Abstract
GRP78 is a major endoplasmic reticulum chaperone as well as a master regulator of the unfolded protein response. In addition to playing an essential role in early embryonic development, recent studies have emerged specifically implicating GRP78 and chaperone integrity in the aging process and age-related diseases. Another exciting discovery is the regulation of GRP78 by insulin/IGF-1 signaling pathways impacting cell proliferation and survival. Mouse models of cancer, in combination with cell culture studies, validate the critical role of GRP78 in tumorigenesis and tumor angiogenesis. Further, these studies demonstrate the ability of GRP78 to suppress oncogenic PI3K/AKT signaling. The discovery of cell surface GRP78, in cancer cells and cells undergoing ER stress, presents a novel therapeutic strategy.
Introduction
The 78 kilodalton glucose regulated protein 78 (GRP78), also referred to as BiP or HSPA5, is a highly abundant endoplasmic reticulum (ER) chaperone. Along with its role in protein folding, GRP78 is also known to be an important component in modulating the unfolded protein response (UPR) [1]. Under conditions of ER homeostasis, GRP78 constitutively binds to and maintains the three UPR transmembrane sensors, ATF6, PERK, and IRE1 in an inactive form [2]. Under conditions of ER stress when unfolded proteins accumulate in the lumen of the ER, GRP78 is released from the UPR sensors, leading to their activation. The activated UPR relieves ER stress by decreasing protein translation and increasing the folding capacity of the ER, which includes the upregulation of GRP78. Importantly, if ER homeostasis cannot be restored, the UPR is capable of inducing apoptosis.
Recent research has demonstrated that normal physiologic processes induce ER stress and require GRP78, as well as an intact UPR, to restore and maintain homeostasis. At the most basic level, GRP78 is required for embryonic growth and development [3]. Mouse embryos with homozygous knockout of GRP78 exhibit severe proliferative defects, apoptosis of the inner cells mass, and embryonic lethality at day 3.5 [3]. Transgenic mouse strain harboring the Grp78 promoter driving the expression of the LacZ reporter gene further showed that Grp78 induction was prominent in the embryonic heart at day 11 and the induction was mediated through the ER stress response element [4]. Other mouse genetic mouse models targeting various UPR components have also shown that an intact UPR is critical for survival [5]. Taken together, these studies demonstrate that normal physiologic processes are capable of producing ER stress, and that the ability to respond to this stress is vital. Beyond its essential role in embryonic development, GRP78 has been implicated in adaptive responses to a diverse array of cellular processes [1,6,7]. Thus, the purpose of this review is to discuss recent discoveries on the emerging roles and the regulation of GRP78 under both physiologic and pathologic conditions. Specifically, we will focus on GRP78 in the context of aging, the regulation of GRP78 as both a downstream target and upstream effector of the insulin and IGF-1 signaling pathway, and the involvement of GRP78 in pathological conditions such as metabolic disorders and cancer.
The role of GRP78 and ER stress in aging
Aging is an independent risk factor for diseases such as cancer, Alzheimer’s and Parkinson’s diseases. Evidence is emerging that ER stress plays a causative role in the aging process. For example, ablation of the UPR protein, IRE1, completely reversed the increased longevity normally observed in C. elegans bearing a mutation in the insulin like growth factor-1 receptor [8•]. IRE1 expression was required for the activation of adaptive genes which promote longevity in the IGF-1r mutants [8•]. Further, the adaptive response stabilized ER homeostasis and enhanced resistance to ER stress [8•]. These findings suggest that ER homeostasis and an increased capacity to maintain homeostasis are important factors in mitigating the detrimental effects of aging.
Aging leads to a significant decline in the protein expression and activity of several ER chaperones, including GRP78. A reduction in GRP78 expression was observed in both brain and hepatic tissues of old vs. young rodents, and is thought to contribute to age-related impairments in cellular function [9–13]. For example, decreased GRP78 and other chaperone proteins in the brain of old rodents were associated with increased susceptibility to ER stress induced apoptosis vs. the young [9]. Old mice exhibited lower GRP78 expression in brain tissue as well as an inability to adapt and overcome ER stress induced by acute sleep deprivation compared to young mice [10]. In hepatic tissue, old mice exhibited increased oxidative damage to GRP78 as well as significantly reduced GRP78 activity [11,12]. Additionally, despite no age-associated reduction in overall ER protein content, hepatic GRP78 protein was reduced by a striking 39% in mice that were observed over a lifetime [13]. Taken together, age related declines in chaperone level or activity and the UPR can compromise adaptive capacity and lead to ER stress (Figure 1). Furthermore, unmitigated ER stress over time is likely to be an important factor in age-associated pathologies. However, the majority of studies regarding GRP78 and aging at this point have been largely observational. Thus, further study is required to uncover the precise mechanisms on how loss of GRP78 can mediate aging and age-related diseases.
