Abstract
Human adenylosuccinate lyase (ASL) deficiency is an inherited metabolic disease in which the majority of the patients are compound heterozygotes for the mutations that occur in the ASL gene. Starting with purified wild type and single mutant human ASL, we generated in vitro hybrids which mimic compound heterozygote ASL. For this study, we used His-tag WT/non-His-tag WT, His-tag WT/non-His tag R396C, His-tag WT/non-His tag R396H, His-tag R194C/non-His tag R396C, and His-tag L311V/non-His tag R396H enzyme pairs. We generated various hybrids by denaturing pairs of enzymes in 1 M guanidinium chloride and renaturing them by removing the denaturant. The hybrids were separated on a Ni-nitrilo-triacetic acid-agarose column based on the number of His-tags present in the enzyme tetramer. Analytical ultracentrifuge data indicate that the hybrids have predominant amounts of heterotetramers. Analysis of the Vmax values of the hybrids indicate that most of the subunits behave independently; however, the hybrid tetramers retain weak positive cooperativity indicating that there is some interaction between the different subunit types. The interactions between WT and mutant subunits may be advantageous to the parents of ASL deficient patients, while the interactions between some mutant subunits may assist heterozygote ASL deficient patients.
Adenylosuccinate lyase (ASL)1 is an important enzyme (E.C. 4.3.2.2) in the de novo purine biosynthesis pathway and the purine nucleotide cycle (1). The reactions catalyzed by ASL are shown in Figure 1. Mutations in the adenylosuccinate lyase gene (the majority of which are missense mutations) lead to adenylosuccinate lyase deficiency, which is associated with mental retardation and autism. In these cases accumulation of succinyl adenosine (S-Ado) and succinyl aminoimidazole carboxamide riboside (SAICAr) (the dephosphorylated products of ASL substrates) in the cerebrospinal fluid, plasma, and urine is used to diagnose ASL deficiency (2–4). We have previously studied five purified disease-associated human mutant ASLs, each of which has a single mutation: R194C, L311V, R396C, R396H and K246E (5). The specific activities of the first two mutants are similar to that of wild type ASL, while the specific activities of R396C, R396H and K246E are considerably lower; and all of the mutants exhibit decreased kinetic cooperativity (5).
Figure 1. Schematic representations of reactions catalyzed by adenylosuccinate lyase.
A: Cleavage of SAICAR to AICAR and fumarate. B: Cleavage of SAMP to AMP and fumarate.
Most of the ASL deficient human patients are compound heterozygotes, in which each gene carries a mutation inherited from one parent. Often, these disease-associated point mutations occur at different regions of the enzyme far from the active site (6,7). Two such compound heterozygous combinations that are present in two different patients are R194C/R396C and L311V/R396H (2,8). The patient with the R194C/R396C combination was a boy with a neonatal fatal form of ASL deficiency, which is characterized by neonatal microcephaly, cerebral atrophy, hyperkinesis, and an extremely low body weight percentile (2). The boy died at the age of 6 weeks. In contrast, the patient with the L311V/R396H combination was a girl with a severe childhood form of ASL deficiency, which developed after the first month of life and progressed slowly with age, ultimately resulting in severe mental retardation accompanied by white matter degeneration in the brain, muscle wasting and autistic features (8). At the time of the report the girl was six years of age. As indicated in the two reports, both patients had very low levels of adenylosuccinate lyase activity (2,8).
Adenylosuccinate lyase is a homotetramer, each active site being constituted from different regions of three subunits (Figure 2, I) (9). For B. subtilis ASL, it has been demonstrated that in vitro mixing of two inactive mutant enzymes with replacements for different amino acids can lead to inter-subunit complementation with partial regain of activity (10–12). Accordingly, we have hypothesized that in human compound heterozygote patients inter-subunit complementation may occur, which may be advantageous to the patient as compared to homozygote patients. As shown in Figure 2, II and III, in compound heterozygote patients only one type of mutation is present in each subunit; it is postulated that in some of the enzyme species if the R194C mutation is present in one subunit, the R396C mutation may be present in the adjacent subunit; or if the L311V mutation occurs in one subunit, the R396H mutation may be located on the adjacent subunit. This hypothesis has not yet been critically evaluated. Therefore, this study focuses on in vitro hybridization using wild type and four purified mutant proteins, separation and characterization of hybrid mutant proteins in the cases of the R194C/R396C pair and L311V/R396H pair. A preliminary version of this work has been presented (13).
Figure 2. Representation of the mutated subunits in compound heterozygote human ASL deficient patients.
(I) The crystal structure of the human ASL, which was crystallized with the products (AMP + fumarate) and substrate (SAMP) (PDB # = 2VD6) (14). Each individual subunit is color coded and one active site of four is designated by a circle. (II) and (III) represent, respectively, the compound heterozygote pairs of R194C/R396C and L311V/R396H, in which each mutation is present only in one subunit.
EXPERIMENTAL PROCEDURES
Materials
SAMP, HEPES, imidazole, and Sephadex (G-100) were purchased from Sigma Chemicals. Nickel nitrilotriacetic acid-agarose (Ni-NTA) was obtained from QIAGEN. Precast NuPAGE Bis-Tris 4–12 % gels, NuPAGE MOPS SDS running buffer (pH 7.7), LDS sample buffer, antioxidant, and reducing agent were obtained from Invitrogen. Human alpha thrombin was from Enzyme Research Laboratories, IN. Silver stain kit was purchased from Pierce Inc. All other reagents were from Fisher Scientific and were of reagent grade. SAICAR was prepared enzymatically from AICAR and fumarate by Dr. Sharmila Sivendran as described in (15).
Site-directed Mutagenesis, Enzyme Expression and Purification
We have previously described site-directed mutagenesis and the introduction of mutations (R194C, L311V, R396H, and R396C) into the pETN25HASL plasmid (5). The human WT and mutant ASLs were expressed as N-terminal His6-tagged proteins in E. coli Rosetta 2 (DE3) (pLysS) strain.
The WT and mutant enzymes were purified to homogeneity using chromatography on Qiagen nickel nitrilotriacetic acid-agarose (16). The purity of the enzymes was assessed electrophoretically at pH 8.6 using 12 % polyacrylamide gels containing 0.1 % sodium dodecyl sulfate (17). The protein concentration of human ASL was determined by absorbance at 280 nm using E 2801% = 14.1 (18). After purification, each enzyme was aliquoted and stored at −80 °C in 50 mM potassium phosphate buffer, pH 7.0, containing 150 mM KCl, 1 mM EDTA, 1 mM DTT, and 10% glycerol (Enzyme Storage Buffer). Frozen enzyme samples were preincubated at 25 °C for ~1 h prior to any experiment since it has been shown both for the B. subtilis ASL (19) and for the human ASL (16) that the restoration of full activity is a slow process.
The specific activity of the purified protein was measured continuously by assaying in 1 ml (over a 1 min period) at 25 °C using 60 μM SAMP in 50 mM HEPES buffer, pH 7.4 (Standard Assay). The activity was measured from the decrease in absorbance at 282 nm as SAMP is converted to AMP and fumarate. The difference extinction coefficient of 10,000 M−1 cm−1 between SAMP and AMP was used to calculate the specific activity, which was expressed in μmol of substrate converted/min/mg of enzyme used (20).
The specific activity of ASL with respect to SAICAR was measured continuously by assaying in 1 ml (over a 1 min period) at 25 °C using 60 μM SAICAR in 50 mM HEPES buffer, pH 7.4. The activity was measured from the decrease in absorbance at 269 nm as SAICAR is converted to AICAR and fumarate. The difference extinction coefficient of 700 M−1 cm−1 between SAICAR and AICAR was used to calculate the specific activity, which was expressed in μmol of substrate converted/min/mg of enzyme used (21).
