Abstract
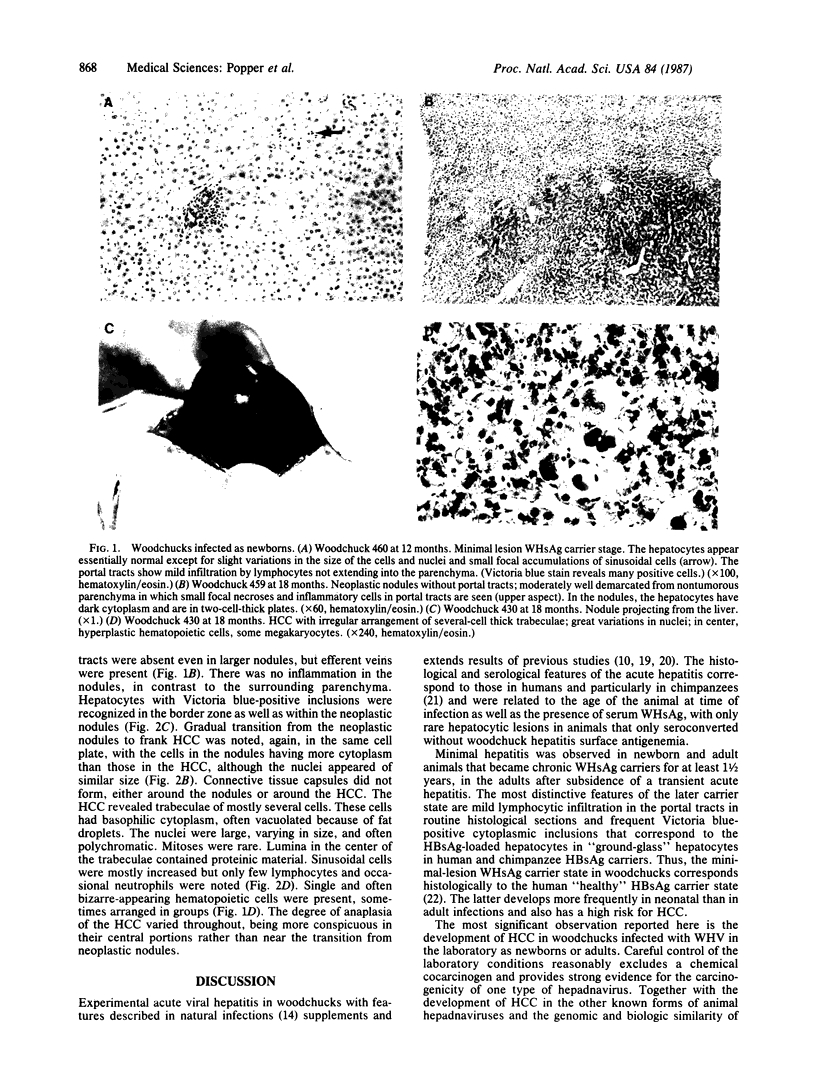
During investigations of the evolution of experimental laboratory infections of woodchucks (Marmota monax) with the woodchuck hepatitis virus (WHV), eight hepatocellular carcinomas (HCC) were observed, six in newborns and two in young adult animals, all within 17-36 months after infection. The absence of an external cocarcinogenic effect in the well-monitored woodchucks indicates the carcinogenicity of WHV and suggests the same for the genetically and biologically similar human hepatitis B virus (HBV). Laboratory infections of woodchucks with two strains of WHV, not reported here in detail, resembled human and chimpanzee HBV infections histologically and serologically. In these studies, eight woodchucks became carriers of surface antigen of WHV for greater than 1 year. All eight woodchucks developed HCC, indicating a 100% risk of HCC in experimentally infected chronic WHV antigen carriers, which is analogous to the high risk of HCC in human hepatitis B surface antigen carriers. Histologically, the absence of cirrhosis in the examined pericarcinomatous tissue permits recognition of gradual transition from normal parenchyma to neoplastic nodules to HCC of rising anaplasia, indicating a continuum of increasingly more malignant neoplastic stages, as known for chemical carcinogenesis. The HCC developed in carrier woodchucks infected as newborns with only minor, if any, hepatitic changes but is associated with antigen-carrying hepatocytes and sometimes with hyperplastic nodules. This stage was preceded in infected adults by an early, acute, weeks-long hepatitis coinciding with the appearance of surface antigen. These findings are also analogous to typical HBV infection in human newborns and young adults, respectively. At the time of HCC development in all animals with adequate histologic material, an acute recent necroinflammation appeared around the tumor, associated with abnormal hematopoietic cells around and within the tumor. A promoting role in carcinogenesis of this necroinflammation of yet unestablished pathogenesis is being postulated, to be confirmed by determination of the status of the WHV DNA in the HCC and by prospective histologic study of the inflammatory reaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg B. S., Larouzé B., London W. T., Werner B., Hesser J. E., Millman I., Saimot G., Payet M. The relation of infection with the hepatitis B agent to primary hepatic carcinoma. Am J Pathol. 1975 Dec;81(3):669–682. [PMC free article] [PubMed] [Google Scholar]
- Cote P. J., Engle R. E., Langer C. A., Ponzetto A., Gerin J. L. Antigenic analysis of woodchuck hepatitis virus surface antigen with site-specific radioimmunoassays. J Virol. 1984 Mar;49(3):701–708. doi: 10.1128/jvi.49.3.701-708.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. L., Hoofnagle J. H., Waggoner J. G. Spontaneous reactivation of chronic hepatitis B virus infection. Gastroenterology. 1984 Feb;86(2):230–235. [PubMed] [Google Scholar]
