Abstract
Background & Aims
Alcohol has been implicated in the development of chronic pancreatitis (CP) in 60%–90% patients, although percentages in the United States are not known. We investigated the frequency of alcohol-related CP at tertiary U.S. referral centers.
Methods
We studied data from patients with CP (n=539) and controls (n=695) enrolled in the North American Pancreatitis Study-2 from 2000 to 2006 at 20 U.S. referral centers. CP was defined by definitive evidence in imaging or histologic analyses. Subjects and physicians each completed a detailed study questionnaire. Using physician-assigned diagnoses, patients were assigned to the following etiology groups: alcohol (with/without other diagnoses), non-alcohol (any etiology of CP from other than alcohol), or idiopathic (no etiology identified).
Results
The distribution of patients among etiology groups were: alcohol (44.5%), non-alcohol (26.9%), and idiopathic (28.6%). Physicians identified alcohol as the etiology more frequently in men (59.4% in men vs 28.1% in women), but non-alcohol (18% in men vs 36.7% in women) and idiopathic etiologies (22.6% in men vs 35.2% in women) more often in women (P<0.01 for all comparisons). Non-alcohol etiologies were equally divided among obstructive, genetic, and other causes. Compared with controls, patients with idiopathic CP were more likely to have ever smoked (58.6% vs 49.7%, P<0.05) or have a history of chronic renal disease or failure (5.2% vs 1.2%, P<0.01). In multivariate analyses, smoking (ever, current, and amount) was independently associated with idiopathic CP.
Conclusions
The frequency of alcohol-related CP at tertiary U.S. referral centers is lower than expected. Idiopathic CP and non-alcohol etiologies represent a large subgroup, particularly among women. Smoking is an independent risk factor for idiopathic CP.
Keywords: Pancreas, Inflammation, alcoholism, tobacco
Introduction
The pathogenesis of chronic pancreatitis (CP) remains elusive. While there are presumably several mechanisms, CP appears to be a disorder of chronic inflammation which leads to irreversible fibrosis and scarring.1, 2 At the time of presentation, the clinical manifestations and disease progression are often unpredictable. Symptoms range from none to intractable pain and weight loss related to exocrine insufficiency with or without endocrine insufficiency.
Historically, alcohol has been implicated as the etiology of CP in 60–90% of cases diagnosed in Western countries.3–11 Until recently, little was known as to the risk of CP based on the amounts of alcohol consumption.12, 13 Contrary to previous data, two recent multicenter studies from Western countries have reported a lower prevalence of heavy alcohol use among CP patients who are evaluated at referral centers.13, 14 In addition to alcohol being less prevalent, smoking not only appears to potentiate the effect of alcohol in acute and chronic pancreatitis but serves as an independent predictor for disease susceptibility13, 15–19 and progression.20, 21
In the past two decades, genetic discoveries using linkage and candidate gene approaches have identified several pancreas-targeting factors that affect susceptibility to acute and CP. These genes target the acinar cells through a trypsin-dependent pathway (PRSS122, PRSS223, 24, CTRC25, 26, CASR27, 28, SPINK129, 30) or the duct cells (CFTR).31, 32 There is a growing recognition that development of CP occurs from a complex interaction between environmental (e.g., alcohol) and genetic factors.33, 34 Moreover, the use of cross-sectional imaging studies to evaluate abdominal symptoms has increased over the past two-three decades. In light of these developments, we hypothesized that the current etiologic profile of CP at U.S. referral centers would be different from historical data and include a more diverse group of etiologies.
The North American Pancreatitis Study 2 (NAPS2) was designed as a molecular genetics study to further our understanding of the role of gene-environment interactions in patients with recurrent acute pancreatitis (RAP) and CP.35 Consisting of a large cohort of well-phenotyped CP patients enrolled by a consortium of U.S. referral centers from 2000–2006, the NAPS2 dataset is uniquely poised to evaluate the current etiologic profile of CP at U.S. referral centers and how it relates to historical data. Therefore, our objective is to evaluate the epidemiology of CP at tertiary care medical centers in the United States using the NAPS2 database. In particular, we focus on the etiology of CP based on the assessment of the treating physician at the time of patient enrollment, and compare the clinical characteristics across different etiologic groups. Finally, we compare the clinical characteristics of patients classified as idiopathic CP with control subjects.
Methods
Overview of North American Pancreatitis Study 2 (NAPS2)
The NAPS2 represents a multicenter effort in which nineteen academic and community practice referral centers with expertise in the evaluation and management of pancreatic disorders across the United States prospectively enrolled patients who met strict criteria for CP or RAP and control subjects from 2000–2006. An additional primary care center enrolled only control subjects. The methodology of NAPS2 has been detailed previously.35 The entry criteria for CP included definitive evidence on CT scan or ERCP using the Cambridge classification.36 While 83% of cases fulfilled one or both of these criteria, other enrollees had documentation of CP using MRCP, EUS or pancreatic histology. Pancreatitis patients completed a detailed questionnaire on personal and family history, risk factors, symptoms and quality of life, and their enrolling physicians and research nurses completed a separate questionnaire containing questions relating to clinical phenotype, working diagnosis, risk factors, diagnostic and therapeutic interventions. Control patients included unaffected first degree relatives, spouses, accompanying friends or unrelated subjects without pancreatitis.35 All control subjects completed the patient questionnaire. All patients and control subjects signed an informed consent. The study was approved by the Institutional Review Board of each participating center.