Figure 1.
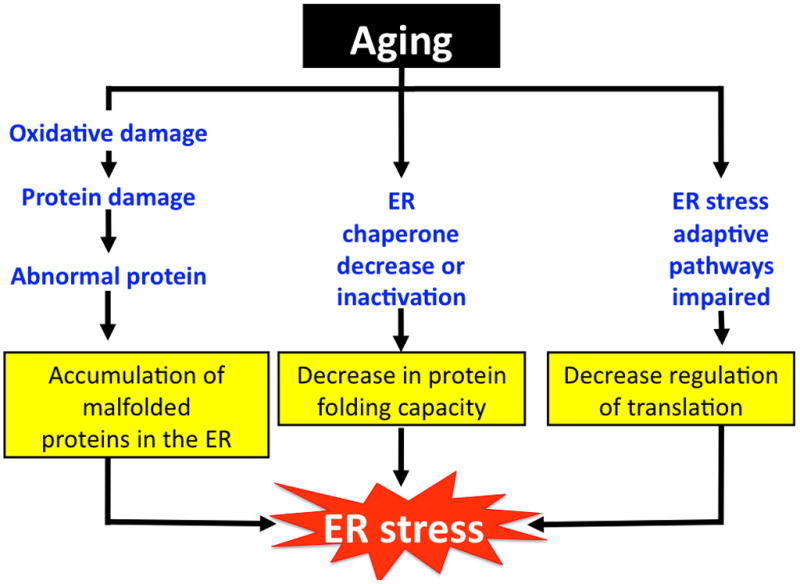
Aging leads to ER stress. The physiological process of aging can lead to ER stress via three main mechanisms; increased oxidative damage, decline in chaperone expression and activity, and reduced adaptive capacity of the ER. Prolonged ER stress contributes in part to age related disease development.
The role of GRP78 in insulin-mediated protection and chaperone balance
Insulin and insulin like growth factor-1 (IGF-1) stimulate protein synthesis, proliferation, and anti-apoptotic signaling via induction of mitogen activated protein kinases (MAPK) and PI3K/AKT/mTOR signaling. An estimated one third of all newly synthesized proteins are folded and processed in the ER. This suggests that insulin and IGF-1 signaling could be coupled to GRP78 expression and the adaptive UPR. In fact, there is a growing body of recent literature which demonstrates that GRP78 level, and the UPR, can be regulated by insulin and IGF-1 under a variety of conditions. For example, feeding, which is characterized by an elevation in plasma insulin and protein synthesis, can activate UPR components in both liver and pancreas, indicating a role for the UPR in the context of a common physiologic perturbation [14,15]. In rat hepatic tissue, the feeding induced increase in GRP78 and the activation of UPR components in response to feeding was mTOR dependent [14]. However, the cellular mechanism(s) by which mTOR regulates the UPR in response to feeding remains to be determined.
Recently, several studies using cell culture systems have demonstrated that insulin can augment the UPR and protect cells against ER stress induced apoptosis. Insulin treatment of human neuroblastoma cells led to a PI3K dependent increase in GRP78 expression and mitigated cell death in response to the ER stress inducer, thapsigargin [16]. In a study using MCF-7 and NIH/3T3 cells, treatment with IGF-1 also conferred protection against ER stress induced apoptosis [17•]. The protective effect of IGF-1 in these cells was attributed to an enhanced activation of the UPR which led to an adaptive increase in folding capacity, exemplified by the induction of GRP78 [17•]. Furthermore, inhibition of either protein translation or MAPK activity, including PI3K, failed to prevent IGF-1 mediated protection against ER stress induced apoptosis [17•]. Thus, the pathway(s) mediating IGF-1 protection under these conditions are yet to be identified. Importantly, two recent independent reports revealed that there is direct interaction between PI3K and UPR signaling [18,19]. The ability to increase GRP78 in response to ER stress was impaired by 50% in PI3K−/− preadipocytes [18]. Taken together, these studies demonstrate that insulin and IGF-1 can regulate GRP78 expression and augment the adaptive capacity of the UPR under ER stress conditions. However, the exact mechanisms by which insulin and IGF-1 mediate this regulation are not clear, and warrant further investigation.
