Abstract
Background & Aims
Weight regain following Roux-en-Y gastric bypass (RYGB) is associated with reductions in health status and quality of life. We evaluated whether gastro-jejunal stoma diameter is a risk factor for weight regain after RYGB.
Methods
We examined data collected over 4 years from consecutive patients referred to a tertiary care bariatric center for upper endoscopy after RYGB. We used linear regression analysis to determine the association between the gastro-jejunal stoma diameter and weight regain. We applied a logistic regression model using clinical and endoscopic parameters to develop a prediction rule for weight gain after RYGB.
Results
Among 165 patients included in our study, 59% had significant weight regain (≥ 20% of maximum weight lost after the RYGB) and 41% did not. The mean percent of maximal weight lost after RYGB that was regained in the entire cohort was 30% ± 22%. Gastro-jejunal stoma diameter was significantly associated with weight regain after RYGB surgery in univariate analysis (β = 0.31, P < 0.0001). This association remained significant after adjusting for several known or purported risk factors for weight regain (β = 0.19, P=0.003). We developed a simple prediction rule for weight regain after RYGB using a 7 point scoring system that includes the gastro-jejunal stoma diameter, race, and percent maximal body weight lost after RYGB; a cut-off score ≥ 4 points had an area under receiver operating characteristic curve of 0.76 and a positive predictive value of 75%.
Conclusion
Increased gastro-jejunal stoma diameter is a risk factor for weight regain after RYGB and can be incorporated in a novel prediction rule.
Keywords: Weight loss, obesity, surgery, ROC, PPV
Introduction
Obesity and its associated conditions including type two diabetes and cardiovascular disease have reached epidemic proportions especially in the western world, and are associated with significant morbidity, mortality, and cost to the health care system. 1 Of the many treatment approaches for obesity and its complications, bariatric surgery shows the most promise in achieving significant and sustained weight loss and resolution of associated metabolic comorbidities, when compared to combinations of medications, dietary, and behavioral modifications. 2 Roux-en-Y gastric bypass (RYGB) is currently the weight loss surgical procedure of choice and results in excess body weight loss of 62% with an 84% diabetes resolution rate. 3–4
Despite its proven efficacy, the majority of patients undergoing RYGB regain about 30% of the weight they had lost, with about 20%–30% of patients regaining most of the weight that had been lost; thus, negatively impacting their health status and quality of life. 5–6 The etiology of weight regain for those patients it affects is likely multifactorial and remains poorly defined. Risk factors cited include preoperative BMI at the time of RYGB, nutritional habits post surgery, self-esteem, mental health, socio-economic status, and the presence of gastrogastric fistula. 7–10
A long held concept in the bariatric surgical community has been that progressive dilatation of the gastrojejunal (GJ) stoma post-RYGB can contribute substantially to weight regain. This concern dates back to the era of the vertical banded gastroplasty and was the basis for reinforcing or “banding” the distal gastric pouch. The concern over GJ stoma dilatation has also been the reasoning behind the use of “banded” distal gastric pouches popularized by Fobi and Capella, who have among the highest post-RYGB weight loss results reported in the literature. 11–12 More recently, dilatation of the GJ stomal diameter is increasingly an indication for revisional bariatric surgical procedures, which carry high rates of major complications. 13–15 This has led to the development of a variety of less invasive endoscopic techniques and devices aimed at the reduction of the gastrojejunal anastomosis diameter and gastric pouch size. These include endoluminal suturing devices, tissue plication platforms, and sclerotherapy techniques. 16–24 Despite these trends, the data to date are indirect, and no studies have directly evaluated the effects of GJ stoma diameter over time upon weight regain post-RYGB.
Thus, in this study, we sought to evaluate the influence of GJ stoma diameter on the risk of weight regain after RYGB. Furthermore, we sought to develop a predictive model based on endoscopic and clinical parameters that would stratify subjects at high risk for this costly complication. To address these questions, we studied a large, consecutive series of patients in which GJ stoma diameter was recorded, and serial weight measurements were available.
