Abstract
In sexual species, fertilization of oocytes produces individuals with alleles derived from both parents. Here we use pluripotent stem cells derived from somatic cells to combine the haploid genomes from two males to produce viable sons and daughters. Male (XY) mouse induced pluripotent stem cells (Father #1) were used to isolate subclones that had spontaneously lost the Y chromosome to become genetically female (XO). These male-derived XO stem cells were used to generate female chimeras that were bred with genetically distinct males (Father #2), yielding progeny possessing genetic information that was equally derived from both fathers. Thus, functional oocytes can be generated from male somatic cells after reprogramming and spontaneous sex reversal. These findings have novel implications for mammalian reproduction and assisted reproductive technology.
Keywords: assisted reproductive technology, induced pluripotent stem cells, sex reversal, stem cells, Turner syndrome, XO karyotype
XY-derived XO pluripotent stem cells derived from somatic cells differentiate into functional oocytes in female chimeras that can be fertilized to produce normal male and female progeny with alleles derived from two fathers.
INTRODUCTION
Natural matings between males and females result in the fertilization of oocytes by spermatozoa and the generation of progeny that inherit alleles from both parents. In mammals, experimentally generated embryos with alleles only processed through oogenesis or only processed through spermatogenesis usually die during development because of imbalances in imprinted gene expression [1–4]. Thus, epigenetic differences derived during male and female gametogenesis are required for normal mouse development. Interestingly, viable bimaternal female mice have been generated by nuclear transfer into oocytes [4]. This required the introduction of nuclei from H19 mutant nongrowing oocytes into wild-type fully grown oocytes. The frequency of obtaining viable mice from these reconstructed oocytes with genetic information derived from two mothers was <1%. However, if the donor nucleus from the nongrowing oocyte carrying the H19 mutation also had a deletion of the Dlk1-Dio3 intergenic region, then there was a high success rate in obtaining bimaternal female mice from the reconstructed oocytes [5]. The resulting bimaternal mice were relatively normal but weighed less than controls, and there were some gene expression differences. These bimaternal mice also had significantly longer life spans compared to controls [6].
Viable progeny with two fathers have yet to be generated. To generate progeny with alleles derived from two males, the cells from one male must undergo meiosis to produce oocytes. This is possible in cases of XY sex-reversal. XY sex-reversed individuals are genetically male but develop as females with variable gonadal phenotypes, from dysgenesis to functional ovaries [7–9]. There are also cases of XY sex-reversal in mice with a Y chromosome ingressed from one genetic background into a different genetic background [10, 11]. XY sex-reversed females are capable of initiating oogenesis and in some cases generate functional oocytes [8, 9, 12]. Thus, two genetic males can generate viable progeny if one is sex-reversed and fertile. In the MRL/MpJ mouse strain, rare XY testicular germ cells of phenotypic males can differentiate into oocytes in spermatogenically active seminiferous tubules although they do not persist in the adult [13]. It has not been determined if these testicular oocytes can be fertilized to produce progeny. Are there other ways for phenotypic males to generate oocytes?
Pluripotent stem cell lines provide a system to differentiate germ cells. Embryonic stem (ES) cells are capable of differentiating into every cell type of the adult body, including oocytes and sperm. Although this has only been demonstrated for mouse and rat, it seems likely that ES cells from other species (e.g., human) would also have the ability to differentiate into germ cells under the appropriate conditions [14–16]. Somatic cells can be reprogrammed using a variety of molecular and chemical reprogramming strategies to generate induced pluripotent stem (iPS) cells [17]. iPS cells have many of the characteristics of ES cells, including pluripotency and the ability to generate germline mouse chimeras [18].
Mouse ES cells that are genetically male spontaneously lose the Y chromosome at a 1%–3% frequency, presumably by nondisjunction, resulting in XO subclones [19, 20]. In humans, X chromosome monosomy (45,X) usually results in embryo lethality, but in rare cases, viable females are born with Turner syndrome, a variable spectrum of pathologies that includes gonadal dysgenesis and infertility [21]. However, in the mouse, XO individuals develop as viable, fertile females [22, 23].