Removal of six-Histidine tag from the WT and Mutant Enzymes
The recombinant human ASL was constructed to have a six His-tag and a thrombin cleavage site before the actual protein sequence. The amino acid sequence of the additional part is: MGSSHHHHHHSSGLVPRGSH and the thrombin cleaves between Arginine and Glycine of the above sequence. The molecular masses of the His-tag protein, non-His-tag protein, and the cleaved peptide portion are ~57,000, ~55,000, and ~1,700 Da, respectively. In order to remove DTT and change the pH, the ASL sample was first dialyzed overnight against 50 mM potassium phosphate buffer, pH 8, containing 300 mM potassium chloride and 10 % glycerol. Subsequently, an appropriate amount of thrombin (10 units of thrombin per 1 mg of ASL) was added to the enzyme sample, which was then incubated at 25 °C for ~24 h. In order to separate the His-tag peptide from the non-His-tag protein, the mixture was loaded on a Sephadex G-100 column (50 ml), which was equilibrated with the Enzyme Storage Buffer, pH 7.0. One mM DTT was added to the enzyme sample before loading on the column to stabilize ASL and inactivate the thrombin. The fractions (1 ml) were eluted using the Enzyme Storage Buffer, pH7.0, and the activity of each fraction was recorded. The fractions with the highest activity were pooled and concentrated. The presence of a pure non His-tag protein was confirmed either by N-terminal sequencing or mass spectroscopy. We have previously demonstrated that His-tagged and non His-tagged human ASL exhibit similar specific activities and kinetic parameters (16).
Concentration Dependence on Guanidinium Chloride of Denaturation and Renaturation of WT ASL
The preincubated WT enzyme sample (6 mg) was dialyzed overnight against Enzyme Storage Buffer, pH 7.0, that has no glycerol (Buffer B). The concentration and the activity of the enzyme sample were measured after dialysis. For each experiment, 1 mg of wild-type enzyme was used. The WT enzyme in Buffer B, pH 7.0, containing various concentrations of guanidinium chloride (0.5 – 3 M) was separately incubated at 25 °C for 1 min. During the incubation, aliquots were withdrawn and assayed at 25 °C, by addition of a 5 μl aliquot of enzyme to 1.0 mL of solution containing 50 mM HEPES, pH 7.4, 60 μM SAMP, and guanidinium chloride to yield each concentration that was used for the incubation. After the 1 min incubation period, the enzyme sample was diluted five times with the 50 mM potassium phosphate Buffer, pH 7.0, containing 150 mm KCl, 0.1 mM DTT, and 10 % glycerol (Nickel Column Buffer) and the diluted mixture was incubated at 25 °C to regain activity. At regular time intervals aliquots were withdrawn and assayed under Standard Assay Conditions to determine the percentage of activity regained.
Denaturation and Renaturation of Mutant Enzymes with Guanidinium Chloride
The wild type and mutant enzyme samples were preincubated at 25 °C for ~ 1 h and each sample was diluted with the Enzyme Storage Buffer, pH 7.0, lacking glycerol, to yield a concentration of 2 mg/ml. To each enzyme sample guanidinium chloride was added to yield a final concentration of 1 M and the enzyme samples were incubated at room temperature for 30 s. During the incubation, an aliquot from each enzyme sample was withdrawn and assayed at 25 °C, by addition of a 5 μl aliquot of enzyme to 1.0 ml of solution containing 50 mM HEPES, pH 7.4, 60 μM SAMP, and guanidinium chloride to yield a final concentration of 1 M. Subsequently, each mixture was diluted 5 times with Nickel Column Buffer, pH 7.0, and dialyzed against the same buffer for 1 h to lower the guanidinium concentration and allow renaturation. The fluorescence intensities of mutant enzymes in fresh Nickel Column Buffer, pH 7.0, were measured on a Perkin-Elmer MPF-3 fluorescence spectrophotometer, which was thermostated at 25 °C. The enzyme solutions were excited at 290 nm, which is specific for tryptophan residues. The emission intensity of each enzyme sample before and after the guanidine treatment was recorded at 335 nm.
Standardization of the Ni-NTA Column using His-tag and non-His-tag WT Human ASL
A mixture containing equal amounts (2 mg of each) of the His-tag WT and non-His-tag WT enzymes that was not treated with guanidinium chloride was loaded onto 1 ml of the Qiagen nickel nitrilotriacetic acid-agarose column equilibrated with the Nickel Column Buffer, pH 7.0. A total volume of 2 ml was loaded onto the column, which was first washed with 100 ml of Nickel Column Buffer, pH 7.0. Subsequently, a linear gradient of imidazole (0 – 250 mM) was used (formed from 100 ml of starting buffer and 100 ml of starting buffer containing 250 mM imidazole) and 2.5 ml fractions were collected in tubes containing 250 μl of the Enzyme Storage Buffer, pH 7.0, containing 10 mM DTT. The absorbance of each fraction at 280 nm was recorded and the blank absorbance of each fraction at 280 nm was subtracted to obtain the elution profile. In order to obtain the absorbance of the blank, a linear gradient of imidazole (0 – 250 mM) was passed through the column that had no bound enzyme. Fractions of 2.5 ml were collected in the tubes containing 250 μl of the Enzyme Storage Buffer, pH 7.0, containing 10 mM DTT. The collected wash flow through and the fractions with high absorbance was concentrated to 1 ml using Amicon Ultra Centrifugation unit, which has a molecular weight cut off of 10,000. The absorbance at 280 nm and enzymatic activity of each pool were measured.
In Vitro Hybridization of WT and Mutant Enzymes and Separation of the Hybrids
The in vitro hybridization and separation of hybrids were performed as described below using the following pairs of enzymes: His-tag WT/non-His-tag WT, His-tag WT/non-His-tag R396C, His-tag WT/non-His-tag R396H, His-tag L311V/non His-tag R396H, and His-tag R194C/non His-tag R396C.
For each hybridization reaction, a mixture (2 ml) was prepared from the two preincubated enzyme samples (2.5 mg of each enzyme), guanidinium chloride to yield a final concentration of 1 M and the Enzyme Storage Buffer, pH 7.0, that has no glycerol. The mixture was incubated at 25 °C for 20 – 30 s. During the incubation, an aliquot was withdrawn and assayed in the presence of 1 M guanidinium chloride as described above. Subsequently, the mixture was diluted five times using the Nickel Column Buffer, pH 7.0, and dialyzed against the same buffer, for 1 h to lower the guanidine concentration and allow renaturation. The mixture was removed from the buffer and centrifuged at 10, 000 rpm at 25 °C for ~1 min to remove any precipitate. The clear supernatant was loaded onto a 1 ml column of Qiagen nickel nitrilotriacetic acid-agarose, which was equilibrated with the Nickel Column Buffer, pH 7.0. The column was first washed using 100 ml of Nickel Column Buffer, pH 7.0. Then a linear gradient of imidazole (0 – 250 mM) was passed through the column and fractions (2.5 ml) were collected in tubes containing 250 μl of the Enzyme Storage Buffer, pH 7.0, with 10 mM DTT. A total volume of 200 ml of gradient was used for the separation. The absorbance at 280 nm was measured in each fraction and each peak was pooled. The wash flow through and each pool were concentrated to 1 ml by centrifugation at 5,000 rpm using an Amicon Ultra Centrifugation unit, which has a molecular weight cut off of 10,000. In order to lower the imidazole concentration, ~15 ml of Enzyme Storage Buffer, pH 7.0 was added to the Amicon Ultra Centrifugation unit, which had 1 ml of concentrated fraction, and was centrifuged at 5,000 rpm to concentrate it to 1 ml. This process was repeated three times for each pool. The pools were kept at 25 °C for ~3 days after eluting from the column. The kinetics, gels, and analytical ultracentrifugation (AUC) were carried out within 2 – 3 days of eluting the fractions. Any remaining pools were aliquoted and stored at −80 °C.
Thermal stability at 60 °C
The pool 2 enzyme samples (0.2 mg/ml) of all the hybrid pairs in fresh Enzyme Storage Buffer, pH 7.0, were incubated at the harsh temperature of 60 °C. Aliquots were removed periodically and assayed for 1 min under Standard Assay conditions at 25 °C. All the enzyme samples were preincubated at 25 °C for ~1 hr before being transferred to 60 °C for stability measurements. The ki value for each reaction was determined from the slope of ln (Et/E0) versus time.