Classification based on etiology of CP
The enrolling physician was asked to provide a working diagnosis for the patient's CP from the following choices: alcohol, idiopathic, hereditary, cystic fibrosis, pancreas divisum, hyperlipidemia, hypercalcemia, trauma, and other. One or more etiologies could be checked by the physician. If the physician checked the “other” category, space was provided to specify a diagnosis. Since the study questionnaire was designed for both CP and RAP, additional etiologies listed in the working diagnosis question specific to RAP (gallstones, medications, post-ERCP pancreatitis) were not considered as etiologies for CP. The working diagnosis of pancreatitis due to hereditary causes or cystic fibrosis was based on a patient's personal and family history with or without formal genetic testing. Physicians were not given any specific instructions on the amount or duration of alcohol consumption for assigning alcohol etiology.
Using the working diagnosis, patients were assigned to an etiology group using a hierarchical algorithm as follows: if alcohol was checked as the sole or in conjunction with other working diagnoses, the patient was assigned to “alcohol” etiology group; among remaining patients, those with hereditary or cystic fibrosis diagnosis by itself or with another diagnosis were assigned to “genetic” etiology group; among remaining patients, those with autoimmune pancreatitis diagnosis by itself or with another diagnoses were assigned to “autoimmune” etiology group; among remaining patients, those with an obstructive etiology (e.g., pancreas divisum,) by itself or with another diagnosis were assigned to “obstructive” etiology group; among remaining patients, those identified with a specific etiology not included into any of the previous group were assigned to “other” etiology group; all the remaining patients were then assigned to “idiopathic” etiology group. Thus, the idiopathic group represents patients in whom no etiology was identified by the physician during their diagnostic evaluation until the time of enrollment into the NAPS2 study. We combined all patients in the “genetic”, “autoimmune”, “obstructive” and “other” etiology groups into a single category of “non-alcohol” etiology group for the purposes of statistical analysis, thereby forming three overall groups: alcohol, non-alcohol and idiopathic for comparison of demographics, risk factors and phenotypic characteristics.
Patient characteristics and disease phenotype
We compare patient characteristics including age, sex, race (defined as white, black or other) and body-mass index (BMI) (kg/m2) in alcohol, non-alcohol and idiopathic CP groups. In addition, we report the prevalence of select risk modifiers and potential risk factors: tobacco and alcohol use, along with a history of renal disease or failure. Tobacco exposure was classified as ever (> 100 cigarettes in their lifetime) or never, and quantified as packs per day (ppd) (<1 or ≥1) and pack years (<12, 12–35, ≥35). Ever alcohol drinkers completed the TWEAK questionnaire, a previously validated measure of at-risk drinking.37 A composite score (range, 0–7) was calculated based on patient responses. Further details on the TWEAK questionnaire within the framework of NAPS2 have been published.35 Based on previous data, At-risk drinking was defined as a score of 3 or higher.13 As previously reported, controls and patients were assigned to five drinking categories based on responses to the quantity and frequency of drinking during the maximum lifetime drinking period.35 The drinking categories included – abstainer (no alcohol use or <20 drinks in lifetime), light (≤0.5 drinks/day), moderate (>0.5–1 drinks/day for females, >0.5–2 drinks/day for males), heavy (>1 – <5 drinks/day for females, >2 –<5 drinks/day for males), and, very heavy (≥5 drinks/day for both genders). The presence of renal disease or failure was determined solely from patient responses.
Statistical analysis
Descriptive analyses are presented as proportions for categorical data and as mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous data, as applicable. Bivariate comparisons for continuous variables were performed using the student's-t test, and for categorical data using chi-squared test or Cochran-Armitage test, as applicable. The association between smoking and idiopathic CP was assessed using multivariable logistic regression analyses. Specifically, we used alcohol consumption (as drinking categories), age (as a continuous variable), sex (male, female), and current or maximum body mass index [BMI]) (normal/low, ≤25kg/m2; overweight, >25–≤30; obese, >30) as covariates. Smoking was assessed as never or ever; never, <1, ≥1 ppd; and never, <12, 12–35, >35 pack years. We used the combination of abstainers and light drinkers as the reference category for alcohol consumption. Regression models were evaluated by the goodness of fit χ2 test. We defined significance using two-sided p-values <0.05. Analysis was performed using the R Project software (www.r-project.org) and SPSS version 16 (SPSS Inc., Chicago, IL.).
Results
Demographics and Individual Working diagnoses
Of the 539 patients with CP, 484 (90%) were enrolled from 11 of the 19 participating centers that recruited pancreatitis patients. The mean age of patients at the time of enrollment was 49.5 ±15 years, 52.5% were males and 84.5% were White. Overall, alcohol was implicated as a working diagnosis in 44.5% of cases, more frequently in males (59.4%) compared to females (28.1%) (p<0.001). An unknown cause (i.e., idiopathic) was suspected more often in females (48%) compared to males (31.8%) (p=0.0001). Obstructive causes were implicated more frequently in females; in particular, pancreas divisum was considered as the working diagnosis more frequently in females (13.7%) than males (5.7%) (p < 0.002). Other commonly cited working diagnoses included hereditary (9.3%), hyperlipidemia (6.1%), cystic fibrosis (2.8%) and autoimmune pancreatitis (2.4%) (Table 1). Physicians cited one working diagnosis in 79.4% of cases (77% of females, 81.3% of males), two diagnoses in 17.4% (19.1% of females, 15.9% of males) and three or more in 3.2% patients (3.5% of females, 2.8% of males). The number of working diagnoses cited was similar for both genders (p = 0.31).
