Abstract
Diabetes mellitus is a primary risk factor for cardiovascular diseases and heart failure. Activation of the retinoic acid receptor (RAR) and retinoid X receptor (RXR) has an anti-diabetic effect; but, a role in diabetic cardiomyopathy remains unclear. Using neonatal and adult cardiomyocytes, we determined the role of RAR and RXR in hyperglycemia-induced apoptosis and expression of renin-angiotensin system (RAS) components. Decreased nuclear expression of RARα and RXRα, activation of apoptotic signaling and cell apoptosis was observed in HG treated neonatal and adult cardiomyocytes and diabetic hearts in ZDF rats. HG-induced apoptosis and reactive oxygen species (ROS) generation was prevented by both RAR and RXR agonists. Silencing expression of RARα and RXRα, by small interference RNA, promoted apoptosis under normal conditions and significantly enhanced HG-induced apoptosis, indicating that RARα and RXRα are required in regulating cell apoptotic signaling. Blocking angiotensin type 1 receptor (AT1R); but, not AT2R, attenuated HG-induced apoptosis and ROS generation. Moreover, HG induced gene expression of angiotensinogen, renin, AT1R and angiotensin II (Ang II) synthesis were inhibited by RARα agonists and promoted by silencing RARα. Activation of RXRα, downregulated the expression of AT1R; and RXRα silencing accelerated HG induced expression of angiotensinogen and Ang II synthesis, whereas there was no significant effect on renin gene expression. These results indicate that reduction in the expression of RARα and RXRα has an important role in hyperglycemia mediated apoptosis and expression of RAS components. Activation of RAR/RXR signaling protects cardiomyocytes from hyperglycemia, by reducing oxidative stress and inhibition of the RAS.
Keywords: Retinoid receptors, Hyperglycemia, Cardiomyocytes, Renin-angiotensin system, Apoptosis
Introduction
Diabetes mellitus (DM) results in numerous cardiovascular complications, which are a major cause of morbidity and mortality in the diabetic population (Ho et al., 1993). Diabetes can result in cardiac damage in the absence of coronary artery disease, leading to diabetic cardiomyopathy (Devereux et al., 2000; Ilercil et al., 2001), characterized by systolic and diastolic dysfunction due to reduced contractility, prolonged relaxation, and decreased compliance of the myocardium. Several factors have been postulated to contribute to the pathogenesis of diabetic cardiomyopathy, including hyperglycemia, insulin resistance, oxidative stress and the renin-angiotensin system (Karnik et al., 2007; Marra et al., 2002; Penckofer et al., 2002). Hyperglycemia-induced apoptosis has an important role in diabetic cardiomyopathy in both animals and humans (Baraka and AbdelGawad, 2010; Kuethe et al., 2007). Attenuation of cardiomyocyte apoptosis has been shown to prevent the progression of cardiac remodeling associated with diabetes (Cai et al., 2006; Katare et al., 2010; Li et al., 2009). It is well known that hyperglycemia causes cardiac remodeling due to increased production of reactive oxygen species (ROS) and an altered cellular redox state (Cai et al., 2006; Fiordaliso et al., 2004). Hyperglycemia-induced ROS generation contributes to the expression of angiotensinogen (Ao), the sole substrate of the renin-angiotensin system (RAS) (Hsieh et al., 2002). Enhanced expression of RAS components has been implicated in the development of diabetic cardiomyopathy (Dzau, 2001). Moreover, activation of AT1R induces the generation of oxygen-derived free radicals, which can have detrimental effects (Nickenig and Harrison, 2002). These data suggest that the interaction between hyperglycemia-induced apoptosis, ROS and the RAS has an important role in the development of diabetic cardiomyopathy.
All-trans retinoic acid (ATRA), the active metabolite of vitamin A, is important for both cardiac development and differentiation into adult muscle cells (Kastner et al., 1997; Subbarayan et al., 2000). The pleiotropic activities of RA in regulating cell proliferation and differentiation are mediated by two classes of nuclear receptors, retinoic acid receptors (RARα, β and γ), which respond to ATRA; and retinoid X receptors (RXRα, β and γ), which are activated by the 9-cis-isomers of RA exclusively. Specific functions of the different retinoid receptors during heart embryogenesis have been identified using genetic approaches. RARα, RARβ and RARγ single mutations in mice had no effect on heart development (Li et al., 1993; Lohnes et al., 1993; Luo et al., 1995). Significant heart malformations were only demonstrated with double mutations of RARα/RARβ and RARα/RARγ; but, not RARβ/RARγ (Lohnes et al., 1994; Mendelsohn et al., 1994), suggesting that RARα may be the major subtype receptor of RAR, in regulating heart formation. Disruption of the RXRα gene in mice, results in cardiac defects and heart failure (Dyson et al., 1995; Sucov et al., 1994), while RXRβ and RXRγ gene knockout mice have normal heart formation. Double mutation of RXRβ-/-/RXRγ-/- or triple mutation of RXRα+/-/RXRβ-/-/RXRγ-/- mice are viable, displaying no obvious congenital heart abnormalities (Krezel et al., 1996). These studies suggest that RXRα is the main RXR isoform implicated in cardiac development and function. During the postnatal stage, we and others have demonstrated that activation of both RAR and RXR suppresses myocardial cell hypertrophy, in response to a variety of hypertrophic stimuli (Palm-Leis et al., 2004; Wang et al., 2002; Wu et al., 1996; Zhou et al., 1995), indicating that RAR/RXR mediated signaling has an important role in regulating the transition from adaptive cardiac hypertrophy to heart failure. However, little is known about the role of RAR and RXR in the development of diabetic cardiomyopathy.
There is evidence that vitamin A metabolism is impaired, especially in poorly controlled diabetes (Basu and Basualdo, 1997; Basualdo et al., 1997). The plasma level of retinol in type 1 diabetic humans and animals is decreased (Baena et al., 2002; Tuitoek et al., 1996), while administration of ATRA improves type 1 diabetes (Van et al., 2009). RXR agonists sensitize insulin action and rescue hyperglycemia, hypertriglyceridemia and hyperinsulinemia in type 2 diabetic mouse models (Leibowitz et al., 2006; Mukherjee et al., 1997). Thus, changes in RA signaling via the extracellular/intracellular RA level or the expression of RAR/RXR, closely correlate with the development of diabetes and insulin resistance. The anti-diabetic effects of RA may have an important role in diabetes induced cardiac remodeling.
Here, we report that RARα and RXRα are downregulated in HG exposed cardiomyocytes and diabetic hearts. The decreased expression of RARα and RXRα contributes to hyperglycemia-induced cardiac remodeling. High glucose-mediated upregulation of RAS components is inhibited by activation of RARα; and promoted by loss of function of RARα. These findings suggest that RAR/RXR mediated signaling may have therapeutic potential, by preventing hyperglycemia induced cardiac remodeling and regulating expression of RAS components.
Materials and Methods
Antibodies and Reagents
Cell culture medium, antibiotics and fetal bovine serum (FBS) were obtained from Invitrogen (Baltimore, MD). The Annexin V-FITC Apoptosis Detection Kit was obtained from Roche Diagnostics (Indianapolis, IN). Total and cleaved caspase-3 antibodies were purchased from Cell Signaling (Boston, MA). RAR (α, β, γ), RXR (α, β, γ), Bax, Bcl-2, and histone antibodies were obtained from Santa Cruz (Delaware, CA). The Ang II EIA kit and Ang II antibody for immunochemistry were purchased from Peninsula Laboratories (Belmont, CA). All-trans retinoic acid (ATRA, pan-RAR agonist), TTNPB (RAR agonist), 9-cis RA (pan-RXR agonist), Ang II, losartan (AT1R blocker), PD123319 (AT2R blocker), Benazepril (angiotensin converting enzyme inhibitor), D-glucose, D-mannitol, actin antibody and other reagents were purchased from Sigma (St. Louis, MO). Am580 (selective RARα agonist) was from Biomol International (Plymouth Meeting, PA). LGD1069 (RXR selective agonist) was from LC Laboratories (Woburn, MA). AGN193109 (pan-RAR antagonist) was from Allergen pharmaceuticals and HX532 (pan-RXR antagonist) was a gift from Dr. Kagechika (University of Tokyo, Japan). DOSPER [1, 3-dioleoyloxy-2-(6-carboxyspermly)-propyl amide] was from Roche (Indianapolis, IN). NAC (N-acetyl cysteine), DPI (diphenyleneiodonium) and Tiron (1, 2-Dihydroxy-3, 5-benzenedisulfonic acid disodium) were purchased from Calbiochem (Gibbstown, NJ).
