Abstract
Galactoxylomannan (GalXM) is a complex polysaccharide produced by the human pathogenic fungus Cryptococcus neoformans that mediates profound immunological derangements in murine models. GalXM is essentially non-immunogenic and produces immune paralysis in mice. Previous studies have attempted to enhance immunogenicity by conjugating GalXM to a protein carrier, but only transient antibody responses were elicited. Here we report the generation of two GalXM conjugates with bovine serum albumin (BSA) and protective antigen (PA) of Bacillus anthracis, respectively, using 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) as the cyanylating reagent. Both conjugates induced potent and sustained antibody responses as detected by both cross antigen-based and CovaLink direct ELISAs. We confirmed the specificity of the response to GalXM by inhibition ELISA and immunofluorescence. The isotype composition analysis revealed that IgG and IgM were abundant in the immune sera against GalXM, consistent with the induction of a T cell-dependent response. IgG1 was the predominant IgG subclass against GalXM, while immunization with Quil A as adjuvant elicited a significantly higher production of IgG2a than with Freund's adjuvant. Immune sera were not opsonic for C. neoformans and there was no survival difference between immune and non-immune mice challenged with C. neoformans. These results demonstrated the effectiveness of the GalXM-protein conjugate to induce robust immune responses although no evidence was obtained that such responses contributed to host defense.
Keywords: galactoxylomannan, Cryptococcus neoformans, capsule, polysaccharide, ELISA, immunoglobin
Introduction
Cryptococcus neoformans is an opportunistic basidiomycete that causes life-threatening infections primarily in immunocompromised patient populations, especially those with HIV infection, cancers, or organ transplant [1]. One of the major virulence factors of C. neoformans is its capsule, which enhances fungal survival by impeding macrophage phagocytosis [2]. The capsular polysaccharide (CPS) consists of glucuronoxylomannan (GXM), galactoxylomannan (GalXM), and mannoprotein [3-5]. Among the three components, GalXM is the most numerous polysaccharide on a molar basis in the capsule, bearing a galactopyranose backbone with xylose and mannose side groups [4,6]. Recent studies on GalXM structures also revealed the presence of glucuronic acid that gives the negative charge to this polysaccharide [7,8].
GalXM causes profound deleterious effects on the immune system. GalXM inhibits proliferation in T cell and peripheral blood mononuclear cell (PBMC), increases IFN-γ and IL-10 production, and induces T cell apoptosis mediated by caspase-8 and glycoreceptors including CD7, CD43, and CD45 [9-11]. GalXM induces TNF-α, NO production, iNOS expression, and Fas/FasL-mediated apoptosis in macrophage [12]. GalXM influences cytokine production and causes caspase-3-dependent apoptosis in B cell [13]. Given its abundance in shed capsular polysaccharide, its potent effects on the immune system, and a unique structure that distinguishes it from host polysaccharide antigens, GalXM is arguably a good target for antibody and vaccine development.
Microbial polysaccharides are generally poorly immunogenic T-cell independent type 2 antigens, which makes them inefficient antigens for inducing antibody responses [13-15]. To circumvent this problem, polysaccharides are often conjugated covalently to proteins such as bovine serum albumin (BSA), tetanus-toxoid (TT), and protective antigen (PA) [16-18]. This approach has formed the basis of several licensed pediatric polysaccharide-based vaccines [19,20], and conjugate-immunized mice have provided rich sources of splenocytes for generating libraries of monoclonal antibodies (mAb) to polysaccharide antigens such as GXM [21-23]. Previously we reported the conjugation of GalXM to PA that elicited antibody in mice [16]. However, the immune responses were transient and no hybridomas were recovered that produced antibodies to GalXM. In the present study we report new conjugates that elicit sustained antibody responses to GalXM and characterize their biological activity.
Materials and Methods
C. neoformans strains
C. neoformans var. neoformans acapsular mutant cap67, a strain derived from strain B3501 (serotype D), was obtained from American Type Culture Collection (Manassas, VA). Strain cap67 is also known as B-4131 in the literature and its capsular phenotype can be restored by complementation with the gene CAP59 [24]. In the immunofluorescence studies, wild type strains H99 (serotype A), 24067 (serotype D), and mutants cap67 and uge1Δ (serotype D) were used. The strain uge1Δ is a mutant in which the UGE gene encoding a putative UDP-glucose epimerase is deficient and does not make GalXM [16,25]. C. neoformans wild type strains H99 and 24067 were obtained from the New York State Herbarium, Albany, NY, and uge1Δ was a kind gift from Dr. Guilhem Janbon at Institut Pasteur.
GalXM isolation
GalXM was isolated from the C. neoformans culture supernatant, as described [4]. Briefly, a 500 ml culture of C. neoformans var. neoformans strain cap67 (serotype D) was grown in peptone supplemented with 2% galactose for 7 d. The culture supernatant was then separated from the cells by centrifugation at 900 g for 15 min at room temperature and passed through a 0.2 μm filter. The supernatant was concentrated and lyophilized. The freeze-dried mixture was dissolved in 60 ml start buffer (CaCl2 and Mn(II)Cl2 [final concentrations: 1 mM] were sequentially added to 0.01 M Tris base and 0.5 M NaCl solution, pH 7.2). To separate the GalXM and mannoproteins the solution was continuously passed through a Concanavalin A-Sepharose 4B column (Sigma Aldrich) overnight at 4 °C using a peristaltic pump with a flow rate of 16 ml/hr. The flow through and 5 column washes with start buffer were collected as 45-ml fractions. Carbohydrate containing fractions were identified using the phenol-sulfuric assay [26]. The fractions were combined, concentrated, and dialyzed against water for 3 d. GalXM was then recovered by lyophilization. The carbohydrate composition analysis of the isolated GalXM was confirmed by combined gas chromatography/mass spectrometry of the per-O-trimethylsilyl derivatives of the monosaccharide methyl glycosides produced from the sample by acidic methanolysis. Composition analysis revealed the mole percentages for glucuronic acid (1.9%), xylose (12.6%), mannose (25.8%), and galactose (56.6%). These numbers closely approximate the mass composition described by previous reports [4,8]. Purified GalXM was tested for possible Con A contamination employing an anti-Con A antibody (Vector Laboratories) by Western blotting, and no Con A was detected (data not shown).