Determination of Subunit Ratios by SDS-PAGE
The precast 4 – 12 % Bis-Tris acrylamide gels from Invitrogen were used to perform SDS-PAGE. The samples for the SDS gels were prepared according to the manufacturer’s protocol using the standards (His-tag and non-His-tag enzymes of each pair) and each pool of 0.1 mg/ml. Each sample (2 μl) was loaded onto the gel and the electrophoresis was performed at 200 V for 2 hrs using SDS-MOPS running buffer. The gels were stained using Silver Stain Kit (Pierce Inc.) according to the manufacturer’s protocol. The stained gels were scanned and the area of each band was measured using the ImageJ free software (22). Each sample was repeated 2–3 times.
Kinetics of Hybrid Pools
The activities of the different pools of enzyme hybrids in fresh Enzyme Storage Buffer, pH 7.0, were measured by assaying for 1 min under Standard Assay conditions. The kinetic parameters were determined by varying the SAMP concentrations (0.2 – 10 μM). Since the data did not obey simple Michaelis-Menten kinetics, they were analyzed using the Hill equation, v = {Vmax * [S]n}/{[S]n + K0.5}, where n = Hill coefficient, and K0.5 = the substrate concentration yielding half the Vmax. The standard error (S.E.) estimates were obtained from the SigmaPlot software (SPSS Inc., Chicago, IL).
Molecular Mass Determination using Analytical Ultracentrifugation (AUC)
In order to assess the species present in each pool, sedimentation equilibrium (SE) was used. SE experiments of the WT and mutant enzymes were conducted using a Beckman Coulter ProteomLab™ XL-I analytical ultracentrifuge equipped with an An-60Ti analytical rotor and six chamber center piece assembly. Each sample was loaded in duplicate or triplicate into each six chamber center piece. Samples (~0.1 mg/ml) were centrifuged at 11,000 rpm at 25 °C. Some of the pure mutant samples (R194C, R396C and L311V) were also centrifuged at 9,000 rpm at 25 °C. Since the results were similar at 9,000 and 11,000 rpm, the comparison was carried out at 11,000 rpm. After equilibrium was reached (~20 h), stepwise radial scans were performed at 280, 240, and 220 nm, using a step size of 0.001 cm (23). Initially the equilibrium time was determined by scanning at 5 h intervals for ~20 h. The species present in each pool was evaluated by analyzing the data using the non-interacting discrete species model in the SEDPHAT program (24), which assumes that there is no interaction among the species during the time of the SE experiment. In all cases the experimental data were fitted to all the possible theoretical models of an oligomer with species of lower molecular mass. The models that gave best fits were chosen as representative of that sample. The density of the buffer at 25 °C was calculated using the SEDNTERP program (25). The partial specific volume of human ASL at 25 °C is 0.73698. We have used a similar approach previously in analyzing the molecular mass of WT and mutant B. subtilis and human ASL (5,26).
RESULTS
Expression and Purity of the Human ASL WT and Mutants
Wild type and mutant enzymes were expressed and purified to homogeneity by the methods described previously (16) and the purity was assessed using SDS-PAGE and mass spectroscopy. Each His-tagged and non-His-tagged enzyme exhibited a single subunit band in the SDS-PAGE (5). The mass analysis of each of the enzymes indicated that the molecular mass of His-tag and non-His-tag enzymes is ~57,000 and ~55,000, respectively.
Denaturation and Renaturation of WT and Mutant Human ASL
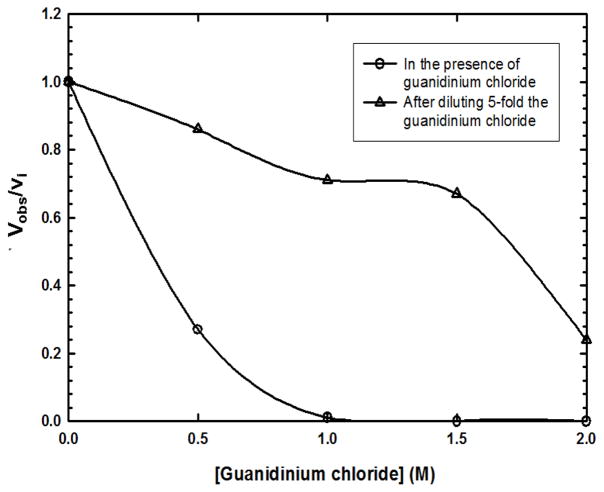
In order to perform in vitro protein hybridization, it is desirable that the two enzymes dissociate to subunits in the presence of a denaturant and then reassociate to form tetramer with minimal conformational changes when the denaturant is removed. If there are two different subunit types present in the denaturant solution, various hetero-tetramers can be formed as the denaturant is removed. We chose guanidinium chloride as the denaturant and, as a screen for conditions, sought to evaluate the concentration dependence of inactivation of human WT ASL by guanidinium chloride. Since glycerol stabilizes proteins, the wild type enzyme was dialyzed overnight to remove glycerol prior to the experiment. As represented in Figure 3, during the 1 min incubation of human wild type ASL in the presence of ≥ 1 M guanidinium chloride there is complete loss of activity. When the guanidinium chloride is diluted five-fold, the activity is regained to various extents (Figure 3). However, some of the mutant enzymes were unstable in the presence of ≥ 1.5 M guanidinium chloride under the same denaturation and renaturation conditions. In contrast, all the mutant enzymes were equally stable when they were diluted directly with the Enzyme Storage Buffer, pH 7.0 lacking glycerol immediately prior to the guanidine treatment followed by denaturation (in the presence of 1.0 M guanidinium chloride for 30 s) and renaturation. This is the procedure that was followed subsequently.
Figure 3. Fraction of specific activity of human wild type ASL at 25 °C in the presence of different concentrations of guanidinium chloride (○) and after diluting the guanidinium chloride 5-fold (Δ).
The vi and vobs are, respectively, the specific activities of the enzyme without guanidinium chloride, and during the denaturing and renaturing process.
The activity and fluorescence emission intensity (which is an indication of the enzyme’s conformational state) of each sample were measured before and after the guanidinium chloride treatment and the results are recorded in Table 1. It is evident that the enzymes regain full activity when the mixture was diluted 5 times and dialyzed for 1 h to lower the guanidinium chloride concentration and allow renaturation. Furthermore, there is minimal change in emission fluorescence intensity when comparing each sample before and after the guanidinium chloride treatment, indicating that denaturation is reversible and does not cause major conformational changes in any of the enzymes.
Table 1.
Activity and fluorescence intensity of wild type and mutant human ASL at 25 °C before and after guanidinium chloride treatment
| Enzyme | Before Gdn.HCl treatment |
After Gdn.HCl treatmenta |
||
|---|---|---|---|---|
| Specific Activityb (μmol/min/mg) | Fluorescence Intensity at 335 nmc | Specific Activityb (μmol/min/mg) | Fluorescence Intensity at 335 nmc | |
| His-WT | 8.5 ± 0.2 | 41 ± 1.0 | 8.3 ± 0.1 | 42 ± 0.5 |
| Non-His-WT | 8.6 ± 0.1 | 42 ± 0.5 | 8.4 ± 0.2 | 42 ± 1.0 |
| His-L311V | 7.8 ± 0.2 | 36 ± 1.0 | 7.5 ± 0.1 | 37 ± 0.5 |
| Non-His-R396H | 1.0 ± 0.2 | 44 ± 1.0 | 1.1 ± 0.1 | 43 ± 0.5 |
| His-R194C | 8.7 ± 0.2 | 42 ± 0.5 | 8.9 ± 0.2 | 41 ± 0.5 |
| Non-His-R396C | 1.8 ± 0.1 | 41 ± 1.0 | 1.6 ± 0.2 | 40 ± 0.5 |
Each enzyme (2 mg/ml) was incubated at 25 °C in the presence of 1 M guanidinium chloride for 30 s and diluted five times followed by dialysis for 1 hr at room temperature.
The specific activity of each enzyme sample was measured continuously by assaying (over a 1 min period) at 282 nm using 60 μM SAMP in 50 mM HEPES buffer, pH 7.4 at 25 °C.
The fluorescence emission intensity of each enzyme sample was measured at 335 nm and the intensities were normalized to the protein concentration of 0.33 mg/ml.
