Abstract
A robust route to 2,4-disubstituted pyrrole heterocycles relying upon a cascade reaction is reported. The reaction benefits from operational simplicity: it is air and moisture tolerant and is performed at ambient temperature. Control over the reaction conditions provides ready access to isopyrroles, 2,3,4- trisubstituted pyrroles and 3- substituted pyrollidin-2-ones.
The finite nature of chemical feedstocks coupled with the negative impacts of manufacturing waste streams necessitates the continued development of increasingly efficient processes for the preparation of valuable synthetic building blocks.1 In this regard, our group has demonstrated that simple addition reactions between differentially substituted alkynes can be interfaced with subsequent isomerizations to generate functional molecules while upholding high levels of atom-economy.2 These one-pot reactions benefit from the ability to conduct multiple chemical transformations in a single reaction vessel, providing their intended target while minimizing waste associated with traditional isolation and purification protocols.3
We envisioned that such a strategy could be applied to the efficient production of valuable pyrrole heterocycles from alkyne starting materials (Scheme 1).4 The addition of terminal alkyne 2 to suitably activated propargyl amine 1 under alkyne cross-coupling conditions5 would result in ynenoate 3, whose isomerization via a 5-endo-dig cyclization and tautomerization would then provide pyrrole 5 (Scheme 1).6
Scheme 1.
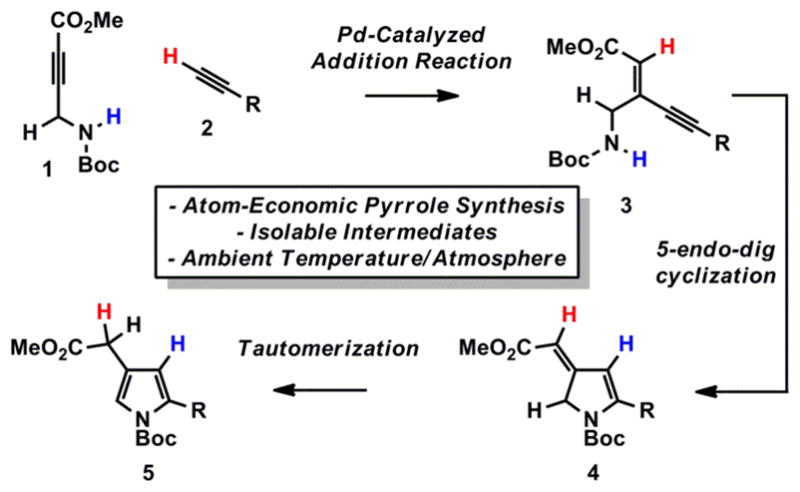
Pyrroles from Alkynes
While this sequence represents an efficient, isohypsic7 entry into 2,4-disubstituted pyrroles,8 we anticipated that intermediates 3 and 4 could serve as strategic points of product diversification if suitable conditions could be found for their selective preparation.9 In this regard, we viewed the design of a flexible route to topologically varied 5-membered nitrogen heterocycles as an intriguing challenge for atom-economic reaction design.10
We anticipated that electron-deficient propargyl amine 111 would serve as a suitable acceptor in a cross-alkyne coupling reaction. It should be noted that propargyl amides similar to 1 are prone to 5-endo-dig cyclization, affording the corresponding oxazole heterocycle.12 In this regard, the current method provides a novel avenue of reactivity for these versatile building blocks while avoiding such an isomerization process.
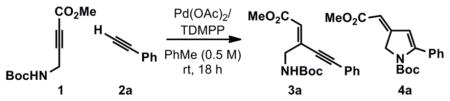
Initial investigations employing phenyl acetylene (2a) as the donor alkyne with toluene as the solvent13 revealed that product distributions depend on the ratio of Pd(OAc)2 to the tris(2,6-dimethoxyphenyl)phosphine (TDMPP) ligand (Table 1).14 Accordingly, an equimolar amount of ligand and metal cleanly afforded ynenoate 3a as a single geometrical isomer (entry 1), whereas decreasing the amount of TDMPP resulted in competitive formation of isopyrrole 4a (entries 2 and 3). Importantly, pyrrole formation was not observed under the reaction conditions and increasing either the reaction time or temperature resulted in complex mixtures and poor mass recovery.