GalXM-protein coupling and its purification
Purified GalXM was activated with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) as described, with modifications [27,28]. In brief, GalXM was dissolved in 0.1 M sodium borate buffer, pH 9 (35 mg/ml). At t = 0 sec, 0.3 ml of CDAP (100 mg/ml in acetonitrile) was added slowly with stirring. At 30 sec, 0.2 ml of 0.5 M NaOH solution was added to the mixture to raise the pH to ∼9.2. At t = 8 min, 15 mg of BSA or 5 mg of PA in 0.15 M HEPES buffer, pH 7, was added to the mixture. The reaction was then run for 3 hr at 25 °C and terminated by 100 μl 1 M ethanolamine in 0.75 M HEPES buffer, pH 7. Polysaccharide-protein conjugate product was purified using an S200HR column (GE Healthcare Life Sciences), equilibrated with saline. The void volume was determined by Blue dextran elution, and the column was calibrated with dextran (100 kDa), BSA (67 kDa), and ribonuclease (13.7 kDa). HPLC was performed on HPLC size exclusion columns monitored at 280 nm. The presence of carbohydrate and protein contents in fractions was confirmed by phenol-sulfuric acid assay and Bradford assay respectively.
Animals and immunization with conjugates
Six- to 8- week-old female BALB/c mice were obtained from the National Cancer Institute (Bethesda, MD), and all the animal experiments were done according to the institutional guidelines. BALB/c mice in groups of three were immunized with the 0.5, 5, and 50 μg of GalXM-BSA or GalXM-PA conjugate subcutaneously in Quil A or intraperitoneally in Freund's complete adjuvant. Control mice were injected with saline, BSA, or PA in the relevant adjuvant. Boosts were performed in Quil A or Freund's incomplete adjuvant on 14 and 28 d after the first immunization. Blood samples were collected on day 0, 14, 28, and 42. Serum titers were then tested.
Serum antibodies
Blood was collected from mice, and serum was analyzed by cross antigen-based enzyme-linked immunosorbent assays (ELISAs). To test the serum reactivity against GalXM from GalXM-BSA immunized mice, Costar plates were coated with GalXM-PA in concentrations indicated in figures. As a negative control, PA was coated to plates separately to test if GalXM-BSA immunized mouse sera cross-react with PA. Vice versa, Costar plates were coated with GalXM-BSA to test the sera obtained from GalXM-PA immunized mice. BSA coated condition was used as the negative control. We used cross conjugate reactivity to detect the presence of antibodies to GalXM, because GalXM did not bind to the polystyrene (see below). This coating method was based on no cross-reactivity between the carrier proteins and the blocking reagent, i.e. ovalbumin [16,29]. Following the coating of antigens, the plates were blocked with 1% ovalbumin, and a 1:100 dilution of serum was serially diluted in a 1:3 ratio along the plate unless specifically stated. A cocktail of alkaline phosphatase-conjugated anti-immunoglobulin M (IgM), -IgA, and -IgG (H+L) polyclonal antibodies (Southern Biotechnology, Birmingham, AL) at 1 μg/ml was used as the secondary antibody for the detection of bound antibodies. For antibody isotype and subclass studies, alkaline phosphatase-conjugated goat anti-mouse IgG, -IgA, -IgM, -IgG1, -IgG2a, -IgG2b, and -IgG3 were used individually at 1 μg/ml. Reactions were developed with p-nitrophenyl phosphate (PNPP), and the absorbance was measured at 405 nm. Ovalbumin-coated plates were used as a negative control. Serum reactivity against GalXM was also tested using the specialized ELISA plate, Nunc CovaLink™ NH Modules (Fisher Scientific), with –NH2 group exposed on the surface to allow covalent binding to GalXM. 12 mg of GalXM was dissolved in 1 ml 0.1 M sodium borate buffer, pH 9. At t = 0 sec, 0.3 ml of CDAP (100 mg/ml in acetonitrile) was added slowly with stirring. At 30 sec, 0.2 ml of 0.5 M NaOH solution was added to the mixture to raise the pH to ∼9.2. At t = 8 min, activated GalXM solution was added to the plate and serially diluted across the plate making 1:10 dilutions. The covalent binding of GalXM to the plate was performed at room temperature for 30 min. 25 μl of 1M ethanolamine in 0.75 M HEPES buffer, pH 7, was added to terminate the reaction. Approximately 60 min later the antigens were removed then blocked with 1% ovalbumin, followed by the ELISA procedures as described above.
Inhibition ELISA
Using Nunc Covalink NH plates where GalXM was covalently bound to the surface, GalXM-BSA conjugate immune sera were diluted 1:100, 1:200, and 1:400, and incubated with various concentrations of free soluble GalXM ranging from 0 to 6 mg/ml. Specific antibody binding was then detected as described above.
Immunofluorescence
C. neoformans strains were grown in Sabouraud dextrose broth (Difco Laboratories, Detroit, MI) for 1 d at 30 °C. The cells were then transferred to capsule inducing media (1:10 Sabouraud broth- MOPS (morpholinepropanesulfonic acid), 50 mM, pH 7.3) for another day of incubation at 30 °C to allow for capsule growth [30]. The cells were washed three times with phosphate-buffered saline (PBS, pH 7.4) and counted with a hemocytometer. For GalXM staining using GalXM-BSA or GalXM-PA immune sera, 2 × 106 cells in 100 μl of IF buffer (1% BSA and 0.05% goat serum in PBS) were incubated with 4 μl of serum for 1 h at room temperature. Cells were washed three times with buffer and incubated with 1:25 dilution of goat anti-mouse IgM-FITC as the secondary antibody for 1 h at room temperature. Cells were then washed and incubated with 1:10000 Uvitex 2B (Polysciences Inc.) in PBS for 20 min. Stained cells were suspended in mounting media (50% glycerol and 50 mM N-propyl gallate in PBS) and imaged by epifluorescence microscopy on a Zeiss Axioskop 200 inverted microscope equipped with a cool charge-coupled device using a 63×, 1.4-numerical-aperture (NA) objective with a 1.6× optovar. Images were acquired using the same exposure time and microscopic setting, and processed by Axio Vision 4.6 software (Carl Zeiss Micro Imaging, New York, NY).
Protection Studies
C. neoformans strain 24067 (serotype D) was used for the protection experiments. This strain was selected because it has been used extensively in prior passive protection studies [23,31] and was of the same serotype as the strain from which GalXM was obtained. Female BALB/c mice (6 to 8 weeks old) were obtained from the National Cancer Institute and divided into three groups. Nine to ten mice per group were immunized intraperitoneally in Freund's adjuvant with 50 μg of GalXM-BSA, BSA, or PBS respectively. Mice were boosted subsequently on day 14, 28, and 42. Each group of mice was then infected intravenously with 1 × 105 of fungal cells in 100 μl of PBS, and was monitored daily for the survival. The route of infection and specific inoculum were selected because this model was effective in demonstrating antibody-mediated immunity in prior vaccine experiments [54] and passive antibody protection experiments [31].