While it appears that GRP78 expression is a downstream target of insulin, recent evidence also suggests that GRP78 and the overall balance of ER chaperone proteins can regulate whole body insulin sensitivity, glucose homeostasis, and protect cells during acute stress. Obesity and type II diabetes are metabolic disorders characterized by insulin resistance and hepatic steatosis. The existence of ER stress in the context of the metabolic syndrome has been documented [20,21], and chaperone balance could be an important regulator of insulin sensitivity and glucose homeostasis. For example, administration of chemical chaperones to ob/ob mice reduced markers of ER stress, restored glucose and insulin homeostasis, mitigated weight gain, and increased hepatic insulin sensitivity [22]. Similarly, a specific increase in GRP78 using adenovirus was able to reduce hepatic steatosis and improve insulin sensitivity in ob/ob mice [23••]. GRP78 mRNA was decreased in adipose tissue of gastric bypass patients after weight loss, implying that the relationship between obesity-related ER stress and metabolism dysfunction exists in humans as well [24].
Given the important role of GRP78 and chaperone proteins in maintaining glucose homeostasis and weight gain resistance, it would be expected that a reduction in GRP78 protein would exacerbate the development of obesity and diabetes in response to a high fat diet. However, depending on the genetic background and specific tissues, loss of GRP78 could elicit different responses when exposed to dietary stress. A recent study showed that compared to wild type siblings, Grp78 heterozygous (+/−) mice in C57/B6 genetic background were protected from the metabolic abnormalities normally observed after long-term exposure to high fat diet. Grp78+/− mice fed a high fat diet for 30 weeks demonstrated less weight gain, greater insulin sensitivity, increased energy expenditure, and improved glucose homeostasis independent of adiposity [25••]. The studies revealed that under high fat diet stress, Grp78 haploinsufficiency activated the adaptive UPR in white adipose tissue which led to a compensatory increase in the expression of other ER chaperone proteins [25••]. This, coupled with attenuation of translational block and upregulation of ER associated protein degradation, leads to improved ER quality control and folding capacity and contributes to the metabolic protection observed in Grp78+/− mice [25••].
The importance of chaperone balance in mitigating the detrimental effects of cellular stress was also demonstrated in the case of acute pancreatitis [26]. Pancreatic acinar cells are professional secretory cells with an extensive ER structure for processing high protein load. Grp78 heterozygosity protected pancreatic acinar cells against cerulein induced pancreatitis in a diet and genetic-background dependent manner [26]. Protection was conferred under conditions in which there was a compensatory rise in ER chaperone proteins, such as GRP94, calreticulin, and calnexin [26]. The adaptive metabolic pathways in response to Grp78 haploinsufficiency are summarized in Figure 2.
Figure 2.