Table 1.
Optimization of Selective Ynenoate Formationa
 | ||||
|---|---|---|---|---|
| entry | Pd(OAc)2 | TDMPP | ratio (3a/4a)b | conversionb |
| 1 | 3.0 mol % | 3.0 mol % | 20/1 | 100% |
| 2 | 3.0 mol % | 1.5 mol % | 3/2 | 100% |
| 3 | 3.0 mol % | 0.8 mol % | 1/5 | 100% |
All reactions were performed using 0.1 mmol of 1 and 2a.
Determined by 1H-NMR.
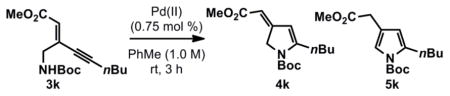
While both free and phosphine-ligated Pd(OAc)2 were ineffective at promoting isomerization to the pyrrole product, we quickly found that Pd(OTFA)2 resulted in clean formation of pyrrole 5k from ynenoate 3k (Table 2).2b In this case, both acetonitrile and benzonitrile complexes of PdCl2 (entries 4 and 5) were not as effective as Pd(OTFA)2, which promoted the desired cyclization and tautomerization in near quantitative yield. Once again, TDMPP was found to inhibit both of these transformations (compare entries 1 and 3), suggesting that a non-phosphine ligated Pd species is responsible for catalysis.15
Table 2.
Optimization of Pyrrole Formationa
 | ||||
|---|---|---|---|---|
| entry | Pd(II) Source | TDMPP | ratio (4k/5k)b | conversionb |
| 1 | Pd(OTFA)2 | --- | 1/20 | 100% |
| 2 | Pd(OTFA)2 | 0.4 mol % | 1/20 | 95% |
| 3 | Pd(OTFA)2 | 0.8 mol % | 1/2 | 72% |
| 4 | PdCl2(CH3CN)2 | --- | 1/1 | 47% |
| 5 | PdCl2(PhCN)2 | --- | 1/20 | 52% |
All reactions performed with 0.1 mmol of 3k.
Determined by 1H-NMR.
The results presented in Tables 1 and 2 led us to adopt a set of optimized conditions for the one-pot synthesis of either pyrrole or enyne products (Table 3). Thus, treatment of 1 with a variety of aromatic alkynes in the presence of Pd(OAc)2 (0.75 mol %) and TDMPP (0.75 mol %) in PhMe at room temperature afforded the corresponding ynenoate 3 in 77 to 97% isolated yields after 6 hours. Non-aromatic donor alkynes generally required slightly longer reaction times (12–24 hours), and provided ynenoates 3 in 64 to 97% isolated yield.
Table 3.
One-Pot Synthesis of Ynenoates or Isopyrroles
 | |||||
|---|---|---|---|---|---|
| Entry | R = | Enyne (Yield)c Pyrrole (Yield)c |
Entry | R = | Enyne (Yield)c Pyrrole (Yield)c |
| 1 |  |
3a (90%) 5a (80%) |
9 |  |
3i (77%) (12 h) 5i (82%)d |
| 2 |  |
3b (83%) 5b (97%) |
10 |  |
3j (97%) 5j (96%) |
| 3 |  |
3c (86%) 5c (89%) |
11 | 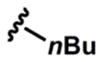 |
3k (83%) (18 h) 5k (97%) |
| 4 | 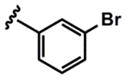 |
3d (95%) 5d (75%) |
12 | 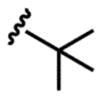 |
3l (74%) (24 h) 5l (60%) |
| 5 | 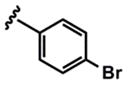 |
3e (74%) 5e (86%) |
13 | 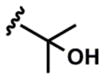 |
3m (95%) (24 h) 5m (70%) |
| 6 | 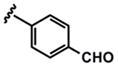 |
3f (78%) 5f (90%) |
14 | 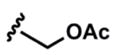 |
3n (74%) 5n (74%) |
| 7 | 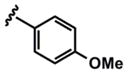 |
3g (84%) 5g (81%) |
15 | 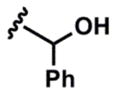 |
3o (64%) 5o (70%) |
| 8 | 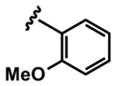 |
3h (78%) (12 h) 5h (99%) |
16 |  |
3p (86%) (24 h) 5p (68%) |
Conditions A: 1 (0.1 mmol, 1 equiv), Donor Alkyne 2 (0.1 mmol, 1 equiv) PhMe (1.0 M), Pd(OAc)2 (0.75 mol %), TDMPP (0.75 mol %), 6 h, rt. Reaction times other than 6 h are included in parentheses.