Phagocytosis Assay
Phagocytosis assay was performed using C. neoformans strain 24067 and macrophage-like cell line J774 at an E:T ratio of 2:1, with 1 × 105 C. neoformans per well. C. neoformans was incubated with J774 in 96 well plate containing sera from PBS control, BSA immunized, or GalXM-BSA immunized mice for 2 h at 37 °C in 10% CO2. mAb 18B7 was used as the positive control [32]. The culture was washed and fixed with ice-cold methanol for 30 min at -20 °C. Cells were then stained with Giemsa diluted 1:20 with water. Cells were counted under an inverted light microscope, and the phagocytosis index was measured by the number of macrophage with internalized C. neoformans divided by the total number of macrophage per field of view. At least 3 field of views and 400 cells per well were analyzed.
Statistics
Statistical differences in survival rates were examined using the log-rank survival test. A p value of <0.05 was considered to be significant.
Results
GalXM activated by CDAP conjugates to BSA and PA respectively
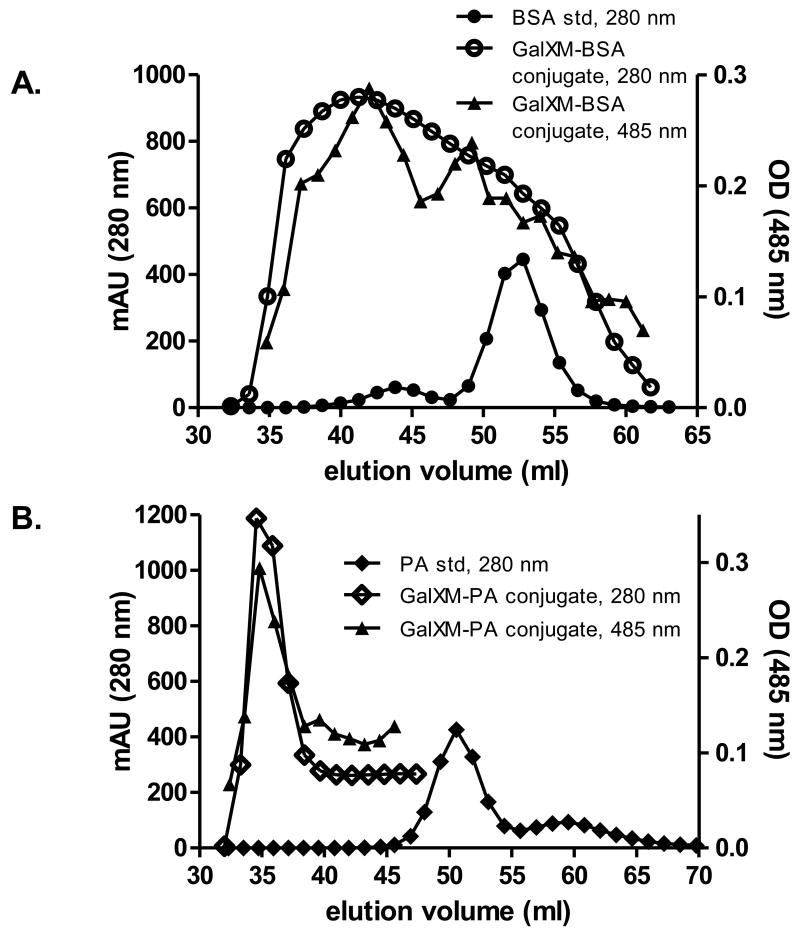
GalXM did not react with proteins without CDAP, such that co-incubating GalXM with CDAP alone yielded no conjugate (data not shown). Unreacted dimeric and monomeric forms of BSA eluted at 44 and 53 ml, respectively, in our chromatography conditions. Addition of BSA to CDAP-activated GalXM resulted in a peak signal from the GalXM-BSA conjugate at 40 ml of the elution volume, a shift of protein signal that came from the conjugated BSA (Fig. 1A). Phenol sulfuric acid test revealed the presence of polysaccharide in the putative conjugate fraction (Fig. 1A). Addition of PA to CDAP-activated GalXM also left-shifted the protein peak signal from 50.5 ml to 34.5 ml that closely paralleled the presence of polysaccharide as detected by the phenol-sulfuric assay (Fig. 1B). Fractions of the conjugate were loaded to SDS gel then stained with Coomassie Blue. The results indicated the presence of protein bands with high-molecular weight (>250 kDa) (data now shown). Taken together, we conclude that GalXM was conjugated to BSA and PA respectively after being activated by CDAP.
Figure 1.
Column chromatography analysis of GalXM conjugates and its components. Absorbance and phenol sulfuric acid reactivity of the reaction mixture of CDAP activated GalXM to carrier proteins and free protein standards. Activated GalXM was conjugated to (A) BSA and (B) PA (open circle and diamond respectively). Same amount of (A) BSA and (B) PA (closed circle and diamond respectively) was used as the standard. Phenol sulfuric acid test of the conjugate mixtures were presented in triangles. Protein and polysaccharide signals were recorded at 280 nm and 485 nm respectively.