In this study, we exploit XY pluripotent stem cells and in vitro sex reversal to efficiently differentiate functional oocytes in female chimeras. Natural matings of these female chimeras result in the generation of viable male and female mice that combine the haploid genomes from two fathers. Thus, iPS cell technologies can be used to bypass sex-specific epigenetic constraints on reproduction. These results have important implications for mammalian reproduction and assisted reproductive technologies.
MATERIALS AND METHODS
Mice
C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Pou5f1-GFP (green fluorescent protein) transgenic mice—official symbol Tg(Pou5f1-EGFP)2Mnn—were obtained from Hans Schöler [24]. All the animal manipulations were conducted in accordance with the National Research Council Guide for Care and Use of Laboratory Animals and approved by the University of Texas M.D. Anderson Cancer Center Institutional Animal Care and Use Committee.
Generation and Characterization of iPS Cells
Pou5f1-GFP mouse embryo fibroblasts (MEFs) were isolated from 13.5 days postcoitus fetuses using standard procedures [25]. MEFs (passage 3) were reprogrammed by retroviral transduction using Pou5f1, Sox2, Klf4, and Myc [26]. iPS cell colonies were picked 28 days after infection based on morphology without selection [27]. Goat polyclonal antibodies to POU5F1, SOX2, and NANOG (Santa Cruz Biotechnology, Inc.) were used at a 1:200 dilution. The secondary antibody was Texas Red conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, Inc.) used at a 1:500 dilution. Subsequently, glass cover slips were applied using mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Nine clones expressed alkaline phosphatase activity and POU5F1, SOX2, and NANOG. These clones were screened for germline potential by injection into albino-B6 blastocysts to generate chimeras. This resulted in the S3 iPS cell line used in this study.
Isolation and Characterization of XO Pluripotent Stem Cells
PC3 ES cells [28] and S3 iPS cells were cultured using standard procedures [25, 26]. Single cell suspensions of ES or iPS cells were plated onto mitomycin C-treated fibroblast feeders at clonal density without selection. Colonies were picked, disaggregated with trypsin, expanded, and frozen using a 96-well format [25]. Clones were genotyped by PCR for Sry using the primers 5′-TCCCAGCATGCAAAATACAGAG-3′ and 5′-TTGGAGTACAGGTGTGCAGCTC-3′ and Rapsn as a positive control using primers 5′-AGGACTGGGTGGCTTCCAACTCCCAGACAC-3′ and 5′-AGCTTCTCATTGCTGCGCGCCAGGTTCAGG-3′ with an annealing temperature of 61°C. Genomic DNA isolated from putative XO ES cell clones identified by Sry PCR was digested with EcoR1 for Southern blot analysis. Blots were hybridized with a 32P-labeled pY353/B riboprobe [29]. XO ES and iPS cell clones were karyotyped by multicolor spectral karyotype (SKY) analysis (Clinical and Research Cytogenetic Laboratory of the Texas Children's Hospital, Houston, TX).
Mouse Chimeras and Progeny Genotyping
Ten to fifteen XO ES or iPS cells were microinjected into B6 blastocysts to generate female chimeras that were bred with B6 males using standard methods. The agouti-pigmented progeny from the ES cell-derived female chimeras were genotyped by PCR for DXMit216 using primers 5′-GTTTCTTCACAATCTATCCAGTTACAGCATTT-3′ and 5′-GTTTCTTATAGCTCTCAAAGCCCATGC-3′ with an annealing temperature of 55°C that recognize polymorphisms between strains 129 and B6. Prm1-cre and Pou5f1-GFP transgenes were genotyped by PCR.