Conditions B: 1 (0.23 mmol, 1 equiv), Donor Alkyne 2 (0.23 mmol, 1 equiv), PhMe (1.0 M), (Entries 1–8, 10): Pd(OAc)2 (0.75 mol %), TDMPP (0.75 mol %), 6 h, rt; then Pd(OTFA)2 (2.0 mol %), 6 h, rt. (Entries 11–16): Pd(OAc)2 (1.5 mol %), TDMPP (1.5 mol %), 24 h, rt; then Pd(OTFA)2 (5.0 mol %), 24 h, rt.
Isolated Yields.
From 3i (0.1 mmol, 1 equiv), Pd(OAc)2 (5.0 mol %), THF (0.25 M), 60 °C, 14 h.
BDMS = Benzyldimethylsilane.
Alternatively, pyrroles can be obtained in yields ranging from 60 to 99% in a two-stage, one-pot process. For aromatic donors, addition of Pd(OTFA)2 (1.5 mol %) following complete conversion to the ynenoate resulted in the cyclized/isomerized product after only 6 hours. Once again, non-aromatic donors require slightly longer reaction times and higher catalyst loadings (5.0 mol % Pd(OTFA)2), but nevertheless returned good to excellent yields of the desired products after 24 hours. Importantly, these reactions are performed in screw-cap vials under an ambient atmosphere, with commercial grade alkynes and bench-top solvents. Furthermore, yields remain consistent upon scale-up, as both entries 1 and 11 have been performed on half-gram and gram scales, respectively.
As evidenced by the breadth of substrates in Table 3, this method tolerates a wide range of substituted donor alkynes. ortho-, meta- and para-Substituted aromatic alkynes with both electron-donating and withdrawing groups participate effectively (entries 7–9 and entries 3 and 6 respectively). Given the involvement of Pd(II) species throughout both the coupling and the isomerization steps, aryl bromides do not interfere with the reaction (entries 4 and 5). The basic nitrogen of an unprotected aniline is also tolerated in the coupling portion of the cascade (entry 9), however, cyclization to the pyrrole requires more rigorous conditions, starting from ynenoate 3i.
In addition to aromatic donors, aliphatic alkynes undergo efficient coupling and isomerization. Importantly, both free- and acetylated propargyl alcohols react smoothly under the standard conditions (entries 13–15). We note the use of a 1,3-enyne as a donor (entry 10) which provides an efficient synthesis of desirable C-vinyl pyrroles.16
Having established a robust set of conditions for the formation of 2,4-disubstituted pyrroles, we turned our attention to the synthesis of additional derivatives by exploiting the reactivity of intermediates 3 and 4. We were particularly intrigued by the utility of isopyrroles, which we identified as suitable donors for ene-type addition reactions (Scheme 2).17 To this end, ynenoate 3k was cyclized to isopyrrole 4k in the presence of Pd(OAc)2 (3 mol %) in THF in quantitative yield.18 Gratifyingly, 4k underwent addition to both Echenmoser’s salt (7) and diazene 8, affording products of C–C and C–N bond formation respectively. In addition, oxygenation adjacent to the methyl ester could be effected by simply stirring 4k overnight open to the atmosphere in the presence of SiO2.19 The ability to intercept isopyrrole 4k provides an attractive, atom-economical avenue for direct derivatization of the pyrrole side-chain.20
Scheme 2.