Antibody responses after GalXM-protein immunization detected by ELISA
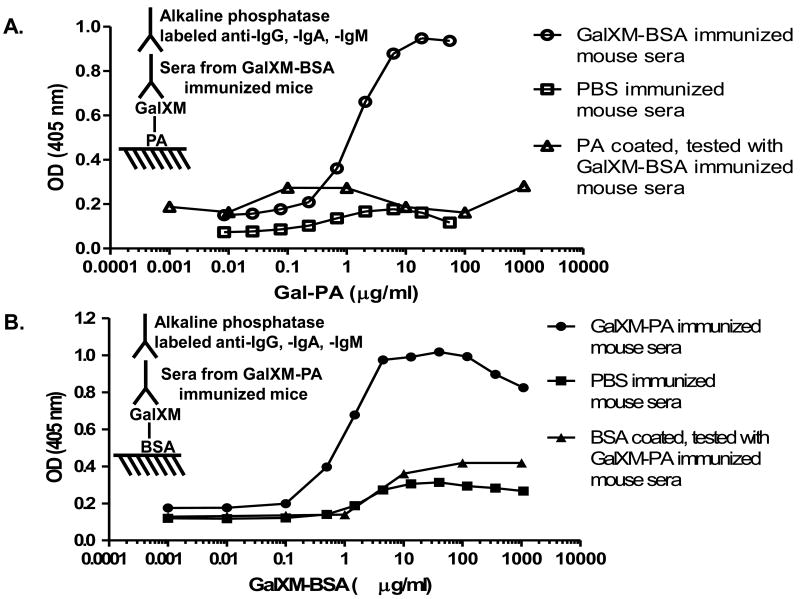
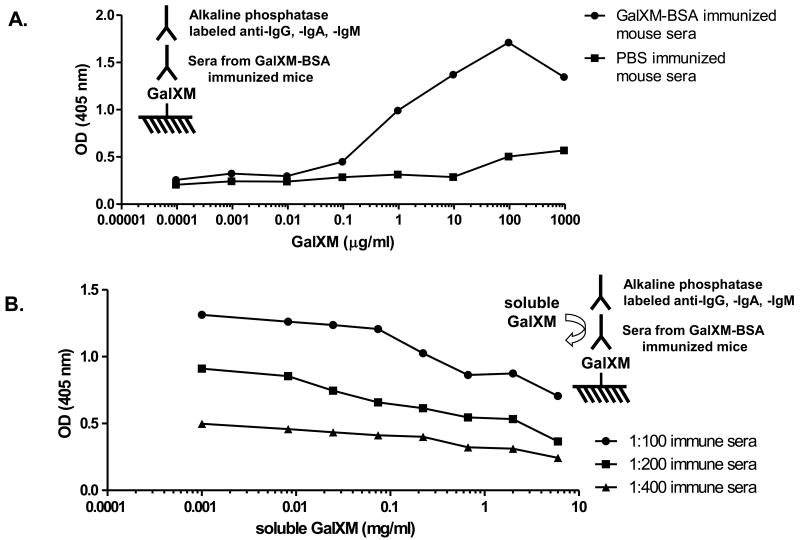
Initially we employed cross antigen-based ELISA to detect antibody responses to GalXM because GalXM did not appear to bind to polystyrene plates. Two conjugates were made by conjugating GalXM to BSA and PA respectively. GalXM-PA was coated on Costar ELISA plate and tested with GalXM-BSA immune sera. The results revealed that only conjugate-immunized mice produced antibodies against GalXM (Fig. 2A). Most importantly, the serum antibodies in mice immunized with GalXM-BSA did not cross-react with PA, indicating that the positive titers were due to GalXM reactivity. On the other hand, immune sera from mice immunized with GalXM-PA reacted only with GalXM-BSA but not BSA (Fig. 2B). To further confirm the specificity of the sera for GalXM we sought to develop a GalXM-only ELISA. GalXM was covalently linked to the amino group on Nunc Covalink NH ELISA plate and then tested against the conjugate immune sera. Strong titers were observed with the experimental but not the control group (Fig. 3A). Moreover, free soluble GalXMs inhibited the binding of immune sera in a dose-dependent manner, providing additional evidence for the specificity of the response (Fig. 3B). The highest titers induced by GalXM-BSA and GalXM-PA conjugates reached 1:24300, and did not drop significantly even 6 months after the last boost. Mice immunized with saline, BSA, or PA did not have antibody responses reactive with GalXM.
Figure 2.
Reactivity of immune sera to GalXM as detected by cross antigen-based ELISAs. (A) GalXM-BSA conjugate immune sera (open circle), and PBS control sera (open square) were tested against GalXM-PA. GalXM-BSA immune sera were tested against PA as a negative control (open triangle). (B) GalXM-PA conjugate immune sera (closed circle), and PBS control sera (closed square) were tested against GalXM-BSA. GalXM-PA immune sera were tested against BSA as a negative control (closed triangle).
Figure 3.
Reactivity of immune sera to GalXM as detected by GalXM ELISA using Nunc Covalink NH plates with varying concentrations of reacting GalXM. (A) GalXM-BSA immune sera (circle) but not the control sera (square) revealed antibody response to GalXM covalently coated to the plate. (B) Inhibition ELISA using various concentrations of free GalXM confirmed the specificity of antibody response to GalXM.
Immunofluorescence labeling of GalXM on C. neoformans
Immunofluorescence using the immune sera on whole C. neoformans cells was used as an additional measure of reactivity. Control sera did not react with either the non-encapsulated mutant strain cap67 or wild type strains 24067 and H99 (Fig. 4A, C, and E). Conjugate immune sera gave a robust labeling on strains cap67, 24067, and H99, each of which is known to make GalXM (Fig. 4B, D, and F). The GalXM labeling patterns for wild type strains versus mutant strains were different, as reported previously [8,16]. For the non-encapsulated cap67 strain immunofluorescence was rim-like and adjacent to the cell wall (Fig. 4B), consistent with the reported presence of GalXM in the cell wall. For the encapsulated wild type strains, immunofluorescence was dot- or punctate-like in the region of the capsule (Fig. 4D and F). No immunofluorescence reactivity was observed when control and conjugate immune sera were tested on C. neoformans GalXM-deficient mutant strain uge1Δ (serotype D) (Fig. 4G and H).
Figure 4.
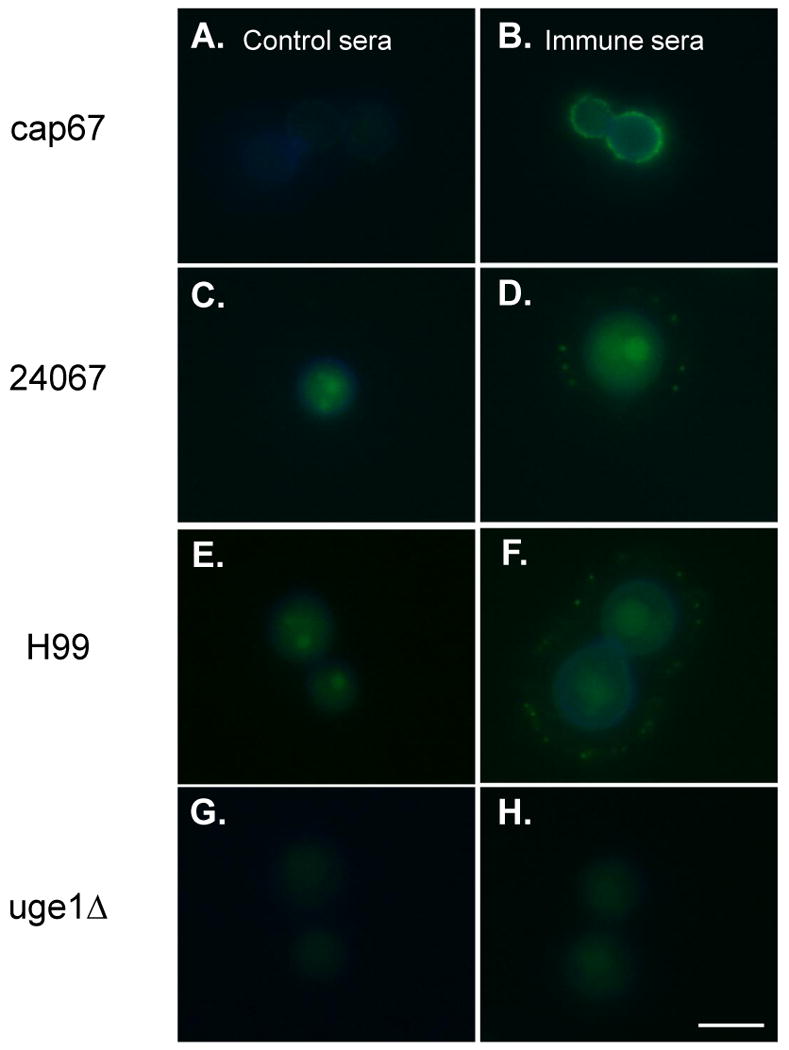
Detection of antibody responses against GalXM using indirect immunofluorescence staining. Control sera were incubated with (A) cap67, (C) 24067, (E) H99, and (G) uge1Δ. GalXM-BSA conjugate immune sera were incubated with (B) cap67, (D) 24067, (F) H99, and (H) uge1Δ. H99 is serotype A strain, and the others are serotype D strains. The blue rim around the cell body is the result of calcofluor staining. The green fluorescence in the cell body reflects autofluorescence [16]. Scale bar, 5 μm.