Methods
We reviewed data for all patients referred to our tertiary care bariatric center to undergo upper endoscopy post RYGB between 01/01/2006 to 09/01/2010. The indication for the upper endoscopy varied and included work-up for epigastric abdominal pain, nausea and vomiting, evaluation of possible gastric fistula and marginal ulcerations, as well as weight regain after RYGB. As a standard practice, all bariatric patients referred to our center for upper endoscopy post RYGB were examined by the same single expert endoscopist, who measured all GJ stoma diameters in an identical protocol using a novel direct reading endoscopic measuring instrument (Olympus, Center Valley, PA, USA). This flexible calibrated measuring instrument is introduced through the upper endoscope working channel and placed in proximity to the anatomical structure to be measured. Using this measurement, the endoscopist recorded the maximal diameter of the GJ stoma (figure 1).
Figure 1.
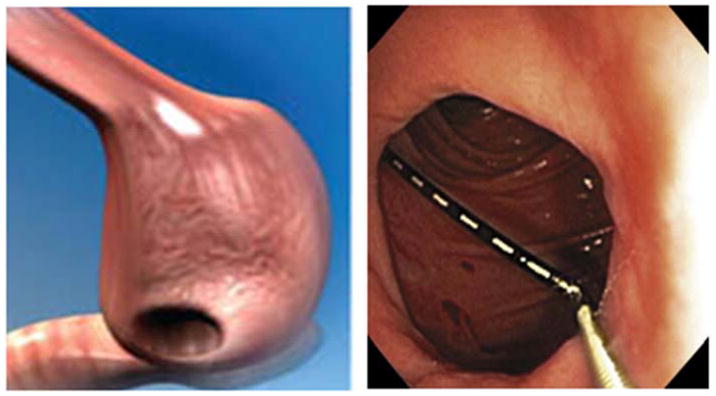
Endoscopic measurement technique of the gastrojejunal stoma diameter. A flexible calibrated measuring instrument is introduced through the upper endoscope working channel and placed in proximity to the GJ stoma. The endoscopist recorded its maximal diameter.
165 consecutive patients, at least one year after RYGB, who underwent upper endoscopy with recorded GJ stoma diameter, and had documented post RYGB clinical profiles and weight data were included in the study. We excluded subjects with gastrogastric fistulae since the pathophysiology of their weight regain likely differs.
Statistical Methodology
Linear regression analysis was performed to determine the association between the GJ stoma diameter (in millimeters) and the percent of weight regained from maximal weight loss nadir after RYGB. In the multivariate analyses, we adjusted for known or purported risk factors for weight regain, including age, gender, race, percent of body weight lost at nadir after RYGB, time since the RYGB in years, type of the RYGB (open vs. laproscopic), diabetes status, selective Serotonin re-uptake inhibitors (SSRI) use, anti-psychotic medications use, and presences of marginal ulcers on upper endoscopy. Standardized β coefficients were reported and significant two-sided p-value was set at ≤ 0.05. Pearson correlation was used to study the correlation between the length of the gastric pouch and the GJ stoma diameter.
We also used a logistic regression model to develop a prediction rule which consists of significant clinical and endoscopic parameters associated with weight regain after RYGB. For this, we divided the cohort in half, 82 subjects were used to derive the prediction rule and 83 subjects to valid it. We converted odds ratio estimates of different predictors used in the derivation model into a point system based on their strength of association with weight regain after RYGB. We compared the area under the receiver operating characteristic (ROC) curve for the derivation and validation models, as well as the positive predictive values (PPV) of weight regain associated with a score ≥ to 4 points. Statistical significance was set at a two-sided p-value ≤ 0.05. The Hosmer-Lemeshow test was used to assess the goodness-of-fit of the predictive model.
All statistical modeling was done using SAS version 9.2 software (Cary, NC, USA). Graphing was done using the JMP version 8 software (SAS Institute, Cary, NC, USA).
Results
Among the 165 patients included in our study, the average age of the population was 44 years with 91% females, 68% Caucasians, 16% Blacks, 16% Hispanics, 17.5% with a diagnosis of diabetes prior to the RYGB, 36% on SSRI therapy, 5% on anti-psychotic medications, 20% with known marginal ulcers or erosions, and 50% underwent an open RYGB procedure. The mean pre-RYGB BMI for the cohort was 49 (SD=8). After RYGB, the mean maximal percent total body weight lost was 38% (SD = 9), representing a maximal percent excess body weight lost (EBWL) of 73% (SD = 19). Fifty nine percent of the cohort (97 subjects) had significant weight regain (≥ 20% of maximal weight lost after the RYGB), and 41% (68 subjects) did not. The mean percent of maximal weight lost after RYGB that was regained in the entire cohort was 30% (SD = 22). The average time between the RYGB surgery and endoscopic measurement of the GJ stoma diameter was 4.6 years (SD = 3).