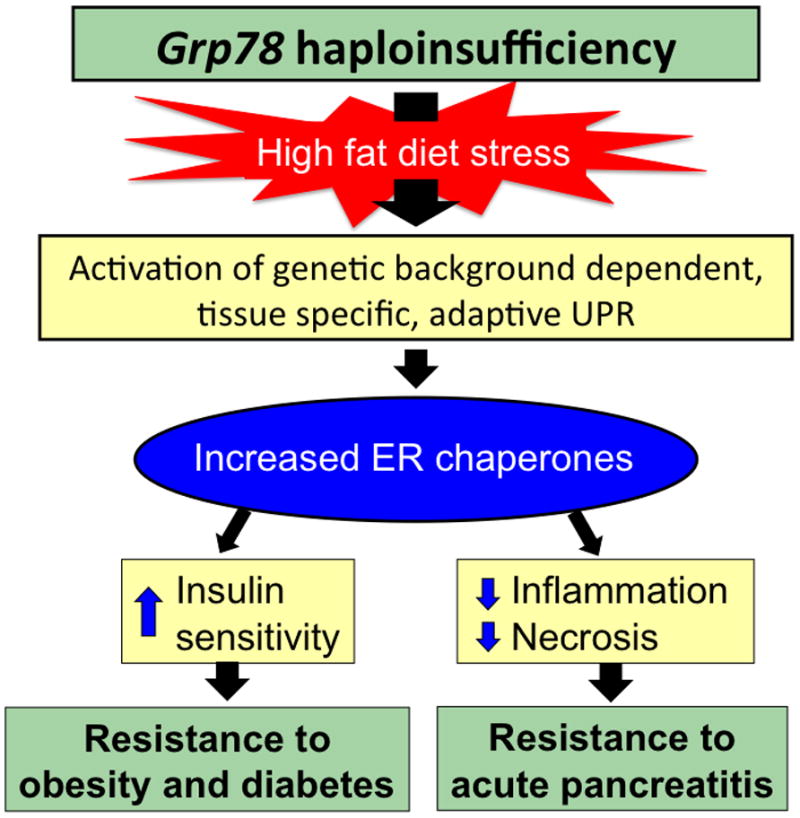
Triggering of adaptive UPR by Grp78 haploinsufficiency suppresses detrimental effects of diet induced stress. Grp78+/− mice subjected to chronic ER stress induced by high fat diet activate the adaptive UPR in a tissue and genetic background dependent manner, leading to increased ER chaperones and improved metabolic phenotypes.
The role of GRP78 in cancer diagnosis and tumorigenesis
Cancer cells are characterized by altered glucose metabolism and the tumor microenvironment is marked by impaired blood flow and hypoxia, all of which can cause ER stress. Under such conditions, tumor cells overexpress GRP78 and the pro-survival characteristics of GRP78 aid in tumor progression and chemoresistance [27]. Several recent studies in humans have demonstrated an important association between GRP78 and patient outcome. For example, in 219 prostate cancer patients, GRP78 expression was strongly associated with castration resistant tumor cells [28]. In this and other studies, increased GRP78 was associated with greater risk for cancer recurrence and an overall decrease in patient survival [28,29], suggesting that GRP78 could be an important novel prognostic indication for development of castration resistance and recurrence in prostate cancer patients. Similarly, overexpression of GRP78 is correlative with progression of melanoma [30]. For breast cancer, the discovery of predictive factors for chemoresistance is critical for improving adjuvant therapy for cancer patients. Retrospective analysis showed that about 67% of study subjects expressed high level of GRP78 in their tumors prior to chemotherapy and for patients treated with adriamycin alone GRP78 positivity correlated with a shorter time to recurrence [31]. Interestingly, this and another study revealed that high GRP78 level may predict greater response to adjuvant taxane therapy in breast cancer [31,32]. Nonetheless, the interaction between GRP78 and/or the UPR pathways with adriamycin/taxane therapy remains to be determined.
The utilization of knockdown and overexpression techniques, as well as genetic mouse models, have furthered our understanding of the role GRP78 plays in tumor development, progression, and chemoresistance. Malignant gliomas are among the most chemoresistant tumors. GRP78 is expressed at very low levels in normal brain but is highly elevated in malignant glioma specimens and human malignant glioma cell lines, correlating with their rate of proliferation. Knockdown of GRP78 by siRNA reduced the growth rate and increased chemotherapy induced apoptosis in multiple glioma cell lines [33]. Similarly, GRP78 downregulation by siRNA or treatment with a GRP78-specific subtilase toxin produced a synergistic enhancement with ER stress inducing chemotherapy agents, enhancing cell death in melanoma cells [34]. Not only is GRP78 important for tumor cell survival, it also supports the growth and chemoresistance of tumor-associated endothelial cells (TuBECs). TuBEC cells demonstrated increased GRP78 expression compared to blood vessel cells from non-malignant tissues (BECs) [35]. Further, siRNA mediated knockdown of GRP78 sensitized TuBEC cells to chemotherapy; whereas BEC cells overexpressing GRP78 acquired chemoresistance [35]. Recently, it was reported that distinct solid tumor cells were able to secrete GRP78 into the microenvironment and recombinant GRP78 can confer chemoresistance to endothelial cells [36]. Thus, GRP78 may have roles beyond the ER compartment.