Rat cardiomyocyte culture
Animal use was approved by the Institutional Animal Care and Use Committee of the Texas A&M Health Science Center and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Pub. No.85-23, 1996). Primary cultures of neonatal cardiomyocytes were prepared from ventricles of 1- to 2-day-old Sprague-Dawley rat pups, as previously described (Palm-Leis et al., 2004). Adult female rats (Sprague-Dawley, 6 weeks old) were anesthetized and hearts were removed from the chest cavity, rinsed with Dulbecco's modified essential medium (DMEM) and cardiomyocytes isolated by enzymatic dissociation with collagenase (Claycomb and Palazzo, 1980). Freshly isolated cardiomyocytes were plated on laminin (20 μg/ml) coated chamber slides at a density of 2×104 cells/cm2 and incubated overnight in DMEM, containing 10% FBS and 0.1 % penicillin-streptomycin, at 37°C, in an atmosphere containing 5% CO2. After 12 h of plating, the medium was changed and myocytes were cultured for 24 h in DMEM containing 5 % FBS, 5.5 mM (normal glucose, NG) or 25 mM glucose (HG), in the absence or presence of different agonists for RAR and RXR. To prevent growth of nonmyocytes, the medium was also supplemented with 10 mM cytosine-β-D-arabinofuranoside.
Animal groups
Male Zucker diabetic fatty (ZDF) rats and age matched lean Zucker rats (Charles River Laboratories) were used. All animals were randomized into 6 groups (6 rats/group) at the age of 8 weeks: (1) untreated lean rats; (2) untreated ZDF rats; (3) Lean rats treated with ATRA (20 mg/kg body weight/day); (4) Lean rats treated with LGD1069 (20 mg/kg body weight/day); (5) ZDF rats + ATRA and (6) ZDF rats + LGD1069. Rats were given vehicle (corn oil, 1 μL/g body), ATRA or LGD1069, orally by gastric gavage, daily. All rats had free access to rat chow (Purina 5008, Charles River Laboratories) and water. Body weight was monitored daily and fasting blood glucose measured weekly using a commercially available glucometer (Elite’ Bayer, Newbury, U.K.). Rats were sacrificed after 2 weeks of treatment, blood collected by direct cardiac puncture and hearts arrested in Krebs-Henselite solution and immediately placed into liquid nitrogen.
Cardiomyocyte apoptosis
Apoptotic cardiomyocytes were detected using the terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL) assay, caspase-3 activity and Annexin V binding analysis (Choudhary et al., 2008a). TUNEL assay was performed using a commercial kit (Millipore, Temecula, CA), following the manufacturer's instructions. Myocyte cytoplasm and nuclei were counterstained with phalloidin and DAPI, respectively. Caspase-3 activity was determined by both Western blotting and immunostaining. For cleaved caspase-3 staining, cardiomyocytes cultured in 2-well chamber slides were washed with cold PBS. After fixation with 4% paraformaldehyde solution, cells were incubated with anti-cleaved caspase-3 antibody (1:200) overnight at 4°C, followed by fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (1:200; Molecular Probes). The number of positively stained cells was counted from 20 fields per slide. Annexin V binding and FACS analysis were performed. Quantitative results of apoptotic cells were averaged from three independent experiments. Total cell lysates were collected from treated and untreated cells and the expression of Bcl2, Bax and caspase-3 activity were determined by Western blotting, using specific antibodies against Bcl2, Bax and caspase-3 (Choudhary et al., 2008a).
Intracellular ROS generation
Intracellular generation of ROS was determined by flow cytometry with 2, 7-dichlorodihydrofluorescein diacetate (DCF) staining (Choudhary et al., 2008a). Data were expressed as F/FO (fluorescence of test sample/fluorescence of control), and analyzed by CellQuest software on a BD FACScan.
Ang II measurement
Ang II was measured in cell lysates and the culture medium by a quantitative, competitive EIA kit (Peninsula Laboratories), as previously described (Baker et al., 2004; Sakoda et al., 2010; Singh et al., 2007). Briefly, cell culture medium was collected after treatment, and cells were washed three times with ice-cold phosphate-buffered saline. Cells were extracted in lysis buffer containing 20 mM Tris-HCl, 10 mM EDTA, 5 mM EGTA, 5 mM mercaptoethanol, 1 mM PMSF, 1 μg/ml aprotinin and pepstatin. Cell lysate was homogenized and sedimented at 4 °C, 13,000 rpm, for 15 min. The supernatant was collected and passed over a DSC-18 column (Supelco, Bellefonte, PA) and eluted with methanol. The methanol was evaporated and the peptide reconstituted in PBS. To purify Ang II from the culture medium, the medium was passed through Biomax 10K filters before applying over a DSC-18 column.
Real time RT-PCR
Gene expression of cardiac RARα, RXRα, Ao, renin, ACE, ACE2, AT1R and AT2R was determined by real time RT-PCR, as described previously (Choudhary et al., 2008b). PCR was performed using the Mx3005P Real-time PCR System (Stratagene, TX). The relative amount of mRNAs was calculated using the comparative CT method. GAPDH mRNA was used as an internal control for all experiments.
Transfection of cardiomyocytes by small interfering RNA (siRNA)
Cardiomyocytes were transfected with Stealth/ siRNA oligoribonucleotides (50 nM for RXRα, No. 34, 35 and 36; and 100 nM for RARα, No.57, 58 and 59, Invitrogen), using 3 μg/ml DOSPER for 12 h, in OPTIMEM I medium (Gibco, Invitrogen). Scrambled probe was used as a negative control. After washing, cells were maintained in DMEM medium with 5 % FBS, and subjected to different treatments. The siRNA probes were as follows: RARα (57) sense 5’-UAAUCUGGUCGGCAAUGGUGAGGGU-3’, anti-sense 5’-ACCCUCACCAUUGCCGACCAGAUUA-3’; RXRα (34) sense 5’-UUCUCU ACAGGCAUGUCCUCGUUGG-3’, anti-sense 5’-CCAACGAGGACAUGCCUGUAGAGAA-3’.
Nuclear expression of RAR and RXR
Nuclear proteins were extracted from cardiomyocytes or left ventricular myocardium, using NE-PER reagents (Thermo Scientific). The purity of nuclear protein extraction was determined before performing experiments. The same amount of cytoplasmic and nuclear proteins (40 μg) were separated by SDS–PAGE and transferred to a PVDF membrane for Western blotting using a specific HSP90 (heat shock protein 90) antibody, which provided detection in the cytoplasmic but not nuclear extraction. The absence of expression of HSP90 in the nuclear proteins, demonstrates the absence of contamination of cytoplasmic protein in the nuclear extraction. Nuclear expression of RAR and RXR was determined by Western blotting, using antibodies against RARα, -β, -γ, and RXRα, -β and -γ.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical significance was determined using one-way ANOVA, combined with the Tukey-Kramer Multiple Comparisons test. P < 0.05 was considered statistically significant.
RESULTS
RAR and RXR expression in high glucose treated cardiomyocytes and diabetic hearts
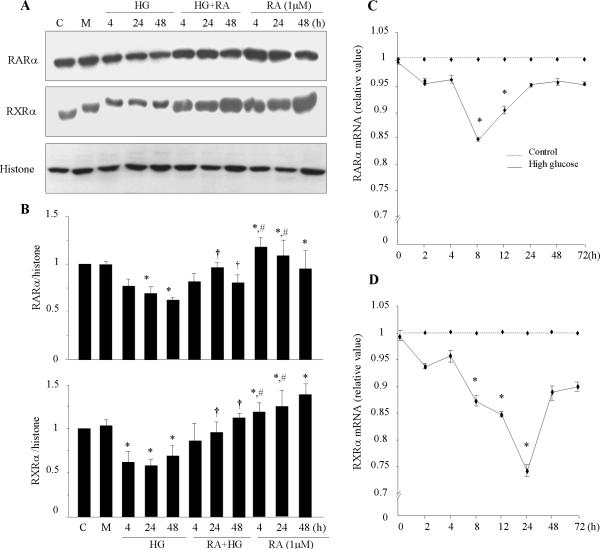
To determine the effect of high glucose (HG) on cardiac expression of RAR and RXR, primary cultured cardiomyocytes were exposed to normal glucose (5.5 mM) or HG (25 mM), with or without pretreatment with 1 μM of ATRA for different time periods. Nuclear protein expression of RAR and RXR was determined. Significant downregulation of the expression of RARα and RXRα was observed following 4 - 48 h of HG stimulation (Fig. 1A & B), whereas no significant changes were observed in the expression of other receptors (data not shown). ATRA treatment induced the expression of RARα and RXRα in cardiomyocytes cultured in normal glucose medium and prevented the downregulation in HG treated cells. Mannitol did not have any significant effect. We next determined whether the decrease in RARα and RXRα protein levels was preceded by a decrease in mRNA levels. As shown in Fig. 1C & D, HG produced a modest decrease in the levels of RARα and RXRα mRNA. A rapid decrease (5%) was observed from 2 h of HG exposure, with an observed maximal decrease in RARα mRNA (15%) at 8 h, and RXRα mRNA (25%) at 24 h. These decreases were sustained for 72 h. With the limitation of in vitro studies, changes in the expression of RAR and RXR in cultured cardiomyocytes may not represent in vivo, chronic diabetic conditions. To determine the effect of diabetes on cardiac expression of RAR and RXR, ZDF rats and age matched Zucker lean rats were sacrificed at 10 weeks of age. Compared to Zucker lean rats, a significant decrease in nuclear expression of RARα and RXRα proteins (Fig. 1E) and mRNA levels (Fig. 1F) was observed in ZDF rat hearts. These results are consistent with the in vitro data and suggest that cardiac expression of RARα and RXRα is impaired at both gene and protein levels, directly and/or indirectly by hyperglycemia.