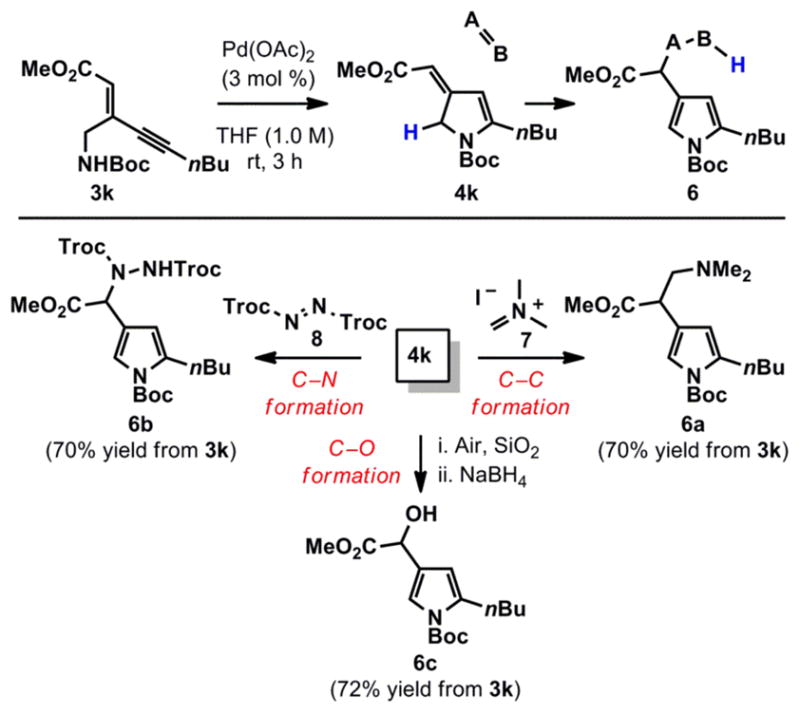
Reactivity of Isopyrrole Intermediates
The use of Pd-catalysis to effect the cyclization of 3k offers additional avenues for substitution of the pyrrole nucleus. For example, we reasoned that 2,3,4-trisubstituted heterocycles 9 could be accessed by trapping vinyl-palladium intermediate 4′, which is generated during the 5-endo-dig cyclization (Scheme 3).21 Thus, exposure of ynenoate 3k to Pd(OAc)2 in the presence of acrolein and LiBr afforded 9a via a reductive Heck-type addition reaction.22 Alternatively, allylation in the 3-position could be effected with allyl chloride in the presence of PdCl2(CH3CN)2 and propylene oxide as a suitable acid scavenger.23 This method compliments current strategies for the functionalization of 2,4-disubstitued pyrroles, which remains a challenging transformation.24
Scheme 3.
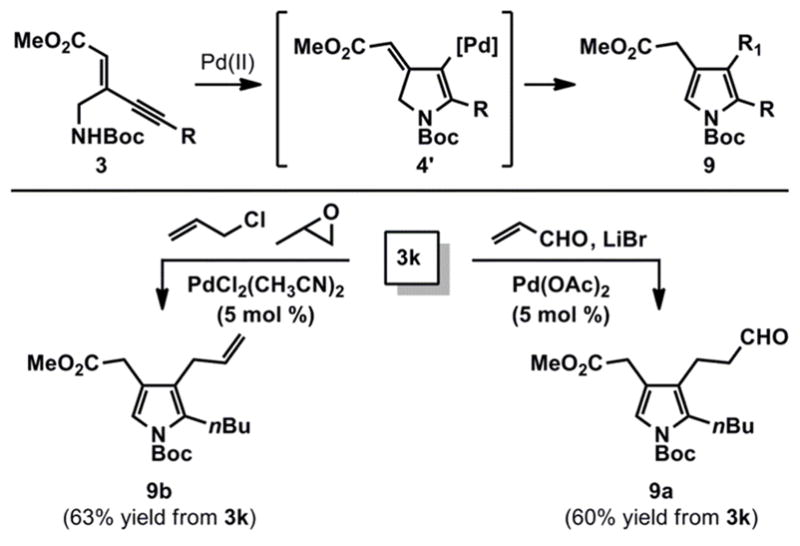
Synthesis of 2,3,4-Trisubstituted Pyrroles
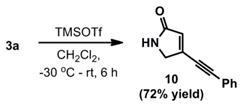
In addition to pyrrole heterocycles, 3-substituted pyrollidin-2-ones are available via a one-pot deprotection, cyclization sequence (equation 1). Thus, exposure of ynenoate 3a to TMS-OTf in CH2Cl2 afforded 10 in 72% isolated yield, demonstrating an alternative, chemoselective cyclization that highlights the versatility of our overall strategy in accessing structurally distinct 5-membered nitrogen heterocycles.