In a transgene-induced endogenous mammary tumor model, Grp78 haploinsufficiency exhibited delayed tumor latency, decreased tumor proliferation, increased apoptosis, and decreased tumor angiogenesis [37]. Mutation or inactivation of the tumor suppressor gene Pten is commonly observed in human cancer. Mouse with homozygous deletion of Pten in the prostate epithelium presents an excellent model for prostate cancer development as it mimics human pathology [38••]. Strikingly, in mice harboring biallelic conditional knockout of both GRP78 and PTEN in the prostate epithelium, prostate tumorigenesis was potently arrested [38••]. This provides the first evidence that GRP78 is required for tumorigenesis driven by loss of PTEN, which leads to activation of the PI3K/AKT oncogenic pathway.
Recent studies support an interaction between PI3K/AKT signaling and GRP78 as a mechanism by which GRP78 can facilitate tumor growth and resistance to apoptosis. Evidence suggests that in the context of cancer, GRP78 can serve as both a downstream target and an upstream regulator of PI3K/AKT signaling. The oncoprotein p28gank, which is overexpressed in human hepatocellular carcinoma, was recently demonstrated to upregulate GRP78 protein expression and suppress ER stress induced apoptosis in an AKT dependent manner [39]. Suppression of p28gank decreased phospho-AKT levels, whereas overexpression of p28gank increased phospho-AKT, in ER stressed hepatoma cells [39]. Furthermore, inhibition of AKT signaling significantly reduced the p28gank dependent increase in GRP78 expression in stressed cells, and sensitized cells to apoptosis [39]. Other reports demonstrate that GRP78 can act as an upstream regulator of AKT activation in cancer. ER stress resistant lung cancer cells displayed GRP78 and phospho-AKT dependent chemoresistance [40]. Further, in this model GRP78 knockdown decreased phospho-AKT expression, but neither overexpression nor chemical inhibition of phospho-AKT affected GRP78 expression [40]. In mice with the tissue specific knockout of Grp78 and Pten in the prostate epithelium, immunofluorescence staining of the epithelium showed a dramatic reduction in phospho-AKT levels compared to mice harboring only the Pten deletion [38••]. Furthermore, siRNA knockdown of GRP78 protein led to a reduction in phospho-AKT levels in both ER-stressed and non-stressed PC3 prostate cancer cells [38••].
Taken together, GRP78 plays an important role in tumor development and chemoresistance in a wide variety of cancer. Given the relative abundance of GRP78 protein level, the availability of commercially available antibodies against GRP78 and established staining protocols for GRP78 in paraffin-fixed sections [31,41], measuring GRP78 expression in tumors has the potential to be an accessible and useful method for assessing and planning cancer treatment strategies. Additionally, despite recent progress in identifying key mechanisms by which GRP78 can regulate tumor progression and its obligatory role in the tumor microenvironment, more research is needed to expand our understanding of pathways and mechanisms regulated by GRP78 in cancer.
The emerging role of cell surface GRP78 in cancer and cancer treatment
Recent research regarding the expression of GRP78 at the cell surface presents exciting new areas in GRP78 regulation and cancer therapeutics. Cancer cells, as well as cells undergoing ER stress, express GRP78 on the outer plasma membrane [7,42–44]. Cell surface GRP78 has been demonstrated to act as a receptor, and several small proteins which are upregulated in cancer, can bind to surface GRP78 and modulate proliferation [44–47]. For example, the oncoprotein Cripto was shown to bind to cell surface GRP78 and stimulate growth [46,48••]. Several studies implicated that cell surface GRP78 signaling acts through activation of AKT [44,47]. Thus, ligation of cell surface GRP78 by antibody was reported to block growth rate and PI3K/AKT signaling [47]. The observation that cell surface GRP78 is largely expressed in cancer cells but not in normal organs, coupled with antibody targeting, make cell surface GRP78 a potential anti-cancer target. Indeed, several studies have begun to address this possibility with promising results [42,49–53].