Fig. 1. Expression of RARα and RXRα in high glucose treated cardiomyocytes and diabetic hearts.

A. Neonatal rat cardiomyocytes were exposed to normal (5.5 mM) or high glucose (HG, 25 mM) for the times indicated, with or without 1μM of ATRA (RA). Mannitol (M, 19.5 mM) was used for osmolarity control. Equal amounts of extracted nuclear proteins (40 μg) were separated on 10% SDS-PAGE. Expression of RARα and RXRα was detected using anti-RARα and -RXRα antibodies. The blots were reprobed using anti-histone antibody, to verify equal loading. B. The intensity of the bands was measured by densitometry and relative intensity calculated, after subtraction of the histone level in each sample. *, p<0.01, versus control (C); †, p<0.001, versus HG; #, p<0.001, versus RA+HG. C & D. RARα and RXRα mRNA expression. Cardiomyocytes were exposed to HG for different time periods and total RNA was isolated. Real-time RT-PCR was performed, as described in “Material and Methods”. The mRNA levels were normalized to GAPDH. Data (mean ± SEM, n = 3) are expressed as a relative value compared to control. *, p<0.05, versus control. E. Nuclear protein expression of RARα and RXRα in diabetic myocardium. Nuclear proteins were extracted from (10 week of age) ZDF and Zucker lean rat hearts. The expression of RARα and RXRα was determined by Western blot. Histone was used to verify equal loading. F. RARα and RXRα mRNA expression. Total RNA was isolated from lean and ZDF rat hearts, and expression of RARα and RXRα mRNA was determined by Real-time RT-PCR and normalized to GAPDH. Data (mean ± SEM, n = 6) are expressed as a relative value, compared to lean control.*, p<0.01, versus lean group.
ATRA protects cardiomyocytes from hyperglycemia-induced apoptosis
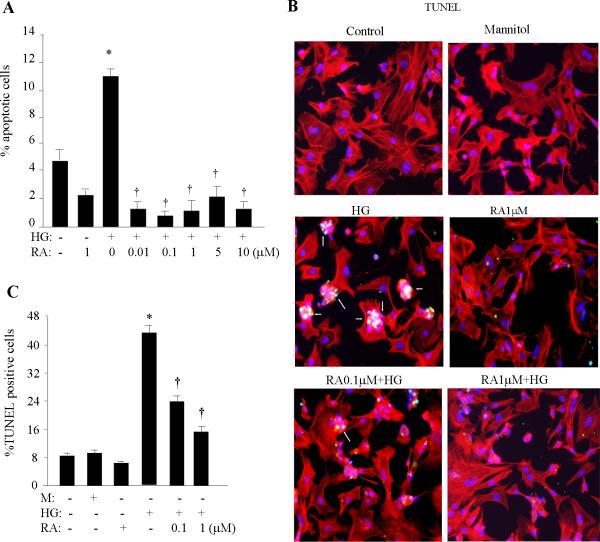
After 24 h of serum starvation, neonatal cardiomyocytes were pretreated with different doses of ATRA for 2 h and then exposed to HG for 24h. Apoptosis was analyzed by flow cytometry, using an Annexin V staining kit. Flowcytometric data demonstrated increases of the Annexin V positive population following HG stimulation (Fig. 2A) and ATRA significantly inhibited the percentage of Annexin V positive cells, even at lower doses (0.01-1 μM). The increased TUNEL positive cell population (Fig. 2B & C) and caspase-3 activity (Fig. 2D) in HG-stimulated cells was dose-dependently inhibited by ATRA. A mild apoptotic response (~5% apoptotic cells) was also observed in untreated cells, due to serum starvation (we compared with non-starved cells, data not shown). ATRA partially attenuated this basal apoptotic response. To confirm our findings, we further examined caspase-3 cleavage and expression of pro-apoptotic and anti-apoptotic Bcl2 protein family members (Fig. 2E). HG resulted in cleavage of the caspase-3 holoenzyme (30 kDa) to form a smaller, active p17 subunit. HG also reduced the protein level of the anti-apoptotic member Bcl2 and increased the level of pro-apoptotic Bax, resulting in a decreased Bcl2/Bax ratio. ATRA prevented the hyperglycemic effects on caspase-3 cleavage, upregulation of Bax and downregulation of Bcl2.
Fig. 2. Effect of RA on hyperglycemia-induced apoptosis.
A. After 24 h of serum starvation, cardiomyocytes were pretreated with ATRA (RA) for 2h and exposed to HG for 24 h. Apoptosis was quantified by Annexin V-based flow cytometric analysis. The percent of Annexin V+ cells was calculated from three independent experiments and expressed as an apoptosis ratio. *, p < 0.001 versus control; †, p < 0.001 versus HG. TUNEL analysis (B) and caspase-3 activity (D) were performed as described in methods. Red color, Texas-red labeled phalloidin staining; blue, DAPI staining; green, TUNEL and active caspase-3 staining; yellow, merged color. Results shown are merged pictures representative of three separate experiments. Five hundred cardiomyocytes were counted and the number of TUNEL-positive cells (C) and caspase-3 positive cells (D) presented as a percentage from three independent experiments. *, p < 0.001 versus control; †, p < 0.001 versus HG. E. Following 2 h of RA pretreatment, cardiomyocytes were exposed to HG for 8 h. Cell lysates were prepared and expression of Bax, Bcl2 and caspase -3 was determined by Western blotting. The blots were reprobed using anti-actin antibody, to verify equal loading. Results are representative of three independent experiments. The intensity of the bands was analyzed by densitometry. Data are presented as the mean ± SEM (n=3). *, p < 0.001 versus control; †, p < 0.001 versus HG.
Using cultured adult cardiomyocytes, we determined whether there was a similar apoptotic response to HG as observed in neonatal cardiomyocytes. Primary cultured adult rat cardiomyocytes were exposed to normal or HG for 24 h, in the presence or absence of 1 μM of ATRA, 9-cis RA, Am580 or SR11345. 9-cis RA is a pan-RXR ligand that activates all RXR subtypes (Dawson, 2004); Am580 is a selective high affinity RARα ligand that activates RARα (Delescluse et al., 1991); and SR11345 is a selective ligand for RXRα (Dawson and Zhang, 2002). Cell apoptosis was determined by immunostaining, using specific antibody against cleaved caspase-3. As shown in Fig. 3A, a significant increased caspase-3 signal was observed in HG-stimulated adult cardiomyocytes, which was significantly inhibited by ATRA and Am580, and partially inhibited by 9-cis RA or SR11345. These data suggest that activation of RAR and RXR, especially RARα/RXRα mediated signaling, inhibits the hyperglycemia-induced apoptotic response in both neonatal and adult cardiomyocytes.
Fig. 3. Effect of RA on cardiomyocyte apoptosis in diabetic heart.
A. Adult rat cardiomyocytes were cultured in normal or HG medium, in the presence or absence of ATRA (0.1 and 1 μM), 9-cis RA (9-cis, 0.1 and 1 μM), Am580 (1 μM) or SR11345 (SR, 1μM) for 24 hours. Caspase-3 activity was determined, as described in methods. Red color, Texas-red labeled phalloidin staining; blue, DAPI staining; green, active caspase-3 staining. B. Left ventricle was collected from ZDF and Zucker lean rat heats, following 2 weeks of treatment with ATRA and LGD1069. Cardiac expression of Bax and Bcl2 was determined by Western blotting. The blots were reprobed using anti-actin antibody, to verify equal loading. The intensity of the bands was analyzed by densitometry and the Bcl2/Bax ratio calculated. Data are presented as the mean ± SEM (n=6). *, p < 0.001 versus Zucker lean group; †, p < 0.001 versus ZDF group; #, p < 0.001 versus lean + LGD1069 group.
Activation of RAR and RXR inhibits apoptotic signaling in diabetic hearts
To confirm our in vitro findings, we determined the role of RAR and RXR in the diabetic heart, using ZDF rats. Consistent with a diabetic phenotype, ZDF rats were obese and hyperglycemic (body weight: 381 ± 12.4 mg; blood glucose: 528 ± 28 mg/dl), compared with Zucker lean rats (body weight: 279 ± 2.8 mg; blood glucose: 85 ± 1.7 mg/dl). The increased body weight in ZDF rats was reduced following 2 weeks of ATRA treatment (328 ± 7.4 mg, p <0.001 vs ZDF group); but, not in LGD1069 treated animals (379 ± 5.5 mg, p> 0.05 vs ZDF group). ATRA and LGD1069 treatment, both significantly reduced the increased blood glucose level in ZDF rats (223 ± 25 mg/dl and 260 ± 12 mg/dl, respectively; p<0.001 vs ZDF group). We further determined the effect of RA on the expression of Bcl2 and Bax proteins in diabetic heart. Myocardium lysates were prepared and protein expression of Bcl2 and Bax determined by Western blotting. As shown in Fig. 3B, increased Bax and decreased Bcl2 expression was observed in ZDF rat (10 weeks of age) hearts, resulting in a decreased Bcl2/Bax ratio, indicating increased apoptotic cell death signaling in the diabetic heart. The decreased Bcl2/Bax ratio was significantly improved by ATRA and LGD1069 treatment, suggesting that activation RAR/RXR-mediated signaling can protect the myocardium from diabetes-induced cell death.