In summary, we have developed an atom-economic synthesis of 2,4-disubstituted pyrroles. The method utilizes
 |
(1) |
readily available alkynes and employs a Pd(II) mediated cascade reaction. By exerting control over the conditions, we have also shown that several intermediates along the pathway can be intercepted for further functionalization. These include an ene addition reaction with an isopyrrole, as well as access to 2, 3, 4-trisubstituted pyrroles and 3-substituted pyrollidin-2-ones. This method benefits from operational simplicity as all reactions were performed using bench-top solvents under an ambient atmosphere at room temperature. Current efforts are directed towards the further functionalization of these intermediates, and results will be presented in due course.
Supplementary Material
Acknowledgments
We thank the National Science Foundation (CHE 0846427) and the National Institutes of Health (GM33049) for their generous support of our programs. J.-P. L. is grateful for a NIH postdoctoral fellowship (GM083428). J.M.A. thanks the American Chemical Society and the Barry M. Goldwater Foundation for pre-doctoral fellowships. We thank Jacob Stern for technical assistance. We thank Johnson-Matthey for generously providing us with palladium salts.
Footnotes
Supporting Information Available. Experimental procedures and analytical data for all new compounds are available free of charge via the Internet at: http://pubs.acs.org.
References
- 1.(a) Li CJ, Trost BM. Proc Natl Acad Sci USA. 2008;105:13197. doi: 10.1073/pnas.0804348105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]; (c) Trost BM. Angew Chem, Int Ed. 1995;34:259. [Google Scholar]; (d) Anastas PT, Warner JC. Green Chemistry Theory and Practice. Oxford University Press; New York: 1998. [Google Scholar]
- 2.For the synthesis of furans and butenolides see: Trost BM, McIntosh MC. J Am Chem Soc. 1995;117:7255.For the synthesis of pyrans and related 7-membered oxygen heterocycles see: Trost BM, Frontier AJ. J Am Chem Soc. 2000;122:11727.
- 3.Andraos J. In: Green Chemistry Metrics: Measuring and Monitoring Sustainable Processes. Lapkin A, Constable DJC, editors. John Wiley & Sons; West Sussex, United Kingdom: 2009. pp. 69–200.These and related 1-pot reactions have been classified as “cascade reactions.” For a recent review on their application in total synthesis see: Nicolaou KC, Edmonds DJ, Bulger PG. Angew Chem, Int Ed. 2006;45:7134. doi: 10.1002/anie.200601872.
- 4.For a general review of pyrrole heterocycle syntheses see: Bergman J, Janosik T. In: Comprehensive Heterocyclic Chemistry III. Jones G, Ramsden CA, editors. Vol. 3. Elsevier; Amsterdam: 2008. pp. 269–351.For reviews regarding the prevalence and biological importance of pyrrole heterocycles see: Lipkus AH, Yuan Q, Lucas KA, Funk SA, Bartelt WF, III, Schenk RJ, Trippe AJ. J Org Chem. 2008;73:4443. doi: 10.1021/jo8001276.Fan H, Peng J, Hamann MT, Hu JF. Chem Rev. 2008;108:264. doi: 10.1021/cr078199m.
-
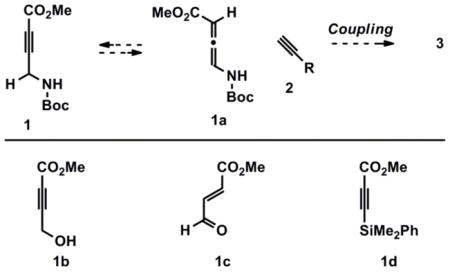
5.For the addition of terminal alkynes to electron poor, internal alkynes see: Trost BM, Sorum MT, Chan C, Rühter G. J Am Chem Soc. 1997;119:698.A reviewer has suggested that coupling of 1 and 2 may involve allene tautomer 1a (see below). Our previous success utilizing acceptors 1b and 1d, where competitive formation of 1c was not observed, and where formation of an allene intermediate is not possible respectively, suggests that allene intermediates are not involved. For the use of 1b see reference 2a, and for the use of 1d see: Trost BM, Gunzner JL, Yasukata T. Tetrahedron Lett. 2001;42:3775.For a review on the isomerization of alkynoates to enoates see: Kwong CKW, Fu MY, Lam CSL, Toy PH. Synthesis. 2008:2307.