- Farber E. The multistep nature of cancer development. Cancer Res. 1984 Oct;44(10):4217–4223. [PubMed] [Google Scholar]
- Frommel D., Crevat D., Vitvitsky L., Pichoud C., Hantz O., Chevalier M., Grimaud J. A., Lindberg J., Trépo C. G. Immunopathologic aspects of woodchuck hepatitis. Am J Pathol. 1984 Apr;115(1):125–134. [PMC free article] [PubMed] [Google Scholar]
- Korba B. E., Wells F., Tennant B. C., Yoakum G. H., Purcell R. H., Gerin J. L. Hepadnavirus infection of peripheral blood lymphocytes in vivo: woodchuck and chimpanzee models of viral hepatitis. J Virol. 1986 Apr;58(1):1–8. doi: 10.1128/jvi.58.1.1-8.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo Y., Okuda K., Musha H., Nakashima T. Detection of hepatocellular carcinoma during a clinical follow-up of chronic liver disease: observations in 31 patients. Gastroenterology. 1978 Mar;74(3):578–582. [PubMed] [Google Scholar]
- Marion P. L., Knight S. S., Ho B. K., Guo Y. Y., Robinson W. S., Popper H. Liver disease associated with duck hepatitis B virus infection of domestic ducks. Proc Natl Acad Sci U S A. 1984 Feb;81(3):898–902. doi: 10.1073/pnas.81.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Knight S. S., Salazar F. H., Popper H., Robinson W. S. Ground squirrel hepatitis virus infection. Hepatology. 1983 Jul-Aug;3(4):519–527. doi: 10.1002/hep.1840030408. [DOI] [PubMed] [Google Scholar]
- Marion P. L., Van Davelaar M. J., Knight S. S., Salazar F. H., Garcia G., Popper H., Robinson W. S. Hepatocellular carcinoma in ground squirrels persistently infected with ground squirrel hepatitis virus. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4543–4546. doi: 10.1073/pnas.83.12.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman I., Southam L., Halbherr T., Simmons H., Kang C. M. Woodchuck hepatitis virus: experimental infection and natural occurrence. Hepatology. 1984 Sep-Oct;4(5):817–823. doi: 10.1002/hep.1840040503. [DOI] [PubMed] [Google Scholar]
- Okuda K. Primary liver cancer. Quadrennial review lecture. Dig Dis Sci. 1986 Sep;31(9 Suppl):133S–146S. doi: 10.1007/BF01295995. [DOI] [PubMed] [Google Scholar]
- Omata M., Uchiumi K., Ito Y., Yokosuka O., Mori J., Terao K., Wei-Fa Y., O'Connell A. P., London W. T., Okuda K. Duck hepatitis B virus and liver diseases. Gastroenterology. 1983 Aug;85(2):260–267. [PubMed] [Google Scholar]
- Omata M., Yokosuka O., Imazeki F., Matsuyama Y., Uchiumi K., Ito Y., Mori J., Okuda K. Transmission of duck hepatitis B virus from Chinese carrier ducks to Japanese ducklings: a study of viral DNA in serum and tissue. Hepatology. 1984 Jul-Aug;4(4):603–607. doi: 10.1002/hep.1840040404. [DOI] [PubMed] [Google Scholar]