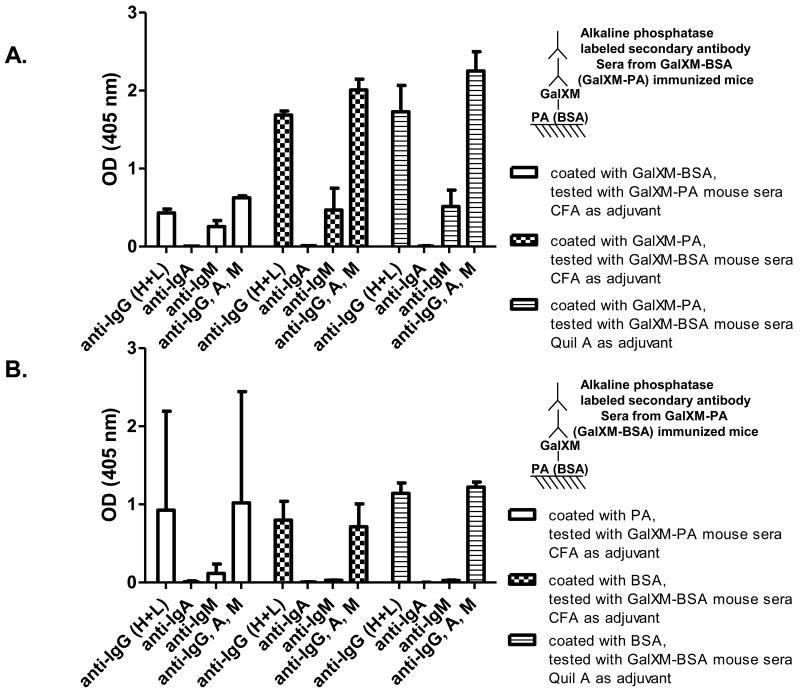
Isotypes composition of the antibody response to GalXM
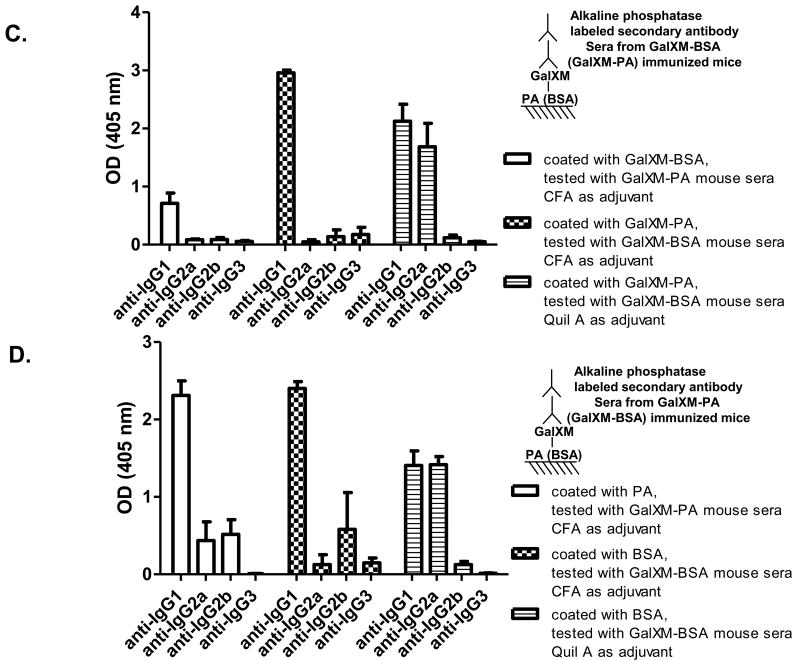
GalXM-BSA and GalXM-PA conjugate immunized mice produced antibodies to both the polysaccharide and the protein (Fig. 5). The serum response to GalXM included both IgG and IgM, with IgG being the predominant isotype (Fig. 5A). In contrast the antibody response against the protein antigen was primarily IgG (Fig. 5B). Usage of different adjuvants did not affect the proportion between IgG, IgA, and IgM against the polysaccharide and protein components (Fig. 5A and B). However, immunization with Quil A as adjuvant induced significantly stronger IgG2a response than that with Freund's adjuvant (Fig. 5C and D). In all cases, IgG1 was the predominant IgG subclass found in the serum antibodies.
Figure 5.
Antibody isotypes and IgG subclasses in conjugate immune sera. Conjugate immune sera were tested against (A) GalXM and (B) carrier proteins using ELISA to determine IgG, IgA, IgM proportions. Conjugate immune sera were tested against (C) GalXM and (D) carrier proteins to reveal IgG subclass proportions. Bars represent averages (n = 3) and brackets denote standard deviations.