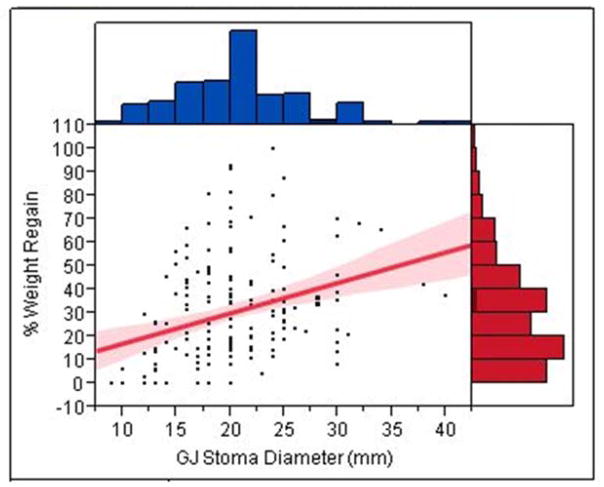
Table 1 shows the univariate and multivariate associations between different variables and weight regain after RYGB. The GJ stoma diameter was significantly associated with weight regain after RYGB (standardized β coefficient = 0.31, p = <0.0001) in univariate analysis. This association remained significant after adjusting for multiple potential risk factors in a multivariate linear regression model (standardized β coefficient = 0.19, p = 0.003). Figure 2 shows a linear relationship with a positive slope between the GJ stoma diameter in millimeters and percent of maximal weight lost after RYGB that was regained. At 5 years after the RYGB each 10 mm increase in the GJ stoma diameter was associated with an 8% increase in the percent of maximal weight lost after RYGB that was regained (figure 3).
Table 1.
Univariate and multivariate analysis of variables associated with weight regain after RYGB in a linear regression model
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Standardized β coefficient | P-Value | Standardized β coefficient | P-Value | |
| Age (years) | 0.04 | 0.62 | 0.02 | 0.8 |
| Gen (Male vs. Female) | − 0.14 | 0.08 | − 0.03 | 0.67 |
| Race (Caucasians vs. African Americans and Hispanics with Caucasians as reference) | − 0.23 | 0.003 | − 0.1 | 0.17 |
| Maximal percent body weight lost after RYGB (%) | 0.06 | 0.4 | − 0.06 | 0.4 |
| Time from RYGB (years) | 0.66 | <0.0001 | 0.6 | <0.0001 |
| Type of the RYGB (open vs. laproscopic with laproscopic as reference) | 0.44 | <0.0001 | − 0.01 | 0.88 |
| Diabetes | −0.09 | 0.26 | 0.09 | 0.15 |
| SSRI use | 0.21 | 0.008 | − 0.01 | 0.86 |
| Anti-psychotic medications use | 0.18 | 0.02 | 0.07 | 0.23 |
| Marginal ulcers presence | − 0.29 | 0.0002 | − 0.19 | 0.004 |
| Gastric pouch (cm) | 0.09 | 0.27 | − 0.003 | 0.95 |
| Gastrojejunal stoma diameter in (mm) | 0.31 | <0.0001 | 0.19 | 0.003 |
Figure 2.
Scatter plot with best fit linear regression line and 95% confidence intervals of the association between the gastrojejunal stoma diameter in millimeters and percent of maximal weight lost after RYGB that was regained. The normal distribution of each of the continuous variables is also shown.
Figure 3.