Table 1.
Distribution of individual working diagnoses for chronic pancreatitis patients (all and stratified by gender) in the North American Pancreatitis Study 2
| Working diagnosis (%) | All (N=539) | Female (N=256) | Male (N=283) |
|---|---|---|---|
| Alcohol | 240 (44.5) | 72 (28.1) | 168 (59.4) |
| Idiopathic | 213 (39.5) | 123 (48.0) | 90 (31.8) |
| Pancreas divisum | 51 (9.5) | 35 (13.7) | 16 (5.7) |
| Hereditary | 50 (9.3) | 30 (11.7) | 20 (7.1) |
| Hyperlipidemia | 33 (6.1) | 16 (6.3) | 17 (6.0) |
| Cystic fibrosis | 15 (2.8) | 9 (3.5) | 6 (2.1) |
| Autoimmune pancreatitis | 13 (2.4) | 7 (2.7) | 6 (2.1) |
| Trauma | 11 (2.0) | 4 (1.6) | 7 (2.5) |
| Sphincter of Oddi dysfunction | 9 (1.7) | 6 (2.3) | 3 (1.1) |
| Hypercalcemia | 3 (0.6) | 1 (0.4) | 2 (0.7) |
| Radiation | 2 (0.4) | 0 (0.0) | 2 (0.7) |
| Intraductal Papillary Neoplasm | 2 (0.4) | 2 (0.8) | 0 (0.0) |
| Post-necrotic | 1 (0.2) | 1 (0.4) | 0 (0.0) |
| Duct obstruction | 1 (0.2) | 0 (0.0) | 1 (0.4) |
| Other autoimmune diseases | 1 (0.2) | 1 (0.4) | 0 (0.0) |
| Other | 27 (5.0) | 18 (7.0) | 9 (3.2) |
Numbers do not add up to the total “n” as patients may have been assigned more than one working diagnosis.
Etiology groups
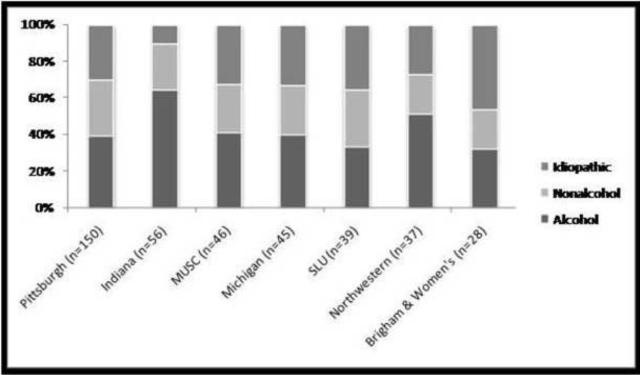
For the purposes of subsequent analysis in this manuscript, the physician's working diagnoses were used to classify patients into etiology groups (Table 2) using the hierarchical algorithm defined a priori as outlined in the methods section. Alcohol was listed as the sole working diagnosis in 34.7% and as a contributing factor in an additional 9.8%. Subjects in whom alcohol was not considered as a diagnosis (55.5%) were equally distributed in the non-alcohol (genetic, autoimmune, obstructive, and other) and idiopathic etiology groups. While more males were assigned to the alcohol etiology group, the proportion of females within the alternate etiology groups was higher compared to males (Table 2). A considerable number of CP patients were assigned to the idiopathic etiology group (28.6%), more often in females compared to males (35% versus 23%, p=0.0001). The distribution of patients in alcohol, non-alcohol and idiopathic groups was similar across the top recruiting medical centers in NAPS2 (Figure 1).
Table 2.
Distribution of etiologic groups for chronic pancreatitis patients (all and stratified by gender) in the North American Pancreatitis Study 2
| Etiology group (%) | All (n=539) | Female (n=256) | Male (n=283) |
|---|---|---|---|
| Alcohol | 240 (44.5) | 72 (28.1) | 168 (59.4) |
| Genetic | 47 (8.7) | 32 (12.5) | 15 (5.3) |
| Autoimmune | 12 (2.2) | 6 (2.3) | 6 (2.1) |
| Obstructive | 47 (8.7) | 33 (12.9) | 14 (4.9) |
| Other | 39 (7.2) | 23 (9.0) | 16 (5.7) |
| Idiopathic | 154 (28.6) | 90 (35.2) | 64 (22.6) |
Patients were assigned to an etiologic group based on the physician's working diagnosis using a hierarchical algorithm (see methods section for details). Patients with genetic, autoimmune, obstructive and other etiologies were combined into the “non-alcohol” etiology group.
Figure 1. Distribution of etiologic groups in chronic pancreatitis patients enrolled at different participating centers in the North American Pancreatitis Study 2.
Data restricted to NAPS2 medical centers that recruited 25 or more patients.