In vitro Hybridization and Separation of Hybrids
In vitro hybridization was carried out by mixing equal amounts of the appropriate non-His-tag and His-tag enzyme pairs as described in Experimental Procedures. Since human ASL is a tetramer, three different types of hybrid tetramers with the following ratios of non-His-tag:His-tag: 3:1, 2:2, and 1:3 can form during hybridization, as well as the 4:0 and 0:4 of the original His-tag and non-His-tag proteins. Separation of the mixture was accomplished by using a nickel column; affinity for the column depends on the number of His-tags present on the tetramer. Theoretically the non-His-tag protein should elute first and the several hybrids should be separated by the imidazole gradient as follows: earliest should be the 3:1 mixture (non-His-tag:His-tag subunits), followed by the 2:2 mixture, then the 1:3 mixture, and last should be the pure His-tag protein.
The 1.0 ml nickel column was standardized using a mixture of an equal amount of His-tag WT and non-His-tag WT enzyme that had not undergone the guanidinium chloride treatment. Once the mixture was loaded onto the column, it was washed with the Nickel Column Buffer, pH 7.0, that lacks imidazole. No ASL activity was detected in the wash fraction after concentrating, implying that even the non-His-tag WT enzyme binds to the nickel column. In the imidazole gradient, the non-His-tag WT enzyme elutes between volumes 12 – 40 ml with a maximum at ~20 ml; while the pure His-tag enzyme elutes between 130 and 175 ml with a maximum at ~140 ml (Figure 4). Absorbance and ASL activity were not detected between effluent volumes 40 – 130 ml, suggesting that hybridization does not occurr without the guanidinium treatment. Furthermore, these results indicate that there is a relatively large distinction between the pure non-His-tag and His-tag protein, which allows good separation of the hybrids.
Figure 4. The elution profiles of the enzyme pairs treated with guanidine in the presence of the imidazole gradient.
(A) The elution profiles related to the R194C/R396C pair. (B) The elution profiles related to the L311V/R396H pair. The fraction that had highest absorbance for elution of pure non-His-tag WT and His-tag WT are shown by two arrows on top of the graph. The four peaks in each elution profile are numbered from 1 – 4. The elution profile of His-tag WT/non-His-tag WT is shown in both graphs. The volume of each fraction is 2.5 mL.
To assess the feasibility of the hybridization protocol, a mixture of His-tag WT and non-His-tag WT enzymes was used to perform the in vitro hybridization and separation of the hybrids. Since R396H and R396C mutant enzymes have very low (~20 % that of WT activity) activity, we sought to evaluate whether high activity hybrids are formed between WT and the R396C/H mutants. This scenario mimics the situation of parents of the ASL deficient patients, as each parent has only one mutation in one gene while the other gene corresponds to the WT protein. In order to mimic the human ASL deficient patients, appropriate enzyme pairs (R194C/R396C and L311V/R396H) were used to perform the in vitro hybridization. The elution profiles of His-tag WT/non-His-tag WT, His-tag WT/non-His-tag R396C, and His-tag R194C/non-His-tag R396C in the presence of the imidazole gradient are shown in Figure 4A. The elution profiles of His-tag WT/non-His-tag WT, His-tag WT/non-His-tag R396H, and His-tag L311V/non-His-tag R396H in the presence of the imidazole gradient are shown in Figure 4B. The peak position of pure non-His-tag WT ASL and pure His-tag WT ASL are indicated above the graph.
The elution profiles of all the enzyme pairs show four peaks compared to the two peaks of the mixture of His-tag WT and non-His-tag WT enzymes without the guanidinium treatment. Hence, the two middle pools, pools 2 and 3 contain different types of hybrid mixtures. The amount of hybrid in the final preparations is represented by the areas under peaks 2 + 3 as compared to the total area under peaks 1 + 2 + 3 + 4. We estimate that about 33% of the protein recovered are hybrids. (Although the amount of the hybrids formed under these in vitro conditions may not seem large compared with the original enzymes, the extent of dissociation of the ASL tetramer depends on the harshness of the conditions used for denaturation. We sought to optimize the solubility and preservation of activity while forming sufficient amounts of hybrids to allow their characterization.) N-terminal sequencing of pool 2 and 3 of each enzyme pair indicates that each pool consists of His-tag and non-His-tag proteins, implying that hybrids are present. However, quantitative results of the precise ratios of the His-tag and non-His-tag proteins were hard to obtain because of the poor yield upon Edman sequencing of the six His residues in the His-tag enzyme.
AUC data of hybrid pools
In order to assess the molecular mass of each enzyme pool, analytical ultracentrifugation (AUC) was used. We have previously shown that the ASLs exist as a mixture of species; e.g., monomer-tetramer, dimer-tetramer, trimer-tetramer, etc.(5,26). A constant concentration of ~0.1 mg/ml was used for all the enzymes and the experimental AUC data at 25 °C were fitted to various theoretical models for a mixture of an oligomer with species of different molecular masses. Since the results at 280, 240, and 220 nm are similar, only the representative experimental data and residuals at 280 nm, which illustrate goodness of fit for various models [e.g. monomer-tetramer (M-Tet) or dimer-tetramer (D-Tet)], are shown in the Supporting Information section (Figures 1S–3S). Results of the molecular mass analyses of pools 1 – 3 are summarized in Table 2. The subunit molecular mass of the pure non-His-tag and His-tag enzymes are ~55,000 and ~57,000, respectively; therefore, a tetramer of non-His-tag enzyme would be expected to have a molecular mass of ~220,000, while a pure His-tag enzyme would have a molecular mass of ~228,000. The five original purified His-tag individual enzymes are shown at the top of the Table 2, and exhibit tetramers of ~228 kDa.
Table 2.
Molecular mass data of hybrid pools of human ASL at 25 °C and pH 7.0
| Enzyme | Molecular mass by AUCa (kDa) | |
|---|---|---|
| M-Tet | D-Tet | |
| His-tag WT | 58 ± 1 (23%) | |
| 228 ± 0.5 (77%) | ||
| His-tag R194C | 57 ± 1 (27%) | |
| 229 ± 2 (73%) | ||
| His-tag L311V | 57 ± 0.5 (32%) | |
| 230 ± 1 (68%) | ||
| His-tag R396H | 116 ± 1(20%) | |
| 227 ± 2(80%) | ||
| His-tag R396C | 56 ± 2 (22%) | |
| 227 ± 1 (78%) | ||
| non-His-tag WT/His-tag WT | ||
| Pool 1 | 55 ± 0.8(32%) | |
| 219 ± 1 (68%) | ||
| Pool 2b | 56 ± 3 (27%) | |
| 223 ± 2 (73%) | ||
| Pool 3b | 55 ± 2 (25%) | |
| 230 ± 2 (75%) | ||
| non-His-tag 396C/His-tag WT | ||
| Pool 1 | 55 ± 1 (24%) | |
| 218 ± 2 (76%) | ||
| Pool 2 | 56 ± 0.6 (27%) | |
| 222 ± 1 (72%) | ||
| Pool 3c | 57 ± 1 (30%) | |
| 227 ± 3 (70%) | ||
| non-His-tag R396H/His-tag WT | ||
| Pool 1 | 54 ± 1 (25%) | |
| 221 ± 2 (75%) | ||
| Pool 2d | 58 ± 1 (27%) | |
| 225 ± 2 (73%) | ||
| Pool 3 | 57 ± 1 (28%) | |
| 229 ± 2 (72%) | ||
| non-His-tag R396C/His-tag R194C | ||
| Pool 1 | 54 ± 1 (20%) | |
| 217 ± 3 (80%) | ||
| Pool 2e | 57 ± 2 (22%) | |
| 223 ± 3 (78%) | ||
| Pool 3e | 56 ± 2 (20%) | |
| 227 ± 2 (80%) | ||
| non-His-tag R396H/His-tag L311V | ||
| Pool 1 | 55 ± 0.5 (26%) | |
| 220 ± 2 (74%) | ||
| Pool 2f | 57 ± 2 (26%) | |
| 224 ± 2 (74%) | ||
| Pool 3f | 58 ± 3 (26%) | |
| 229 ± 2 (74%) | ||
The molecular mass by AUC was measured at 25 °C, using ~0.1 mg/ml sample in fresh Enzyme Storage Buffer, pH 7.0, at 11,000 rpm. The experiments were performed within 48 hrs after eluting all the pools. M = monomer, D = dimer, Tri = trimer, Tet = tetramer, and the dash (−) is used to indicate that different species are in equilibrium. The percentage of each species is given in parenthesis. The AUC data of the following pools are fit equally well to the following models:
pools 2 and 3 of non-His-tag WT/His-tag WT also fit to D-Tet (27%,73%) model,
pool 3 of non-His-tag R396C/His-tag WT also fits to M-Tri (30%,70%) model,
pool 2 of non-His-tag R396H/His-tag WT also fits to M-5mer (29%, 71%) model,
in non-His-tag R396C/His-tag R194C pair, pool 2 also fits to M-5mer (25%,75%) model, while pool 3 also fits to the M-Tri (18%,82%) model, and
pool 2 and 3 of non-His-tag R396H/His-tag L311V also fits to M-5mer (30%, 70%) model.