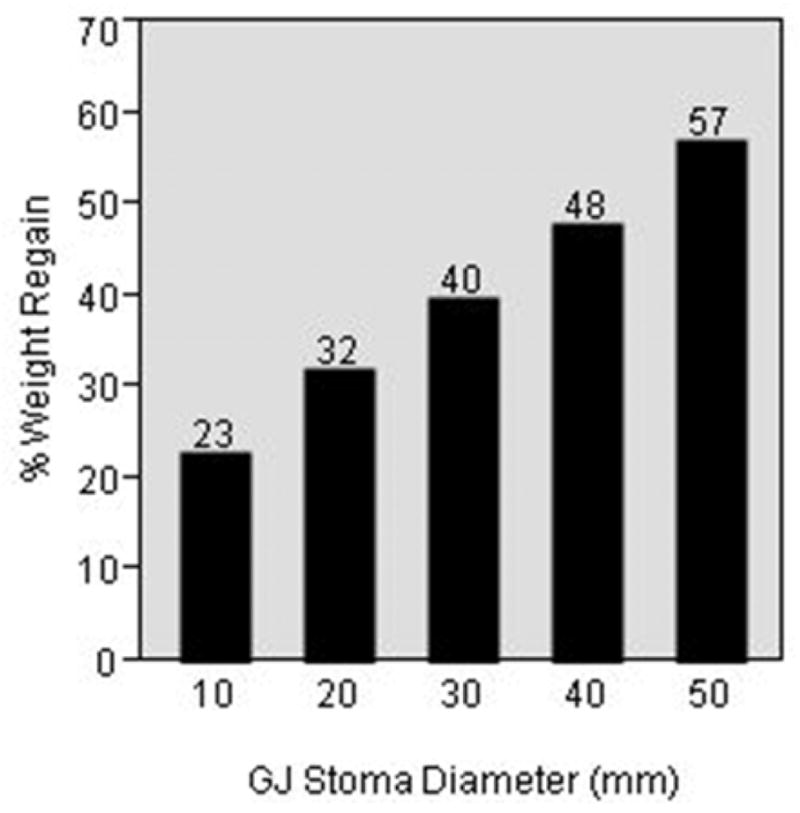
Predicted percent of maximal weight lost after RYGB that was regained in 5 years after the procedure at different gastrojejunal stoma diameters based on the linear regression model.
Other than the GJ stoma diameter, other variables that were significantly associated with weight regain after RYGB in the multivariate linear regression model included the time elapsed since the RYGB (standardized β coefficient = 0.65, p = <0.0001), and the presence of marginal ulcerations on endoscopy (standardized β coefficient = − 0.17, p = 0.01). The length of the gastric pouch was not associated with weight regain on both univariate and multivariate analyses.
In order to determine if the size of the gastric pouch correlates with the GJ stoma diameter, we calculated a Pearson correlation coefficient between them. The Pearson correlation coefficient was 0.1 (p = 0.06); indicating no correlation between these two measurements.
Given the positive association observed between the GJ stoma diameter and percent weight regain after RYGB in our linear regression model, we sought to develop a simple prediction rule based on clinical and endoscopic variables to estimate the probability of weight regain. In order to do so, we divided the cohort in half, 82 subjects were used to derive the prediction rule and 83 subjects to valid it in a multivariate logistic regression model. The area under the ROC curve for a prediction model including GJ stoma diameter, race, and maximal percent weight lost after RYGB was 0.76 (Hosmer-Lemeshow goodness-of-fit test = 0.6) (figure 4).
Figure 4.
