- Kavanagh J, Kelly AJ, Thomas J. Corticosteroids for cervical ripening and induction of labour. Cochrane Database Syst Rev 2006; CD003100 [DOI] [PMC free article] [PubMed]
- Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol 1999; 9: 313 325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester H, Allchorne AJ, Woolf CJ. Recovery of C-fiber-induced extravasation following peripheral nerve injury in the rat. Exp Neurol 1998; 154: 628 636 [DOI] [PubMed] [Google Scholar]
- Challis JRG. Mechanism of parturition and preterm labor. Obstet Gynecol Surv 2000; 55: 650 660 [DOI] [PubMed] [Google Scholar]
- Charlifue SW, Gerhart KA, Menter RR, Whiteneck GG, Manley MS. Sexual issues of women with spinal cord injuries. Paraplegia 1992; 30: 192 199 [DOI] [PubMed] [Google Scholar]
- Cross LL, Meythaler JM, Tuel SM, Cross AL. Pregnancy, labor and delivery post spinal cord injury. Paraplegia 1992; 30: 890 902 [DOI] [PubMed] [Google Scholar]
- Westgren N, Hultling C, Levi R, Westgren M. Pregnancy and delivery in women with a traumatic spinal cord injury in Sweden, 1980–1991. Obstet Gynecol 1993; 81: 926 930 [PubMed] [Google Scholar]
- Chaliha C. Postpartum pelvic floor trauma. Curr Opin Obstet Gynecol 2009; 21: 474 479 [DOI] [PubMed] [Google Scholar]
- Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol 1999; 162: 7198 7207 [PubMed] [Google Scholar]