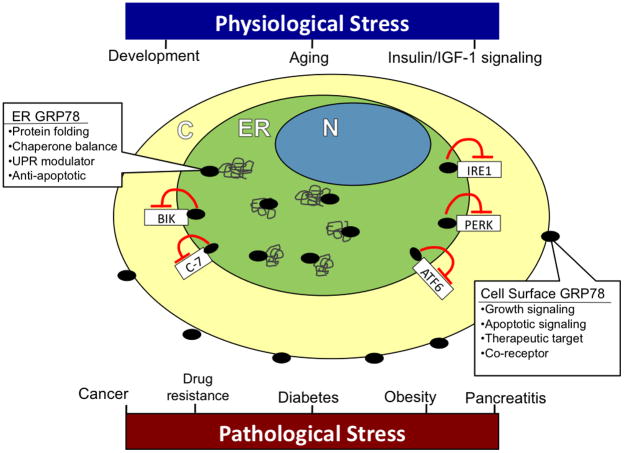
In conclusion, while the overt role of GRP78 in ER stress signaling and its increased expression in various types of cancer are well established, the regulation of GRP78 under various physiologic conditions and its many roles in disease are just emerging. The studies reviewed here mark an exciting expansion in our understanding of GRP78 signaling and regulation (Figure 3). Furthermore, insight into the mechanisms by which GRP78 acts will provide valuable information regarding processes such as aging and insulin signaling, as well as present viable therapeutic strategies for the treatment of a number of pathologic conditions.
Figure 3.
The role of GRP78 in physiologic and pathologic stress. GRP78 in the ER plays a critical role in physiologic processes such as development, aging, and insulin/IGF-1 signaling. ER GRP78 serves to properly fold proteins, regulate UPR signaling, maintain chaperone homeostasis, and protect against apoptosis. GRP78 also plays an important role in a number of pathological conditions, including cancer, diabetes, obesity, and acute pancreatitis. Under ER stress conditions, and particularly in cancer, a subfraction of GRP78 translocates to the plasma membrane and is expressed on the cell surface. Cell surface GRP78 can function as a receptor, augment growth signaling, apoptotic signaling, and presents a potential therapeutic target in cancer treatment. N nucleus; ER endoplasmic reticulum; C cytoplasm; Bik Bcl-2-interacting killer; C-7 pro-caspase-7; IRE1 inositol-requiring enzyme 1; PERK PKR-like endoplasmic reticulum kinase; ATF6 activating transcription factor 6;  GRP78;
GRP78;  nascent protein.
nascent protein.
Acknowledgments
We thank members of the Lee laboratory for helpful discussions. Due to space limitations, we apologize that many important primary articles cannot be cited.
Grant Support
K.P. is supported by a National Institutes of Health fellowship 5T32 CA009320 and USC Norris Comprehensive Cancer Center Postdoctoral Supplemental Award. This work was supported in part by National Institutes of Health grants CA027607, CA111700 and National Institute of Diabetes and Digestive and Kidney Diseases DK079999 to A.S.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kyle T. Pfaffenbach, Email: kyle.t.pfaffenbach@usc.edu.
Amy S. Lee, Email: amylee@ccnt.usc.edu.
References and recommended reading
- 1.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao C, Dong D, Little E, Luo S, Lee AS. Transgenic mouse models for monitoring endoplasmic reticulum stress in vivo. Nat Med. 2004;10:1013–1014. doi: 10.1038/nm1004-1013. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 6.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee SJ, Cary M, Kenyon C. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A. 2010;107:9730–9735. doi: 10.1073/pnas.1002575107. In C. elegans with insulin/IGF-1 mutations, lifespan extension requires IRE and XBP-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paz Gavilan M, Vela J, Castano A, Ramos B, del Rio JC, Vitorica J, Ruano D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging. 2006;27:973–982. doi: 10.1016/j.neurobiolaging.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabek JP, Boylston WH, 3rd, Papaconstantinou J. Carbonylation of ER chaperone proteins in aged mouse liver. Biochem Biophys Res Commun. 2003;305:566–572. doi: 10.1016/s0006-291x(03)00826-x. [DOI] [PubMed] [Google Scholar]
- 12.Nuss JE, Choksi KB, DeFord JH, Papaconstantinou J. Decreased enzyme activities of chaperones PDI and BiP in aged mouse livers. Biochem Biophys Res Commun. 2008;365:355–361. doi: 10.1016/j.bbrc.2007.10.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson RR, Dunning LM, Holtzman JL. The effect of aging on the chaperone concentrations in the hepatic, endoplasmic reticulum of male rats: the possible role of protein misfolding due to the loss of chaperones in the decline in physiological function seen with age. J Gerontol A Biol Sci Med Sci. 2006;61:435–443. doi: 10.1093/gerona/61.5.435. [DOI] [PubMed] [Google Scholar]