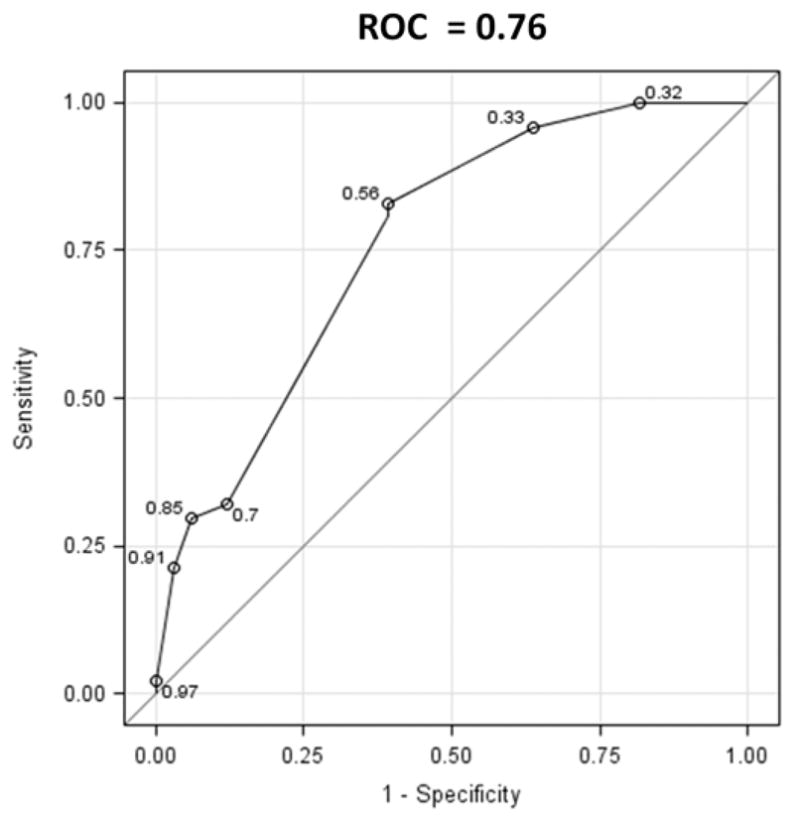
Area under ROC curve for weight regain after RYGB prediction model including gastojejunal stoma diameter, race, and percent of maximal weight loss after RYGB.
The odds ratio estimates of the different predictors used in this model were then converted to a simple 7 point system based on their strength of association with greater than 20% weight regain after RYGB (table 2). The positive predictive value (PPV) in predicting significant weight regain after RYGB for a cut-off score ≥ to 4 points in this model was 75%. Scores lower than 4 points were indeterminate.
Table 2.
Prediction model for weight regain after RYGB
| Risk Factors | Points | Cut-off point | Positive predictive value (PPV) |
|---|---|---|---|
| Gastrojejunal stoma diameter (mm) | ≥ 4 points | 75 % | |
| • ≤ 15 | 0 | ||
| • 15–25 | 2 | ||
| • ≥ 25 | 4 | ||
| Race | |||
| • Other | 0 | ||
| • White | 2 | ||
| Percent maximal body weight lost after RYGB (%) | < 4 points | Indeterminate | |
| • ≤ 50% | 0 | ||
| • > 50% | 1 | ||
| Maximum points | 7 | ||
The performance of the predication rule in the validation cohort was similar. The area under the ROC curve was 0.70, and the PPV in predicting significant weight regain after RYGB for a cut-off score ≥ to 4 points was 72%.
Conclusions
In this study, we demonstrate that an enlarged GJ stoma diameter is a significant risk factor for weight regain after RYGB; thus, establishing it as a legitimate delayed post-surgical complication that explains a significant proportion of variability in long term weight outcomes after RYGB surgery. Stoma dilation should thus be regarded in a manner similar to gastro-gastric fistulae or other postsurgical anatomic complications.
This finding is corroborated by earlier observations from vertical banded gastroplasty procedures 11–12, 25, previous data supporting the reinforcing or “banding” the GJ stoma in RYGB patients 26, and the early results of endoscopic procedures to narrow the GJ stoma in RYGB patients who have gained weight. 16, 19–23
Weight regain after RYGB is multi-factorial and likely involves a complex interplay between a permissive psychosocial environment, nutritional habits, and a complex genetic and anatomic milieu that effect many physiological regulatory pathways controlling food-intake behavior and energy metabolism after the procedure. 5, 27–30 Some in the bariatric surgical community have claimed that weight regain post-RYGB is largely attributed to patient non-compliance, and as such, surgical or endoscopic revisional procedures in such patients should not be routinely considered. However, if a dilated GJ stoma results in early emptying of a gastric pouch and loss of post-prandial satiety, then such an anatomic alteration would present in the same manner as simple non-compliance. Thus, there is likely an interplay between the patient’s anatomy and the complex physiologic and psychosocial factors that regulate their eating behavior. The difficulty in treating such patients lies in the same difficulties that they face with non-operative measures to lose weight prior to their bariatric surgical procedure. Therefore, the ability to identify key modifiable risk factors for weight regain, and target them effectively with less invasive and cost-saving endoscopic approaches, could offer a substantial potential benefit to patients with this complication.