Role of RAR and RXR in hyperglycemia-mediated apoptosis
To determine the role of RAR and RXR in HG-induced apoptosis, cells were pretreated with or without 1 μM of AGN193109 or HX531 for 1 h, and exposed to HG for 24 h, in the absence or presence of 1 μM of ATRA, TTNPB, Am580 or 9-cis RA. AGN193109 exhibits potent antagonism of ATRA-mediated transactivation of RARα, RARβ and RARγ (Johnson et al., 1995; Klein et al., 1996). HX531, an antagonist of RXRs, antagonizes the activation of RAR-RXR heterodimmer induced by 9-cis RA (Ebisawa et al., 1999). As shown in Fig. 4A, HG-induced increases in the percentage of Annexin V-positive cells were attenuated by all of the agonists and the anti-apoptotic effect of ATRA was abrogated by the antagonists AGN193109 and HX531, indicating the participation of both RAR and RXR in the cardioprotective effect of ATRA. RARα and RXRα are the two major receptor subtypes that have an important role in developmental heart formation. Expression of these receptor subtypes was reduced by HG in both neonatal cardiomyocytes and the diabetic adult heart (as shown in Fig. 1). To determine the contribution of RARα and RXRα in HG-induced cell apoptosis, the expression of RARα and RXRα was silenced by siRNA. Cells were transfected with a final concentration of 50-200 nM Stealth/siRNA oligoribonucleotides for RARα or RXRα. Scrambled siRNA probe was used as a non specific control. After 12 h of transfection, cells were washed and cultured for another 24 h. Nuclear protein was extracted and the expression of RARα and RXRα determined. Transfection of siRNA probes 57 and 34, significantly knocked down the expression of RARα and RXRα, respectively, compared to scrambled siRNA (Fig. 4B). Following 12 h of transfection, cardiomyocytes were exposed to HG for 24 h, in the presence or absence of selective agonists for RARα and RXRα, and cell apoptosis was evaluated by TUNEL. As shown in Fig 4C, HG-induced increases in the TUNEL positive cell population were significantly inhibited by either Am580 or SR11345. Interestingly, silencing RARα or RXRα resulted in a modest apoptotic effect (lane 9 and 13), compared to scrambled normal control. HG-induced apoptosis was further accelerated by silencing RARα or RXRα (lane 10 and 14). Am580 and SR11345 had no significant effect on HG-induced cellular apoptosis, when the expression of RARα or RXRα was silenced. Similar results were observed in HG-induced caspase-3 activity (data not shown). These data suggest that both RARα and RXRα are required for maintaining cell survival and that downregulation of RARα or RXRα has an important role in hyperglycemia-induced cardiomyocyte apoptosis.
Fig. 4. Role of RARα and RXRα in HG-mediated apoptosis.
A. Cardiomyocytes were pretreated with or without 1μM of AGN193109 (AGN193), or HX531 for 1 h, exposed to 1 μM of RA, TTNPB (TTN), 9-Cis RA (9-cis), Am580 or mannitol (M) for 2h, under normal or HG conditions. Apoptosis was quantified by flow cytometric analysis. *, p < 0.001 versus control; †, p<0.001 versus HG. B. Silencing of RARα or RXRα was achieved by transfecting cardiomyocytes with individual, pre-designed RARα and RXRα siRNA probes or scrambled siRNA (Scram) as a negative control. Nuclear proteins were extracted and expression of RARα and RXRα was determined by Western blot. C. Scrambled or RARα and RXRα siRNA transfected cells were exposed to HG, in the presence or absence of Am580 (Am) or SR11345 (SR), for 24 h, followed by TUNEL assay. TUNEL positive cell counting was presented as the mean ± SEM. *, p < 0.001 versus control; †, p < 0.001 versus HG or HG + scrambled probe; #, p < 0.01 versus siRARα; §, p < 0.01 versus siRXRα.
Activation of RAR and RXR signaling inhibits HG-mediated ROS generation
To determine whether the protective effect of RA on HG-induced apoptosis is regulated by reducing oxidative stress, cardiomyocytes were pretreated with or without ATRA (10 nM to 10 μM) for 2 h, exposed to HG for 1 h, and intracellular oxidant production monitored by DCF staining and flow cytometry. An increased DCF fluorescence intensity was observed in HG stimulated cells (Fig. 5A), which was blocked by ATRA. To determine whether activation of RAR and RXR has an effect on ROS generation, cells cultured in normal or HG medium were treated with different antagonists or agonists for RAR and RXR and the contribution of RAR and RXR on the RA-mediated inhibitory effect on ROS generation determined. As shown in Fig. 5B, the HG-induced increase in DCF fluorescence intensity was inhibited by ATRA, TTNPB, Am580 and 9-cis RA. ATRA-mediated inhibition of DCF fluorescence intensity was reversed by AGN193109 and HX531, suggesting that both RAR and RXR are involved in regulating hyperglycemia-induced intracellular ROS generation.
Fig. 5. RA inhibits HG-mediated intracellular ROS generation.
Cardiomyocytes were pretreated with different doses of ATRA (RA), and exposed to HG for 1h (A). Cells were preincubated with or without 1 μM of AGN193109 (AGN), or HX531 for 1 h and treated with 1 μM of RA, TTNPB, 9-Cis RA or Am580 for 2h, followed by exposure to HG for 1h (B). Cardiomyocytes were pretreated with or without 10 mM of NAC, 5 mM Tiron, 10 μM of rotenone or DPI, following 1 h of HG exposure (C). Intracellular ROS production was determined by flow cytometry with DCF staining. Results are expressed as the mean ± SEM (n=3). *, p < 0.001 versus control; †, p < 0.001 versus HG.
Both NADPH oxidase and mitochondrial-derived ROS have been proposed to contribute to the development of diabetic cardiomyopathy (Shen et al., 2009; Song et al., 2007). To determine the source of increased ROS in HG-stimulated cardiomyocytes, we used N-acetyl cysteine (NAC, 10 mM, a ROS scavenger); 1, 2-dihydroxybenzene-3, 5-disulfonate (Tiron, 5 mM, a superoxide scavenger); diphenyleneiodonium (DPI, 10 μM, NADPH-oxidase inhibitor) and rotenone (10 μM, inhibitor of mitochondrial complex I). The addition of these agents to cardiomyocytes prevented HG-induced ROS generation (Fig 5C), indicating that both NADPH oxidase and mitochondrial-derived ROS are involved. These results suggest that activation of RAR/RXR signaling has a protective role, by inhibiting HG-induced oxidative stress.
Ang II/AT1R signaling is involved in HG-mediated apoptosis and ROS generation
To determine if the local renin-angiotensin system was involved in HG-mediated cellular injury, cardiomyocytes were treated with losartan (Los, AT1R antagonist, 1 μM), PD123319 ditrifluoroacetate (PD, AT2R antagonist, 10 μM) or benazepril (BE, ACE inhibitor, 100 nM) for 1 h, before HG stimulation. As shown in Fig 6A & B, HG-induced apoptosis and ROS generation were significantly inhibited by losartan; but, not by PD123319, indicating that Ang II via AT1R contributes to HG-mediated cellular injury. Benazepril only partially inhibited HG-induced apoptosis and ROS generation. We next determined the effect of RA on Ang II-induced cellular injury. Cells were exposed to HG, Ang II, or HG+Ang II, with or without ATRA treatment. As shown in Fig. 6C & D, both HG and Ang II promoted cell apoptosis and intracellular ROS generation. Ang II had an additional effect on HG-mediated intracellular ROS generation; however, no additional effect was observed on HG-mediated apoptosis. ATRA prevented both Ang II and HG induced cell apoptosis and oxidative stress. These data indicate that Ang II/AT1R signaling is involved in the hyperglycemic effects on cell apoptosis and ROS generation, while ATRA largely prevented the cellular effects caused by HG and Ang II.
Fig. 6. Role of Ang II in HG-mediated apoptosis and ROS generation.
Cardiomyocytes were pretreated with or without 10 μM of losartan (Los), PD123319 (PD) or benazepril (BE) for 1 h, and exposed to HG for 24 h, to determine cell apoptosis (A) and 1 h for intracellular ROS generation (B). Cardiomyocytes were treated with or without Ang II (100 nM) and HG, in the presence or absence of ATRA (RA, 1μM). Cell apoptosis (C, 24 h treatment) and intracellular ROS generation (D, 1 h treatment) were determined. Data are expressed as the mean ± SEM (n=3). *, p < 0.001 versus LG; †, p < 0.001 versus HG; ††, p < 0.001 versus Ang II; #, p < 0.001 versus HG+Ang II.