- 6.For a review of transition-metal-mediated pyrrole syntheses see: Patil NT, Yamamoto Y. ARKIVOC. 2007. p. 121.. For related examples see: Peng HM, Zhao J, Li X. Adv Synth Catal. 2009;351:1371.Gabriele B, Salerno G, Fazio A. J Org Chem. 2003;68:7853. doi: 10.1021/jo034850j.Egi M, Azechi K, Akai S. Org Lett. 2009;11:5002. doi: 10.1021/ol901942t.Rakshit S, Patureau FW, Glorius F. J Am Chem Soc. 2010;132:9585. doi: 10.1021/ja104305s.Staurt DR, Alsabeh P, Kuhn M, Fagnou J. Am Chem Soc. 2010 doi: 10.1021/ja1082624.. Article ASAP.
- 7.Hendrickson JB. J Am Chem Soc. 1971;93:6947.For a recent review of isohypsic reactions in total synthesis see: Burns NZ, Baran PS, Hoffmann RW. Angew Chem, Int Ed. Vol. 48. 2009. p. 2854.
- 8.For a review on the synthesis of substituted pyrroles see: Schmuck C, Rupprecht D. Synthesis. 2007:3095.
- 9.Isopyrroles related to 4 have been reported: Larock RC, Doty MJ, Han X. Tetrahedron Lett. 1998;39:5143.In addition, isofurans were observed as intermediates in previous work (see ref. 2a). In both cases however, the reactivity of these intermediates was not explored.
- 10.Recent efforts in drug discovery have underscored the importance of accessing distinct molecular architectures from a single reaction sequence: Gray BL, Schreiber SL. J Comb Chem. 2007;9:1028. doi: 10.1021/cc7001028.
- 11.Acceptor 1 is prepared in 2-steps from commercially available Boc-propargyl amine. See supporting information for details.
- 12.Wipf P, Aoyama Y, Benedum TE. Org Lett. 2004;6:3593. doi: 10.1021/ol0485058. [DOI] [PubMed] [Google Scholar]
- 13.The reaction is tolerant of a variety of solvents. In this study PhMe displayed the best compromise between reaction time and yield.
- 14.The coordination of TDMPP to Pd has been discussed: Ma JF, Kojima Y, Yamamoto Y. J Organomet Chem. 2000;616:149.
- 15.Attempts to isomerize 3k or 4k to 5k under basic and acidic conditions returned low isolated yields of the desired product. 3k could be cyclized to 5k under thermal conditions, but required unacceptably high-temperatures (> 100 °C) and reaction times (> 48 h).
- 16.Trofimov BA, Sobenina LN, Demenev AP, Mikhaleva AI. Chem Rev. 2004;104:2481. doi: 10.1021/cr020100i. [DOI] [PubMed] [Google Scholar]
- 17.For a related ene reaction using 3-methyleneindolines see: Tidwell JH, Buchwald SL. J Am Chem Soc. 1994;116:11797.
- 18.Following complete conversion, the reaction mixture is filtered through a short column of florosil to provide analytically pure samples of the iso-pyrrole. See Supporting Information for details.
- 19.Reduction of crude reaction mixtures with NaBH4 simplified product mixtures which were typically composed of alcohol 6c and the corresponding ketone.
- 20.Functionalization of 5k would require stoichiometric amounts of activating agents and would reduce the overall atom economy of this process.
- 21.We note that Pd(OAc)2 and PdCl2 alone are not as effective as Pd(OTFA)2 in the conversion of 3k to 5k (Table 2). Nevertheless, under the conditions described in Scheme 3, substituted isopyrrole products were not detected. We speculate that the additional additives present under these conditions promote the isomerization of ispoyrrole intermediates into the corresponding pyrrole products.
- 22.Shen Z, Lu X. Tetrahedron. 2006;62:10896. [Google Scholar]
- 23.Wakabayashi Y, Fukuda Y, Shiragami H, Utimoto K, Nozaki H. Tetrahedron. 1985;41:3655. [Google Scholar]
- 24.Lian Y, Davies HML. Org Lett. 2010;12:924. doi: 10.1021/ol9028385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