Mechanisms by which a dilated GJ stoma increases the risk for weight regain after RYGB are not yet clearly defined. However, a dilated gastrojejunal stoma may be a surrogate for a compliant gastric pouch. This permits accommodation of larger meal boluses prior to the activation of gastric wall mechanoreceptors and inhibition of the gastric hormone ghrelin, which is a 28-amino acid peptide secreted primarily by the A/X cells of the gastric fundus with strong orexigenic and adipogenic actions. 31–36 A dilated GJ stoma may also alter the rate by which the gastric pouch empties partially digested nutrients to the jejunum, which in turn modulate the production of incretins from specialized enteroendocrine cells within the walls of the small bowel that modulate insulin secretion, energy expenditure, and body weight regulation. 35, 37–38
Other risk factors for weight regain after RYGB identified by our linear regression model include the time since RYGB surgery. Time since surgery includes in it many risk factors for weight regain that we could not measure and account for directly in our study, such as a permissive psychosocial environment, nutritional habits, physical activity, and genetic backgrounds of patients. Similar to others, our study did show an association between psychiatric medications use and weight regain. However, these associations were attenuated after multivariate adjustment. 8
Since 1998 the number of bariatric surgery procedures has been on an exponential incline with an estimated 220,000 bariatric operations performed in the USA and Canada in 2008. 39–40 With the continued projected increase in these procedures, identifying patients at increase risk for weight regain, with subsequent application of more aggressive behavioral, nutritional, and endoscopic interventions aimed at reducing the GJ stoma diameter is advisable to prevent or halt this outcome. To that end, our simple prediction rule based on clinical and endoscopic variables, had a positive predictive value of 75% in predicting weight regain at a cut-off score ≥ 4 points.
Our study has multiple strengths. First, this is the first study to directly correlate GJ stoma diameter with weight loss outcomes. Second, we included all consecutive patients who were referred to our center for upper endoscopy after RYGB to avoid confounding by indication. Third, the GJ stoma diameter in all subjects was measured in the same fashion by a single endoscopist to minimize variability. Fourth, we had a reasonable sample size to examine our hypotheses, derive our prediction rule, and validate it.
Several limitations deserve comment. First, although the data was collected prospectively, the analysis was retrospective in nature. Second, we lacked information about nutritional habits and physical activity. Third, we only measured the maximal GJ stoma diameter. Finally, we lacked a reference group with no significant weight regain after RYGB, who also did not have an indication for upper endoscopy. However, the distribution of weight regain in our entire cohort was close to normal with a mean percent weight regain of 30%, which is similar to other studies that followed post RYGB subjects prospectively.6 Hence, it is unlikely that we had a significant selection bias.
All in all, as the obesity epidemic persists and more patients undergo surgical intervention there will be more patients at risk of weight regain and procedural failure. Thus, demonstrating that an enlarged GJ stoma diameter is a risk factor for weight regain after RYGB, and using it to develop a prediction rule for this complication, will enable us to more accurately stratify patients and employ a variety of early intervention strategies. If confirmed, these results suggest that endoscopic interventions designed to reduce gastrojejunal anastomosis aperture may be effective in treating or preventing weight regain. These devices and techniques, including endoluminal suturing devices, tissue plication platforms, and sclerotherapy techniques, have shown promise in managing weight regain after RYGB; however, they are yet to be validated in randomized controlled trials. 18–24
Acknowledgments
The authors would like to thank Raymond Chung M.D. and Andrew Chan M.D. MPH for their critical review of this manuscript.
Funding: This project was supported in part by a T32 NIH training grant to the Massachusetts General Hospital and Barham Abu Dayyeh M.D. (T32DK007191).
Abbreviations
- BMI
Body mass index
- EBWL
Excess body weight lost
- GJ
Gastrojejunal
- PPV
Positive predictive value
- ROC
Receiver operating characteristic
- RYGB
Roux-en-Y gastric bypass
- SSRI
Serotonin re-uptake inhibitors
Footnotes
Disclosures: Christopher C. Thompson is a consultant for USGI medical, BARD medical, Covidien, Boston Scientific, and ValenTx. No conflict of interest exists for Barham K. Abu Dayyeh and David B. Lautz.