MUSC = Medical University of South Carolina
SLU = Saint Louis University
Patient demographics, prevalence of select risk factors and phenotypic characteristics are compared across alcohol, non-alcohol and idiopathic CP groups in Table 3. Alcohol-related CP patients were less likely to be of white race and have a history of cholecystectomy. Renal disease or failure was less frequently reported by patients in the alcohol compared to the non-alcohol group (p=0.02); all other comparisons for this measure were similar between groups. Among patients where the physician reported alcohol as a working diagnosis, 65% self-reported At-risk drinking based on the TWEAK questionnaire. The prevalence of smoking was significantly higher in the alcohol group compared to the idiopathic and non-alcohol etiology groups. The prevalence of obesity, defined as a maximum BMI of ≥ 30kg/m2 and a history of acute pancreatitis were similar across all groups. Phenotypic characteristics including exocrine and endocrine insufficiency and pancreatic duct abnormalities were comparable across groups. However, calcifications, CP-associated common bile duct strictures and pseudocysts were identified more frequently among patients in the alcohol etiology group. Idiopathic CP patients were similar to non-alcohol CP patients in all characteristics except for: 1) a lower rate of pancreatic duct dilation/stricture (56.5% versus 69.0%, p=0.04) and 2) a higher rate of pancreatic calcifications (53.9% versus 38.6%, p=0.01).
Table 3.
Patient characteristics and prevalence of select risk factors in chronic pancreatitis patients (stratified by etiology groups) in the North American Pancreatitis Study 2
| Variable | Etiology Group (n) | p-value | ||||
|---|---|---|---|---|---|---|
| Alcohol (240) | Non-Alcohol (145) | Idiopathic (154) | Alcohol vs. Non-alcohol | Alcohol vs. Idiopathic | Idiopathic vs. Non-alcohol | |
| Patient characteristics | ||||||
| Mean age (± SD) | 50.9±12.0 | 46.5±17.6 | 50±18.5 | 0.01 | 0.62 | 0.09 |
| Males (%) | 168 (70) | 51 (35.2) | 64 (41.6) | <0.001 | <0.001 | 0.31 |
| White Race (%) | 192 (80) | 127 (88.2) | 138 (89.6) | 0.05 | 0.02 | 0.84 |
| History of acute pancreatitis (%) | 151 (63.2) | 100 (69.4) | 94 (61.4) | 0.26 | 0.81 | 0.19 |
| Prior cholecystectomy (%) | 88 (36.7) | 80 (55.2) | 70 (45.5) | 0.001 | 0.10 | 0.12 |
| Prevalence of select risk factors | ||||||
| At-risk drinking (%) | 155 (64.6) | 8 (5.5) | 8 (5.2) | <0.001 | <0.001 | 0.89 |
| Ever smoker (%) | 222 (92.9) | 71 (49.7) | 89 (58.6) | <0.001 | <0.001 | 0.16 |
| Maximum BMI (%) | 0.21 | 0.10 | 0.70 | |||
| Normal/Low | 69 (33) | 36 (27.3) | 26 (21.7) | |||
| Overweight | 69 (33) | 44 (33.3) | 49 (40.8) | |||
| Obese | 71 (34) | 52 (39.4) | 45 (37.5) | |||
| Current BMI >30 (%) | <0.001 | 0.005 | 0.18 | |||
| Normal/Low | 166 (69.7) | 65 (46.1) | 80 (54.1) | |||
| Overweight | 50 (21) | 52 (36.9) | 48 (32.4) | |||
| Obese | 22 (9.2) | 24 (17) | 20 (13.5) | |||
| Renal disease or failure (%) | 4 (1.7) | 10 (6.9) | 8 (5.2) | 0.02 | 0.09 | 0.71 |
| Phenotypic characteristics | ||||||
| Exocrine insufficiency (%) | 70 (30.8) | 30 (22.2) | 40 (28.6) | 0.10 | 0.73 | 0.29 |
| Endocrine insufficiency (%) | 68 (29.2) | 32 (22.7) | 38 (26.4) | 0.21 | 0.64 | 0.56 |
| Pancreatic duct dilation/stricture (%) | 145 (60.4) | 100 (69.0) | 87 (56.5) | 0.11 | 0.51 | 0.04 |
| Calcifications (%) | 159 (66.2) | 56 (38.6) | 83 (53.9) | <0.001 | 0.02 | 0.01 |
| Pseudocyst(s) (%) | 92 (38.3) | 28 (19.3) | 20 (13.0) | <0.001 | <0.001 | 0.18 |
| Common bile duct dilation/stricture (%) | 52 (21.7) | 17 (11.7) | 13 (8.4) | 0.02 | 0.001 | 0.45 |
At-risk drinking = Individuals who met criteria for at-risk drinking using the TWEAK questionnaire in the months before getting pancreatitis. The presence of renal disease or failure was extracted from the patient questionnaire.
Maximum BMI = Calculated based on the patient's self-reported highest weight (ever).
Current BMI = Calculated based on the patient's self-reported weight at the time of enrollment into NAPS2
BMI (body-mass index) (kg/m2) categories: normal/low: <25; overweight: 25–30; obese: ≥30
Comparison of idiopathic CP with NAPS2 controls
Characteristics of idiopathic CP patients were compared against NAPS2 controls (Table 4). On univariate analysis, At-risk drinking was more prevalent in the control population (12.2%) compared to idiopathic CP patients (5.2%, p < 0.02). The frequency of tobacco exposure and renal disease were higher among idiopathic CP patients (p < 0.05 and < 0.01 respectively), while no differences were seen in age, race and sex distribution between the two groups. After controlling for age, sex, BMI and alcohol intake (drinking categories), ever-smoking (OR 1.65, 95%CI 1.08–2.52), current smoking (1.8, 1.10–3.05) and smoking ≥ 1 pack per day (1.87, 1.10–3.12) were independently associated with idiopathic CP.