RA inhibits HG induced cardiac Ang II synthesis
To determine the effect of RA on HG induced Ang II synthesis, cardiomyocytes were exposed to HG for 24 h, with or without ATRA. Ang II was measured in cell lysates and culture medium, using a quantitative, competitive EIA kit (Singh et al., 2007). Significantly increased levels of Ang II were observed in lysates of HG-stimulated cardiomyocytes, compared to control (Fig. 7A). Interestingly, Ang II levels in the culture medium were not affected by HG (data not shown), suggesting that the HG effects were exhibited only on the intracellular RAS. Mannitol had no effect on Ang II levels. RA inhibited Ang II levels in cell lysates, in a dose-dependent fashion. We further determined the contribution of RAR and RXR in the regulation of Ang II synthesis, using different receptor agonists. As shown in Fig. 7B, the increased Ang II levels were inhibited by RAR agonists (ATRA, TTNPB and Am580); but, not by 9-cis RA, indicating that RAR mediated signaling contributes to the inhibitory effect of RA on intracellular Ang II synthesis.
Fig. 7. Effect of RA on HG-induced Ang II synthesis.
Cardiomyocytes were exposed to HG for 24 h, in the presence or absence of ATRA (A), TTNPB, Am580 (Am) or 9-cis RA (9-cis) (B). Cell lysates were extracted and Ang II levels determined, using an EIA kit. Data are expressed as the mean ± SEM, from three separate experiments. *, p<0.05, versus control; †, p<0.05, versus HG.
Role of RARα and RXRα in the regulation of expression of RAS components
De novo synthesis of Ang II necessitates the presence of precursor components of the RAS. To determine the role of RARα and RXRα in regulation of HG-stimulated gene expression of Ao, renin, ACE (angiotensin converting enzyme), ACE2, AT1R and AT2R, cardiomyocytes were exposed to HG, in the presence or absence of Am580 and SR11345, for 24 h. Gene expression of these components was analyzed by real-time RT-PCR. As shown in Fig. 8, Ao and renin mRNA were significantly increased in HG stimulated cells and inhibited by low doses (0.1 and 1 μM) of Am580; but, not by SR11345. The inhibitory effect of SR11345 was only observed at a higher dose (5 μM). Expression of AT1R was increased following HG stimulation and inhibited by both Am580 and SR11345. No significant changes were observed in ACE, ACE2 and AT2R, following HG exposure. Expression of ACE2 and AT2R was slightly increased in Am580 treated cells, and ACE expression was unaffected by either Am580 or SR11345.
Fig. 8. Effect of RA on HG-induced expression of RAS components.
Cardiomyocytes were exposed to HG for 24 h, in the presence or absence of 0.1 and 1 μM of Am580 (Am) or SR11345 (SR, 0.1-5 μM). Total RNA was isolated and gene expression of cardiac angiotensinogen (Ao), renin, ACE, ACE2, AT1R and AT2R was determined by real-time RT-PCR. Data were normalized to a housekeeping gene (GAPDH). Data (mean ± SEM; n=3) were expressed as a relative value compared to control. *, p<0.01, versus control; †, p<0.01, versus HG.
To confirm the role of RARα and RXRα in the regulation of expression of RAS components, we silenced expression of RARα and RXRα, with the appropriate siRNA. As shown in Fig. 9A and B, silencing RARα, enhanced expression of Ao and renin in cardiomyocytes (lane 7), an increase comparable to HG stimulation. HG stimulated expression of Ao and renin (lane 4) was synergistically increased in RARα silenced cells (lane 9) and these increases were not inhibited by Am580 and SR11345. Silencing of RXRα promoted Ao gene expression, under normal and HG conditions (lane 8 & 9) and the increased Ao expression was partially inhibited by Am580 (lane 13). Renin expression was not affected by silencing RXRα. We further determined the contribution of RARα and RXRα in HG-induced intracellular synthesis of Ang II. As shown in Fig. 9C, in control cells, cytoplasmic Ang II staining was very faint. A dramatically elevated level of staining was observed in HG exposed cells, which correlated with the intracellular Ang II levels detected by ELISA. The majority of staining was in the perinuclear and nuclear regions. The increased Ang II staining was significantly suppressed by Am580, and partially inhibited by SR11345. Increased Ang II staining was also observed in RARα and RXRα silenced cells, and an additional effect was observed in HG plus siRARα or siRXRα treated cells. Silencing of RARα, promoted synthesis of Ang II, which was not affected by either Am580 or SR11345. However, Am580 partially inhibited the increased Ang II staining in RXRα silenced cells, suggesting that RARα is likely the major receptor subtype contributing to the regulation of RAS expression and intracellular Ang II synthesis.
Fig. 9. Role of RARα and RXRα in HG-mediated RAS component expression.
The expression of RARα and RXRα in cardiomyocytes was silenced by transfecting individual pre-designed RARα and RXRα siRNA. Scrambled siRNA was used as a negative control. Cells were exposed to HG, in the presence or absence of 1μM of Am580 (Am) or SR11345 (SR), for 24 h. Gene expression of Ao (A) and renin (B) was determined by Real-time RT-PCR. *, p<0.001, versus control; †, p<0.001, versus HG; §, p<0.05, versus HG+siRXRα. Intracellular Ang II distribution was determined by fluorescence immunostaining (C). Red, Texas-red labeled phalloidin staining; blue, DAPI staining; green, Ang II staining; yellow, merged color. Results shown are merged pictures, representative of three separate experiments.
DISCUSSION
In the present study, we observed a reduction in RARα and RXRα expression in diabetic myocardium and HG treated neonatal and adult cardiomyocytes. Activation of RAR and RXR mediated signaling prevented the hyperglycemic effects on cell apoptosis and ROS generation. Silencing the expression of RARα and RXRα accelerated HG-induced apoptosis. HG increased the expression of RAS components, which was further promoted by silencing RARα and RXRα, and inhibited by activation of RARα and RXRα. These data suggest that decreased expression of RARα and RXRα contributes to HG-induced cardiac remodeling, through regulation of the expression of RAS components and oxidative stress related pathways.
High glucose toxicity is an important initiator of cardiovascular disease, contributing to the development of cardiomyocyte death and diabetic complications. Apoptotic cell death has been found in the hearts of diabetic patients and animal models (Li et al., 1993; Lohnes et al., 1993; Luo et al., 1995), and has an important role in initiating the early pathogenic changes that lead to the development of diabetic cardiomyopathy (Baraka and AbdelGawad, 2010; Cai and Kang, 2003). However, hyperglycemia is not always associated with cardiomyocyte death or apoptosis. It has also been reported that hyperglycemia or diabetes protects cardiomyocytes or the heart from various pathological insults, such as ischemia and hypoxia (Schaffer et al., 2000; Wang and Chatham, 2004). The pro- or anti-apoptotic effects of high glucose may be associated with the presence or absence of insulin, or the exposure time (Ricci et al., 2008; Xu et al., 2004). These data suggest that hyperglycemia may initiate distinct mechanisms that can precondition cardiomyocytes under stress conditions. In the present study, we have focused on determining the direct effect of hyperglycemia on cardiomyocytes under normal conditions. We observed cell apoptosis in neonatal and adult cardiomyocytes, in response to high glucose exposure (24 hours). The apoptotic response was also observed in hearts from 12 week old ZDF rats. Hyperglycemia or diabetes-induced apoptosis were associated with a decrease in the anti-apoptotic protein Bcl2 and increased pro-apoptotic protein Bax. These data indicate that acute hyperglycemia exposure or early diabetes can directly initiate pro-apoptotic signaling, resulting in cardiomyocyte apoptosis. Though our data are consistent with other studies (Rajamani and Essop, 2010; Shen et al., 2009); the complexity of high glucose effects on cardiomyocytes necessitates further evaluation of the role of hyperglycemia in cardiac remodeling, in different experimental conditions and pathological insults.
Activation of RXR-mediated signaling improves insulin resistance in type 2 DM (Mukherjee et al., 1997) and activation of RAR prevents the development of type 1 DM (Stosic-Grujicic et al., 2009; Van et al., 2009), indicating that activation of RAR/RXR signaling has an important role in the development of DM. A recent study has shown that activation of RXR improves insulin responsiveness in cardiomyocytes (Montessuit et al., 2008), suggesting that RXR mediated signaling may have a role in diabetic cardiomyopathy, through regulation of glucose transport and the metabolic profile. We observed that hyperglycemia induced cardiomyocyte apoptosis was prevented by activation of RAR and RXR, while abrogated by inhibiting the activation of these receptors, indicating that RAR/RXR heterodimer mediated signaling is involved in regulating hyperglycemia-induced cell apoptosis. The apoptotic response in ZDF diabetic rat heart was also attenuated by ATRA and LGD1069 treatment. We observed anti-diabetic effects (decreased blood glucose level) in ATRA and LGD1069 treated ZDF rats. Though we didn't study changes in cardiac glucose metabolism or activation of insulin/PI3k/Akt signaling in these animals, it has previously been shown that 9-cis RA, which induces activation of RXR signaling, increased insulin stimulated glucose transport and activation of Akt in cardiomyocytes (Montessuit et al., 2008), indicating that the anti-apoptotic effect of ATRA and LGD1069 on diabetic heart, may be through activation of PI3KAkt signaling. However, the anti-diabetic effect of ATRA and LGD1069, may also be related to the anti-apoptotic effects of these ligands in ZDF rat hearts, by improvement in local cardiac glucose metabolism. We have previously demonstrated that ATRA inhibits intracellular ROS generation by increasing the anti-oxidant defense system in mechanical stretch-induced cardiac injury (Choudhary et al., 2008a). Here, we demonstrated that this mechanism also applies in hyperglycemia-induced apoptosis. Superoxide generation is one of the earliest detectable cellular responses to HG. HG causes mitochondrial membrane depolarization and a loss of uncoupling proteins, resulting in increased oxidative stress, as well as the release of cytochrome c and activation of caspase-3 (Green and Reed, 1998; Wang, 2001). We observed that both rotenone and DPI inhibited HG-mediated intracellular ROS generation, suggesting that HG may induce both mitochondrial, as well as NADPH-oxidase derived ROS. HG-induced ROS generation was prevented by pharmacological activation of RAR and RXR, indicating that RAR/RXR heterodimer may serve as an important mediator of the endogenous cellular defense system, against oxidative stress-induced cardiovascular dysfunction.