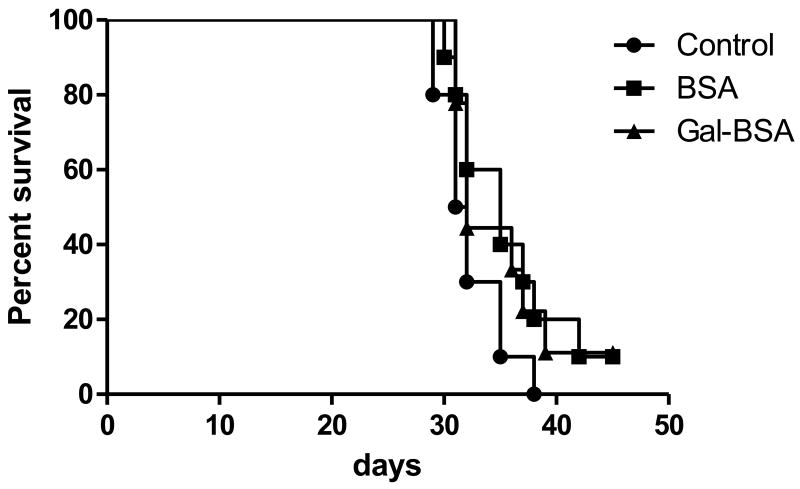
Mice immunized with GalXM-BSA conjugate vaccine manifested comparable survival to non-vaccinated mice when challenged with C. neoformans despite the presence of serum antibodies to this polysaccharide (Fig. 6). In vitro phagocytosis assays with immune sera revealed that the antibodies were not opsonic (data not shown).
Figure 6.
Survival of BALB/c mice infected 1 × 105 C. neoformans. Mice immunized with PBS (circle), BSA (square), or Gal-BSA (triangle) were infected with 1 × 105 C. neoformans (9 to 10 mice per group). p value obtained by log-rank analysis was 0.2184.
Discussion
Serological reagents have proved useful tools to study GalXM localization in the capsule, antigenic difference of GalXMs among serotypes, and the dynamics of GalXM production during fungal growth [8,16]. However, the generation of GalXM immune sera has been difficult because of the very poor immunogenicity of this polysaccharide. In this study we explored the generation of different GalXM- conjugates. The GalXM-PA conjugate used in previous studies was generated using the traditional cyanogen bromide method [8,16]. To increase the yield of conjugate and reduce the complexity of the experimental procedure, a less toxic cyanylating reagent known as CDAP was used in this study to make two new conjugates, GalXM-BSA and GalXM-PA. Both conjugates elicited antibodies to GalXM as measured by direct ELISA, inhibition studies with soluble GalXM, and immunofluorescence on whole cells.
Some of the capsular polysaccharides of C. neoformans, such as serotype B and C GXMs, do not bind to polystyrene ELISA plate [33,34]. Differences in molar ratios of carbohydrate residues, degree of substitution, and charge of the molecule in GXMs may explain their variable affinity for polystyrene surfaces [35-37]. Like the GXM from C. gattii strains, GalXM does not appear to bind well to the polystyrene in regular ELISA microtiter plates. Also, the high structural heterogeneity of GalXM may increase the batch to batch variation, so that absorption of GalXM to microtiter plates by itself is less reproducible [8,38,39]. Protocols to enhance antigen binding usually involve modification of the binding surface. Pretreatment of the hydrophobic plate with polylysine makes it more hydrophilic to favor the binding of a charged polysaccharide [40]. In this study we employed cross antigen-based ELISA to detect antibody responses to GalXM, and obtained comparable titer to the one measured with direct Covalink NH-based ELISA. This protocol thus allows flexibility on choosing carrier proteins to help anchor the target polysaccharide to the plate surface and to elicit T-cell dependence response.
In previous studies we conjugated GalXM to PA using cyanogen bromide as the cyanylating reagent, and obtained antibody response to GalXM through immunization of mice [13,16]. However, the immune responses were short-lived, and the hybridomas producing antibodies to GalXM ceased to grow at the stage of soft agar cloning. This was thought to be caused by the presence of excess free GalXM in the vaccine preparation that could not be separated from the conjugate moiety, such that the free GalXM continued to mediate B-cell depletion and immunological paralysis [13]. In this study, we were able to separate the conjugate product from free GalXM, and this step was associated with a more effective vaccine that elicited persistently high titers in mice.
To further dissect the antibody responses against GalXM, immunoglobin isotype and IgG subclass composition of the GalXM-specific antibodies was measured. Immuniazation with GalXM conjugates elicited antibodies against both the polysaccharide and protein components. For all conditions, no IgA was measured, while the IgG response was the strongest. IgM to GalXM was measured but not against the protein carriers, supporting its association with polysaccharide antigens [41]. Choice of carrier protein and adjuvant did not alter the Ig-G, -A, and -M proportion in the sera. In contrast, use of different adjuvants resulted in different IgG subclass production. IgG1 was the predominant IgG subclass against GalXM and the two carrier proteins. Immunization using Quil A as adjuvant led to significantly higher production of IgG2a. IgG1 is associated with Th2-like response, while IgG2a, IgG2b, and IgG3 are associated with Th1-like response [51]. The usage of Quil A as adjuvant has been shown to trigger Th1-like response including the induction of high IgG2a response and the increase of CD8+ cells [41-44].
Previous studies have shown the correlation between the immunofluorescence pattern and the protective ability of antibodies against GXM of C. neoformans. Antibodies labeling with a punctate pattern are consistently non-protective, while those with annular pattern can be protective or non-protective depending on their isotype and binding specificity [45,46]. In vitro phagocytosis assay indicates that non-protective antibodies with punctate pattern are also not opsonic [47], which agrees with the notion that ingestion of particles by macrophage requires sequential, circumferential interaction between the ligand and receptor [48,49]. Although those studies were focused on antibodies to GXM they could be relevant to this work because antibodies to GalXM also bind with a punctate pattern on the capsule [8,16]. Antibodies elicited by the conjugate vaccine were not opsonic, and conjugate vaccinated mice did not live longer than control mice. Hence, the vaccines described here do not appear to modify the course of experimental cryptococcal infection despite their high immunogenicity.
When viewed from the historical context of polysaccharide-based vaccines against C. neoformans we note that first attempt to develop conjugate vaccines against cryptococcosis involved the conjugation of total polysaccharide from C. neoformans to bovine γ-globulin (BGG), in which the conjugate induced robust immune responses but no protection against fungal infection [50]. Three decades later a vaccine composed of GXM conjugated to tetanus toxoid (TT) was effective in eliciting protective antibodies [17]. Subsequently, a GXM peptide mimetic, P13 (GMDGTQLDRW) selected from a peptide phage display library, was conjugated to TT or BSA, and shown to elicit an antibody response that was associated with prolonged survival in mice infected with C. neoformans [52]. Recently, we conjugated a heptasaccharide oligosaccharide representing the M2 structural motif of GXM to human serum albumin (HSA), but no protection was observed despite its high immunogenicity [53]. In contrast to those studies, which focused on eliciting antibody responses to GXM, this study used GalXM as the target antigen.