RESULTS
XY-Derived XO ES Cells Produce Functional Oocytes in Female Chimeras
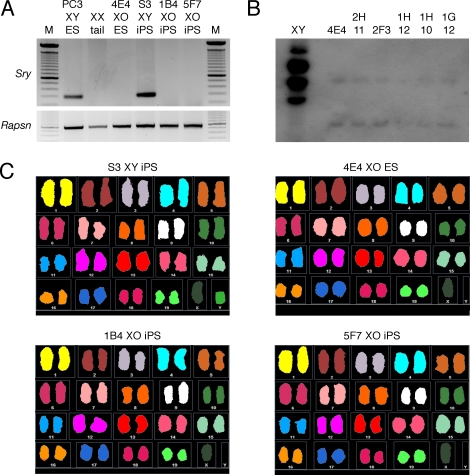
We devised an iPS cell strategy to generate oocytes from the somatic cells of genetically male mice (Fig. 1). Initially, we modeled our strategy using mouse embryonic stem (ES) cells because previous studies that mentioned the germ line potential of XY-derived XO ES cells was not their primary focus or the approach taken differed from our proposed strategy [19, 20, 30–32]. PC3 ES cells are genetically male (XY), homozygous for a Prm1-cre transgene, and derived from an agouti-pigmented (A/A) 129 inbred mouse strain [28]. PC3 ES cells were plated at clonal density and cultured without selection. Colonies were then picked for expansion, genotype analysis, and cryopreservation. PCR genotyping of each clone was performed to detect the presence of the Y chromosome, using the Y chromosome-linked testis-determining gene Sry [33]. Of the 840 clones that were screened, 11 (1.3%) were found to be negative for Sry, suggesting that they had lost the Y chromosome, becoming XO (Fig. 2A). Subsequently, Southern blot analysis of these putative XO clones was performed using a Y chromosome repetitive sequence probe, pY353/B [29]. Of the 11 putative XO clones, 10 were negative for pY353/B hybridization (Fig. 2B). Finally, multicolor SKY analysis of clones 4E4 and 2H11 showed that they lacked the Y chromosome but were disomic for all autosomes without any detectable chromosomal abnormalities, demonstrating the successful derivation of XO clones from the original XY ES cell line (Fig. 2C).
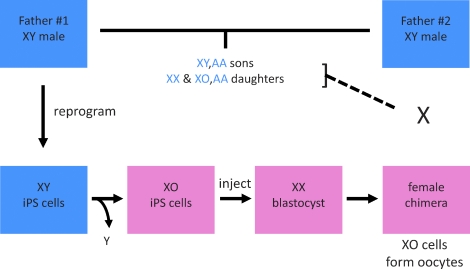
FIG. 1.
Scheme to generate mice from two fathers using iPS cells. Somatic cells from Father #1 are reprogrammed to generate iPS cells that are screened for the spontaneous loss of the Y chromosome to isolate XO clones. These XY-derived XO iPS cells are injected into blastocysts to generate female chimeras. The female chimeras serve as surrogates to differentiate oocytes from the XY-derived XO iPS cells and carry the progeny to term (dashed line). Genetically, the progeny from the female chimera are sons and daughters of Father #1 and Father #2 (horizontal line). Blue boxes, male phenotype; pink boxes, female phenotype; blue letters, alleles derived from Fathers #1 and #2.
FIG. 2.
Generation and characterization of XY-derived XO mouse ES and iPS cell lines. A) Sry PCR genotyping of mouse ES and iPS cell clones. Rapsn PCR genotyping, positive control. M, 100 bp ladder. B) Southern blot analysis of putative XY-derived XO mouse ES cell clones. EcoRI-digested genomic DNA. The blot was hybridized with the Y chromosome repeat sequence probe pY353/B. C) SKY analysis of mouse ES and iPS cell lines.