To our knowledge, there are no reports regarding the expression profile of RAR and RXR in cardiomyocytes exposed to high glucose. We have reported previously that all six subtypes of retinoid receptors are expressed in cardiomyocytes, though RARα and RXRα are the two subtypes predominantly expressed (Palm-Leis et al., 2004). In the present study, we demonstrated that RARα and RXRα are the major receptor subtypes downregulated in cardiomyocytes, in response to HG and in diabetic rat hearts. Moreover, silencing the expression of RARα or RXRα resulted in cell apoptosis and enhanced HG-induced cellular injury. Activation of RARα/RXRα mediated signaling protects cardiomyocytes from hyperglycemia-induced apoptosis, through inhibition of pro-apoptotic signaling and by enhancing anti-apoptotic signaling, indicating that both RARα and RXRα are required for maintaining the balance between cell survival and apoptotic signaling. These data indicate that hyperglycemia impairs RA signaling at the receptor level, and that decreased expression of RARα/RXRα is involved in hyperglycemia-mediated dysregulation of RAR/RXR transactivation and downstream signaling activity. We studied the expression of RARα and RXRα in the early stage diabetic heart in the present study, and whether the changes of the expression/activation of RAR and RXR during the development of DM are correlated with the progressive structural and functional deterioration of cardiac muscle associated with diabetes induced cardiomyopathy, needs to be addressed.
Little information is presently available regarding the intracellular signaling regulating retinoid receptors in cardiomyocytes. Based on previous reports and our data, we present several hypotheses regarding the mechanisms for regulation of the expression/activation of RAR and RXR in diabetic hearts. First, it has been demonstrated that oxidative stress is an important contributor to the onset and progression of diabetic cardiomyopathy (Marra et al., 2002; Penckofer et al., 2002), and can induce downregulation of functional RXR protein in H9C2 cardiomyocytes (Shan et al., 2008), indicating that high glucose-induced ROS generation may contribute to the downregulated expression of RARα and RXRα in diabetic cardiomyocytes. Pharmacological activation of RAR and RXR prevented the generation of ROS, suggesting that a negative feedback between ROS and the expression/activation of RAR and RXR may be involved. Second, the metabolic availability of vitamin A and the carrier proteins are affected in poorly controlled type 1 and 2 diabetic patients (Baena et al., 2002; Cho et al., 2006; Tuitoek et al., 1996; Yang et al., 2005). This may result in less ATRA synthesis or intracellular transportation, leading to reduced stimulation of normal expression and transactivation of retinoid receptors. Third, we have observed that both protein and mRNA levels of RARα and RXRα were decreased; however, we cannot exclude the possibility of additional post-translational modifications. It has been reported that the transcriptional activity of RAR/RXR can be modulated through phosphorylation by various kinases, such as PKC, JNK and p38 MAP kinase (Bastien and Rochette-Egly, 2004; Bruck et al., 2005; Delmotte et al., 1999). The expression of RAR and RXR can also regulated by proteasome-mediated degradation (Boudjelal et al., 2002; Kopf et al., 2000). Furthermore, HG or advanced glycation end products may directly and/or indirectly result in reduced RAR and RXR protein synthesis or increased degradation. Since downregulation of RARα and RXRα might represent the initial predisposing step leading to metabolic and structural changes during the development of cardiac remodeling, it will be important to further determine the precise mechanisms mediating the downregulation of these receptors.
Expression/activation of the RAS has been implicated in the development of diabetic cardiomyopathy and cardiac remodeling. The interaction between RA signaling and regulation of RAS components has previously been reported. RA negatively regulates renal RAS components in rats with experimental nephritis (Dechow et al., 2001). Downregulation of AT1R is observed in RA treated vascular smooth muscle cells (Haxsen et al., 2001; Takeda et al., 2000) and in hearts of SHR rats, which is accompanied by a decrease in blood pressure (Zhong et al., 2005; Zhong et al., 2004). We have recently demonstrated that the increased activation/expression of cardiac RAS components was inhibited by ATRA, in pressure overloaded rats and mechanically stretched cardiomyocytes (Choudhary et al., 2008b), indicating that activation of RAR/RXR negatively regulates the RAS, contributing to the protective effects of RA. Based on these results, we hypothesized that the protective effects of RA on hyperglycemia-induced cardiac remodeling were mediated via regulation of RAS components. As demonstrated in this study, hyperglycemia-induced apoptosis and ROS production were inhibited by an AT1R blocker, indicating that AT1R signaling contributed to hyperglycemia mediated cellular injury. ATRA inhibited HG- and Ang II-induced cell apoptosis and ROS production and hyperglycemia stimulated synthesis of Ang II, indicating an interaction between RA and Ang II signaling. ACE inhibitor only partially inhibited hyperglycemia mediated cell apoptosis and ROS generation, consistent with a previous study, demonstrating that HG-induced intracellular Ang II synthesis was chymase-dependent in cardiomyocytes (Singh et al., 2007). It was previously demonstrated that HG increased intracellular Ang II in cardiomyocytes, in the presence of candesartan (specific AT1R blocker), indicating that the increase was due to intracellular synthesis and not uptake via AT1R-mediated internalization (Singh et al., 2007). Corroborating these data, we again observed that HG stimulated intracellular, but not extracellular Ang II synthesis. The increased levels of Ang II were prevented by ATRA, suggesting that RA inhibits HG-induced Ang II production, by regulating intracardiac Ang II synthesis.
We have shown that RXR agonist has no inhibitory effect on HG-induced Ang II synthesis, suggesting that RAR; but not RXR, may be the major receptor involved in intracellular regulation of Ang II synthesis. Using selective RARα and RXRα agonists, we observed that activation of RARα significantly inhibited HG-induced gene expression of Ao and renin, and that activation of RXRα alone had no significant effect. However, activation either RARα or RXRα markedly suppressed the increased expression of AT1R. Silencing the expression of RARα or RXRα, increased the basal level of gene expression of Ao and dramatically increased Ao expression in combination with HG stimulation. In regard to renin expression, silencing RARα; but, not RXRα, significantly increased basal level expression and had a synergistic effect on HG-stimulated upregulation. Our results suggest the following: (1) a certain level of expression of RARα and RXRα is required in the regulation of expression of Ao and renin, under normal and hyperglycemic conditions; (2) RARα/RXRα heterodimer-mediated signaling, negatively regulates RAS components; (3) downregulation of RARα and RXRα contributes to hyperglycemia-induced expression/activation of RAS components; (4) RARα is the major receptor subtype responsible for regulating Ang II synthesis and gene expression of Ao and renin; and that RXRα has a coordinate role; and (5) activation of RARα and RXRα leads to a reduction in AT1R mediated signaling events, through downregulation of expression of AT1R.
In summary, our findings have several conceptual and therapeutic implications. The fact that activation of RAR and RXR and silencing the expression of RARα and RXRα, regulated cardiomyocyte apoptosis, provides evidence supporting a role of RARα and RXRα in hyperglycemia-induced development of cardiac remodeling. The negative regulatory effects of RARα and RXRα signaling, on hyperglycemia-induced expression of RAS components, indicate that pharmacologic manipulation of RARα and RXRα activity, may be beneficial in the treatment of heart dysfunction associated with increased expression of the renin-angiotensin system. Thus, selective activation of RARα and RXRα mediated signaling, may result in a cardioprotective effect, by inhibiting hyperglycemia/oxidative stress-induced apoptosis and cardiac expression of RAS components, providing an important strategy for therapeutic development in treating diabetic cardiomyopathy.