In summary, we describe two GalXM conjugates that have superior immunogenicity to those previously described, possibly because removal of free GalXM eliminated the detrimental effects of this powerful immunomodulator. The conjugates are useful for generating serum immune responses, but the antibody responses are not protective. We caution that our inability to demonstrate that our GalXM conjugates elicit protective responses does not imply that all antibodies to GalXM are non-protective. In this regard, we note that early conjugate vaccines against C. neoformans based on capsular polysaccharide were not protective [50], while subsequent conjugates were effective in eliciting protective antibodies [17]. However, we did not feel that additional animal experimentation was justified with this vaccine given that the antibody response elicited was non-opsonic, punctuate by immunofluorescence, and not protective against C. neoformans. Hence, we consider the GalXM conjugate vaccines described here an advance in establishing that GalXM can elicit strong antibody responses that are likely to yield useful serological reagents while acknowledging that at this time there is no evidence that antibodies to GalXM contribute to protection.
Acknowledgments
This work was supported in part by the Northeastern Biodefense Center under grant U54-AI057158-Lipkin through the protein synthesis facility that produced protective antigen for conjugation. Arturo Casadevall is supported by the following grants: AI33774-11, HL59842-07, AI33142-11 and AI52733-02. The data in this paper are from a thesis submitted by Siu-Kei Chow in partial fulfillment of the requirements for a Ph.D. from the Albert Einstein College of Medicine, Yeshiva University, Bronx, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casadevall A, Perfect JR. Cryptococcus neoformans. ASM Press; 1998. [Google Scholar]
- 2.Bose I, Reese AJ, Ory JJ, Janbon G, Doering TL. A yeast under cover: the capsule of Cryptococcus neoformans. Eukaryot Cell. 2003 Aug;2(4):655–63. doi: 10.1128/EC.2.4.655-663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blandamer A, Danishefsky I. Investigations on the structure of the capsular polysaccharide from Cryptococcus neoformans type B. Biochim Biophys Acta. 1966 Apr 25;117(2):305–13. doi: 10.1016/0304-4165(66)90081-x. [DOI] [PubMed] [Google Scholar]
- 4.Vaishnav VV, Bacon BE, O'Neill M, Cherniak R. Structural characterization of the galactoxylomannan of Cryptococcus neoformans Cap67. Carbohydr Res. 1998 Jan;306(1-2):315–30. doi: 10.1016/s0008-6215(97)10058-1. [DOI] [PubMed] [Google Scholar]
- 5.Levitz SM, Specht CA. The molecular basis for the immunogenicity of Cryptococcus neoformans mannoproteins. FEMS Yeast Res. 2006 Jun;6(4):513–24. doi: 10.1111/j.1567-1364.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 6.McFadden DC, De Jesus M, Casadevall A. The physical properties of the capsular polysaccharides from Cryptococcus neoformans suggest features for capsule construction. J Biol Chem. 2006 Jan 27;281(4):1868–75. doi: 10.1074/jbc.M509465200. [DOI] [PubMed] [Google Scholar]
- 7.Heiss C, Klutts JS, Wang Z, Doering TL, Azadi P. The structure of Cryptococcus neoformans galactoxylomannan contains beta-D-glucuronic acid. Carbohydr Res. 2009 May 12;344(7):915–20. doi: 10.1016/j.carres.2009.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jesus M, Chow SK, Cordero RJ, Frases S, Casadevall A. Galactoxylomannans from Cryptococcus neoformans varieties neoformans and grubii are structurally and antigenically variable. Eukaryot Cell. 2010 Jul;9(7):1018–28. doi: 10.1128/EC.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pericolini E, Cenci E, Monari C, De Jesus M, Bistoni F, Casadevall A, et al. Cryptococcus neoformans capsular polysaccharide component galactoxylomannan induces apoptosis of human T-cells through activation of caspase-8. Cell Microbiol. 2006 Feb;8(2):267–75. doi: 10.1111/j.1462-5822.2005.00619.x. [DOI] [PubMed] [Google Scholar]
- 10.Pericolini E, Gabrielli E, Bistoni G, Cenci E, Perito S, Chow SK, et al. Role of CD45 signaling pathway in galactoxylomannan-induced T cell damage. PLoS One. 2010;5(9):e12720. doi: 10.1371/journal.pone.0012720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pericolini E, Gabrielli E, Cenci E, De Jesus M, Bistoni F, Casadevall A, et al. Involvement of glycoreceptors in galactoxylomannan-induced T cell death. J Immunol. 2009 May 15;182(10):6003–10. doi: 10.4049/jimmunol.0803833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villena SN, Pinheiro RO, Pinheiro CS, Nunes MP, Takiya CM, DosReis GA, et al. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol. 2008 Jun;10(6):1274–85. doi: 10.1111/j.1462-5822.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 13.De Jesus M, Nicola A, Fraser S, Lee IR, Mieses S, Casadevall A. Galactoxylomannan-mediated immunological paralysis results from specific B cell depletion in the context of widespread immune system damage. Journal of Immunology. 2009;183(6):3885–94. doi: 10.4049/jimmunol.0900449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson B, Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–24. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- 15.Feldmann M, Basten A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. J Exp Med. 1971 Jul 1;134(1):103–19. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Jesus M, Nicola AM, Rodrigues ML, Janbon G, Casadevall A. Capsular localization of the Cryptococcus neoformans polysaccharide component galactoxylomannan. Eukaryot Cell. 2009 Jan;8(1):96–103. doi: 10.1128/EC.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devi SJ, Schneerson R, Egan W, Ulrich TJ, Bryla D, Robbins JB, et al. Cryptococcus neoformans serotype A glucuronoxylomannan-protein conjugate vaccines: synthesis, characterization, and immunogenicity. Infect Immun. 1991 Oct;59(10):3700–7. doi: 10.1128/iai.59.10.3700-3707.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dromer F, Salamero J, Contrepois A, Carbon C, Yeni P. Production, characterization, and antibody specificity of a mouse monoclonal antibody reactive with Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Mar;55(3):742–8. doi: 10.1128/iai.55.3.742-748.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002 Sep;21(9):810–5. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Ellis RW, Granoff DM. Development and clinical uses of Haemophilus B conjugate vaccines. Informa Healthcare. (1) 1994 [Google Scholar]