Table 4.
Comparison of demographic and select risk factors between idiopathic chronic pancreatitis and control subjects in the North American Pancreatitis Study 2
| Variable | Idiopathic chronic pancreatitis | NAPS 2 controls | Univariate p- value | ||
|---|---|---|---|---|---|
| n* | n* | ||||
| Mean age (sd) | 50.0±18.5 | 154 | 52.2 ±14.5 | 694 | 0.17 |
| Male sex (%) | 64 (41.6) | 154 | 249 (35.8) | 695 | 0.21 |
| White race (%) | 138 (89.6) | 154 | 606 (87.4) | 693 | 0.54 |
| Maximum BMI (kg/m2) (%) | 120 | 643 | 0.18 | ||
| Normal/Low | 26 (21.7) | 128 (19.9) | |||
| Overweight | 49 (40.8) | 220 (34.2) | |||
| Obese | 45 (37.5) | 295 (45.9) | |||
| Current BMI (kg/m2) (%) | 148 | 682 | <0.001 | ||
| Normal/Low | 80 (54.1) | 233 (34.2) | |||
| Overweight | 48 (32.4) | 253 (37.1) | |||
| Obese | 20 (13.5) | 196 (28.7) | |||
| At-risk drinking (%) | 8 (5.2) | 154 | 85 (12.2) | 695 | < 0.02 |
| Ever smoker (%) | 89 (58.6) | 152 | 342 (49.7) | 688 | < 0.05 |
| Current smoker (%) | 45 (29.6) | 152 | 140 (20.3) | 688 | 0.01 |
| Renal disease or failure (%) | 8 (5.2) | 154 | 8 (1.2) | 695 | < 0.01 |
Specific data points were missing for some patients.
At-risk drinking - Individuals who met criteria for at-risk drinking using the TWEAK questionnaire. A reference period (in the months before getting pancreatitis) was used for pancreatitis subjects but not for control subjects.
The presence of renal disease or failure was extracted from the patient questionnaire.
Maximum BMI = Calculated based on the patient's self-reported highest weight (ever).
Current BMI = Calculated based on the patient's weight at the time of enrollment into NAPS2
BMI (body-mass index) (kg/m2) categories: normal/low: <25; overweight: 25–30; obese: ≥30
Discussion
In this largest epidemiologic study on CP from the United States, we observe that the current etiologic profile of CP patients evaluated at U.S. referral centers is quite different from historical data. Although alcohol continues to be the most common etiology, the proportion of patients in whom physicians identified this to be the sole or contributing cause of CP was much lower than the traditional belief. A larger fraction of patients were considered to have non-alcoholic etiologies, and more than a quarter of all CP patients had no identifiable cause for their disease (idiopathic CP). Among the risk factors assessed, smoking was independently associated with idiopathic CP.
Lower prevalence of alcohol etiology in CP in NAPS2
Historically, the overwhelming majority of patients with CP have been attributed to heavy alcohol use3–11. In a previous epidemiological analysis from the United States consisting of CP patients evaluated at the Mayo Clinic from 1976–1982, the proportion with any and heavy alcohol consumption (defined as >50 gms/day) was 84% and 58% respectively.9, 38 Recent studies from Europe and Asia suggest that over 50% of CP cases may be secondary to non-alcohol etiologies.14, 39–41 Frulloni et al14 noted a shift in the etiologic profile of CP in Italy from being predominantly alcohol-related (74%) between 1971–9511 to a wider spectrum of etiologies among patients evaluated from 2000–2006 with only 43% cases attributed to alcohol (34% as sole, 9% as contributing cause). Data from the NAPS2 study corroborates this, where only 45% of CP patients evaluated at U.S. referral centers were believed to be alcohol-related.
There are several potential explanations for the lower prevalence of alcoholic etiology in our cohort of CP patients. Firstly, patients were enrolled from secondary and tertiary care centers which may be subject to a referral bias. Patients typically seek referral to expert centers if the cause of their disease is unclear or when treatments are not available at community centers. Patients who are actively drinking may be less likely to seek a referral and physicians who believe alcohol consumption can explain their patient's disease may not seek additional consultation for diagnosis. Therefore, it is possible that the prevalence of alcoholic etiology among CP patients is higher at the community level. However, this is difficult to corroborate due to a lack of population-level data on the epidemiology of CP in the United States. Data are available on the epidemiologic trends for alcohol consumption in the United States in the past several decades. The results are mixed and do not explain the impressive shift in the etiologic profile.42, 43
Secondly, discoveries over the past 15 years have improved our ability to detect genetic factors that can potentially explain the cause of CP. For example, mutations in PRSS122, CFTR31, 32, SPINK129, 44 and Chymotypsin C25, 26 genes can be detected in a significant subset of patients previously considered to have idiopathic disease. Therefore, patients in whom the etiology of CP is unclear or those who do not drink heavily may be selectively referred to tertiary centers for evaluation in search of potential explanations for their disease.
Thirdly, improvement in the imaging technology over the past 2–3 decades may have led to uncovering of anatomical abnormalities as the potential explanation of patient's disease (e.g., a stricture or pancreas divisum). Improved imaging techniques may also lead to detection of morphological changes at an earlier stage. In fact, obstructive causes were considered as individual working diagnosis in over 10% of all CP patients in our study.