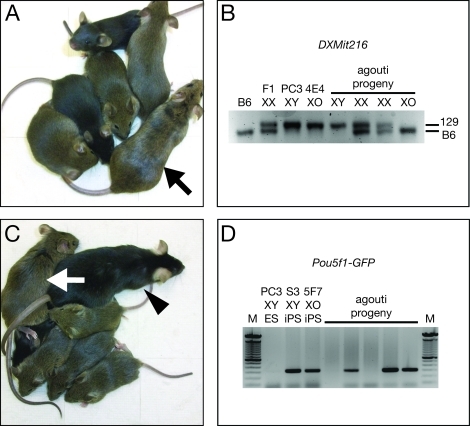
The 4E4 XO ES cell clone was injected into C57BL/6J (B6) blastocysts to generate mouse chimeras. Approximately 50% of the blastocysts injected will be female (XX) and yield female chimeras [20]. Three female chimeras were obtained with substantial ES cell-derived agouti pigmentation and bred with black-pigmented (i.e., non-agouti, a/a) B6 males. Two of the female chimeras proved to be fertile and generated litters containing both black (blastocyst-derived) and agouti-pigmented (XO ES cell-derived) progeny that appeared normal (Fig. 3A). The crosses of these XO ES cell-derived female chimeras with wild-type males should produce XY male and XX or XO female progeny. Therefore, the X chromosomes of the agouti-pigmented progeny from the female chimeras were genotyped for PCR polymorphisms between strains 129 and B6 (Fig. 3B). Male progeny (Sry positive by PCR, not shown) had their X chromosome derived from strain 129 (XO ES cell-derived, Father #1), XX female progeny had one X chromosome derived from strain 129 and the other from B6 (Father #2), and XO female progeny had their single X chromosome derived from B6 (Father #2). To date, three males, two XX females, and five XO females have been produced. All of these agouti-pigmented progeny from the female chimeras were PCR positive for the ES cell-derived Prm1-cre transgene (not shown), consistent with the homozygosity of the Prm1-cre transgene in the parental PC3 ES cell line. These results demonstrate that alleles (agouti and Prm1-cre) originally derived from an individual male (i.e., the embryo that gave rise to the PC3 ES cell line) were transmitted to progeny by undergoing meiosis during oogenesis (Supplemental Fig. S1; all the supplemental data are available online at www.biolreprod.org).
FIG. 3.
Generation of viable progeny with two fathers. A) Clone 4E4 XO ES cell-derived female chimera (arrow) and ES cell-derived progeny (agouti pigmented). B) DXMit216 PCR genotype analysis for X chromosome origin in progeny from 4E4 XO ES cell-derived female chimeras. Strain 129 generates a 145-bp fragment; B6 generates a 139-bp fragment. C) Clone 5F7 XO iPS cell-derived female chimera (arrow), B6 father (arrowhead), and iPS cell-derived progeny (agouti pigmented). D) PCR genotyping of iPS-derived progeny for Pou5f1-GFP transgene. The S3 iPS cell line is hemizygous for Pou5f1-GFP; therefore, ∼50% of the progeny inherit the transgene.
Generation of Progeny with Two Fathers Using XY-Derived XO iPS Cells
ES cell lines are derived by disassembling the inner cell mass of a blastocyst; thus, the individual that would have been generated from that particular blastocyst does not exist. Therefore, the utility of ES cells for generating progeny from two males is limited. However, iPS cells are derived from somatic cells in which the individual can be maintained [26]. Thus, iPS cell technologies extend the utility of our strategy.
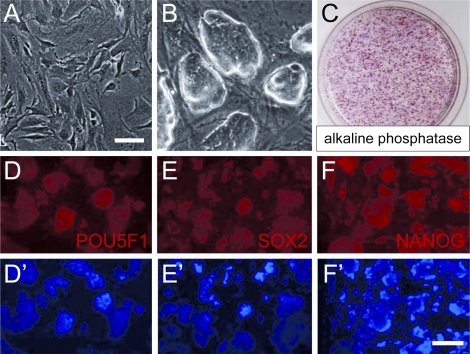
MEFs were isolated from a male fetus of mixed genetic background (B6/129/CD1) that was hemizygous for a Pou5f1-GFP transgene [24]. These MEFs were reprogrammed by forced POU5F1, SOX2, KLF4, and MYC expression into iPS cells using methods developed by Takahashi and Yamanaka [26]. iPS cell clones were isolated, expanded, and characterized for markers of pluripotency (Fig. 4). The efficiency of generating alkaline phosphatase positive staining cells, an indicator of pluripotency, was ∼1%. One of the clones, designated S3, was genetically male (XY karyotype, Fig. 2C), heterozygous for agouti (A/a), and proved to be very efficient at generating male germline chimeras.