- 14.Pfaffenbach KT, Nivala AM, Reese L, Ellis F, Wang D, Wei Y, Pagliassotti MJ. Rapamycin inhibits postprandial-mediated X-box-binding protein-1 splicing in rat liver. J Nutr. 2010;140:879–884. doi: 10.3945/jn.109.119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Xue Z, He Y, Sun S, Chen H, Qi L. A Phos-tag-based approach reveals the extent of physiological endoplasmic reticulum stress. PLoS One. 2010;5:e11621. doi: 10.1371/journal.pone.0011621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inageda K. Insulin modulates induction of glucose-regulated protein 78 during endoplasmic reticulum stress via augmentation of ATF4 expression in human neuroblastoma cells. FEBS Lett. 2010;584:3649–3654. doi: 10.1016/j.febslet.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 17•.Novosyadlyy R, Kurshan N, Lann D, Vijayakumar A, Yakar S, LeRoith D. Insulin- like growth factor-I protects cells from ER stress-induced apoptosis via enhancement of the adaptive capacity of endoplasmic reticulum. Cell Death Differ. 2008;15:1304–1317. doi: 10.1038/cdd.2008.52. IGF-1 protects cancer and fibroblast cells against ER stress induced apoptosis through augmentation of UPR signaling and GRP78 upregulation. [DOI] [PubMed] [Google Scholar]
- 18.Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med. 2010;16:438–445. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;16:429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 21.Flamment M, Kammoun HL, Hainault I, Ferre P, Foufelle F. Endoplasmic reticulum stress: a new actor in the development of hepatic steatosis. Curr Opin Lipidol. 2010;21:239–246. doi: 10.1097/MOL.0b013e3283395e5c. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. Overexpression of GRP78 in liver of ob/ob mice using an adenoviral vector reduces ER stress and leads to metabolic improvements. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Ye R, Jung DY, Jun JY, Li J, Luo S, Ko HJ, Kim JK, Lee AS. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. Grp78 haploinsufficiency protects mice against high fat diet induced obesity and insulin resistance through adpative UPR in the white adipose tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye R, Mareninova OA, Barron E, Wang M, Hinton DR, Pandol SJ, Lee AS. Grp78 heterozygosity regulates chaperone balance in exocrine pancreas with differential response to cerulein-induced acute pancreatitis. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.100368. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 28.Pootrakul L, Datar RH, Shi SR, Cai J, Hawes D, Groshen SG, Lee AS, Cote RJ. Expression of stress response protein Grp78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12:5987–5993. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 29.Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG, et al. Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol. 2007;38:1547–1552. doi: 10.1016/j.humpath.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Zhuang L, Scolyer RA, Lee CS, McCarthy SW, Cooper WA, Zhang XD, Thompson JF, Hersey P. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462–470. doi: 10.1111/j.1365-2559.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 32.Lee E, Nichols P, Groshen S, Spicer D, Lee AS. GRP78 as potential predictor for breast cancer response to adjuvant taxane therapy. Int J Cancer. 2010 doi: 10.1002/ijc.25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 34.Martin S, Hill DS, Paton JC, Paton AW, Birch-Machin MA, Lovat PE, Redfern CP. Targeting GRP78 to enhance melanoma cell death. Pigment Cell Melanoma Res. 2010 doi: 10.1111/j.1755–1148X.2010.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virrey JJ, Dong D, Stiles C, Patterson JB, Pen L, Ni M, Schonthal AH, Chen TC, Hofman FM, Lee AS. Stress chaperone GRP78/BiP confers chemoresistance to tumor-associated endothelial cells. Mol Cancer Res. 2008;6:1268–1275. doi: 10.1158/1541-7786.MCR-08-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kern J, Untergasser G, Zenzmaier C, Sarg B, Gastl G, Gunsilius E, Steurer M. GRP-78 secreted by tumor cells blocks the antiangiogenic activity of bortezomib. Blood. 2009;114:3960–3967. doi: 10.1182/blood-2009-03-209668. [DOI] [PubMed] [Google Scholar]