Higher prevalence of non-alcohol etiologies in CP in NAPS2
One of the more remarkable findings from the NAPS2 study is that in over 50% patients physicians did not consider alcohol as the etiologic factor. Moreover, a quarter of patients were believed to have no identifiable etiology. A diagnosis of “idiopathic” CP may reflect the extent of the diagnostic evaluation which may include genetic testing, newer imaging modalities such as MRCP and/or EUS, and pancreatic function testing. The assignment of diagnosis by the NAPS2 enrolling physician was based on a review of the patient's evaluation to the time of enrollment. Being a cross-sectional study, NAPS2 data reflect patient status and diagnosis until enrollment, which could have changed during the follow up period or after further diagnostic testing. For example, if genetic testing was performed after NAPS2 enrollment, the impact of the results on etiological classification is unknown. It is likely that further genetic testing in patients with idiopathic CP patients may identify novel or unrecognized genetic mutations. Interestingly, subsequent change in etiology in patients with CP has not been addressed in previous studies. NAPS2 is currently collecting follow up data which could be analyzed for diagnostic trends throughout the natural history of the disease.
Greater knowledge of the clinical characteristics of idiopathic CP patients may highlight previously unrecognized risk factors for CP. Demographic and phenotypic characteristics of patients with idiopathic CP were fairly similar to other non-alcohol etiologies but different from patients believed to have alcohol-related disease. Similar to previous observations9, 45, when compared to alcohol-related CP, patients with idiopathic CP were more likely to be female, have a higher current BMI but less likely to be smokers or have morphological features of calcifications, pseudocysts and common bile duct strictures. However, there were no features that consistently distinguished idiopathic CP patients from both alcohol and non-alcohol etiologies.
We further explored the idiopathic CP population by comparing them to NAPS2 control patients. Among the assessed risk factors, perhaps the most intriguing was the association between smoking and idiopathic CP. In conjunction with prior analyses from NAPS2 and other studies13, 16–21, this association and stresses the need to incorporate smoking cessation into the treatment algorithm for patients with CP. On univariate analyses, an association was also observed for the presence of renal disease or failure in patients with idiopathic CP; however the overall prevalence of patients with self-reported renal disease was low (n=16), limiting our analysis of this variable.
These results provide important information on proximal etiologies that are linked by unknown mechanisms to various pancreatic pathologies, including inflammation, calcifications, fibrosis, exocrine insufficiency, diabetes mellitus, recurrent or chronic pain and risk for pancreatic cancer that are part of the CP syndrome. We previously demonstrated the importance of etiological classification of patients in understanding the risk of SPINK1 mutations, which clarified the role of trypsin-activation in relation to tropical CP, idiopathic CP and alcoholic CP.46 The present study is also important because it suggest that smoking is a risk factor for CP regardless of etiological classification. Finally, these results are valuable for future studies so that alcohol-dependent, trypsin-dependent, smoking-dependent and other gene-dependent pathways can be identified in homogenous groups. Thus, it is anticipated that these findings will enhance our understanding of the genetics of CP, which may help in targeting preventative and therapeutic interventions.
Generalizability of results
The generalizability of NAPS2 data to the spectrum of disease seen at community level has been discussed previously. On the other hand, the data appear to be reproducible across academic centers since the distribution of etiologies and morphological features were similar across the top-recruiting sites. Since we focused on the working diagnosis based on the physician's impression at the time of enrollment, our results are susceptible to the extent of diagnostic evaluation until the time of enrollment and to individual physician biases; for example, physician interpretation of “alcohol-induced” CP may be variable. Finally, there is limited data on CP among minority populations in NAPS2, an area of need for future research.
Conclusions
The epidemiologic profile of CP currently seen at U.S. referral centers is distinctly different from historical data. Although alcohol remains the most common etiology, a larger subset of patients is considered to have non-alcoholic etiologies, and in over a quarter of patients, no identifiable cause of disease is apparent. Smoking is an independent risk factor for idiopathic CP. Future genetic analyses from NAPS2 and other CP cohorts will likely identify previously unrecognized genetic factors and/or interaction between genes and environmental factors as potential explanations of disease development. In the meantime, the era of dismissing all cases of CP as alcohol-induced has undoubtedly come to a close.
Acknowledgements
The following physicians and centers also contributed patients to the NAPS2 study: Joseph Romagnuolo, MD, Peter B. Cotton, MD, Digestive Disease Center, Medical University of South Carolina, Charleston, SC, Simon K. Lo MD, Department of Medicine, Cedars-Sinai Medical Center, University of California, Los Angeles; Mark T. DeMeo MD, Department of Medicine, Rush University Medical Center, Chicago, IL; William M. Steinberg MD, Washington Hospital Center, Washington DC; Michael L. Kochman MD, Department of Medicine, University of Pennsylvania, Philadelphia, PA; Babak Etemad MD, Department of Gastroenterology and Hepatology, Ochsner Medical Center, New Orleans, LA; Christopher E. Forsmark MD, Department of Medicine, University of Florida, Gainesville, FL.
The authors thank Elizabeth D. Kennard, PhD, Epidemiology Data Center, University of Pittsburgh, PA for reviewing the manuscript, and assisting with statistical analyses.
Funding Source: National Institutes of Health –(NIDDK) - DK061451 (DCW), the National Pancreas Foundation (DCW), Robert and Vicki Hall, and Andrew and Michelle Aloe.