Ethics committee approval: This study was reviewed and approved by the Partner’s Institutional Review Board (IRB) at the Brigham and Women’s Hospital. The IRB protocol number is 2003P001597. This study was exempt from written informed consent requirement.
Authors Contributions:
Barham K. Abu Dayyeh: Study concept and design, acquisition of data, statistical analysis and interpretation of data, and drafting of the manuscript.
David B. Lautz: Study concept, critical revision of the manuscript for important intellectual content.
Christopher C. Thompson: Study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, and study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010 Jan 20;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007 Aug 23;357(8):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004 Oct 13;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 4.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009 Mar;122(3):248–56. e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006 Nov;244(5):734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004 Dec 23;351(26):2683–93. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 7.van Hout GC, Verschure SK, van Heck GL. Psychosocial predictors of success following bariatric surgery. Obes Surg. 2005 Apr;15(4):552–60. doi: 10.1381/0960892053723484. [DOI] [PubMed] [Google Scholar]
- 8.Rutledge T, Groesz LM, Savu M. Psychiatric Factors and Weight Loss Patterns Following Gastric Bypass Surgery in a Veteran Population. Obes Surg. 2009 Jul 15; doi: 10.1007/s11695-009-9923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perugini RA, Mason R, Czerniach DR, et al. Predictors of complication and suboptimal weight loss after laparoscopic Roux-en-Y gastric bypass: a series of 188 patients. Arch Surg. 2003 May;138(5):541–5. doi: 10.1001/archsurg.138.5.541. [DOI] [PubMed] [Google Scholar]
- 10.Odom J, Zalesin KC, Washington TL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010 Mar;20(3):349–56. doi: 10.1007/s11695-009-9895-6. [DOI] [PubMed] [Google Scholar]
- 11.Fobi MA. Vertical Banded Gastroplasty vs Gastric Bypass: 10 years follow-up. Obes Surg. 1993 May;3(2):161–4. doi: 10.1381/096089293765559511. [DOI] [PubMed] [Google Scholar]
- 12.Capella JF, Capella RF. The weight reduction operation of choice: vertical banded gastroplasty or gastric bypass? Am J Surg. 1996 Jan;171(1):74–9. doi: 10.1016/S0002-9610(99)80077-4. [DOI] [PubMed] [Google Scholar]
- 13.Spyropoulos C, Kehagias I, Panagiotopoulos S, et al. Revisional bariatric surgery: 13-year experience from a tertiary institution. Arch Surg. 2010 Feb;145(2):173–7. doi: 10.1001/archsurg.2009.260. [DOI] [PubMed] [Google Scholar]
- 14.Inabnet WB, 3rd, Belle SH, Bessler M, et al. Comparison of 30-day outcomes after non-LapBand primary and revisional bariatric surgical procedures from the Longitudinal Assessment of Bariatric Surgery study. Surg Obes Relat Dis. 2010 Jan–Feb;6(1):22–30. doi: 10.1016/j.soard.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallowell PT, Stellato TA, Yao DA, et al. Should bariatric revisional surgery be avoided secondary to increased morbidity and mortality? Am J Surg. 2009 Mar;197(3):391–6. doi: 10.1016/j.amjsurg.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Thompson CC, Slattery J, Bundga ME, et al. Peroral endoscopic reduction of dilated gastrojejunal anastomosis after Roux-en-Y gastric bypass: a possible new option for patients with weight regain. Surg Endosc. 2006 Nov;20(11):1744–8. doi: 10.1007/s00464-006-0045-0. [DOI] [PubMed] [Google Scholar]
- 17.Herron DM, Birkett DH, Thompson CC, et al. Gastric bypass pouch and stoma reduction using a transoral endoscopic anchor placement system: a feasibility study. Surg Endosc. 2008 Apr;22(4):1093–9. doi: 10.1007/s00464-007-9623-z. [DOI] [PubMed] [Google Scholar]
- 18.Tang SJ, Olukoga CO, Provost DA, et al. Gastrojejunal stomal reduction with the T-tag device in porcine models (with videos) Gastrointest Endosc. 2008 Jul;68(1):132–8. doi: 10.1016/j.gie.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Mullady DK, Lautz DB, Thompson CC. Treatment of weight regain after gastric bypass surgery when using a new endoscopic platform: initial experience and early outcomes (with video) Gastrointest Endosc. 2009 Sep;70(3):440–4. doi: 10.1016/j.gie.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 20.Ryou M, Mullady DK, Lautz DB, et al. Pilot study evaluating technical feasibility and early outcomes of second-generation endosurgical platform for treatment of weight regain after gastric bypass surgery. Surg Obes Relat Dis. 2009 Jul–Aug;5(4):450–4. doi: 10.1016/j.soard.2009.03.217. [DOI] [PubMed] [Google Scholar]