FIG. 4.
Generation and characterization of S3 mouse iPS cell line. A) MEFs prior to reprogramming. B) iPS cells colonies. C) Alkaline phosphatase staining of iPS cell colonies. D–F) S3 iPS cell colonies immunostained for POU5F1 (D), SOX2 (E), and NANOG (F). D′–F′) 4′,6-diamidino-2-phenylindole (DAPI) staining of D–F. Scale bars: A, B = 50 μm; D–F′ = 100 μm.
XO subclones with normal sets of autosomes were isolated from the S3 iPS cell line at a 0.9% frequency (2 of 220 clones screened) by limiting dilution using the same Sry PCR and SKY criteria described above for the derivation of XO ES cell clones (Fig. 2, A and C). These two XO iPS cell clones (1B4 and 5F7) were injected into B6 blastocysts to generate 10 female chimeras (4 from 1B4 and 6 from 5F7) as judged by agouti fur pigmentation (iPS cell-derived). These female iPS cell chimeras were then bred with B6 males; 27 agouti-pigmented male (15) and female (12) progeny were obtained from female chimeras derived from both clones that appeared normal (Fig. 3C). The S3 iPS cell line was found to carry a B6 X chromosome, thus we could not distinguish XX and XO female progeny by PCR. Consistent with the hemizygous genotype of the S3 iPS cell line, a little more than half (17/27) of the agouti-pigmented progeny were positive for the Pou5f1-GFP transgene (Fig. 3D). Furthermore, 7 of 23 black-pigmented progeny screened were positive for the Pou5f1-GFP transgene, demonstrating that the original S3 iPS cell line is heterozygous for agouti (A/a). These results demonstrate that somatic cells isolated from an XY male can be reprogrammed to pluripotency to isolate XO iPS cell sublines that can be used to generate functional oocytes through female chimeras. These results indicate that both oocytes and spermatozoa can be generated from the somatic cells of a genetic male. In addition, alleles (agouti and Pou5f1-GFP) originally derived from an individual male (i.e., the MEFs that gave rise to the S3 iPS cell line) were transmitted to progeny by undergoing meiosis during oogenesis.
DISCUSSION
Previous studies have shown that XY mouse ES cells spontaneously lose the Y chromosome at a 1.4%–2.7% frequency to become XO [19, 20]. The frequency of Y chromosome loss from the PC3 XY mouse ES cell line used in this study was 1.3%, which is consistent with these previous reports. We also demonstrated that the XY-derived XO ES cell line (4E4) was capable of generating female chimeras that transmitted ES cell-derived alleles to their progeny. This is also consistent with previous reports [20, 30–32]. In addition, fertile XO mice can be produced from XY-derived XO ES cells by tetraploid embryo complementation in a scheme to accelerate the production of homozygous mutant mice [19]. Thus, the basic idea that XY-derived XO ES cells can contribute to the germ line of female chimeras has been established. However, none of these previous studies discussed how this stem cell strategy could be used to generate progeny with genetic information from two distinct fathers. Perhaps this is because these ES cell studies focused on other topics. In addition, the generation of ES cell lines does not preserve the individual (i.e., blastocyst disassembly), whereas in the derivation of iPS cell lines the individual can be maintained (i.e., somatic cells are used).