According to the best fit models shown in Table 2, all the pools have predominant amounts of tetramer, indicating that the enzyme mixtures, which were initially denatured with guanidinium chloride, indeed renatured and reassociated when guanidinium chloride was removed. The AUC results indicate that the experimental AUC data of pools 1–4 of all the hybrid pairs have a good fit with monomer-tetramer model. In some of the cases, the results can also be fit either to monomer-5mer models (Table 2), which may be indicative of some instability of the hybrids, or to monomer-trimer model (Table 2) suggesting that there may be unfavorable interactions between the two types of enzyme subunits.
SDS-PAGE
Denaturing gels were used for evaluation of the approximate amounts of His-tag and non-His-tag protein in each pool, since a His-tagged subunit of ASL exhibits a molecular mass of ~57,000, whereas a non-His-tagged subunit of ASL has a molecular mass of ~55,000. These gels gave reproducible bands. Representative gels of each pair are shown in Figure 5. The subunit ratios estimated from these gels (represented as non-His-tag:His-tag subunit ratios are shown in Table 3, column 2.
Figure 5. Representative SDS-PAGE gels of each enzyme pair. Standard (S1 and S2) 1 and 2 are, respectively, His-tag and non-His-tag protein of each pair.
The P 1 – P 4 represent the pools in the elution profile of each pair. (A) The gel of the non-His-tag WT/His-tag WT pair. (B) The gel of the non-His-tag R396C/His-tag WT pair. (C) The gel of the non-His-tag R396H/His-tag WT pair. (D) The gel of the non-His-tag R396C/His-tag R194C pair. (E) The gel of the non-His-tag R396H/His-tag L311V pair. The band for the P4 pool from all the pairs is similar to that of WT/WT pair and is omitted from gels B – E for clarity.
Table 3.
Subunit ratios of the enzyme from SDS-PAGE and the enzymatic activities of the heterotetramer at 25 ºC
| Enzyme | Subunit ratios (Non-His-tag: His-tag) | Enzymatic activitiesa |
|||
|---|---|---|---|---|---|
| SAMP (% of WT) | SAICAR (% of WT) | ||||
| Emeas | Ecalc | Emeas | Ecalc | ||
| WT | 100 | 100 | |||
| R194C | 125 | 107 | |||
| L311V | N.A. | 90 | N.A. | 86 | N.A. |
| R396H | 20 | 22 | |||
| R396C | 21 | 29 | |||
| non-His-tag WT/His-tag WT | |||||
| Pool 2 | 1.3:1 | 95 | 100 | 93 | 100 |
| Pool 3 | 1:3.7 | 95 | 100 | 93 | 100 |
| non-His-tag R396C/His-tag WT | |||||
| Pool 2 | 1.2:1 | 45 | 50 | 60 | 56 |
| Pool 3 | 1:2.3 | 100 | 76 | 82 | 79 |
| non-His-tag R396H/His-tag WT | |||||
| Pool 2 | 1.1:1 | 59 | 57 | 62 | 58 |
| Pool 3 | 1:1.6 | 102 | 68 | 85 | 69 |
| non-His-tag R396C/His-tag R194C | |||||
| Pool 2 | 1.2:1 | 65 | 68 | 64 | 65 |
| Pool 3 | 1:3.2 | 113 | 100 | 103 | 88 |
| non-His-tag R396H/His-tag L311V | |||||
| Pool 2 | 1.8:1 | 57 | 44 | 56 | 45 |
| Pool 3 | 1:1 | 85 | 56 | 80 | 54 |
The activity of each pool for SAMP and SAICAR was measured separately at 25 °C by addition of a 10 μl aliquot of enzyme to 1.0 mL solution containing 50 mM HEPES buffer, pH 7.4 and 60 μM of each substrate. Ecalc is the calculated specific activity of each pool relative to that of WT and Emeas is the measured specific activity relative to that of WT. The specific activities of WT enzyme with respect to SAMP and SAICAR are 9.2 and 15 μmol/min/mg, respectively. N.A. = not applicable.
It is apparent from the SDS gels that, along the imidazole gradient, initially there is pure non-His-tag protein (pool 1) followed by pool 2, which is a hybrid with either approximately equal amounts of non-His-tag and His-tag protein or more non-His-tag protein compared to that of His-tag protein. Pool 3 is also a hybrid and has more of His-tag protein (either double or triple the amount of non-His-tag enzyme). Pool 4 contains predominantly His-tag protein with a small amount of non-His-tag protein. The gel pattern of pool 4 of WT/mutant and mutant/mutant pairs was similar to that of WT/WT pair. Polyacrylamide gels run under conditions which maintained the enzyme in its native state (i.e., no SDS) have also demonstrated the existence of several bands in pools 2 and 3. However, these gels were more difficult to quantify.
SAMP and SAICAR activity of the hybrid pools
The two substrates for ASL are SAMP and SAICAR. Therefore, we sought to evaluate separately the activity of the hybrid enzyme pools with each of the two substrates under saturating conditions (60 μM substrate) at pH 7.4 in the direction of fumarate formation. The Emeas (the measured specific activity relative to that of WT) and Ecalc (calculated specific activity relative to that of WT) values for each pool of the enzyme pairs are summarized in Table 3. The expected activity of each hybrid pool was calculated using the subunit ratios obtained by SDS-PAGE and the Vmax of the homotetramers (Table 5).
Table 5.
Kinetic parameters of human ASL hybrid pairs at 25 °C
| Enzyme | Vmax ± S.Ea (μmol/min/mg) | K0.5 ± S.Ea (μM) | Hill Coefficienta |
|---|---|---|---|
| WT | 9.2 ± 0.1 | 1.9 ± 0.05 | 1.8 ± 0.1 |
| R194C | 12.0 ± 0.9 | 2.2 ± 0.3 | 1.3 ± 0.2 |
| L311V | 8.6 ± 0.7 | 3.4 ± 0.5 | 1.3 ± 0.1 |
| R396H | 1.9 ± 0.2 | 3.8 ± 0.8 | 0.9 ± 0.2 |
| R396C | 2.0 ± 0.2 | 1.1 ± 0.2 | 0.9 ± 0.1 |
| non-His-tag WT/His-tag WT | 9.8 ± 0.3 | 2.2 ± 0.1 | 1.8 ± 0.2 |
| non-His-tag R396C/His-tag WT | |||
| Pool 1 | 2.1 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Pool 2 | 4.2 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.2 |
| Pool 3 | 9.2 ± 0.7 | 3.2 ± 0.3 | 1.6 ± 0.1 |
| non-His-tag R396H/His-tag WT | |||
| Pool 1 | 1.9 ± 0.1 | 3.2 ± 0.9 | 0.7 ± 0.06 |
| Pool 2 | 5.5 ± 0.2 | 1.9 ± 0.2 | 1.4 ± 0.1 |
| Pool 3 | 9.4 ± 0.6 | 2.7 ± 0.3 | 1.5 ± 0.2 |
| non-His-tag R396C/His-tag R194C | |||
| Pool 1 | 2.2 ± 0.1 | 1.3 ± 0.3 | 0.8 ± 0.1 |
| Pool 2 | 6.0 ± 0.2 | 1.9 ± 0.2 | 1.5 ± 0.2 |
| Pool 3 | 10.4 ± 0.4 | 1.9 ± 0.2 | 1.2 ± 0.1 |
| non-His-tag R396H/His-tag L311V | |||
| Pool 1 | 1.9 ± 0.1 | 3.2 ± 0.9 | 0.7 ± 0.06 |
| Pool 2 | 5.3 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.1 |
| Pool 3 | 7.9 ± 0.6 | 2.4 ± 0.4 | 1.1 ± 0.1 |
The Vmax, K0.5, and Hill coefficient values were determined by varying the concentration of SAMP and fitting the data to the Hill equation using Sigma Plot. The values are shown along with their standard errors.