Abbreviations
- (CP)
chronic pancreatitis
- (NAPS2)
North American Pancreatitis Study 2
- (RAP)
recurrent acute pancreatitis
- (ERCP)
endoscopic retrograde cholangiopancreatography
- (MRCP)
magnetic resonance cholangiopancreatography
- (EUS)
endoscopic ultrasound
- (CT)
computed tomography
- (NA)
nonalcohol
- (iCP)
idiopathic chronic pancreatitis
- (BMI)
body-mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration: These data were presented at the annual meeting of the American Gastroenterological Association, May, 2010, New Orleans, LA.
Disclosures: Potential conflict of interest relevant to this manuscript: None
Authorship Criteria: All authors had access to the data, had a role in writing the manuscript and meet the authorship criteria.
References
- 1.Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557–73. doi: 10.1053/j.gastro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nature reviews. 7:131–45. doi: 10.1038/nrgastro.2010.6. [DOI] [PubMed] [Google Scholar]
- 3.Gastard J, Joubaud F, Farbos T, et al. Etiology and course of primary chronic pancreatitis in Western France. Digestion. 1973;9:416–28. doi: 10.1159/000197470. [DOI] [PubMed] [Google Scholar]
- 4.Marks IN, Bank S, Louw JH. Chronic pancreatitis in the Western Cape. Digestion. 1973;9:447–53. doi: 10.1159/000197473. [DOI] [PubMed] [Google Scholar]
- 5.Durbec JP, Sarles H. Multicenter survey of the etiology of pancreatic diseases. Relationship between the relative risk of developing chronic pancreaitis and alcohol, protein and lipid consumption. Digestion. 1978;18:337–50. doi: 10.1159/000198221. [DOI] [PubMed] [Google Scholar]
- 6.Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820–8. [PubMed] [Google Scholar]
- 7.Dani R, Mott CB, Guarita DR, Nogueira CE. Epidemiology and etiology of chronic pancreatitis in Brazil: a tale of two cities. Pancreas. 1990;5:474–8. doi: 10.1097/00006676-199007000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Robles-Diaz G, Vargas F, Uscanga L, Fernandez-del Castillo C. Chronic pancreatitis in Mexico City. Pancreas. 1990;5:479–83. doi: 10.1097/00006676-199007000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481–7. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 10.Lankisch PG, Assmus C, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic diseases in Luneburg County. A study in a defined german population. Pancreatology. 2002;2:469–77. doi: 10.1159/000064713. [DOI] [PubMed] [Google Scholar]
- 11.Cavallini G, Frulloni L, Pederzoli P, et al. Long-term follow-up of patients with chronic pancreatitis in Italy. Scandinavian journal of gastroenterology. 1998;33:880–9. doi: 10.1080/00365529850171567. [DOI] [PubMed] [Google Scholar]
- 12.Kristiansen L, Gronbaek M, Becker U, Tolstrup JS. Risk of pancreatitis according to alcohol drinking habits: a population-based cohort study. American journal of epidemiology. 2008;168:932–7. doi: 10.1093/aje/kwn222. [DOI] [PubMed] [Google Scholar]
- 13.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169:1035–45. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frulloni L, Gabbrielli A, Pezzilli R, et al. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis. 2009;41:311–7. doi: 10.1016/j.dld.2008.07.316. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Tamakoshi A, Hayakawa T, Ogawa M, Ohno Y. Cigarette smoking as a risk factor for chronic pancreatitis: a case-control study in Japan. Research Committee on Intractable Pancreatic Diseases. Pancreas. 2000;21:109–14. doi: 10.1097/00006676-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Maisonneuve P, Frulloni L, Mullhaupt B, et al. Impact of smoking on patients with idiopathic chronic pancreatitis. Pancreas. 2006;33:163–8. doi: 10.1097/01.mpa.0000227916.94073.fc. [DOI] [PubMed] [Google Scholar]
- 17.Morton C, Klatsky AL, Udaltsova N. Smoking, coffee, and pancreatitis. The American Journal of Gastroenterology. 2004;99:731–8. doi: 10.1111/j.1572-0241.2004.04143.x. [DOI] [PubMed] [Google Scholar]
- 18.Talamini G, Bassi C, Falconi M, et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Digestive diseases and sciences. 1999;44:1303–11. doi: 10.1023/a:1026670911955. [DOI] [PubMed] [Google Scholar]
- 19.Tolstrup JS, Kristiansen L, Becker U, Gronbaek M. Smoking and risk of acute and chronic pancreatitis among women and men: a population-based cohort study. Arch Intern Med. 2009;169:603–9. doi: 10.1001/archinternmed.2008.601. [DOI] [PubMed] [Google Scholar]
- 20.Maisonneuve P, Lowenfels AB, Mullhaupt B, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut. 2005;54:510–4. doi: 10.1136/gut.2004.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talamini G, Bassi C, Falconi M, et al. Smoking cessation at the clinical onset of chronic pancreatitis and risk of pancreatic calcifications. Pancreas. 2007;35:320–6. doi: 10.1097/mpa.0b013e31812e965e. [DOI] [PubMed] [Google Scholar]
- 22.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nature genetics. 1996;14:141–5. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 23.Santhosh S, Witt H, te Morsche RH, et al. A loss of function polymorphism (G191R) of anionic trypsinogen (PRSS2) confers protection against chronic pancreatitis. Pancreas. 2008;36:317–20. doi: 10.1097/MPA.0b013e31815db4b3. [DOI] [PubMed] [Google Scholar]