We were able to isolate XY-derived XO iPS cell lines at a frequency of 0.9%. Although the number of iPS cell clones that we screened was relatively low, the frequency of Y chromosome loss was similar to that for XY mouse ES cells [19, 20]. This suggests that XY mouse pluripotent stem cells have a relatively high rate of male sex chromosome loss during clonal isolation. It is not clear if XY mouse pluripotent stem cells grown in mass culture have this same relatively high rate of Y chromosome loss. Our results demonstrate that XO subclones can be derived from XY pluripotent stem cell lines reprogrammed from somatic cells and that these sex-reversed stem cell lines are capable of differentiating into functional oocytes in female chimeras.
Our study exploits iPS cell technologies to combine the alleles from two males to generate male and female progeny, that is, a new form of mammalian reproduction. In our study, the progeny with two fathers presumably develop normally because the genome from Father #1 undergoes meiosis during oogenesis in the female chimera, resulting in maternal imprinting. In the scenario presented here, one male serves as the oocyte-producing or so-called maternal father, while the other father provides the sperm. The situation could be reversed, providing a choice for which male will be mother or father for progeny to inherit specific alleles (e.g., imprinted or sex chromosome genes). It is also possible that one male could produce both oocytes and sperm for self-fertilization to generate male and female progeny. This was demonstrated previously using XY mouse ES cells and tetraploid complementation [19]. The authors used a single XY mouse ES cell line to derive XY males directly through tetraploid complentation. They also isolated XY-derived XO subclones to generate XO females by tetraploid complementation. These male and female ES cell-derived mice were then bred together to generate progeny. This stem cell-mediated self-fertilization strategy could be useful in species preservation schemes when there is only a single remaining male. This could be performed through chimeras or in vitro differentiation of iPS cells into gametes (see below). However, as with any live-bearing mammal, there still must be a female to carry the fetus to term. Our strategy could potentially be applied to agriculturally important species to combine a desired set of alleles from two males without the necessity of outcrossing to genetically diverse females. Our strategy may also be useful for studying other forms of inheritance. In natural matings, progeny inherit mitochondrial DNA exclusively from their mothers. In our scheme, the mitochondrial genome is inherited from the maternal father. Variations of our stem cell-mediated reproduction strategy to generate mice with two fathers could conceivably provide a way to bypass the inheritance of mitochondrial diseases.
What are the implications of our results for human reproduction? iPS cells have been generated from human somatic cells [34, 35]. However, the generation of human iPS cells still requires significant refinements prior to their use for therapeutic purposes [36]. Interestingly, one 45,X subclone has been isolated from an XY human ES cell line (1/67 screened, 1.5%) [37]. Thus, it seems likely that 45,X iPS cell lines could be derived from the somatic cells of human males. Mouse ES and iPS cells have the ability to differentiate into oocytes or sperm through chimeras [14, 18]. However, the efficient derivation of functional oocytes and sperm cells from pluripotent stem cells in vitro has yet to be achieved [38]. It is also not clear if in vitro differentiated germ cells would acquire the appropriate epigenetic marks required for normal development [1–3, 39]. Perhaps in the future, it may be possible to generate human oocytes from iPS cells in vitro or through human-animal chimeras [38, 40–42]. However, in humans, a 45,X karyotype results in infertility. To overcome this X chromosome deficiency, one could conceivably create 45,X iPS cell lines from male somatic cells that could be made competent to generate oocytes by adding an isogenic X chromosome using microcell transfer to produce an XX genotype (Supplemental Fig. S2) [31, 43, 44]. If this is possible, then some day two men could produce their own genetic sons and daughters. In addition, variations of our stem cell strategy could be used to treat various forms of human sex chromosome-associated infertility, for example, Turner syndrome. Finally, viable bimaternal female mice have been generated by physical reconstruction of oocytes that possess two haploid genomes derived from oocytes [4, 5]. If sex chromosome transfer is feasible (e.g., the Y chromosome), then it may also be possible using iPS cells to generate sperm from a female donor (converting XX to XO to XY) and produce viable male and female progeny with two mothers (Supplemental Fig. S3).