- POPPER H., STERNBERG S. S., OSER B. L., OSER M. The carcinogenic effect of aramite in rats. A study of hepatic nodules. Cancer. 1960 Sep-Oct;13:1035–1046. doi: 10.1002/1097-0142(196009/10)13:5<1035::aid-cncr2820130526>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Peers F. G., Linsell C. A. Dietary aflatoxins and liver cancer--a population based study in Kenya. Br J Cancer. 1973 Jun;27(6):473–484. doi: 10.1038/bjc.1973.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto A., Cote P. J., Ford E. C., Engle R., Cicmanec J., Shapiro M., Purcell R. H., Gerin J. L. Radioimmunoassay and characterization of woodchuck hepatitis virus core antigen and antibody. Virus Res. 1985 Jun;2(4):301–315. doi: 10.1016/0168-1702(85)90027-9. [DOI] [PubMed] [Google Scholar]
- Popper H., Dienstag J. L., Feinstone S. M., Alter H. J., Purcell R. H. The pathology of viral hepatitis in chimpanzees. Virchows Arch A Pathol Anat Histol. 1980;387(1):91–106. [PubMed] [Google Scholar]
- Popper H., Shih J. W., Gerin J. L., Wong D. C., Hoyer B. H., London W. T., Sly D. L., Purcell R. H. Woodchuck hepatitis and hepatocellular carcinoma: correlation of histologic with virologic observations. Hepatology. 1981 Mar-Apr;1(2):91–98. doi: 10.1002/hep.1840010202. [DOI] [PubMed] [Google Scholar]
- Rogler C. E., Sherman M., Su C. Y., Shafritz D. A., Summers J., Shows T. B., Henderson A., Kew M. Deletion in chromosome 11p associated with a hepatitis B integration site in hepatocellular carcinoma. Science. 1985 Oct 18;230(4723):319–322. doi: 10.1126/science.2996131. [DOI] [PubMed] [Google Scholar]
- Rogler C. E., Summers J. Cloning and structural analysis of integrated woodchuck hepatitis virus sequences from a chronically infected liver. J Virol. 1984 Jun;50(3):832–837. doi: 10.1128/jvi.50.3.832-837.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. L., Tyler G., Summers J. Chronic hepatitis and hepatocellular carcinoma associated with woodchuck hepatitis virus. Am J Pathol. 1982 Jun;107(3):422–425. [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J. Three recently described animal virus models for human hepatitis B virus. Hepatology. 1981 Mar-Apr;1(2):179–183. doi: 10.1002/hep.1840010215. [DOI] [PubMed] [Google Scholar]
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- Tanaka K., Mori W., Suwa K. Victoria blue-nuclear fast red stain for HBs antigen detection in paraffin section. Acta Pathol Jpn. 1981 Jan;31(1):93–98. doi: 10.1111/j.1440-1827.1981.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Tyler G. V., Snyder R. L., Summers J. Experimental infection of the woodchuck (Marmota monax monax) with woodchuck hepatitis virus. Lab Invest. 1986 Jul;55(1):51–55. [PubMed] [Google Scholar]
- Wogan G. N. Dietary factors and special epidemiological situations of liver cancer in Thailand and Africa. Cancer Res. 1975 Nov;35(11 Pt 2):3499–3502. [PubMed] [Google Scholar]
- Ying T. S., Sarma D. S., Farber E. Role of acute hepatic necrosis in the induction of early steps in liver carcinogenesis by diethylnitrosamine. Cancer Res. 1981 Jun;41(6):2096–2102. [PubMed] [Google Scholar]
- zur Hausen H. Intracellular surveillance of persisting viral infections. Human genital cancer results from deficient cellular control of papillomavirus gene expression. Lancet. 1986 Aug 30;2(8505):489–491. doi: 10.1016/s0140-6736(86)90360-0. [DOI] [PubMed] [Google Scholar]