- 21.Casadevall A, Mukherjee J, Devi SJ, Schneerson R, Robbins JB, Scharff MD. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992 Jun;165(6):1086–93. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee J, Casadevall A, Scharff MD. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J Exp Med. 1993 Apr 1;177(4):1105–16. doi: 10.1084/jem.177.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee S, Lee S, Mukherjee J, Scharff MD, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994 Mar;62(3):1079–88. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YC, Kwon-Chung KJ. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994 Jul;14(7):4912–9. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyrand F, Janbon G. UGD1, encoding the Cryptococcus neoformans UDP-glucose dehydrogenase, is essential for growth at 37 degrees C and for capsule biosynthesis. Eukaryot Cell. 2004 Dec;3(6):1601–8. doi: 10.1128/EC.3.6.1601-1608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–56. [Google Scholar]
- 27.Lees A, Nelson BL, Mond JJ. Activation of soluble polysaccharides with 1-cyano-4-dimethylaminopyridinium tetrafluoroborate for use in protein-polysaccharide conjugate vaccines and immunological reagents. Vaccine. 1996 Feb;14(3):190–8. doi: 10.1016/0264-410x(95)00195-7. [DOI] [PubMed] [Google Scholar]
- 28.Suarez N, Massaldi H, Franco Fraguas L, Ferreira F. Improved conjugation and purification strategies for the preparation of protein-polysaccharide conjugates. J Chromatogr A. 2008 Dec 12;1213(2):169–75. doi: 10.1016/j.chroma.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 29.Lees A, Morris SC, Thyphronitis G, Holmes JM, Inman JK, Finkelman FD. Rapid stimulation of large specific antibody responses with conjugates of antigen and anti-IgD antibody. J Immunol. 1990 Dec 1;145(11):3594–600. [PubMed] [Google Scholar]
- 30.Zaragoza O, Casadevall A. Experimental modulation of capsule size in Cryptococcus neoformans. Biol Proced Online. 2004;6:10–5. doi: 10.1251/bpo68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect Immun. 2007 Jun;75(6):2729–39. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukherjee S, Feldmesser M, Casadevall A. J774 murine macrophage-like cell interactions with Cryptococcus neoformans in the presence and absence of opsonins. J Infect Dis. 1996 May;173(5):1222–31. doi: 10.1093/infdis/173.5.1222. [DOI] [PubMed] [Google Scholar]
- 33.Casadevall A, Mukherjee J, Scharff MD. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992 Sep 18;154(1):27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- 34.Cherniak R, Cheeseman MM, Reyes GH, Reiss E, Todaro F. Enhanced binding of capsular polysaccharides of Cryptococcus neoformans to polystyrene microtitration plates for enzyme-linked immunosorbent assay. Diagn Clin Immunol. 1988;5(6):344–8. [PubMed] [Google Scholar]
- 35.Bhattacharjee AK, Bennett JE, Glaudemans CP. Capsular polysaccharides of Cryptococcus neoformans. Rev Infect Dis. 1984 Sep-Oct;6(5):619–24. doi: 10.1093/clinids/6.5.619. [DOI] [PubMed] [Google Scholar]
- 36.Cherniak R, Sundstrom JB. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994 May;62(5):1507–12. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonseca FL, Frases S, Casadevall A, Fischman-Gompertz O, Nimrichter L, Rodrigues ML. Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet Biol. 2009 Jun-Jul;46(6-7):496–505. doi: 10.1016/j.fgb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callahan LT, 3rd, Woodhour AF, Meeker JB, Hilleman MR. Enzyme-linked immunosorbent assay for measurement of antibodies against pneumococcal polysaccharide antigens: comparison with radioimmunoassay. J Clin Microbiol. 1979 Oct;10(4):459–63. doi: 10.1128/jcm.10.4.459-463.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James PG, Cherniak R. Galactoxylomannans of Cryptococcus neoformans. Infect Immun. 1992 Mar;60(3):1084–8. doi: 10.1128/iai.60.3.1084-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckert TF, Kozel TR. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Aug;55(8):1895–9. doi: 10.1128/iai.55.8.1895-1899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleuridor R, Zhong Z, Pirofski L. A human IgM monoclonal antibody prolongs survival of mice with lethal cryptococcosis. J Infect Dis. 1998 Oct;178(4):1213–6. doi: 10.1086/515688. [DOI] [PubMed] [Google Scholar]
- 42.Hacariz O, Sayers G, McCullough M, Garrett M, O'Donovan J, Mulcahy G. The effect of Quil A adjuvant on the course of experimental Fasciola hepatica infection in sheep. Vaccine. 2009 Jan 1;27(1):45–50. doi: 10.1016/j.vaccine.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 43.Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009 Mar 13;27(12):1787–96. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi H, Takeshita T, Morein B, Putney S, Germain RN, Berzofsky JA. Induction of CD8+ cytotoxic T cells by immunization with purified HIV-1 envelope protein in ISCOMs. Nature. 1990 Apr 26;344(6269):873–5. doi: 10.1038/344873a0. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee J, Nussbaum G, Scharff MD, Casadevall A. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J Exp Med. 1995 Jan 1;181(1):405–9. doi: 10.1084/jem.181.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nussbaum G, Cleare W, Casadevall A, Scharff MD, Valadon P. Epitope location in the Cryptococcus neoformans capsule is a determinant of antibody efficacy. J Exp Med. 1997 Feb 17;185(4):685–94. doi: 10.1084/jem.185.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaragoza O, Casadevall A. Antibodies produced in response to Cryptococcus neoformans pulmonary infection in mice have characteristics of nonprotective antibodies. Infect Immun. 2004 Jul;72(7):4271–4. doi: 10.1128/IAI.72.7.4271-4274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin FM, Jr, Griffin JA, Leider JE, Silverstein SC. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–82. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw DR, Griffin FM., Jr Phagocytosis requires repeated triggering of macrophage phagocytic receptors during particle ingestion. Nature. 1981 Jan 29;289(5796):409–11. doi: 10.1038/289409a0. [DOI] [PubMed] [Google Scholar]
- 50.Goren MB. Experimental murine cryptococcosis: effect of hyperimmunization to capsular polysaccharide. J Immunol. 1967 May;98(5):914–22. [PubMed] [Google Scholar]
- 51.Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, et al. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995 Mar;25(3):823–9. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 52.Fleuridor R, Lees A, Pirofski L. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. J Immunol. 2001 Jan 15;166(2):1087–96. doi: 10.4049/jimmunol.166.2.1087. [DOI] [PubMed] [Google Scholar]
- 53.Nakouzi A, Zhang T, Oscarson S, Casadevall A. The common Cryptococcus neoformans glucuronoxylomannan M2 motif elicits non-protective antibodies. Vaccine. 2009 Jun 2;27(27):3513–8. doi: 10.1016/j.vaccine.2009.03.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devi SJ. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine. 1996 Jun;14(9):841–4. doi: 10.1016/0264-410x(95)00256-z. [DOI] [PubMed] [Google Scholar]