Supplementary Material
Acknowledgments
We thank Steve O'Gorman for PC3 ES cells, Allan Bradley for SNL 76/7 STO cells, Colin Bishop for the pY353/B probe, Frank Probst and Angabin Matin for advice on PCR genotyping, Robert Milczarek for assistance with karyotyping, and Xueping Xu and Xiaohong Wang for excellent technical assistance. We are grateful to our colleagues for encouragement and helpful discussions.
Footnotes
Supported by grants from the National Institutes of Health (NIH) GM81627 to A.J.C. and R.R.B., and the Ben F. Love Chair and the Kleberg Foundation to R.R.B. Veterinary animal care was supported by the NIH Cancer Center Support Grant CA16672.
REFERENCES
- McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 1984; 37: 179 183 [DOI] [PubMed] [Google Scholar]
- Surani MA, Barton SC, Norris ML. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 1984; 308: 548 550 [DOI] [PubMed] [Google Scholar]
- Mann JR, Lovell-Badge RH. Inviability of parthenogenones is determined by pronuclei, not egg cytoplasm. Nature 1984; 310: 66 67 [DOI] [PubMed] [Google Scholar]
- Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, Park ES, Seo JS, Ogawa H. Birth of parthenogenetic mice that can develop to adulthood. Nature 2004; 428: 860 864 [DOI] [PubMed] [Google Scholar]
- Kawahara M, Wu Q, Takahashi N, Morita S, Yamada K, Ito M, Ferguson-Smith AC, Kono T. High-frequency generation of viable mice from engineered bi-maternal embryos. Nat Biotechnol 2007; 25: 1045 1050 [DOI] [PubMed] [Google Scholar]
- Kawahara M, Kono T. Longevity in mice without a father. Hum Reprod 2010; 25: 457 461 [DOI] [PubMed] [Google Scholar]
- Jäger RJ, Anvret M, Hall K, Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature 1990; 348: 452 454 [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 1990; 109: 635 646 [DOI] [PubMed] [Google Scholar]
- Dumic M, Lin-Su K, Leibel NI, Ciglar S, Vinci G, Lasan R, Nimkarn S, Wilson JD, McElreavey K, New MI. Report of fertility in woman with predominantly 46,XY karyotype in family with multiple disorders of sexual development: review of prismatic case. Mt Sinai J Med 2008; 75: 168 169 [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL, Whitney JB, III, Morrow KE. Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science 1982; 217: 535 537 [DOI] [PubMed] [Google Scholar]
- Nagamine CM, Taketo T, Koo GC. Studies on the genetics of tda-1 XY sex reversal in the mouse. Differentiation 1987; 33: 223 231 [DOI] [PubMed] [Google Scholar]
- Park EH, Taketo T. Onset and progress of meiotic prophase in the oocytes in the B6.YTIR sex-reversed mouse ovary. Biol Reprod 2003; 69: 1879 1889 [DOI] [PubMed] [Google Scholar]
- Otsuka S, Konno A, Hashimoto Y, Sasaki N, Endoh D, Kon Y. Oocytes in newborn MRL mouse testes. Biol Reprod 2008; 79: 9 16 [DOI] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 1984; 309: 255 256 [DOI] [PubMed] [Google Scholar]
- Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying QL, Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell 2008; 135: 1287 1298 [DOI] [PubMed] [Google Scholar]
- Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL, Pera MF, Ying QL. Germline competent embryonic stem cells derived from rat blastocysts. Cell 2008; 135: 1299 1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Ng JH, Heng JC, Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell 2009; 4: 301 312 [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 2007; 448: 313 317 [DOI] [PubMed] [Google Scholar]
- Eggan K, Rode A, Jentsch I, Samuel C, Hennek T, Tintrup H, Zevnik B, Erwin J, Loring J, Jackson-Grusby L, Speicher MR, Kuehn R, Jaenisch R. Male and female mice derived from the same embryonic stem cell clone by tetraploid embryo complementation. Nat Biotechnol 2002; 20: 455 459 [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Shawlot W, Kania A, Behringer RR. Requirement of Lim1 for female reproductive tract development. Development 2004; 131: 539 549 [DOI] [PubMed] [Google Scholar]