If the subunits of the heterotetramer act independently, the Emeas should be similar to that of Ecalc. The Emeas values for SAMP and SAICAR of pool 2 is similar to that of Ecalc values of the respective pairs implying that there is little or no influence from one type of subunit over the other when there are equal amounts of non-His-tag enzyme (in these cases, the less active enzyme) and His-tag enzyme (in these cases, the more active enzyme). However, the Emeas values for SAMP and SAICAR of pool 3 of the respective pairs are higher than that of the corresponding Ecalc values, indicating that when the His-tag enzyme is predominant, it influences the activity of the mutant subunit of lower activity.
Thermal stability at 60 °C
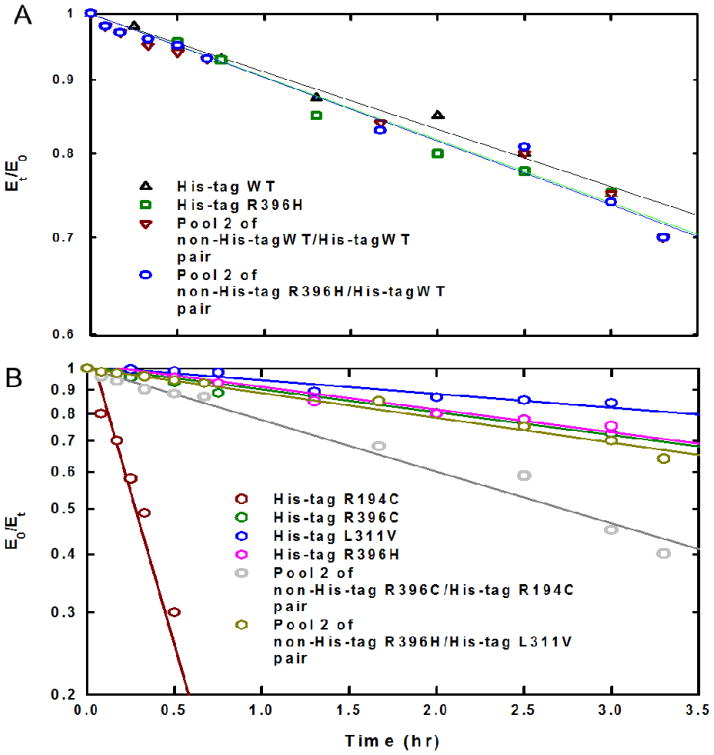
Since we have studied the stability of each individual enzyme under the relatively harsh conditions at 60 °C (5), we sought to evaluate the stability of the hybrids under the same conditions. The time-dependent inactivation plots are illustrated in Figure 6. The inactivation rate constant (ki) value for pool 2 of each enzyme pair was determined from the slope of ln (Et/E0) versus time and the data are summarized in Table 4.
Figure 6. Time dependent thermal inactivation plots of the individual His-tag enzymes and Pool 2 of the enzyme pairs at 60 °C.
(A) The ln (Et/E0) vs time plots of His- tag WT, His-tag R396H, Pool 2 of non-His-tag WT/His-tag WT pair and non-His-tag R396H/His-tag WT pair. (B) The ln (Et/E0) vs time plots of His- tag R194C, His-tag R396C, His-tag L311V, His-tag R396H, Pool 2 of non-His-tag R396C/His-tag R194C pair and non-His-tag R396H/His-tag R396H pair. The Et is the specific activity at a time t and E0 is the specific activity at time zero.
Table 4.
Inactivation rate constants of pure human ASL enzymes and Pool 2 of the hybrid pairs at 60°Ca
| Enzyme | ki ± S.E (hr−1)b |
|---|---|
| His-tag WT | 0.086 ± 0.004 |
| His-tag L311V | 0.059 ± 0.005 |
| His-tag R396H | 0.096 ± 0.007 |
| His-tag R396C | 0.103 ± 0.01 |
| His-tag R194C | 2.76 ± 0.1 |
| Pool 2 of non-His-tag R396C/His-tag R194C | 0.255 ± 0.02 |
| Pool 2 of non-His-tag WT/His-tag WT | 0.099 ± 0.003 |
| Pool 2 of non-His-tag R396H/His-tag WT | 0.101 ± 0.007 |
| Pool 2 of non-His-tag R396H/His-tagL311V | 0.122 ± 0.003 |
The enzyme samples (0.2 mg/ml) in fresh Enzyme Storage Buffer, pH 7.0, were incubated at 60 °C. Aliquots were removed periodically and the activity of was measured at 25 °C by addition of a 10 μl aliquot of enzyme to 1.0 ml solution containing 50 mM HEPES buffer, pH 7.4 and 60 μM of each substrate (SAMP).
The ki value for each reaction was determined from the slope of ln (Et/E0) versus time. The Et and E0 are the specific activity at time t and zero, respectively. The values are shown along with their standard errors.
It is evident that the pools 2 (with approximately equal amounts of non-His-tag and His-tag or more of non-His-tag enzyme subunits) are relatively stable under the harsh conditions at 60 °C and no insoluble aggregate formation was observed. There is a significant increase in the stability of pool 2 of the non-His-tag R396C/His-tag R194C pair as compared to that of His-tag R194C (Figure 6B); this is shown by the 10-fold lower inactivation rate constant for the hybrid non-His-tag R396C/His-tag R194C as compared to that of homotetrameric R194C (Table 4).
Kinetics of hybrid pools
All the pools of each enzyme pair were characterized kinetically using SAMP. The kinetic data were analyzed using the Hill equation and representative plots of velocity vs [SAMP] from each enzyme pair are shown in the Supporting Information section (Figures 4S and 5S). The data at pH 7.4 in the direction of AMP formation for the pools 1 – 3 in each enzyme pair are summarized in Table 5.
The kinetic data of pool 1 of each enzyme pair are similar to that of corresponding non-His-tag enzyme indicating that pool 1 contains pure non-His-tag enzyme. All the pools of His-tag WT/non-His-tag WT enzyme pair have similar kinetic parameters to that of the pure WT. Furthermore, the pools have a Hill coefficient of 1.8 indicating that there is positive cooperativity. In contrast, pool 2 of WT/mutant and mutant/mutant enzyme pairs has an intermediate Vmax (average of the two pure enzymes), while the Vmax of pool 3 is similar to that of the corresponding His-tag enzyme. There is minimal change in the K0.5 values in pools 2 and 3 of all the enzyme pairs indicating that hybridization has less effect on substrate affinity. The Hill coefficients of pool 2 and 3 of WT/mutant pairs are ~1.5 suggesting that the positive cooperativity is slightly reduced as compared to that of pure WT enzyme.
DISCUSSION
Human adenylosuccinate lyase deficiency is a metabolic disorder that is caused by mutations to the ASL gene, most of which are missense mutations. Furthermore, the majority of these missense mutations occur far away from the active site. Since many ASL deficient human patients are compound heterozygotes, the existence of various hybrids has been hypothesized. In the present study we evaluated the feasibility of in vitro hybridization and separation of different hybrid mixtures followed by characterization of the hybrid pools. For this study we chose the R194C/R396C and L311V/R396H mutant pairs. We also used the WT/R396C and WT/R396H pairs to mimic the situation of parents of ASL deficient patients. As a control we performed hybridization using His-tag WT and non-His-tag WT. Since ASL is a tetramer, mixing of two types of mutant enzymes can produce three different types of hybrid tetramers with a ratio of non-His-tag:His-tag of 3:1, 2:2, and 1:3.
The elution profiles of WT/WT and the other enzyme pairs indicate that hybrids have formed and for the first time we have been successful in separating the hybrids. Previously it has been shown that hybrids of ASL are formed when two different types of mutant enzymes are co-expressed, but the authors did not separate the various hybrids (27). It is evident from the SDS-PAGE results that pools 2 and 3 of all the enzyme pairs contain hybrids.