Acknowledgments
Contract grant sponsor: NIH
Contract grant number: 1R01 HL091902
REFERENCES
- Baena RM, Campoy C, Bayes R, Blanca E, Fernandez JM, Molina-Font JA. Vitamin A, retinol binding protein and lipids in type 1 diabetes mellitus. Eur J Clin Nutr. 2002;56(1):44–50. doi: 10.1038/sj.ejcn.1601279. [DOI] [PubMed] [Google Scholar]
- Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul Pept. 2004;120(1-3):5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Baraka A, AbdelGawad H. Targeting apoptosis in the heart of streptozotocin-induced diabetic rats. J Cardiovasc Pharmacol Ther. 2010;15(2):175–181. doi: 10.1177/1074248409356557. [DOI] [PubMed] [Google Scholar]
- Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Basu TK, Basualdo C. Vitamin A homeostasis and diabetes mellitus. Nutrition. 1997;13(9):804–806. doi: 10.1016/s0899-9007(97)00192-5. [DOI] [PubMed] [Google Scholar]
- Basualdo CG, Wein EE, Basu TK. Vitamin A (retinol) status of first nation adults with non-insulin-dependent diabetes mellitus. J Am Coll Nutr. 1997;16(1):39–45. doi: 10.1080/07315724.1997.10718647. [DOI] [PubMed] [Google Scholar]
- Boudjelal M, Voorhees JJ, Fisher GJ. Retinoid signaling is attenuated by proteasome-mediated degradation of retinoid receptors in human keratinocyte HaCaT cells. Exp Cell Res. 2002;274(1):130–137. doi: 10.1006/excr.2001.5450. [DOI] [PubMed] [Google Scholar]
- Bruck N, Bastien J, Bour G, Tarrade A, Plassat JL, Bauer A, Adam-Stitah S, Rochette-Egly C. Phosphorylation of the retinoid x receptor at the omega loop, modulates the expression of retinoic-acid-target genes with a promoter context specificity. Cell Signal. 2005;17(10):1229–1239. doi: 10.1016/j.cellsig.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Cai L, Kang YJ. Cell death and diabetic cardiomyopathy. Cardiovasc Toxicol. 2003;3(3):219–228. doi: 10.1385/ct:3:3:219. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang Y, Zhou G, Chen T, Song Y, Li X, Kang YJ. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48(8):1688–1697. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29(11):2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- Choudhary R, Baker KM, Pan J. All-trans retinoic acid prevents angiotensin II- and mechanical stretch-induced reactive oxygen species generation and cardiomyocyte apoptosis. J Cell Physiol. 2008a;215(1):172–181. doi: 10.1002/jcp.21297. [DOI] [PubMed] [Google Scholar]
- Choudhary R, Palm-Leis A, Scott RC, 3rd, Guleria RS, Rachut E, Baker KM, Pan J. All-trans retinoic acid prevents development of cardiac remodeling in aortic banded rats by inhibiting the renin-angiotensin system. Am J Physiol Heart Circ Physiol. 2008b;294(2):H633–644. doi: 10.1152/ajpheart.01301.2007. [DOI] [PubMed] [Google Scholar]
- Claycomb WC, Palazzo MC. Culture of the terminally differentiated adult cardiac muscle cell: a light and scanning electron microscope study. Dev Biol. 1980;80(2):466–482. doi: 10.1016/0012-1606(80)90419-4. [DOI] [PubMed] [Google Scholar]
- Dawson MI. Synthetic retinoids and their nuclear receptors. Curr Med Chem Anticancer Agents. 2004;4(3):199–230. doi: 10.2174/1568011043352975. [DOI] [PubMed] [Google Scholar]
- Dawson MI, Zhang XK. Discovery and design of retinoic acid receptor and retinoid X receptor class- and subtype-selective synthetic analogs of all-trans-retinoic acid and 9-cis-retinoic acid. Curr Med Chem. 2002;9(6):623–637. doi: 10.2174/0929867023370789. [DOI] [PubMed] [Google Scholar]
- Dechow C, Morath C, Peters J, Lehrke I, Waldherr R, Haxsen V, Ritz E, Wagner J. Effects of all-trans retinoic acid on renin-angiotensin system in rats with experimental nephritis. Am J Physiol Renal Physiol. 2001;281(5):F909–919. doi: 10.1152/ajprenal.2001.281.5.F909. [DOI] [PubMed] [Google Scholar]
- Delescluse C, Cavey MT, Martin B, Bernard BA, Reichert U, Maignan J, Darmon M, Shroot B. Selective high affinity retinoic acid receptor alpha or beta-gamma ligands. Mol Pharmacol. 1991;40(4):556–562. [PubMed] [Google Scholar]
- Delmotte MH, Tahayato A, Formstecher P, Lefebvre P. Serine 157, a retinoic acid receptor alpha residue phosphorylated by protein kinase C in vitro, is involved in RXR.RARalpha heterodimerization and transcriptional activity. J Biol Chem. 1999;274(53):38225–38231. doi: 10.1074/jbc.274.53.38225. [DOI] [PubMed] [Google Scholar]
- Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101(19):2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- Dyson E, Sucov HM, Kubalak SW, Schmid-Schonbein GW, DeLano FA, Evans RM, Ross J, Jr., Chien KR. Atrial-like phenotype is associated with embryonic ventricular failure in retinoid X receptor alpha -/- mice. Proc Natl Acad Sci U S A. 1995;92(16):7386–7390. doi: 10.1073/pnas.92.16.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzau VJ. Theodore Cooper Lecture: Tissue angiotensin and pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37(4):1047–1052. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- Ebisawa M, Umemiya H, Ohta K, Fukasawa H, Kawachi E, Christoffel G, Gronemeyer H, Tsuji M, Hashimoto Y, Shudo K, Kagechika H. Retinoid X receptor-antagonistic diazepinylbenzoic acids. Chem Pharm Bull (Tokyo) 1999;47(12):1778–1786. doi: 10.1248/cpb.47.1778. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Bianchi R, Staszewsky L, Cuccovillo I, Doni M, Laragione T, Salio M, Savino C, Melucci S, Santangelo F, Scanziani E, Masson S, Ghezzi P, Latini R. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol. 2004;37(5):959–968. doi: 10.1016/j.yjmcc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Haxsen V, Adam-Stitah S, Ritz E, Wagner J. Retinoids inhibit the actions of angiotensin II on vascular smooth muscle cells. Circ Res. 2001;88(6):637–644. doi: 10.1161/01.res.88.6.637. [DOI] [PubMed] [Google Scholar]
- Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002;143(8):2975–2985. doi: 10.1210/endo.143.8.8931. [DOI] [PubMed] [Google Scholar]
- Ilercil A, Devereux RB, Roman MJ, Paranicas M, O'Grady M J, Welty TK, Robbins DC, Fabsitz RR, Howard BV, Lee ET. Relationship of impaired glucose tolerance to left ventricular structure and function: The Strong Heart Study. Am Heart J. 2001;141(6):992–998. doi: 10.1067/mhj.2001.115302. [DOI] [PubMed] [Google Scholar]
- Johnson AT, Klein ES, Gillett SJ, Wang L, Song TK, Pino ME, Chandraratna RA. Synthesis and characterization of a highly potent and effective antagonist of retinoic acid receptors. J Med Chem. 1995;38(24):4764–4767. doi: 10.1021/jm00024a003. [DOI] [PubMed] [Google Scholar]
- Karnik AA, Fields AV, Shannon RP. Diabetic cardiomyopathy. Curr Hypertens Rep. 2007;9(6):467–473. doi: 10.1007/s11906-007-0086-3. [DOI] [PubMed] [Google Scholar]
- Kastner P, Messaddeq N, Mark M, Wendling O, Grondona JM, Ward S, Ghyselinck N, Chambon P. Vitamin A deficiency and mutations of RXRalpha, RXRbeta and RARalpha lead to early differentiation of embryonic ventricular cardiomyocytes. Development. 1997;124(23):4749–4758. doi: 10.1242/dev.124.23.4749. [DOI] [PubMed] [Google Scholar]
- Katare RG, Caporali A, Oikawa A, Meloni M, Emanueli C, Madeddu P. Vitamin B1 analog benfotiamine prevents diabetes-induced diastolic dysfunction and heart failure through Akt/Pim-1-mediated survival pathway. Circ Heart Fail. 2010;3(2):294–305. doi: 10.1161/CIRCHEARTFAILURE.109.903450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein ES, Pino ME, Johnson AT, Davies PJ, Nagpal S, Thacher SM, Krasinski G, Chandraratna RA. Identification and functional separation of retinoic acid receptor neutral antagonists and inverse agonists. J Biol Chem. 1996;271(37):22692–22696. doi: 10.1074/jbc.271.37.22692. [DOI] [PubMed] [Google Scholar]
- Kopf E, Plassat JL, Vivat V, de The H, Chambon P, Rochette-Egly C. Dimerization with retinoid X receptors and phosphorylation modulate the retinoic acid-induced degradation of retinoic acid receptors alpha and gamma through the ubiquitin-proteasome pathway. J Biol Chem. 2000;275(43):33280–33288. doi: 10.1074/jbc.M002840200. [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupe V, Mark M, Dierich A, Kastner P, Chambon P. RXR gamma null mice are apparently normal and compound RXR alpha +/-/RXR beta -/-/RXR gamma -/- mutant mice are viable. Proc Natl Acad Sci U S A. 1996;93(17):9010–9014. doi: 10.1073/pnas.93.17.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuethe F, Sigusch HH, Bornstein SR, Hilbig K, Kamvissi V, Figulla HR. Apoptosis in patients with dilated cardiomyopathy and diabetes: a feature of diabetic cardiomyopathy? Horm Metab Res. 2007;39(9):672–676. doi: 10.1055/s-2007-985823. [DOI] [PubMed] [Google Scholar]