- Sybert VP, McCauley E. Turner's syndrome. N Engl J Med 2004; 351: 1227 1238 [DOI] [PubMed] [Google Scholar]
- Russell WL, Russell LB, Gower JS. Exceptional inheritance of a sex-linked gene in the mouse explained on the basis that the X/O sex-chromosome constitution is female. Proc Natl Acad Sci U S A 1959; 45: 554 560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach BM. XO mice. Genet Res 1962; 3: 487 490 [Google Scholar]
- Szabó PE, Hübner K, Schöler H, Mann JR. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev 2002; 115: 157 160 [DOI] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual, 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663 676 [DOI] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol 2007; 25: 1177 1181 [DOI] [PubMed] [Google Scholar]
- O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A 1997; 94: 14602 14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop CE, Hatat D. Molecular cloning and sequence analysis of a mouse Y chromosome RNA transcript expressed in the testis. Nucleic Acids Res 1987; 15: 2959 2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida M, Tokunaga T, Aizawa S, Niwa K, Imai H. Isolation and nature of mouse embryonic stem cell line efficiently producing female germ-line chimeras. Anim Sci Technol (Jpn) 1995; 66: 361 367 [Google Scholar]
- Tomizuka K, Yoshida H, Uejima H, Kugoh H, Sato K, Ohguma A, Hayasaka M, Hanaoka K, Oshimura M, Ishida I. Functional expression and germline transmission of a human chromosome fragment in chimaeric mice. Nat Genet 1997; 16: 133 143 [DOI] [PubMed] [Google Scholar]
- Sato H, Amagai K, Shimizukawa R, Tamai Y. Stable generation of serum- and feeder-free embryonic stem cell-derived mice with full germline-competency by using a GSK3 specific inhibitor. Genesis 2009; 47: 414 422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 1990; 346: 245 250 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861 872 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318: 1917 1920 [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev 2010; 24: 2239 2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach A, Benvenisty N. Studying early lethality of 45,XO (Turner's syndrome) embryos using human embryonic stem cells. PLoS One 2009; 4: e4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou GB, Meng QG, Li N. In vitro derivation of germ cells from embryonic stem cells in mammals. Mol Reprod Dev 2010; 77: 586 594 [DOI] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Ogonuki N, Miki H, Inoue K, Morimoto T, Morimoto H, Ogura A, Shinohara T. Heritable imprinting defect caused by epigenetic abnormalities in mouse spermatogonial stem cells. Biol Reprod 2009; 80: 518 527 [DOI] [PubMed] [Google Scholar]
- Hübner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, Wood J, Strauss JF, III, Boiani M, Schöler HR. Derivation of oocytes from mouse embryonic stem cells. Science 2003; 300: 1251 1256 [DOI] [PubMed] [Google Scholar]
- Nicholas CR, Haston KM, Grewall AK, Longacre TA, Reijo Pera RR. Transplantation directs oocyte maturation from embryonic stem cells and provides a therapeutic strategy for female infertility. Hum Mol Genet 2009; 18: 4376 4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer RR. Human-animal chimeras in biomedical research. Cell Stem Cell 2007; 1: 259 262 [DOI] [PubMed] [Google Scholar]
- Meaburn KJ, Parris CN, Bridger JM. The manipulation of chromosomes by mankind: the uses of microcell-mediated chromosome transfer. Chromosoma 2005; 114: 263 274 [DOI] [PubMed] [Google Scholar]
- O'Doherty A, Ruf S, Mulligan C, Hildreth V, Errington ML, Cooke S, Sesay A, Modino S, Vanes L, Hernandez D, Linehan JM, Sharpe PT, et al. An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science 2005; 309: 2033 2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.