- 21.Catalano MF, Rudic G, Anderson AJ, et al. Weight gain after bariatric surgery as a result of a large gastric stoma: endotherapy with sodium morrhuate may prevent the need for surgical revision. Gastrointest Endosc. 2007 Aug;66(2):240–5. doi: 10.1016/j.gie.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 22.Loewen M, Barba C. Endoscopic sclerotherapy for dilated gastrojejunostomy of failed gastric bypass. Surg Obes Relat Dis. 2008 Jul–Aug;4(4):539–42. doi: 10.1016/j.soard.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Spaulding L, Osler T, Patlak J. Long-term results of sclerotherapy for dilated gastrojejunostomy after gastric bypass. Surg Obes Relat Dis. 2007 Nov–Dec;3(6):623–6. doi: 10.1016/j.soard.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Mikami D, Needleman B, Narula V, et al. Natural orifice surgery: initial US experience utilizing the StomaphyX device to reduce gastric pouches after Roux-en-Y gastric bypass. Surg Endosc. 2010 Jan;24(1):223–8. doi: 10.1007/s00464-009-0640-y. [DOI] [PubMed] [Google Scholar]
- 25.Howard L, Malone M, Michalek A, et al. Gastric Bypass and Vertical Banded Gastroplasty- a Prospective Randomized Comparison and 5-Year Follow-up. Obes Surg. 1995 Feb;5(1):55–60. doi: 10.1381/096089295765558169. [DOI] [PubMed] [Google Scholar]
- 26.Fobi MA, Lee H, Felahy B, et al. Fifty consecutive patients with the GaBP ring system used in the banded gastric bypass operation for obesity with follow up of at least 1 year. Surg Obes Relat Dis. 2005 Nov–Dec;1(6):569–72. doi: 10.1016/j.soard.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Vetter ML, Cardillo S, Rickels MR, et al. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009 Jan 20;150(2):94–103. doi: 10.7326/0003-4819-150-2-200901200-00007. [DOI] [PubMed] [Google Scholar]
- 28.Wang PY, Caspi L, Lam CK, et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008 Apr 24;452(7190):1012–6. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 29.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity. 2009 Oct;17(10):1839–47. doi: 10.1038/oby.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Froguel P, Blakemore AI. The power of the extreme in elucidating obesity. N Engl J Med. 2008 Aug 28;359(9):891–3. doi: 10.1056/NEJMp0805396. [DOI] [PubMed] [Google Scholar]
- 31.Phillips RJ, Powley TL. Gastric volume rather than nutrient content inhibits food intake. Am J Physiol. 1996 Sep;271(3 Pt 2):R766–9. doi: 10.1152/ajpregu.1996.271.3.R766. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz GJ, Salorio CF, Skoglund C, et al. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am J Physiol. 1999 Jun;276(6 Pt 2):R1623–9. doi: 10.1152/ajpregu.1999.276.6.R1623. [DOI] [PubMed] [Google Scholar]
- 33.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999 Dec 9;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 34.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001 Jan 11;409(6817):194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 35.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007 Jan;117(1):13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002 May 23;346(21):1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 37.Theodorakis MJ, Carlson O, Michopoulos S, et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006 Mar;290(3):E550–9. doi: 10.1152/ajpendo.00326.2004. [DOI] [PubMed] [Google Scholar]
- 38.Hansotia T, Maida A, Flock G, et al. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007 Jan;117(1):143–52. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kohn GP, Galanko JA, Overby DW, et al. Recent trends in bariatric surgery case volume in the United States. Surgery. 2009 Aug;146(2):375–80. doi: 10.1016/j.surg.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009 Dec;19(12):1605–11. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]