- Leibowitz MD, Ardecky RJ, Boehm MF, Broderick CL, Carfagna MA, Crombie DL, D'Arrigo J, Etgen GJ, Faul MM, Grese TA, Havel H, Hein NI, Heyman RA, Jolley D, Klausing K, Liu S, Mais DE, Mapes CM, Marschke KB, Michellys PY, Montrose-Rafizadeh C, Ogilvie KM, Pascual B, Rungta D, Tyhonas JS, Urcan MS, Wardlow M, Yumibe N, Reifel-Miller A. Biological characterization of a heterodimer-selective retinoid X receptor modulator: potential benefits for the treatment of type 2 diabetes. Endocrinology. 2006;147(2):1044–1053. doi: 10.1210/en.2005-0690. [DOI] [PubMed] [Google Scholar]
- Li CJ, Zhang QM, Li MZ, Zhang JY, Yu P, Yu DM. Attenuation of myocardial apoptosis by alpha-lipoic acid through suppression of mitochondrial oxidative stress to reduce diabetic cardiomyopathy. Chin Med J (Engl) 2009;122(21):2580–2586. [PubMed] [Google Scholar]
- Li E, Sucov HM, Lee KF, Evans RM, Jaenisch R. Normal development and growth of mice carrying a targeted disruption of the alpha 1 retinoic acid receptor gene. Proc Natl Acad Sci U S A. 1993;90(4):1590–1594. doi: 10.1073/pnas.90.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73(4):643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120(10):2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Luo J, Pasceri P, Conlon RA, Rossant J, Giguere V. Mice lacking all isoforms of retinoic acid receptor beta develop normally and are susceptible to the teratogenic effects of retinoic acid. Mech Dev. 1995;53(1):61–71. doi: 10.1016/0925-4773(95)00424-6. [DOI] [PubMed] [Google Scholar]
- Marra G, Cotroneo P, Pitocco D, Manto A, Di Leo MA, Ruotolo V, Caputo S, Giardina B, Ghirlanda G, Santini SA. Early increase of oxidative stress and reduced antioxidant defenses in patients with uncomplicated type 1 diabetes: a case for gender difference. Diabetes Care. 2002;25(2):370–375. doi: 10.2337/diacare.25.2.370. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120(10):2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Montessuit C, Papageorgiou I, Lerch R. Nuclear receptor agonists improve insulin responsiveness in cultured cardiomyocytes through enhanced signaling and preserved cytoskeletal architecture. Endocrinology. 2008;149(3):1064–1074. doi: 10.1210/en.2007-0656. [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR, Jr., Heyman RA. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386(6623):407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Harrison DG. The AT(1)-type angiotensin receptor in oxidative stress and atherogenesis: part I: oxidative stress and atherogenesis. Circulation. 2002;105(3):393–396. doi: 10.1161/hc0302.102618. [DOI] [PubMed] [Google Scholar]
- Palm-Leis A, Singh US, Herbelin BS, Olsovsky GD, Baker KM, Pan J. Mitogen-activated protein kinases and mitogen-activated protein kinase phosphatases mediate the inhibitory effects of all-trans retinoic acid on the hypertrophic growth of cardiomyocytes. J Biol Chem. 2004;279(52):54905–54917. doi: 10.1074/jbc.M407383200. [DOI] [PubMed] [Google Scholar]
- Penckofer S, Schwertz D, Florczak K. Oxidative stress and cardiovascular disease in type 2 diabetes: the role of antioxidants and pro-oxidants. J Cardiovasc Nurs. 2002;16(2):68–85. doi: 10.1097/00005082-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Rajamani U, Essop MF. Hyperglycemia-mediated activation of the hexosamine biosynthetic pathway results in myocardial apoptosis. Am J Physiol Cell Physiol. 2010;299(1):C139–147. doi: 10.1152/ajpcell.00020.2010. [DOI] [PubMed] [Google Scholar]
- Ricci C, Jong CJ, Schaffer SW. Proapoptotic and antiapoptotic effects of hyperglycemia: role of insulin signaling. Can J Physiol Pharmacol. 2008;86(4):166–172. doi: 10.1139/Y08-021. [DOI] [PubMed] [Google Scholar]
- Sakoda M, Ichihara A, Kurauchi-Mito A, Narita T, Kinouchi K, Murohashi-Bokuda K, Saleem MA, Nishiyama A, Suzuki F, Itoh H. Aliskiren inhibits intracellular angiotensin II levels without affecting (pro)renin receptor signals in human podocytes. Am J Hypertens. 2010;23(5):575–580. doi: 10.1038/ajh.2009.273. [DOI] [PubMed] [Google Scholar]
- Schaffer SW, Croft CB, Solodushko V. Cardioprotective effect of chronic hyperglycemia: effect on hypoxia-induced apoptosis and necrosis. Am J Physiol Heart Circ Physiol. 2000;278(6):H1948–1954. doi: 10.1152/ajpheart.2000.278.6.H1948. [DOI] [PubMed] [Google Scholar]
- Shan P, Pu J, Yuan A, Shen L, Chai D, He B. RXR agonists inhibit oxidative stress-induced apoptosis in H9c2 rat ventricular cells. Biochem Biophys Res Commun. 2008;375(4):628–633. doi: 10.1016/j.bbrc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- Shen E, Li Y, Shan L, Zhu H, Feng Q, Arnold JM, Peng T. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes. 2009;58(10):2386–2395. doi: 10.2337/db08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Le B, Bhat VB, Baker KM, Kumar R. High-glucose-induced regulation of intracellular ANG II synthesis and nuclear redistribution in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293(2):H939–948. doi: 10.1152/ajpheart.00391.2007. [DOI] [PubMed] [Google Scholar]
- Song Y, Du Y, Prabhu SD, Epstein PN. Diabetic Cardiomyopathy in OVE26 Mice Shows Mitochondrial ROS Production and Divergence Between In Vivo and In Vitro Contractility. Rev Diabet Stud. 2007;4(3):159–168. doi: 10.1900/RDS.2007.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stosic-Grujicic S, Cvjeticanin T, Stojanovic I. Retinoids differentially regulate the progression of autoimmune diabetes in three preclinical models in mice. Mol Immunol. 2009;47(1):79–86. doi: 10.1016/j.molimm.2008.12.028. [DOI] [PubMed] [Google Scholar]
- Subbarayan V, Mark M, Messadeq N, Rustin P, Chambon P, Kastner P. RXRalpha overexpression in cardiomyocytes causes dilated cardiomyopathy but fails to rescue myocardial hypoplasia in RXRalpha-null fetuses. J Clin Invest. 2000;105(3):387–394. doi: 10.1172/JCI8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM. RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8(9):1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- Takeda K, Ichiki T, Funakoshi Y, Ito K, Takeshita A. Downregulation of angiotensin II type 1 receptor by all-trans retinoic acid in vascular smooth muscle cells. Hypertension. 2000;35(1 Pt 2):297–302. doi: 10.1161/01.hyp.35.1.297. [DOI] [PubMed] [Google Scholar]
- Tuitoek PJ, Ziari S, Tsin AT, Rajotte RV, Suh M, Basu TK. Streptozotocin-induced diabetes in rats is associated with impaired metabolic availability of vitamin A (retinol). Br J Nutr. 1996;75(4):615–622. doi: 10.1079/bjn19960164. [DOI] [PubMed] [Google Scholar]
- Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes. 2009;58(1):146–155. doi: 10.2337/db08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Zhu YC, Yao T. Effects of all-trans retinoic acid on angiotensin II-induced myocyte hypertrophy. J Appl Physiol. 2002;92(5):2162–2168. doi: 10.1152/japplphysiol.01192.2001. [DOI] [PubMed] [Google Scholar]
- Wang P, Chatham JC. Onset of diabetes in Zucker diabetic fatty (ZDF) rats leads to improved recovery of function after ischemia in the isolated perfused heart. Am J Physiol Endocrinol Metab. 2004;286(5):E725–736. doi: 10.1152/ajpendo.00295.2003. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15(22):2922–2933. [PubMed] [Google Scholar]
- Wu J, Garami M, Cheng T, Gardner DG. 1,25(OH)2 vitamin D3, and retinoic acid antagonize endothelin-stimulated hypertrophy of neonatal rat cardiac myocytes. J Clin Invest. 1996;97(7):1577–1588. doi: 10.1172/JCI118582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Takashi E, Kudo M, Ishiwata T, Naito Z. Contradictory effects of short- and long-term hyperglycemias on ischemic injury of myocardium via intracellular signaling pathway. Exp Mol Pathol. 2004;76(1):57–65. doi: 10.1016/j.yexmp.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436(7049):356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Zhong JC, Huang DY, Liu GF, Jin HY, Yang YM, Li YF, Song XH, Du K. Effects of all-trans retinoic acid on orphan receptor APJ signaling in spontaneously hypertensive rats. Cardiovasc Res. 2005;65(3):743–750. doi: 10.1016/j.cardiores.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Zhong JC, Huang DY, Yang YM, Li YF, Liu GF, Song XH, Du K. Upregulation of angiotensin-converting enzyme 2 by all-trans retinoic acid in spontaneously hypertensive rats. Hypertension. 2004;44(6):907–912. doi: 10.1161/01.HYP.0000146400.57221.74. [DOI] [PubMed] [Google Scholar]
- Zhou MD, Sucov HM, Evans RM, Chien KR. Retinoid-dependent pathways suppress myocardial cell hypertrophy. Proc Natl Acad Sci U S A. 1995;92(16):7391–7395. doi: 10.1073/pnas.92.16.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]