- 37.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, Mao C, Ye R, Wang M, Pen L, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68:498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 38••.Fu Y, Wey S, Wang M, Ye R, Liao CP, Roy-Burman P, Lee AS. Pten null prostate tumorigenesis and AKT activation are blocked by targeted knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc Natl Acad Sci USA. 2008;105:19444–19449. doi: 10.1073/pnas.0807691105. Biallelic deletion of Pten and Grp78 in the mouse prostate epithelium suppresses prostate tumorigenesis mediated by activation of the AKT oncogenic pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai RY, Chen Y, Fu J, Dong LW, Ren YB, Yang GZ, Qian YW, Cao J, Tang SH, Yang SL, et al. p28GANK inhibits endoplasmic reticulum stress-induced cell death via enhancement of the endoplasmic reticulum adaptive capacity. Cell Res. 2009;19:1243–1257. doi: 10.1038/cr.2009.104. [DOI] [PubMed] [Google Scholar]
- 40.Lin Y, Wang Z, Liu L, Chen L. Akt is the downstream target of GRP78 in mediating cisplatin resistance in ER stress-tolerant human lung cancer cells. Lung Cancer. 2010 doi: 10.1016/j.lungcan.2010.1006.1004. [DOI] [PubMed] [Google Scholar]
- 41.Lee AS. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Arap MA, Lahdenranta J, Mintz PJ, Hajitou A, Sarkis AS, Arap W, Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–284. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Liu R, Ni M, Gill P, Lee AS. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J Biol Chem. 2010;285:15065–15075. doi: 10.1074/jbc.M109.087445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-{kappa}B, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem. 2006;281:13694–13707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- 45.Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM. The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell. 2009;138:377–388. doi: 10.1016/j.cell.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC. GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor beta signaling and enhance cell growth. Mol Cell Biol. 2008;28:666–677. doi: 10.1128/MCB.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Misra UK, Pizzo SV. Modulation of the unfolded protein response in prostate cancer cells by antibody-directed against the carboxyl-terminal domain of GRP78. Apoptosis. 2010;15:173–182. doi: 10.1007/s10495-009-0430-y. [DOI] [PubMed] [Google Scholar]
- 48••.Kelber JA, Panopoulos AD, Shani G, Booker EC, Belmonte JC, Vale WW, Gray PC. Blockade of Cripto binding to cell surface GRP78 inhibits oncogenic Cripto signaling via MAPK/PI3K and Smad2/3 pathways. Oncogene. 2009;28:2324–2336. doi: 10.1038/onc.2009.97. Developmental oncoprotein Cripto binds to cell surface GRP78 and promotes prosurvival signaling. Immunoneutralization of the Cripto-GRP78 complex at the cell surface prevents Cripto-dependent increase in cellular proliferation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misra UK, Pizzo SV. Ligation of cell surface GRP78 with antibody directed against the COOH-terminal domain of GRP78 suppresses Ras/MAPK and PI 3-kinase/AKT signaling while promoting caspase activation in human prostate cancer cells. Cancer Biol Ther. 2010;9:142–152. doi: 10.4161/cbt.9.2.10422. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Steiniger SC, Kim Y, Kaufmann GF, Felding-Habermann B, Janda KD. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol Pharm. 2007;4:435–447. doi: 10.1021/mp060122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez-Gronow M, Cuchacovich M, Llanos C, Urzua C, Gawdi G, Pizzo SV. Prostate cancer cell proliferation in vitro is modulated by antibodies against glucose-regulated protein 78 isolated from patient serum. Cancer Res. 2006;66:11424–11431. doi: 10.1158/0008-5472.CAN-06-1721. [DOI] [PubMed] [Google Scholar]
- 52.Misra UK, Mowery Y, Kaczowka S, Pizzo SV. Ligation of cancer cell surface GRP78 with antibodies directed against its COOH-terminal domain up-regulates p53 activity and promotes apoptosis. Mol Cancer Ther. 2009;8:1350–1362. doi: 10.1158/1535-7163.MCT-08-0990. [DOI] [PubMed] [Google Scholar]
- 53.McFarland BC, Stewart J, Jr, Hamza A, Nordal R, Davidson DJ, Henkin J, Gladson CL. Plasminogen Kringle 5 induces apoptosis of brain microvessel endothelial cells: sensitization by radiation and requirement for GRP78 and LRP1. Cancer Res. 2009;69:5537–5545. doi: 10.1158/0008-5472.CAN-08-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]