- 24.Witt H, Sahin-Toth M, Landt O, et al. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nature genetics. 2006;38:668–73. doi: 10.1038/ng1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masson E, Chen JM, Scotet V, Le Marechal C, Ferec C. Association of rare chymotrypsinogen C (CTRC) gene variations in patients with idiopathic chronic pancreatitis. Human genetics. 2008;123:83–91. doi: 10.1007/s00439-007-0459-3. [DOI] [PubMed] [Google Scholar]
- 26.Rosendahl J, Witt H, Szmola R, et al. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nature genetics. 2008;40:78–82. doi: 10.1038/ng.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muddana V, Lamb J, Greer JB, et al. Association between calcium sensing receptor gene polymorphisms and chronic pancreatitis in a US population: role of serine protease inhibitor Kazal 1type and alcohol. World J Gastroenterol. 2008;14:4486–91. doi: 10.3748/wjg.14.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murugaian EE, Premkumar RM, Radhakrishnan L, Vallath B. Novel mutations in the calcium sensing receptor gene in tropical chronic pancreatitis in India. Scand J Gastroenterol. 2008;43:117–21. doi: 10.1080/00365520701580413. [DOI] [PubMed] [Google Scholar]
- 29.Pfutzer RH, Barmada MM, Brunskill AP, et al. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–23. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 30.Witt H, Luck W, Becker M, et al. Mutation in the SPINK1 trypsin inhibitor gene, alcohol use, and chronic pancreatitis. JAMA. 2001;285:2716–7. doi: 10.1001/jama.285.21.2716-a. [DOI] [PubMed] [Google Scholar]
- 31.Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. The New England journal of medicine. 1998;339:653–8. doi: 10.1056/NEJM199809033391002. [DOI] [PubMed] [Google Scholar]
- 32.Sharer N, Schwarz M, Malone G, et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. The New England journal of medicine. 1998;339:645–52. doi: 10.1056/NEJM199809033391001. [DOI] [PubMed] [Google Scholar]
- 33.Whitcomb DC. Genetic aspects of pancreatitis. Annu Rev Med. 2010;61:413–24. doi: 10.1146/annurev.med.041608.121416. [DOI] [PubMed] [Google Scholar]
- 34.Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am J Gastroenterol. 2004;99:2256–70. doi: 10.1111/j.1572-0241.2004.40694.x. [DOI] [PubMed] [Google Scholar]
- 35.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2) Pancreatology. 2008;8:520–31. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756–9. doi: 10.1136/gut.25.7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan AW, Pristach EA, Welte JW, Russell M. Use of the TWEAK test in screening for alcoholism/heavy drinking in three populations. Alcohol Clin Exp Res. 1993;17:1188–92. doi: 10.1111/j.1530-0277.1993.tb05226.x. [DOI] [PubMed] [Google Scholar]
- 38.Imoto M, DiMagno EP. Cigarette smoking increases the risk of pancreatic calcification in late-onset but not early-onset idiopathic chronic pancreatitis. Pancreas. 2000;21:115–9. doi: 10.1097/00006676-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Balakrishnan V, Unnikrishnan AG, Thomas V, et al. Chronic pancreatitis. A prospective nationwide study of 1,086 subjects from India. JOP. 2008;9:593–600. [PubMed] [Google Scholar]
- 40.Garg PK, Tandon RK. Survey on chronic pancreatitis in the Asia-Pacific region. J Gastroenterol Hepatol. 2004;19:998–1004. doi: 10.1111/j.1440-1746.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- 41.Ryu JK, Lee JK, Kim YT, et al. Clinical features of chronic pancreatitis in Korea: a multicenter nationwide study. Digestion. 2005;72:207–11. doi: 10.1159/000089414. [DOI] [PubMed] [Google Scholar]
- 42. [Accessed June 14, 2010];National Institute on Alcohol Abuse and Alcoholism: Apparent per capita ethanol consumption for the United States, 1850–2007. http://www.niaaa.nih.gov/Resources/DatabaseResources/QuickFacts/AlcoholSales/consum01.htm.
- 43. [Accessed June 14, 2010];National Institute on Alcohol Abuse and Alcoholism: Percent reporting heavy alcohol use[6] in the past month by age group and demographic characteristics: NSDUH (NHSDA), 1994–2002. http://www.niaaa.nih.gov/Resources/DatabaseResources/QuickFacts/AlcoholConsumption/dkpat21.htm.
- 44.Witt H, Hennies HC, Becker M. SPINK1 mutations in chronic pancreatitis. Gastroenterology. 2001;120:1060–1. doi: 10.1053/gast.2001.23086. [DOI] [PubMed] [Google Scholar]
- 45.Ammann RW, Buehler H, Muench R, Freiburghaus AW, Siegenthaler W. Differences in the natural history of idiopathic (nonalcoholic) and alcoholic chronic pancreatitis. A comparative long-term study of 287 patients. Pancreas. 1987;2:368–77. doi: 10.1097/00006676-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Aoun E, Chang CC, Greer JB, Papachristou GI, Barmada MM, Whitcomb DC. Pathways to injury in chronic pancreatitis: decoding the role of the high-risk SPINK1 N34S haplotype using meta-analysis. PLoS One. 2008;3:e2003. doi: 10.1371/journal.pone.0002003. [DOI] [PMC free article] [PubMed] [Google Scholar]