The subunit ratio of pool 2 is approximately 1:1 (non-His-tag:His-tag) in most cases. For each substrate the measured activity (Emeas) of pool 2 of all the enzyme pairs approximates the average of the corresponding activities of the two enzymes in the respective pair. Since each active site of ASL is formed from different regions of three subunits (Figure 2I), two active sites of the tetramer of pool 2 have higher contributions from the subunits of the less active mutant whereas the other two active sites have more contributions from the more highly active enzyme (either WT or mutant). This leads to 50% contribution from each type of subunit to the overall activity. Although for each substrate the measured activity (Emeas) of pool 2 of all the enzyme pairs is similar to the respective Ecalc values, the kinetic data reveal that pool 2 of all the pairs have different degrees of positive cooperativity (Table 5) suggesting that there may be very weak positive cooperativity among the subunits. On the other hand, as the amount of His-tag protein increases, as in pool 3, for each active site of the tetramer there is more contribution from the His-tag protein with the higher activity leading to an increase in activity (Tables 3 and 5). Furthermore, the Emeas values of pool 3 of all the enzyme pairs are higher than the corresponding Ecalc values, suggesting that the subunits may have some influence on each other. It is apparent from the kinetic data that pool 3 of all the pairs retains the positive cooperativity of the corresponding His-tag enzyme (Table 5). This observation suggests that there is a disproportionately large influence on the overall activity from the more active His-tag enzyme.
An important observation is that similar differences between Emeas and Ecalc were obtained regardless of the substrate used (either SAMP or SAICAR). This result is consistent with the active site being the same for both substrates (18, 28). Similar results have been reported in an independent study of disease-associated human ASL mutants (27). It has been suggested that the different levels of the two dephosphorylated substrates detected in some ASL-deficient patients are not due to the enzyme having two different active sites for the two substrates; instead, they may be due to either unequal rates of dephosphorylation of the two substrates or different transport rates of the two dephosphorylated substrates out of the cell (18).
Previously, it was shown that the R194C mutant enzyme is the most unstable mutant under harsh conditions (e.g., at 60 °C) due to the rapid oxidation of its cysteines (5). In contrast, the other mutant enzymes were stable for ~ 2–3 hours, after which some insoluble aggregates were observed. Notably, we have now found that the R396C/R194C hybrid pool 2 enzyme is ten times more stable than the pure R194C mutant enzyme (Table 4). Clearly, interactions of the less active R396C subunits with the R194C subunits have stabilized the mutants in the hybrid, although this hybrid is still less stable than WT enzyme. The hybrid pool 2 of the R396C/R194C pair is also less stable than that of the R396H/L311V pair under harsh conditions at 60 °C. These results suggest that under in vivo conditions of ASL deficient patients, it may be possible that the patient with the R194C/R396C mutant pair produces relatively unstable hybrid and non-hybrid mixtures leading to a severe form of ASL deficiency which presents only as a neonatal form. On the other hand, the patient with the L311V/R396H mutant pair may produce more stable hybrid and non-hybrid mixtures leading to ASL deficiency that presents as the childhood severe form. Apparently, there are favorable interactions between the subunits of WT and mutant enzymes, which can lead to the formation of more active and stable hybrid mixtures. This may account for the parents of ASL deficient patients being normal.
The other characteristic of the ASL deficient patients is the presence of higher amounts of dephosphorylated substrates in cerebrospinal fluid than in urine and develop mental retardation. Adenylosuccinate lyase participates in two pathways: the de novo purine biosynthesis pathway and the purine nucleotide cycle. Both pathways are active during development and throughout life (29, 30). However, the demand for purines during development and throughout life is different. Therefore, the two pathways are tightly regulated in distinct ways to meet the demand. It has been shown that all six enzymes in the de novo purine biosynthesis pathway colocalize to form the “purinosome” during purine biosynthesis to channel each product for the next step (31). Mutant enzymes of purine biosynthesis may affect the stability of the purinosome to different extents. Furthermore, different downstream intermediates regulate both pathways; for example an increase in SAICAR levels up regulates the gene expression of de novo purine synthesis (32–34). During the developmental stage there is a high demand for purines and the de novo purine biosynthesis pathway is more highly active than the purine nucleotide cycle (35). Furthermore, purine nucleotides are particularly important as modulators of neurotransmitters and regulators of the synaptic availability of neurotransmitters (36). It may be postulated that more profound brain damage could result in newborns, and present as the neonatal fatal form of ASL deficiency due to accumulation of more SAICAR (with its greater toxicity) than SAMP. However, after birth both pathways become equally active to meet the purine demand. In ASL deficient patients this would lead to accumulation of both substrates, SAMP and SAICAR in similar or slightly different levels, particularly in the cerebrospinal fluid, which may result in slow progression of brain damage. Therefore, in such newborns ASL deficiency presents as the severe childhood form which progresses slowly with age and ultimately results in severe mental retardation.
In conclusion, we have shown that in vitro hybridization of two different enzymes is possible under reversibly denaturing conditions and we were successful in separating various hybrids. The molecular mass data indicate that different types of subunits reassociated to form predominant amounts of heterotetramers and that the extent of favorable interactions between different types of subunits depends upon the mutant enzyme. We have shown that the hybrids are relatively stable under harsh conditions at 60 °C and that the hybrids have positive cooperativity to different degrees. Our results indicate that formation of hybrids is advantageous to ASL deficient patients and as well as their parents. However, under in vivo conditions the formation of hybrids may not be sufficiently effective to prevent symptoms of ASL deficiency disease.
Supplementary Material
Acknowledgments
We thank Dr. Yu-Chu Huang for N-terminal sequencing and Mr. John Dykins for running the mass spectrometer.
Footnotes
This work was supported by NIH grant R01-DK60504 and by a grant from Autism Speaks. The Beckman Optima XL-I analytical ultracentrifuge used in this study was obtained and supported by NIH 2P20 RR016472
Abbreviations: ASL, adenylosuccinate lyase; S-Ado, suucinyl adenosine; SAICAr, succinyl aminoimidazole carboxamide riboside; SAICAR, 5-aminoimidazole-4-(N-succinylocarboxamide ribonucleotide); AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; SAMP, adenylosuucinate; Ni-NTA, Nickel Nitrilo-triacetic Acid; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; MOPS, 3-(N-morpholino) propanesulfonic acid; LDS, lithium dodecylsulfate; AUC, analytical ultracentrifugation; and HEPES, N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid.
Supporting Information Available
Experimental AUC data were fitted to various theoretical models for an oligomer in equilibrium with species of different molecular masses. Representative experimental data and residuals, which illustrate the goodness of fit for hybrid pools 2 and 3 of each enzyme pair, are shown in Figures 1S-3S. Representative kinetic plots of specific activity (v) vs [SAMP] for each enzyme pair are shown in Figures 4S and 5S. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ratner S. Argininosuccinases and adenylosuccinases. In: Boyer PD, editor. The Enzymes. 3. Vol. 7. Academic Press; New York: 1972. pp. 167–197. [Google Scholar]
- 2.Mouchegh K, Zikanova M, Hoffmann GF, Kretzschmar B, Kuhn T, Mildenberger E, Stoltenburg-Didinger G, Krijt J, Dvorakova L, Honzik T, Zeman J, Kmoch S, Rossi R. Lethal fetal and early neonatal presentation of adenylosuccinate lyase deficiency: Observation of 6 Patients in 4 Families. J Pediatr. 2007;150:57–61. doi: 10.1016/j.jpeds.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Jaeken J, Van den Berghe G. An infantile autistic syndrome characterized by the presence of succinylpurines in body fluids. Lancet. 1984;II:1058–1061. [PubMed] [Google Scholar]
- 4.Jaeken J, Wadman SK, Duran M, Van Sprang FJ, Beemer FA, Holl RA, Theunissen PM, de Cock P, Van den Bergh F, Vincent MF, Van den Berghe G. Adenylosuccinase deficiency: an inborn error of purine nucleotide synthesis. Eur J Pediatr. 1988;148:126–131. doi: 10.1007/BF00445919. [DOI] [PubMed] [Google Scholar]
- 5.De Zoysa Ariyananda L, Lee P, Antonopoulos C, Colman RF. Biochemical and biophysical analysis of five disease-associated human adenylosuccinate lyase mutants. Biochemistry. 2009;48:5291–5302. doi: 10.1021/bi802321m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speigel EK, Colman RF, Patterson D. Adenylosuccinate lyase deficiency. Mol Genet Metab. 2006;89:19–31. doi: 10.1016/j.ymgme.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Van den Berghe G, Jaeken J. Adenylosuccinate lyase deficiency. In: Scriver CR, Beaudt AL, Valle D, Sly WS, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Basis of Inherited Diseases. 8. II. McGraw-Hill; New York: 2001. pp. 2653–2662. [Google Scholar]
- 8.Castro M, Perez-Cerda C, Merinero B, Garcia MJ, Bernar J, Gil Nagel A, Torres J, Bermudez M, Garavito P, Marie S, Vincent F, Van den Berghe G, Ugarte M. Screening for adenylosuccinate lyase deficiency: clinical, biochemical and molecular findings in four patients. Neuropediatrics. 2002;33:186–189. doi: 10.1055/s-2002-34493. [DOI] [PubMed] [Google Scholar]
- 9.Brosius JL, Colman RF. Three subunits contribute amino acids to the active site of tetrameric adenylosuccinate lyase: Lys268 and Glu275 are required. Biochemistry. 2002;41:2217–2226. doi: 10.1021/bi011998t. [DOI] [PubMed] [Google Scholar]
- 10.Lee TT, Worby C, Bao Z, Dixon JE, Colman RF. His68 and His141 are critical contributors to the intersubunit catalytic site of adenylosuccinate lyase of Bacillus subtilis. Biochemistry. 1999;38:22–32. doi: 10.1021/bi982299s. [DOI] [PubMed] [Google Scholar]
- 11.Segall ML, Colman RF. Gln212, Asn270, and Arg301 are critical for catalysis by adenylosuccinate lyase from Bacillus subtilis. Biochemistry. 2004;43:7391–7402. doi: 10.1021/bi0494774. [DOI] [PubMed] [Google Scholar]
- 12.Segall ML, Cashman AA, Colman RF. Important roles of hydroxylic amino acid residues in the function of Bacillus subtilis adenylosuccinate lyase. Protein Science. 2007;16:441–448. doi: 10.1110/ps.062650007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonopoulos CH, De Zoysa Ariyananda L, Lee P, Currier JM, Colman RF. Experimental Biology. 2009 Abstract 504.4. [Google Scholar]
- 14.Stenmark P, Moche M, Arrowsmith C, Berglund H, Busam R, Collines R, Dahlgren LG, Edwards A, Flodin S, Flores A, et al. Human adenylosuccinate lyase in complex with its substrate N6-(1,2-dicarboxy-ethyl)-AMP, and its products AMP and fumarate. RCSB protein Data Bank. :2VD6. [Google Scholar]
- 15.Sivendran S, Patterson D, Spiegel E, McGown I, Cowley D, Colman RF. Two novel mutant human adenylosuccinate lyases (ASLs) associated with autism and characterization of the equivalent mutant Bacillus subtilis ASL. J Biol Chem. 2004;279:53789–53797. doi: 10.1074/jbc.M409974200. [DOI] [PubMed] [Google Scholar]
- 16.Lee P, Colman RF. Expression, purification, and characterization of stable, recombinant human adenylosuccinate lyase. Protein Expr Purif. 2007;51:227–234. doi: 10.1016/j.pep.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Sivendran S, Colman RF. Effect of a new non-cleavable substrate analog on wild-type and serine mutants in the signature sequence of adenylosuccinate lyase of Bacillus subtilis and Homo sapiens. Protein Science. 2008;17:1162–1174. doi: 10.1110/ps.034777.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palenchar JB, Colman RF. Characterization of a mutant Bacillus subtilis adenylosuccinate lyase equivalent to a mutant enzyme found in human adenylosuccinate lyase deficiency: Asparagine 276 plays an important structural role. Biochemistry. 2003;42:1831–1841. doi: 10.1021/bi020640+. [DOI] [PubMed] [Google Scholar]
- 20.Tornheim K, Lowenstein JM. The purine nucleotide cycle: The production of ammonia from aspartate by extracts of rat skeletal muscle. J Biol Chem. 1972;247:162–169. [PubMed] [Google Scholar]
- 21.Woodward DO, Bramer HD. Purification and properties of Neurospora adenylosuccinase. J Biol Chem. 1966;241:580–586. [PubMed] [Google Scholar]
- 22.Image processing and analysis using ImageJ program. ( http://rsbweb.nih.gov/ij)
- 23.Hearne JL, Colman RF. Catalytically active monomer of class Mu glutathione transferase from rat. Biochemistry. 2006;45:5974–5984. doi: 10.1021/bi060249e. [DOI] [PubMed] [Google Scholar]
- 24.SEDPHAT program Home Page. ( http://www.analyticalultracentrifugation.com/sedphat/sedphat.htm)
- 25.Laue TM, Shah BD, Ridgeway TM, Pelletier SL. Computer-aided interpretation of analytical sedimentation data for proteins. In: Harding SE, Rowe A, Horton JC, editors. Analytical Ultracentrifugation in Biochemistry and Polymer Science. Royal Society of Chemistry; Cambridge, U.K: 1992. pp. 90–125. [Google Scholar]
- 26.De Zoysa Ariyananda L, Colman RF. Evaluation of types of interactions in subunit association in Bacillus subtilis adenylosuccinate lyase. Biochemistry. 2008;47:2923–2934. doi: 10.1021/bi701400c. [DOI] [PubMed] [Google Scholar]
- 27.Zikanova M, Skopova V, Hnizda A, Krijt J, Kmoch S. Biochemical and structural analysis of 14 mutant ADSL enzyme complexes and correlation to phenotypic heterogeneity of adenylosuccinate lyase deficiency. Hum Mutat. 2010;31:445–455. doi: 10.1002/humu.21212. [DOI] [PubMed] [Google Scholar]
- 28.Stone RL, Zalkin H, Dixon JE. Expression, purification and kinetic characterization of recombinant human adenylosuccinate lyase. J Biol Chem. 1997;268:19710–19716. [PubMed] [Google Scholar]
- 29.Flanagan WF, Holmes EW, Sabina RL, Swain JL. Importance of purine nucleotide cycle to energy production in skeletal muscles. Am J Physiol. 1986;251 (5 Pt 1):C795–802. doi: 10.1152/ajpcell.1986.251.5.C795. [DOI] [PubMed] [Google Scholar]
- 30.Van den Berghe G, Bontemps F, Vincent MF, Van den Bergh F. The purine nucleotide cycle and its metabolic defects. Prog Neurobiol. 1992;39:547–561. doi: 10.1016/0301-0082(92)90006-z. [DOI] [PubMed] [Google Scholar]
- 31.An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science. 2008;320:103–106. doi: 10.1126/science.1152241. [DOI] [PubMed] [Google Scholar]
- 32.Pinson B, Vaur S, Sagot I, Coulpier F, Lemoine S, Daidnan-Fornier B. Metabolic intermediates selectively stimulate transcriptional factor interaction and modulate phosphate and purine pathways. Genes Dev. 2009;23:1399–1407. doi: 10.1101/gad.521809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rébora K, Desmoucelles C, Borne F, Pinson B, Daignan-Fornier B. Cell Biol. 2001;21:7901–7912. doi: 10.1128/MCB.21.23.7901-7912.2001. Yeast AMP pathway genes respond to adenine through regulated synthesis of a metabolic intermediate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rébora K, Laloo B, Daignan-Fornier B. Revisiting purine-histidine cross-pathway regulation in Saccharomyces cerevisiae: a central role for a small molecule. Genetics. 2005;170:61–70. doi: 10.1534/genetics.104.039396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexiou M, Leese HJ. Purine utilization, de novo synthesis and degradation in mouse preimplantation embryos. Development. 1992;114:185–192. doi: 10.1242/dev.114.1.185. [DOI] [PubMed] [Google Scholar]
- 36.Deutsch SI, Long KD, Rosse RB, Mastropaolo J, Eller J. Hypothesized deficiency of guaninebased purines may contribute to abnormalities of neurotransmission in Lesch-Nyhan Syndrome. Clin Neuropharmacol. 2005;28:28–37. doi: 10.1097/01.wnf.0000152043.36198.25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.